Thermal Decrepitation and Thermally-Induced Cracking of Limestone Used in Quicklime Production
Abstract
:1. Introduction
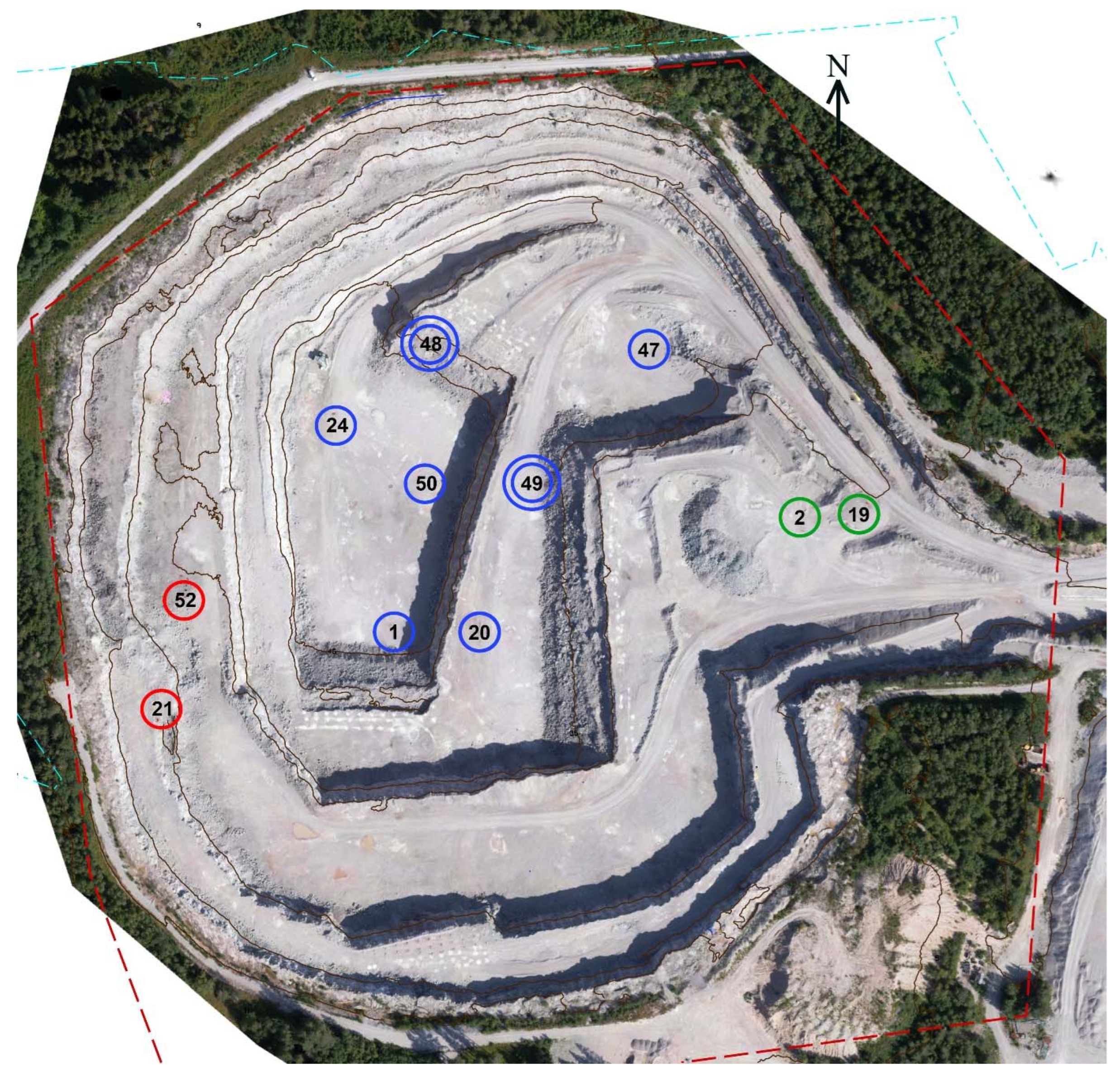
2. Geological Settings
3. Materials
4. Methods
4.1. Chemical Analysis
4.2. Thermal Decrepitation
4.3. Multivariate Analysis
4.4. Scanning Electron Microscopy
4.5. Image Analysis
5. Results and Discussion
5.1. Chemical Composition
5.2. Thermal Decrepitation
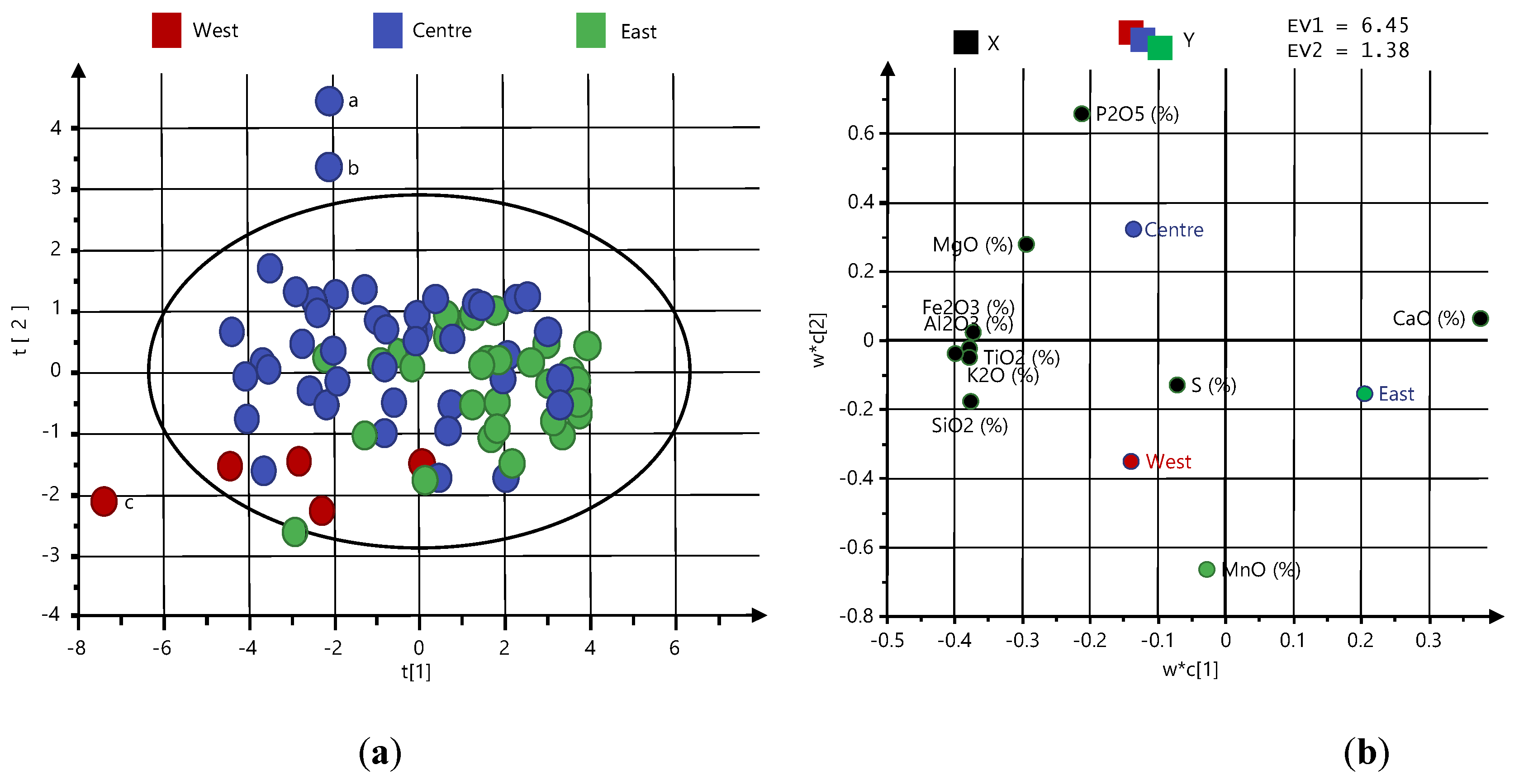
5.3. Multivariate Analysis
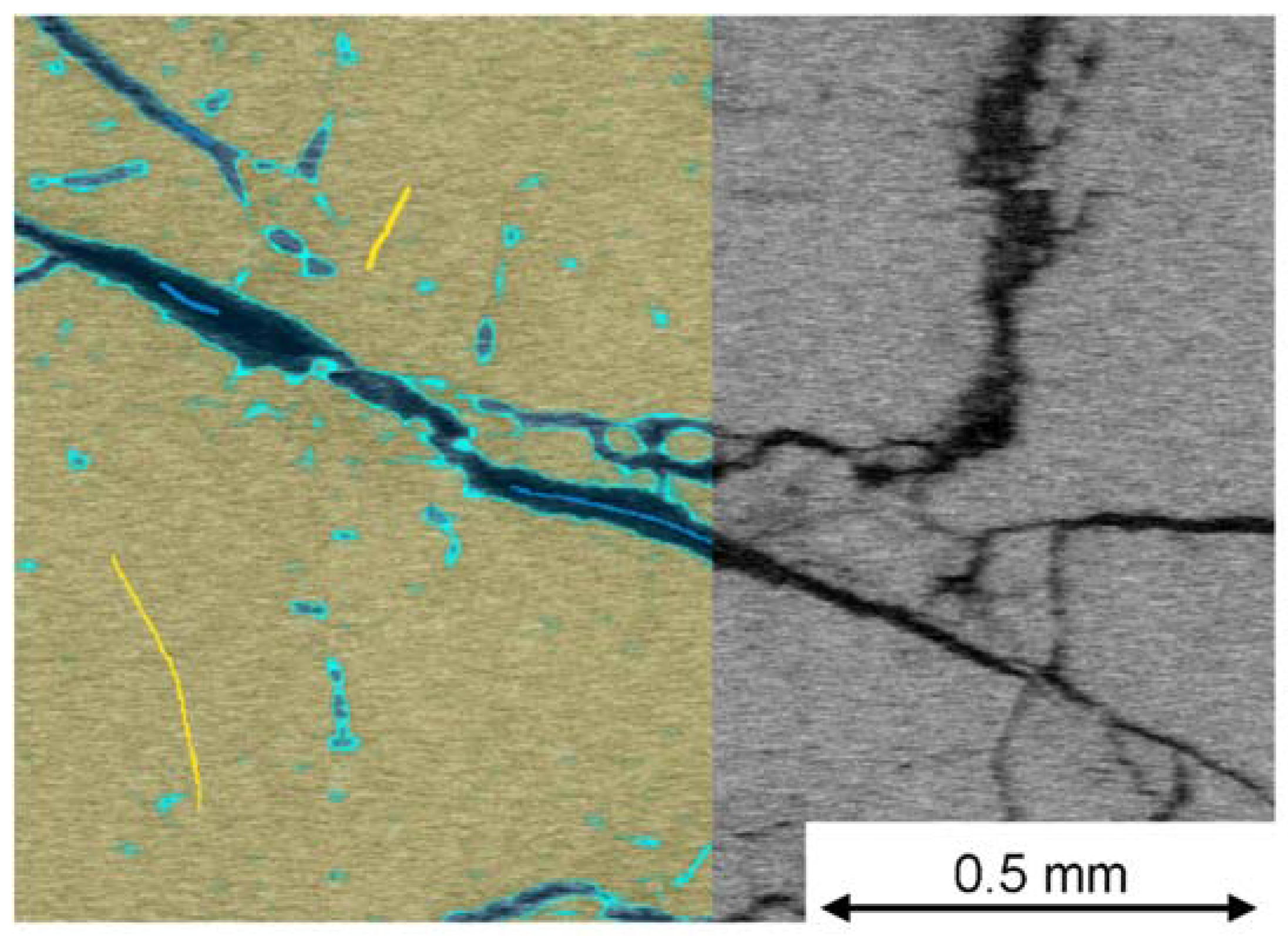
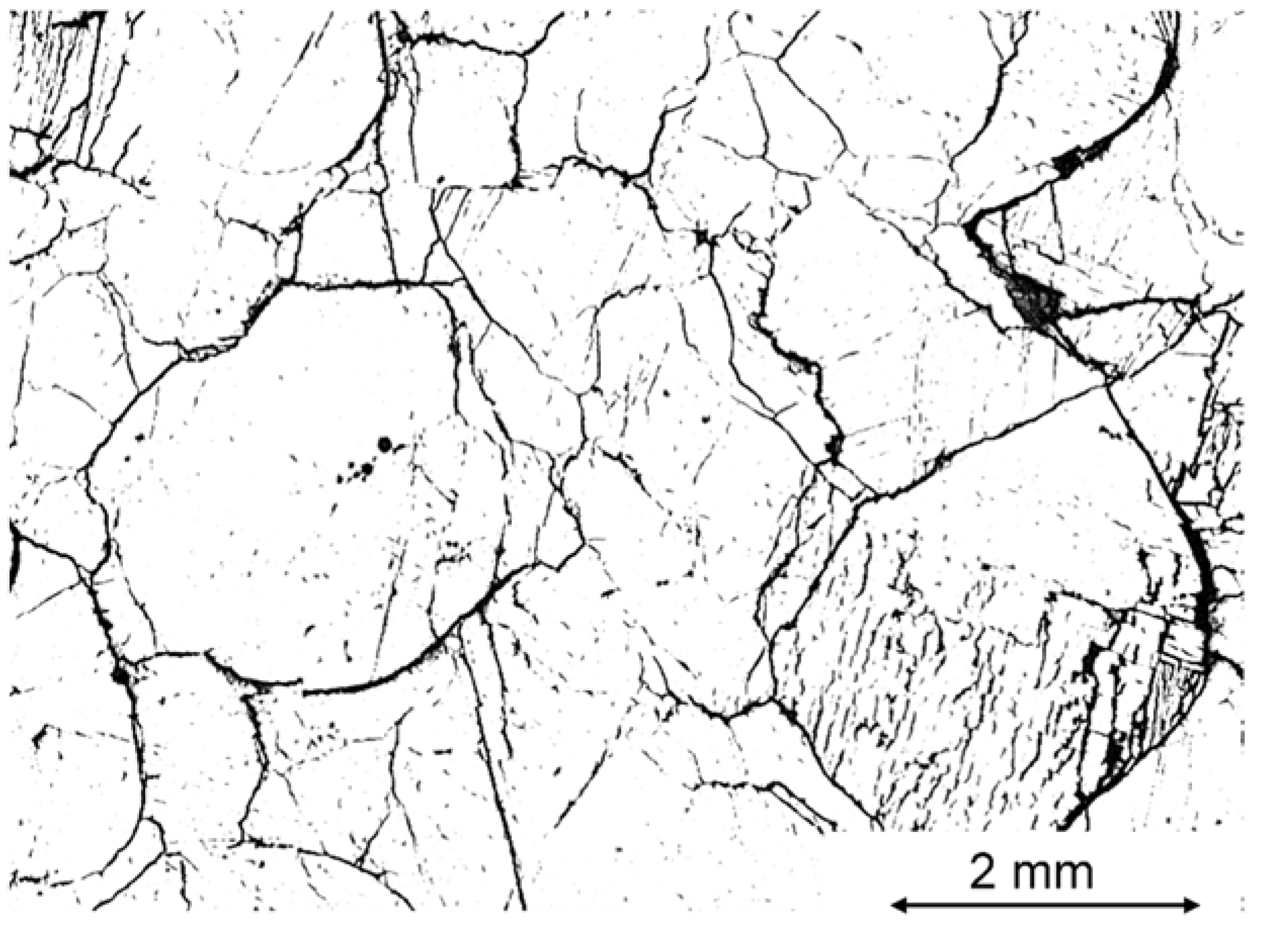
5.4. SEM Image Analysis
6. Conclusions
