Trace Element Geochemistry of Alluvial TiO2 Polymorphs as a Proxy for Sn and W Deposits
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
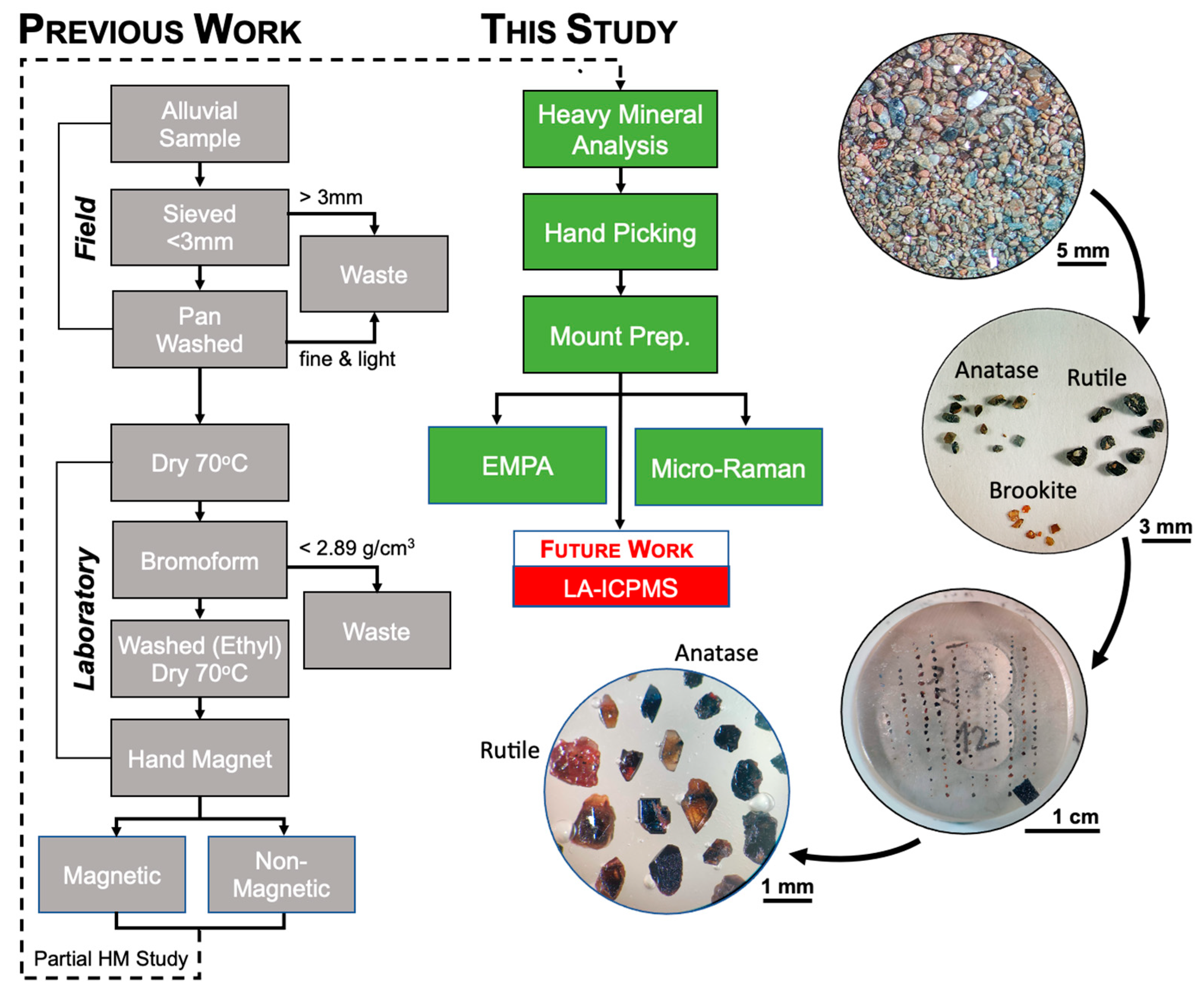
3.1. Heavy Mineral Sample Selection and Sample Preparation
3.2. Heavy Mineral Analyses and Mineral Abundance Mapping
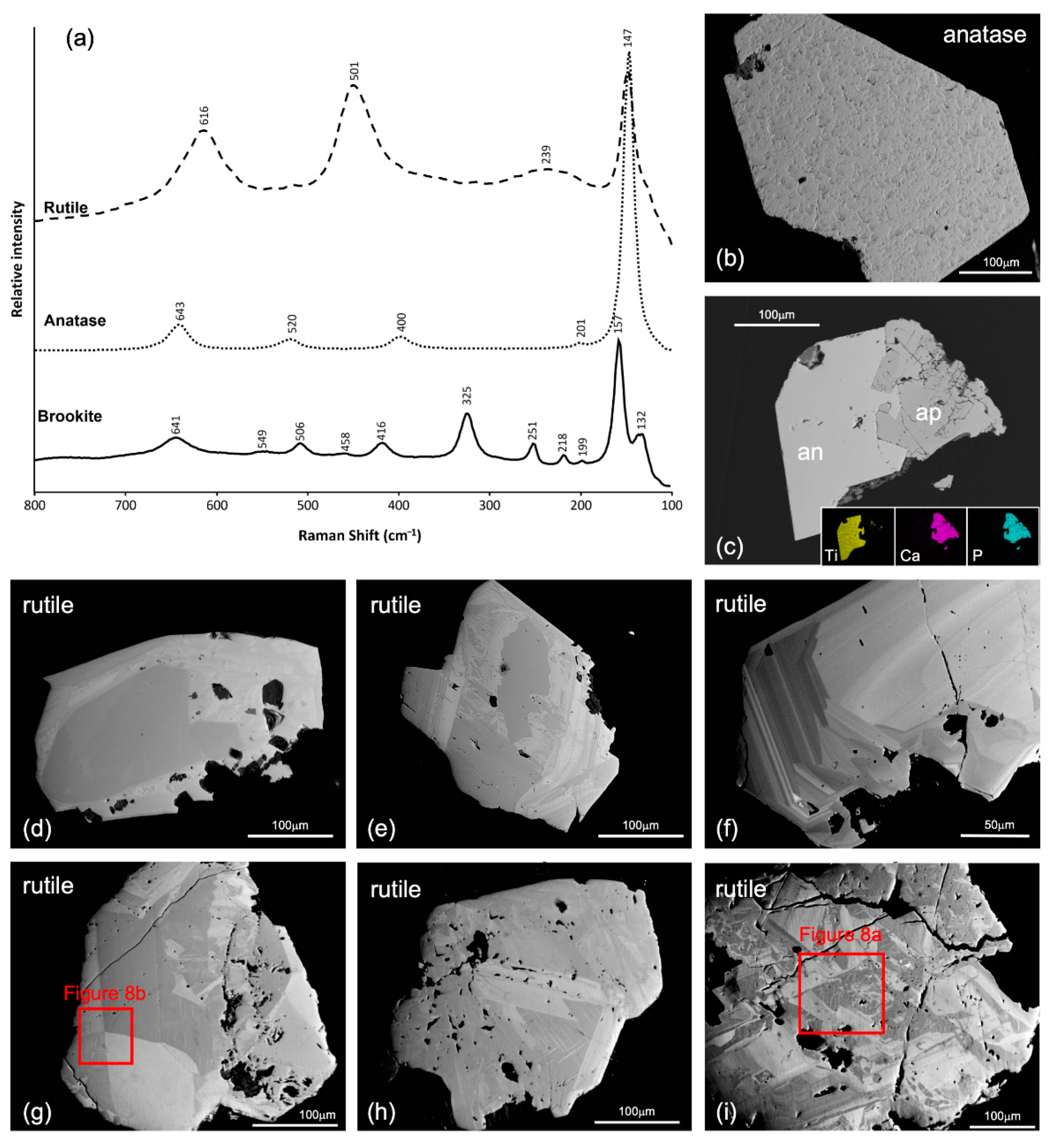
3.3. Micro-Raman Spectroscopy
3.4. Electron Probe Micro-Analyses (EPMA)
4. Results