- Overall, the analyzed limestone had low TD, 78% of the samples had a TD value below 5%, and 18% of the samples had a medium TD of 5–12%. This means that the limestone studied, from a TD perspective, is suitable for shaft kiln operation. One sample exhibited an extreme TD value of 75%, which suggests that quarry quality control and mixing would be required to ensure that no batch fed to a shaft kiln would contain solely this material;
- Very weak correlations were found between TD and the chemical composition (CaO, MgO, Fe2O3, SiO2, Al2O3, MnO, P2O5, TiO2, K2O, and S) of the limestone, and based on that, the TD values were concluded to be connected to the physical properties of the limestone and/or quicklime rather than to the chemical composition;
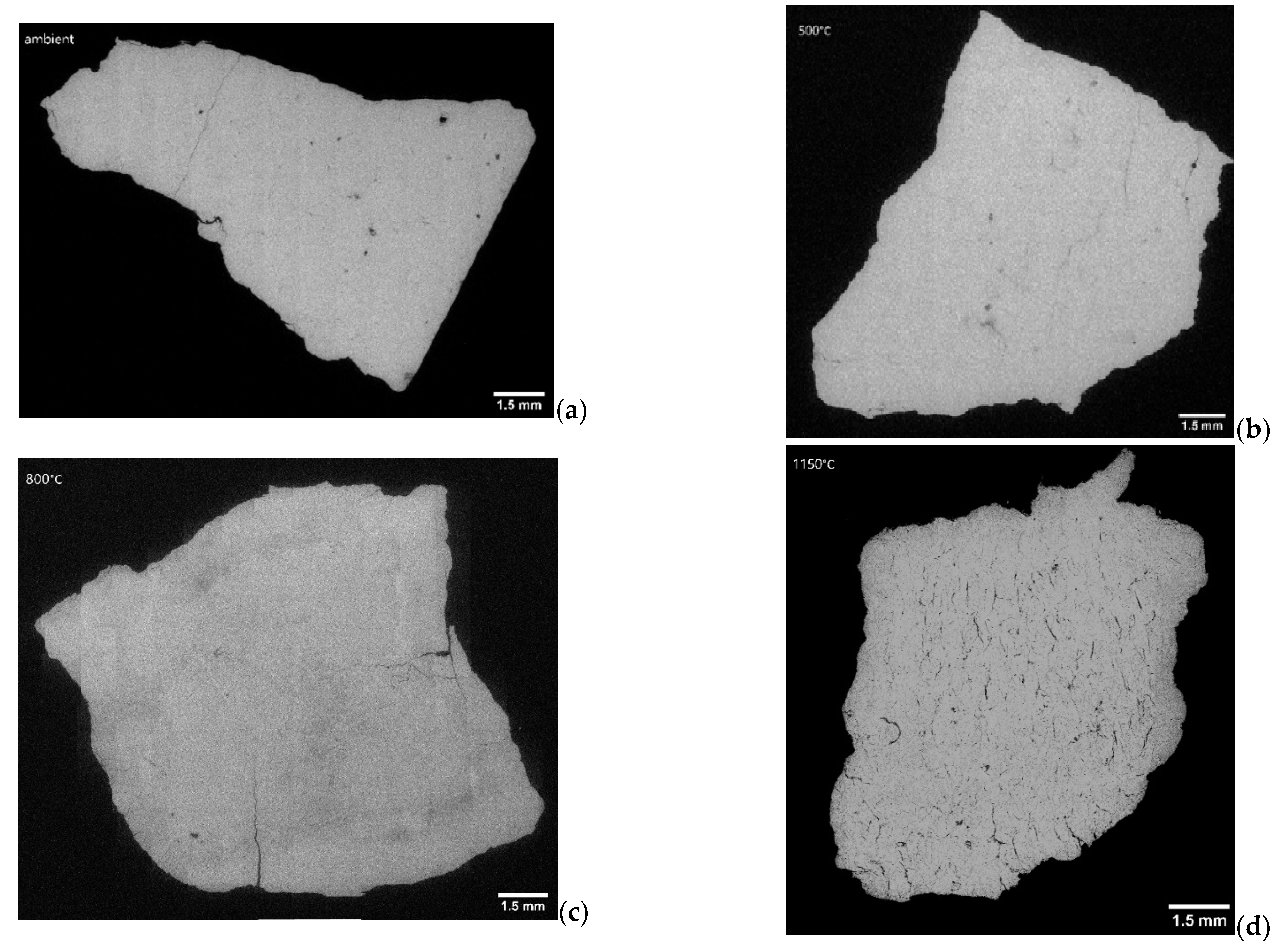
- In a further investigation, the sample with an extreme TD value of 75% was compared to a sample with a similar chemical composition but with a low TD value of 4%. The sample with 75% TD showed significant cracking already at 500 °C, while this was not seen for the corresponding sample with the low TD value. For the low TD sample, after calcination, the pre-existing fractures seemed to grow in length and a large network of small fractures appeared, increasing the total fractured area. This pattern did not occur in the 75% TD sample, in which the already-formed cracks remained few and wide even after 60 min at 1150 °C and full calcination. The network of narrow cracks might favorably influence the internal stresses of the sample, providing an explanation for the low TD overlooked in the previous literature.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manocha, S.; Ponchon, F. Management of lime in steel. Metals 2018, 8, 686. [Google Scholar] [CrossRef]
- Boynton, R.S. Chemistry and Technology of Lime and Limestone, 2nd ed.; Wiley Interscience: New York, NY, USA, 1980. [Google Scholar]
- Vola, G.; Ardit, M.; Sarandrea, L.; Brignoli, G.; Natali, C.; Cavallo, A.; Bianchini, G.; Cruciani, G. Investigation and prediction of sticking tendency, blocks formation and occasional melting of lime at HT (1300 °C) by the overburning test method. Constr. Build. Mater. 2021, 294, 123577. [Google Scholar] [CrossRef]
- Schorcht, F.; Kuorti, I.; Scalet, B.M.; Roudier, S.; Delgado Sancho, L. Best Available Techniques (BAT) Reference Document for the Production of Cement, Lime and Magnesium Oxide; Joint Reasearch Centre of the European Comission: Seville, Spain, 2013. [Google Scholar]
- Cimprogetti. Mechanical Degradation and Drop Test; Cimprogetti Srl: Dalmine, Italy, 2020. [Google Scholar]
- Maerz. Decrepitation Test; Maerz Ofenbau AG: Zurich, Switzerland, 2020. [Google Scholar]
- Allared, J. Determination of Thermal Decrepitation and Abrasion Test; SMA Mineral: Filipstad, Sweden, 2019. [Google Scholar]
- Harrison, D.J. Industrial Minerals Laboratory Manual: Limestone; British Geological Survey: Nottingham, UK, 1993.