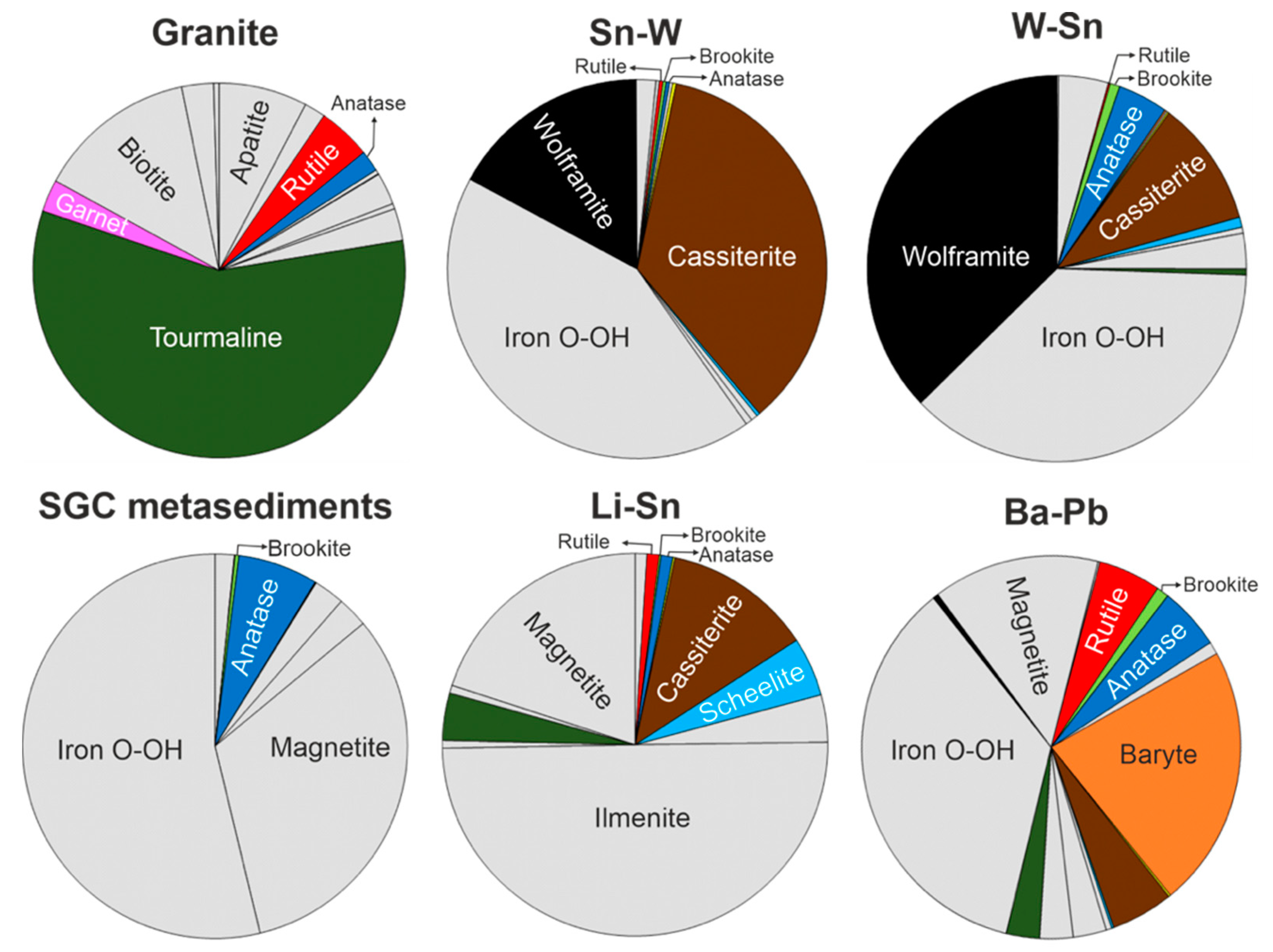
4.1. Alluvial Heavy Mineral Analysis
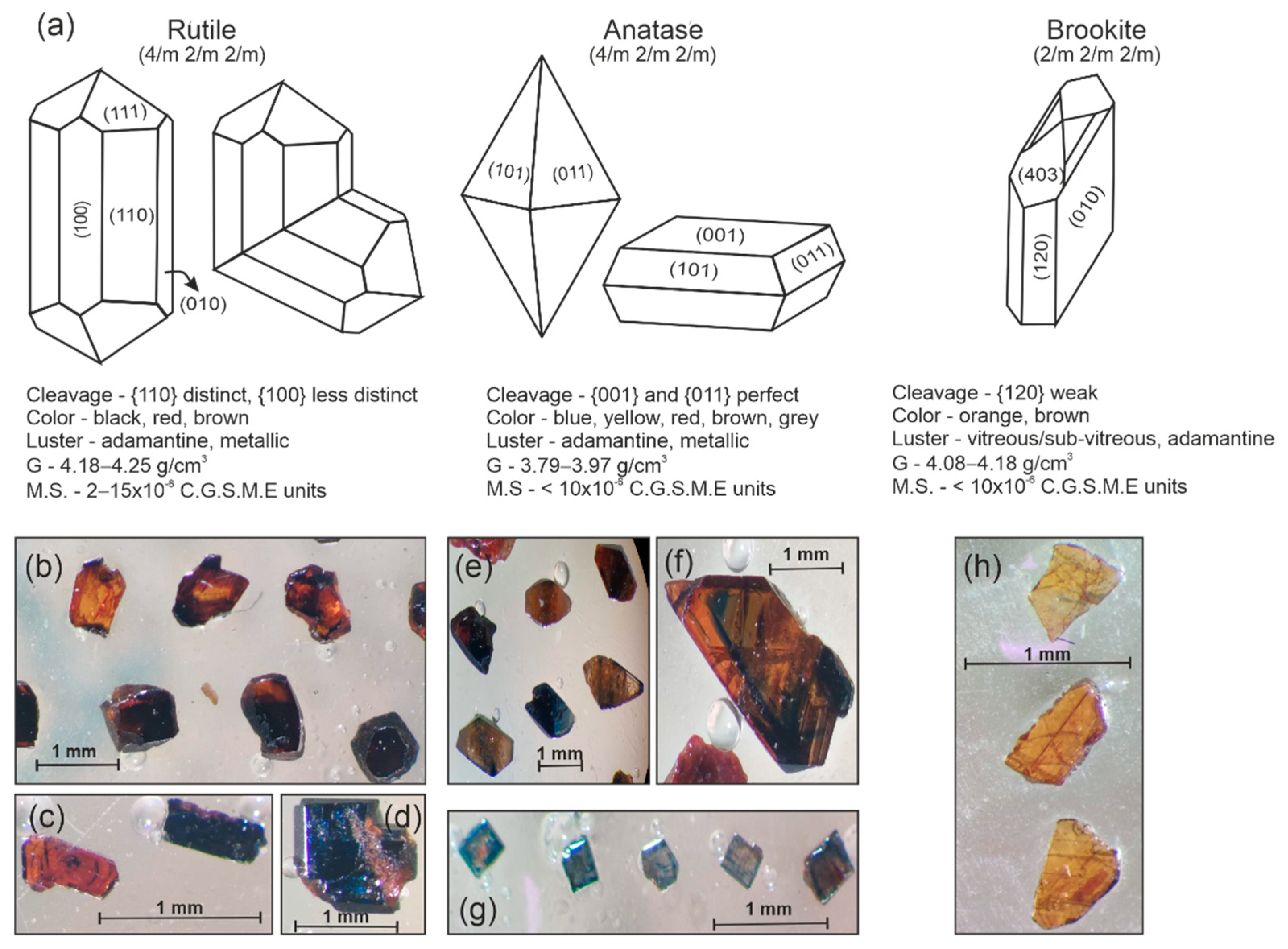
4.1.1. Heavy Mineral Assemblages
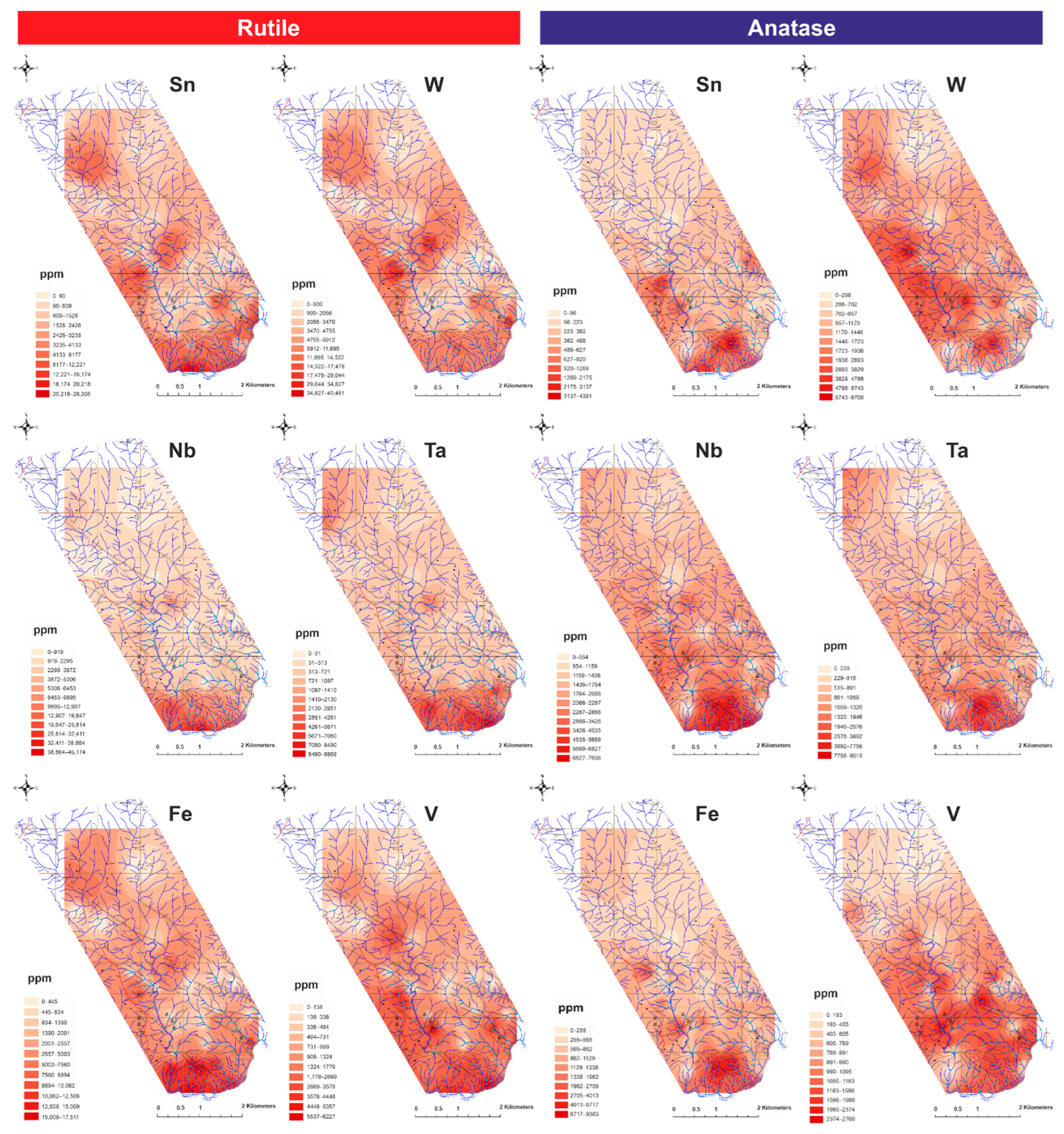
4.1.2. Alluvial Heavy Mineral Abundance Maps
4.2. Trace Elements in TiO2 Minerals—EPMA Data
5. Discussion
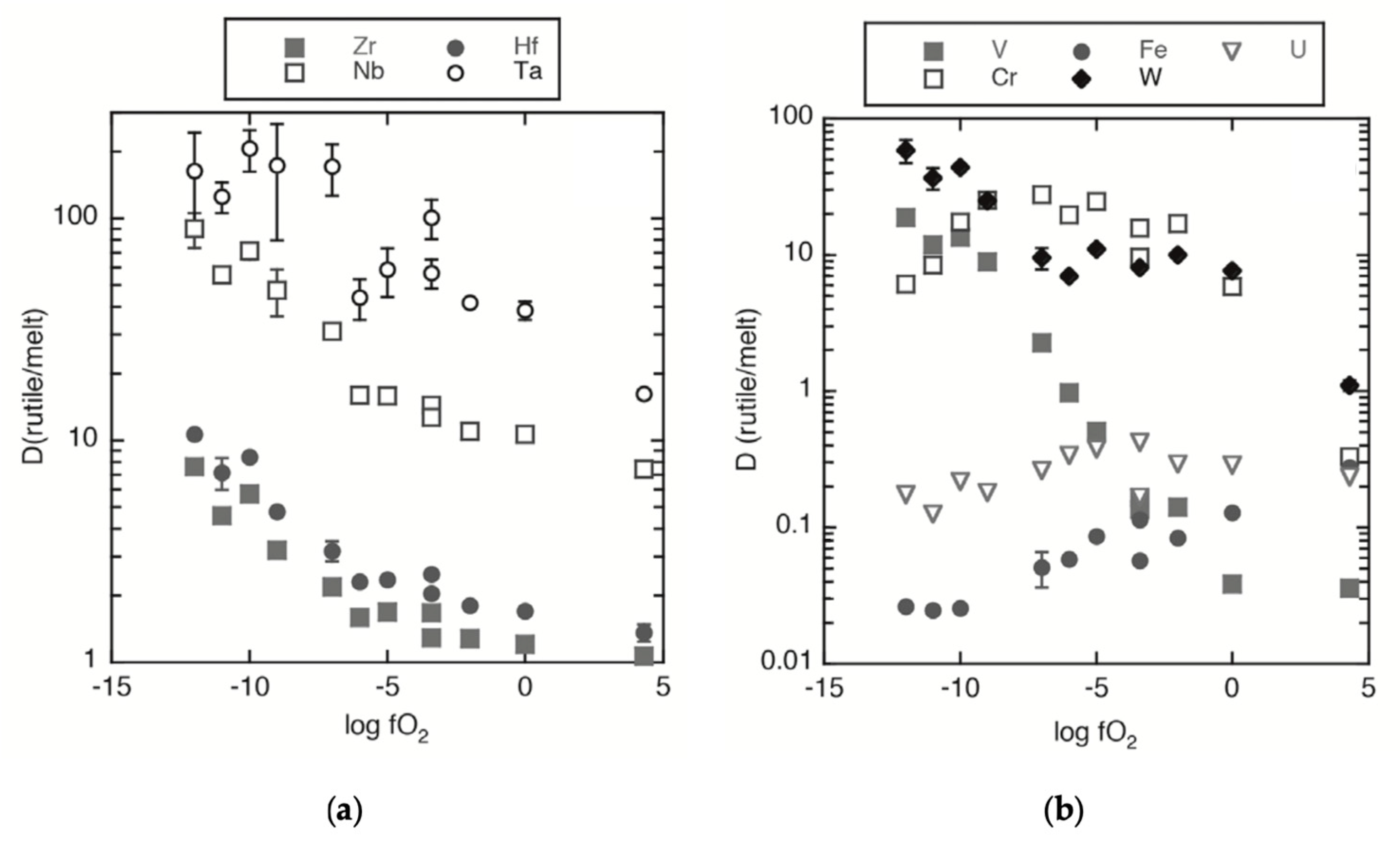
5.1. Controls on TiO2 Polymorphs’ Trace Element Composition
5.1.1. Structure of TiO2 Polymorphs
5.1.2. Trace Element Substitution Mechanisms in TiO2 Minerals
5.1.3. TiO2 Polymorph’s Forming Environments—Genesis and Stability
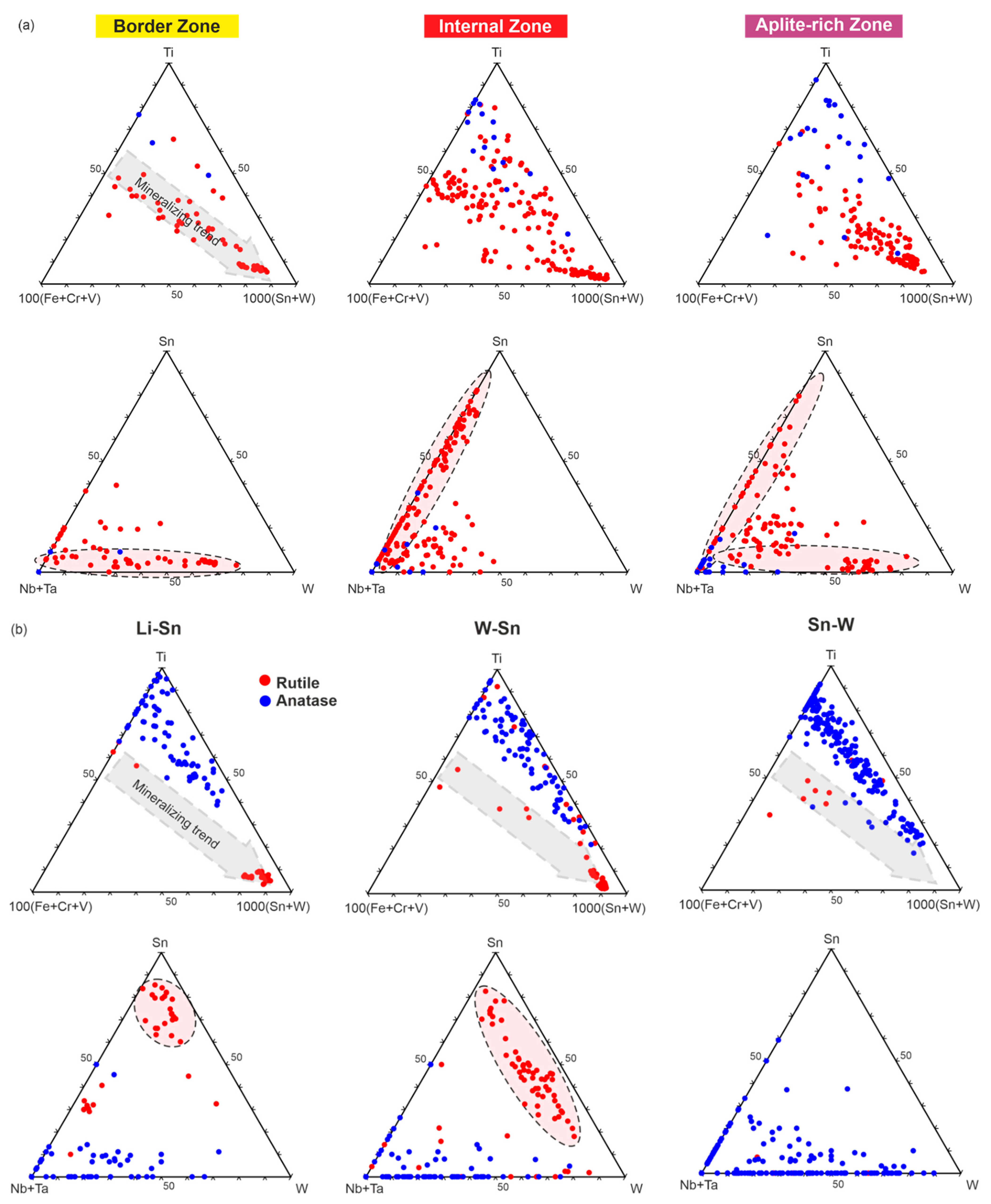
5.2. Trace Element Geochemistry of TiO2 Polymorphs as Tracers of Mineralizing Systems and Its Application to the Exploration for Sn and W Deposits
6. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, W.M. Geochemistry; Wiley-Blackwell: Chichester, UK, 2013; 668p. [Google Scholar]
- Morton, A.C. Heavy minerals. In Sedimentology, Encyclopedia of Earth Science; Springer: Berlin/Heidelberg, Germany, 1978; pp. 574–578. [Google Scholar]
- Stendal, H.; Theobald, P.K. Heavy-Mineral Concentrates in Geochemical Exploration. Handb. Explor. Geochem. 1994, 6, 185–225. [Google Scholar]
- Derby, O.A. On the separation and study of the heavy accessories of rocks. Proc. Rochester. Acad. Sci. 1891, 1, 198–206. [Google Scholar]
- Day, D.T.; Richards, R.H. Investigation of black sands from placer mines. U.S. Geol. Surv. Bull 1905, 285, 150–154. [Google Scholar]
- Fersman, A.Y. Geochemical and Mineralogical Methods of Prospecting for Mineral Deposits; Chapter IV: Special methods of prospecting; Hartsock, L.; Pierce, A.P., Translators; US Geological Survey: Reston, VA, USA, 1952; 127p.