- Nordkalk. Thermal Decrepitation; Nordkalk: Pargas, Finland, 2018. [Google Scholar]
- Sandström, O. Energy Effective Production of Lime Products—Comparing Industrial and Scientific Laboratory Methods for Prediction; Graptolit Geoproject AB: Malmö, Sweden, 2011. [Google Scholar]
- Dollimore, D.; Dunn, J.G.; Lee, Y.F.; Penrod, B.M. The Decrepitation of Dolomite and Limestone. Thermochim. Acta 1994, 237, 125–131. [Google Scholar] [CrossRef]
- McCauley, R.A.; Johnson, L.A. Decrepitation and thermal decomposition of dolomite. Thermochim. Acta 1991, 185, 271–282. [Google Scholar] [CrossRef]
- Kranz, R.L. Microcracks in rocks: A review. Tectonophysics 1983, 100, 449–480. [Google Scholar] [CrossRef]
- Simmons, G.; Richter, D. The Physics and Chemistry of Minerals and Rocks; John Wiley & Sons Inc: New York, NY, USA, 1976; pp. 105–137. [Google Scholar]
- Meng, Q.B.; Wang, C.K.; Liu, J.F.; Zhang, M.W.; Lu, M.M.; Wu, Y. Physical and micro-structural characteristics of limestone after high temperature exposure. Bull. Eng. Geol. Environ. 2020, 79, 1259–1274. [Google Scholar] [CrossRef]
- Chen, L.J.; He, J.; Chao, J.Q.; Qin, B.D. Swelling and breaking characteristics of limestone under high temperatures. Min. Sci. Technol. 2009, 19, 503–507. [Google Scholar] [CrossRef]
- González-Gómez, W.S.; Quintana, P.; May-Pat, A.; Avilés, F.; May-Crespo, J.; Alvarado-Gil, J.J. Thermal effects on the physical properties of limestones from the Yucatan Peninsula. Int. J. Rock Mech. Min. Sci. 2015, 75, 182–189. [Google Scholar] [CrossRef]
- Yavuz, H.; Demirdag, S.; Caran, S. Thermal effect on the physical properties of carbonate rocks. Int. J. Rock Mech. Min. Sci. 2010, 47, 94–103. [Google Scholar] [CrossRef]
- Yang, J.; Fu, L.Y.; Zhang, W.; Wang, Z. Mechanical property and thermal damage factor of limestone at high temperature. Int. J. Rock Mech. Min. Sci. 2019, 117, 11–19. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, Q.; Hao, S.; Wang, B. Experimental Study on the Thermal Damage Characteristics of Limestone and Underlying Mechanism. Rock Mech. Rock Eng. 2016, 49, 2999–3008. [Google Scholar] [CrossRef]
- Oates, J.A.H. Lime and Limestone. Chemistry and Technology, Production and Uses; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Rao, K.V.K.; Naidu, S.V.N.; Murthy, K.S. Precision lattice parameters and thermal expansion of calcite. J. Phys. Chem. Solids 1968, 29, 245–248. [Google Scholar] [CrossRef]
- Johansson, L. Energieffektiv Främstallning av Brända Barbonatprodukter—Orsaker Till Sönderfall; Lund University: Lund, Sweden, 2011. [Google Scholar]
- Kalasová, D.; Dvořák, K.; Slobodník, M.; Všianský, D.; Zikmund, T.; Dluhoš, J.; Váňa, R.; Bureš, J.; Kaiser, J. Characterization of inner structure of limestone by X-ray computed sub-micron tomography. Constr. Build. Mater. 2018, 174, 693–700. [Google Scholar] [CrossRef]
- Olsson, H. Prediction of the Degree of Thermal Breakdown of limestone: A Case Study of the Upper Ordovician Boda Limestone, Siljan District, Central Sweden; Lund University: Lund, Sweden, 2012. [Google Scholar]
- Jennerheim, J. Evaluation of methods to characterise the geochemistry of limestone and its fracturing in connection to heating. Master; Lund University: Lund, Sweden, 2016. [Google Scholar]