- Hawkes, H.E. Principles of geochemical prospecting. U.S. Geol. Surv. Bull. 1957, 1000-F, 133. [Google Scholar]
- Hawkes, H.E. The early days of exploration geochemistry. J. Geochem. Explor. 1976, 6, 1–11. [Google Scholar] [CrossRef]
- Williams, S.A.; Cesbron, F.P. Rutile and apatite: Useful prospecting guides for porphyry copper deposits. Mineral. Mag. 1977, 41, 288–292. [Google Scholar] [CrossRef]
- Morad, S. SEM study of authigenic rutile, anatase and brookite in Proterozoic sandstones from Sweden. Sediment. Geol. 1986, 46, 77–89. [Google Scholar] [CrossRef]
- Averill, S.A. The application of heavy indicator mineralogy in mineral exploration with emphasis on base metal indicators in glaciated metamorphic and plutonic terrains. Geol. Soc. Lond. Spec. Publ. 2001, 185, 69–81. [Google Scholar] [CrossRef]
- McClenaghan, M.B. Indicator mineral methods in mineral exploration. Geochem. Explor. Environ. Anal. 2005, 5, 233–245. [Google Scholar] [CrossRef]
- Barnett, P.J.; Averill, S.A. Heavy mineral dispersal trains in till in the area of the Lac des Iles PGE deposit, northwestern Ontario, Canada. Geochem. Explor. Environ. Anal. 2010, 10, 391–399. [Google Scholar] [CrossRef]
- Averill, S.A. Viable indicator minerals in surficial sediments for two major base metal deposit types: Ni-Cu-PGE and porphyry Cu. Geochem. Explor. Environ. Anal. 2011, 11, 279–291. [Google Scholar] [CrossRef]
- Kelley, K.D.; Eppinger, R.G.; Lang, J.; Smith, S.M.; Fey, D.L. Porphyry Cu indicator minerals in till as an exploration tool: Example from the giant Pebble porphyry Cu-Au-Mo deposit, Alaska, USA. Geochem. Explor. Environ. Anal. 2011, 11, 321–334. [Google Scholar] [CrossRef]
- McMartin, I.; Corriveau, L.; Beaudoin, G.; Averill, S.A.; Kjarsgaard, I. Results from an orientation study of the heavy mineral and till geochemical signatures of the NICO Au-Co-Bi deposit, Great Bear magmatic zone, Northwest Territories, Canada. Geol. Surv. Can. Open File 2011, 6723, 23. [Google Scholar]
- Paulen, R.C.; Paradis, S.; Plouffe, A.; Smith, I.R. Pb and S isotopic composition of indicator minerals in glacial sediments from NW Alberta, Canada: Implications for Zn-Pb base metal exploration. Geochem. Explor. Environ. Anal. 2011, 11, 309–320. [Google Scholar] [CrossRef]
- Decker, M. U-Pb and Trace Element LA-ICP-MS Study of Rutile Grains from the Auriferous Moeda Formation Conglomerate, Quadrilátero Ferrífero of Minas Gerais, Brazil: Provenance and Age Determinations; Julius-Maximilians-University: Würzburg, Germany, 2012. [Google Scholar]
- McClenaghan, M.B.; Kjarsgaard, I.M.; Kjarsgaard, B.A. Kimberlite indicator mineral chemistry of the Bucke and Gravel kimberlites and associated indicator minerals in till, Lake Timiskaming, Ontario. Geol. Surv. Can. Open File 2012, 5815, 30. [Google Scholar]
- Dewaele, S.; Goethals, H.; Thys, T. Mineralogical characterization of cassiterite concentrates from quartz vein and pegmatite mineralization of the Karagwe-Ankole and Kibara Belts, Central Africa. Geol. Belg. 2013, 16, 66–75. [Google Scholar]
- McClenaghan, M.B.; Kjarsgaard, I.M.; Averill, S.A.; Layton-Matthews, D.; Crabtree, D.; Matile, G.; McMartin, I.; Pyne, M. Indicator mineral signatures of magmatic Ni-Cu deposits, Thompson Nickel Belt, Manitoba: Part 2—Till data. Geol. Surv. Can. Open File 2013, 7200, 148. [Google Scholar]
- McClenaghan, M.B.; Parkhill, M.A.; Averill, S.A.; Kjarsgaard, I.M. Indicator mineral signatures of the Sisson W-Mo deposit, New Brunswick: Part 1 bedrock samples. Geol. Surv. Can. Open File 2013, 7431, 29. [Google Scholar]
- Salgueiro, R.; Rosa, D.; Inverno, C.; Oliveira, D.; Solá, A.R.; Guimarães, F. Alluvial xenotime and heavy minerals assemblage from the northern edge of Nisa-Albuquerque Batholith, eastern Portugal: Provenance and geochemical implications. J. Geochem. Explor. 2014, 146, 40–57. [Google Scholar] [CrossRef][Green Version]
- Oviatt, N.M.; Gleeson, S.A.; Paulen, R.C.; McClenaghan, M.B.; Paradis, S. Characterization and dispersal of indicator minerals associated with the Pine Point Mississippi Valley-type (MVT) district, Northwest Territories, Canada. Can. J. Earth Sci. 2015, 52, 776–794. [Google Scholar] [CrossRef]
- Mackay, D.A.R.; Simandl, G.J.; Ma, W.; Redfearn, M.; Gravel, J. Indicator mineral-based exploration for carbonatites and related specialty metal deposits—A QEMSCAN®® orientation survey, British Columbia, Canada. J. Geochem. Explor. 2016, 165, 159–173. [Google Scholar] [CrossRef]