- Luque, A.; Ruiz-Agudo, E.; Cultrone, G.; Sebastián, E.; Siegesmund, S. Direct observation of microcrack development in marble caused by thermal weathering. Environ. Earth Sci. 2011, 62, 1375–1386. [Google Scholar] [CrossRef]
- Shushakova, V.; Fuller, E.R.; Siegesmund, S. Microcracking in calcite and dolomite marble: Microstructural influences and effects on properties. Environ. Earth Sci. 2013, 69, 1263–1279. [Google Scholar] [CrossRef]
- Shushakova, V.; Fuller, E.R.; Heidelbach, F.; Mainprice, D.; Siegesmund, S. Marble decay induced by thermal strains: Simulations and experiments. Environ. Earth Sci. 2013, 69, 1281–1297. [Google Scholar] [CrossRef]
- Vola, G. High-Grade Burnt Lime Products: Impact of Calcination Kinetics on Slaking Reactivity; Sticking Tendency and Blocks Formation at HT (1300 °C); University of Ferrara: Ferrara, Italy, 2019. [Google Scholar]
- Suzuki, Y.; Shiino, Y.; Bergström, J. Stratigraphy, carbonate facies and trilobite associations in the Hirnantian part of the Boda Limestone, Sweden. GFF 2009, 131, 299–310. [Google Scholar] [CrossRef]
- Sjöberg, O. Personal Communication; The Saskatchewan Mining Association: Regina, SK, Canada; p. 2020.
- Allared, J. XRF manual. SMA Miner. 2019. [Google Scholar]
- Eriksson, L.; Johansson, E.; Kettaneh-Wold, N.; Trygg, J.; Wikström, C.; Wold, S. Multi- and Megavariate Data Analysis; Second Revised and Enlarged Edition; Umetrics Academy: Umeå, Sweden, 2006. [Google Scholar]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Vola, G.; Christiansen, T.; Sarandrea, L.; Ferrari, V. Carbonate Rock Characterization for the Industrial Lime Manufacturing: Worldwide Case-Studies. In Proceedings of the 14th Euroseminar on Microscopy Applied to Building Materials, Copenhagen, Denmark, 10–14 June 2013. [Google Scholar]
- Beruto, D.; Barco, L. CO2-Catalyzed Surface Area and Porosity Changes in High-Surface-Area CaO Aggregates. J. Am. Ceram. Soc. 1984, 67, 512–515. [Google Scholar] [CrossRef]
- Borgwardt, R.H. Calcination kinetics and surface area of dispersed limestone particles. AlChE J. 1985, 31, 103–111. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Tian, W.; Wu, X. Investigating and predicting permeability variation in thermally cracked dry rocks. Int. J. Rock Mech. Min. Sci. 2018, 103, 77–88. [Google Scholar] [CrossRef]
- Srinivasan, R. The thermal expansion of calcite from room temperature up to 400 °C. Proc. Indian Acad. Sci. Sect. A 1955, 42, 81–85. [Google Scholar] [CrossRef]
- Siegesmund, S.; Ullemeyer, K.; Weiss, T.; Tschegg, E.K. Physical weathering of marbles caused by anisotropic thermal expansion. Int. J. Earth Sci. 2000, 89, 170–182. [Google Scholar] [CrossRef]

| Type of Test | Weight [g] | Size [mm] | Max. Temperature [°C] | Max. Time [min] | Screen [mm] | Reference | |
|---|---|---|---|---|---|---|---|
| 1 | Decrepitation test | 2 × 250 | 12.5–19 | 1050 | 120 | 10 | Maerz [6] |
| 2 | Thermal decrepitation | 500 | 5–10 | 1000 | 60 | 5 | Nordkalk AB [9] |
| 3 | Thermal decrepitation | 150–400 | 5–10 | 1150 | 60 | 5 | SMA Mineral AB [7] |
| 4 | Thermal decrepitation | 500 | 5–10 | 1150 | 60 | 5 | SMA Mineral AB [10] |
| 5 | Mechanical degradation | ≥500 | 37.5–45 | 1050 | 10 | 10 | Cimprogetti [3,5] |
| 6 | Decrepitation test | 120 | 10–20 | 900, 950, 1000, 1050 | 60 | 4 | D. J. Harrison [8] |
| 7 | Deterioration test | Not specified | 8 | 1050 | 240 | 2 | Lund method [10] |
| 8 | The Pilkington test | 9 | 0.106–1.18 | 1040 | 10 | Weight remaining after calcination | D. Dollimore [11] |
| 9 | TG method | 0.04 | 0.09–2 | 900 | Not specified | Weight loss based on TG-DTG curves | D. Dollimore [11] |
| 10 | TGA | 0.026–0.028 | 0.106–1.18 | 1000 | Not specified | Weight loss based on TG curves | R. A. McCauley [12] |
| CaO [wt.-%] | MgO [wt.-%] | Fe2O3 [wt.-%] | SiO2 [wt.-%] | Al2O3 [wt.-%] | MnO [wt.-%] | P2O5 [wt.-%] | TiO2 [wt.-%] | K2O [wt.-%] | S [wt.-%] | LOI [wt.-%] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | 51.51 | 0.31 | 0.05 | 0.3 | 0.1 | 0.06 | <0.01 | <0.01 | 0.01 | <0.005 | 41.09 |
| Max. | 55.18 | 0.86 | 0.6 | 3.71 | 1.42 | 0.15 | 0.14 | 0.07 | 0.47 | 0.412 | 43.9 |
| Average | 53.84 | 0.52 | 0.21 | 1.42 | 0.49 | 0.09 | 0.02 | 0.02 | 0.14 | 0.03 | 42.97 |
| Std. dev | 0.81 | 0.12 | 0.12 | 0.75 | 0.28 | 0.02 | 0.02 | 0.01 | 0.09 | 0.05 | 0.61 |
| LDL | 0.1 | 0.1 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.005 | 0.01 |
| Sample | CaO [wt.-%] | MgO [wt.-%] | Fe2O3 [wt.-%] | SiO2 [wt.-%] | Al2O3 [wt.-%] | MnO [wt.-%] | P2O5 [wt.-%] | TiO2 [wt.-%] | K2O [wt.-%] | S [wt.-%] | LOI [wt.-%] | TD [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 71 | 54.68 | 0.46 | 0.08 | 0.46 | 0.16 | 0.07 | 0.01 | <0.01 | 0.03 | 0.01 | 43.83 | 75 |
| 74 | 54.78 | 0.43 | 0.10 | 0.61 | 0.21 | 0.08 | 0.04 | 0.01 | 0.05 | <0.005 | 43.50 | 4 |
| T | DOC [%] | Avg. Frac. Area [%] | Avg. Frac. Length [mm] | Avg. Frac. Width [mm] | Structural Characteristics |
|---|---|---|---|---|---|
| Ambient | 0 | 0.6 | 0.179 | 0.018 | Low number of fractures. |
| 500 °C | 0 | 1.1 | 0.166 | 0.018 | Low number of fractures. |
| 800 °C | 76 | 1.9 | 0.252 | 0.021 | Low number of fractures. |
| 1150 °C | 100 | 4.9 | 0.275 | 0.004 | Increased cracking. Dense network of small cracks and some wide cracks. |
| T | DOC [%] | Avg. Frac. Area [%] | Avg. Frac. Length [mm] | Avg. Frac. Width [mm] | Structural Characteristics |
|---|---|---|---|---|---|
| Ambient | 0 | 3.8 | 0.172 | 0.018 | Low number of fractures. |
| 500 °C | 59 | 7.0 | 0.373 | 0.016 | Major cracking with grain boundary and intergranular cracks. |
| 800 °C | 65 | 10.5 | 0.370 | 0.017 | Increased cracking with grain boundary and intergranular cracks. |
| 1150 °C | 100 | 4.0 | 0.287 | 0.020 | Reduced cracking. Less frequent crack network in comparison to samples with 4% TD (Figure 5d). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cwik, K.; Broström, M.; Backlund, K.; Fjäder, K.; Hiljanen, E.; Eriksson, M. Thermal Decrepitation and Thermally-Induced Cracking of Limestone Used in Quicklime Production. Minerals 2022, 12, 1197. https://doi.org/10.3390/min12101197
Cwik K, Broström M, Backlund K, Fjäder K, Hiljanen E, Eriksson M. Thermal Decrepitation and Thermally-Induced Cracking of Limestone Used in Quicklime Production. Minerals. 2022; 12(10):1197. https://doi.org/10.3390/min12101197
Chicago/Turabian StyleCwik, Katarzyna, Markus Broström, Krister Backlund, Kenneth Fjäder, Emil Hiljanen, and Matias Eriksson. 2022. "Thermal Decrepitation and Thermally-Induced Cracking of Limestone Used in Quicklime Production" Minerals 12, no. 10: 1197. https://doi.org/10.3390/min12101197