- Mao, M.; Rukhlov, A.S.; Rowins, S.M.; Spence, J.; Coogan, L.A. Apatite Trace Element Compositions: A Robust New Tool for Mineral Exploration. Econ. Geol. 2016, 111, 1187–1222. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M. Rare earth elements from heavy mineral sands: Assessing the potential of a forgotten resource. Appl. Earth Sci. 2016, 125, 107–113. [Google Scholar] [CrossRef]
- Plouffe, A.; Ferbey, T.; Hashmi, S.; Ward, B.C. Till geochemistry and mineralogy: Vectoring towards Cu porphyry deposits in British Columbia, Canada. Geochem. Explor. Environ. Anal. 2016, 16, 213–233. [Google Scholar] [CrossRef]
- Chapman, R.; Allan, M.; Mortensen, J.; Wrighton, T.; Grimshaw, M. A new indicator mineral methodology based on a generic Bi-Pb-Te-S mineral inclusion signature in detrital gold from porphyry and low/intermediate sulfidation epithermal environments in Yukon Territory, Canada. Miner. Depos. 2018, 53, 815–834. [Google Scholar] [CrossRef]
- Makvandi, S.; Beaudoin, G.; McClenaghan, M.B.; Quirt, D.; Ledra, P. PCA of Fe-oxides MLA data as an advanced tool in provenance discrimination and indicator mineral exploration: Case study from bedrock and till from the Kiggavik U deposits area (Nunavut, Canada). J. Geochem. Explor. 2018, 197, 199–211. [Google Scholar] [CrossRef]
- Manéglia, N.; Beaudoin, G.; Simard, M. Indicator minerals of the Meliadine orogenic gold deposits, Nunavut (Canada), and application to till surveys. Geochem. Explor. Environ. Anal. 2018, 18, 241–251. [Google Scholar] [CrossRef]
- McClenaghan, M.B.; Parkhill, M.A.; Pronk, A.G.; Sinclair, W.D. Indicator mineral and geochemical signatures of the Mount Pleasant W-Mo-Bi and Sn-Zn-In deposits, New Brunswick, Canada. J. Geochem. Explor. 2018, 172, 151–166. [Google Scholar] [CrossRef]
- Poulin, R.S.; Kontak, D.J.; McDonald, A.; McCLenaghan, M.B. Assessing Scheelite As an Ore-deposit Discriminator Using Its Trace-element and REE Chemistry. Can. Mineral. 2018, 56, 265–302. [Google Scholar] [CrossRef]
- Gonc<monospace>̧</monospace>alves, C.; Braga, P. Heavy Mineral Sands in Brazil: Deposits, Characteristics, and Extraction Potential of Selected Areas. Minerals 2019, 9, 176. [Google Scholar]
- Grzeka, D.; Beaudoin, G.; Bédard, E. Tourmaline, scheelite, and magnetite compositions from orogenic gold deposits and glacial sediments of the Val-d’Or district (Québec, Canada): Applications to mineral exploration. J. Geochem. Explor. 2019, 206, 106355. [Google Scholar] [CrossRef]
- Ghosal, S.; Agrahari, S.; Banerjee, S.; Chakrabarti, R.; Sengupta, D. Geochemistry of the Heavy Mineral Sands from the Garampeta to the Markandi beach, southern coast of Odisha, India: Implications of high contents of REE and Radioelements attributed to Placer Monazite. J. Earth Syst. Sci. 2020, 129, 152. [Google Scholar] [CrossRef]
- Grácio, N. Caracterização Mineralométrica e Química de Minerais Pesados Aluvionares: Aplicação à Prospeção de Sn e W na Região de Segura. Master’s Thesis, Faculdade de Ciências da Universidade de Lisboa, Lisboa, Portugal, 2020. [Google Scholar]
- Kyser, T.K.; Leybourne, M.I.; Layton-Matthews, D. Advances in the use of isotopes in geochemical exploration: Instrumentation and applications in understanding geochemical processes. Geochem. Explor. Environ. Anal. 2020, 20, 199–204. [Google Scholar] [CrossRef]
- Lougheed, H.D.; McClenaghan, M.B.; Layton-Matthews, D.; Leybourne, M. Exploration Potential of Fine-Fraction Heavy Mineral Concentrates from Till Using Automated Mineralogy: A Case Study from the Izok Lake Cu–Zn–Pb–Ag VMS Deposit, Nunavut, Canada. Minerals 2020, 10, 310. [Google Scholar] [CrossRef]
- Salgueiro, R.; Inverno, C.; Oliveira, D.; Guimarães, F.; Lencastre, J.; Rosa, D. Alluvial nodular monazite in Monfortinho (Idanha-a-Nova, Portugal): Regional distribution and genesis. J. Geochem. Explor. 2020, 210, 106444. [Google Scholar] [CrossRef]
- Joyce, N.; Layton-Matthews, D.; Kyser, K.; Leybourne, M.; Ansdell, K.; Kotzer, T.; Quirt, D.; Zaluski, G. Alteration mineralogy and pathfinder element inventory in the footprint of the McArthur River unconformity-related uranium deposit, Canada. Can. Mineral. 2021, 59, 985–1019. [Google Scholar] [CrossRef]
- Lee, R.G.; Plouffe, A.; Ferbey, T.; Hart, C.J.R.; Hollings, P.; Gleeson, S.A. Recognizing porphyry copper potential from till zircon composition: A case study from the highland valley porphyry district, south-central british columbia. Econ. Geol. 2021, 116, 1035–1045. [Google Scholar] [CrossRef]
- Lougheed, H.D.; McClenaghan, M.B.; Layton-Matthews, D.; Leybourne, M.I.; Dobosz, A.N. Automated Indicator Mineral Analysis of Fine-Grained Till Associated with the Sisson W-Mo Deposit, New Brunswick, Canada. Minerals 2021, 11, 103. [Google Scholar] [CrossRef]
- Makvandi, S.; Pagé, P.; Tremblay, J.; Girard, R. Exploration for Platinum-Group Minerals in Till: A New Approach to the Recovery, Counting, Mineral Identification and Chemical Characterization. Minerals 2021, 11, 264. [Google Scholar] [CrossRef]
- Plouffe, A.; Acosta-Góngora, P.; Kjarsgaard, I.M.; Petts, D.; Ferbey, T.; Venance, K.E. Detrital epidote chemistry: Detecting the alteration footprint of porphyry copper mineralization in the Quesnel terrane of the Canadian Cordillera, British Columbia. Geol. Surv. Can. Bull. 2021, 616, 137–157. [Google Scholar]
- Plouffe, A.; Wilton, D.H.C.; McNeil, R.; Ferbey, T. Automated indicator-mineral analysis of the fine-sand, heavy-mineral concentrate fraction of till: A promising exploration tool for porphyry copper mineralization. Geol. Surv. Can. Bull. 2021, 616, 203–223. [Google Scholar]
- Balaram, V.; Sawant, S.S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [Google Scholar] [CrossRef]
- Layton-Matthews, D.; McClenaghan, M.B. Current Techniques and Applications of Mineral Chemistry to Mineral Exploration; Examples from Glaciated Terrain: A Review. Minerals 2022, 12, 59. [Google Scholar] [CrossRef]
- Graham, J. Tungsten- and antimony-substituted rutile. Mineral. Mag. 1973, 39, 470–473. [Google Scholar] [CrossRef]
- Czamanske, G.K.; Force, E.R.; Moore, W.J. Some geologic and potential resource aspects of rutile in porphyry copper deposits. Econ. Geol. 1981, 76, 2240–2246. [Google Scholar] [CrossRef]
- Urban, A.J.; Hoskins, B.F.; Grey, I.E. Characterization of V-Sb-W bearing rutile from the Hemlo gold deposit, Ontario. Can. Miner. 1992, 30, 319–326. [Google Scholar]
- Smith, D.C.; Perseil, E.-A. Sb-rich rutile in the manganese concentrations at St. Marcel-Praborna, Aosta Valley, Italy; petrology and crystal-chemistry. Mineral. Mag. 1997, 61, 655–669. [Google Scholar] [CrossRef]
- Rice, C.M.; Darke, K.E.; Still, J.W.; Lachowski, E.E. Tungsten-bearing rutile from the Kori Kollo gold mine, Bolivia. Mineral. Mag. 1998, 62, 421–429. [Google Scholar] [CrossRef]
- Černý, P.; Chapman, R.; Simmons, W.B.; Chackowsky, L.E. Niobian rutile from the McGuire granitic pegmatite, Park County, Colorado: Solid solution, exsolution, and oxidation. Am. Mineral. 1999, 84, 754–763. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Yefimova, E.S. Composition and petrogenesis of Ti-oxides associated with diamonds. Int. Geol. Rev. 2000, 42, 758–767. [Google Scholar] [CrossRef]
- Rabbia, O.M.; Hernández, L.B.; Townley, B.; King, R.W.; Ayers, J.C. Anatase-bearing veins in the El Teniente Cu-Mo porphyry system. In Proceedings of the Special Symposium on Supergiant Andean Porphyry Copper Deposits, Concepción, Chile, 6–10 October 2003. [Google Scholar]
- Clark, J.R.; Williams-Jones, A.E. Rutile as a Potential Indicator Mineral for Metamorphosed Metallic Ore Deposits; Rapport final, sous-projet SC2; DIVEX: Montréal, QC, Canada, 2004. [Google Scholar]
- Scott, K.M. Rutile geochemistry as a guide to porphyry Cu-Au mineralization, Northparkes, New South Wales, Australia. Geochem. Explor. Environ. Anal. 2005, 5, 247–253. [Google Scholar] [CrossRef]
- Scott, K.M.; Radford, N.W. Rutile compositions at the Big Bell Au deposit as a guide for exploration. Geochem. Explor. Environ. Anal. 2007, 7, 353–361. [Google Scholar] [CrossRef]
- Dostal, J.; Kontak, D.; Chatterjee, A. Trace element geochemistry of scheelite and rutile from metaturbidite-hosted quartz vein gold deposits, Meguma Terrane, Nova Scotia, Canada: Genetic implications. Mineral. Petrol. 2009, 97, 95–109. [Google Scholar] [CrossRef]
- Rabbia, O.; Hernández, L.; French, D.; King, R.; Ayers, J. The El Teniente porphyry Cu–Mo deposit from a hydrothermal rutile perspective. Miner. Depos. 2009, 44, 849–866. [Google Scholar] [CrossRef]
- Meinhold, G. Rutile and its applications in earth sciences. Earth-Sci. Rev. 2010, 102, 1–28. [Google Scholar] [CrossRef]
- Scott, K.M.; Radford, N.W.; Hough, R.M.; Reddy, S.M. Rutile compositions in the Kalgoorlie goldfields and their implications for exploration. Aust. J. Earth Sci. 2011, 58, 803–812. [Google Scholar] [CrossRef]
- Rabbia, O.; Hernández, L. Mineral chemistry and potential applications of natural-multi-doped hydrothermal rutile from porphyry copper deposits. In Rutile: Properties, Synthesis and Applications; Low, I.M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2012; pp. 209–228. [Google Scholar]
- 65. Dill, H.G.; Weber, B.; Melcher, F.; Wiesner, W.; Müller, A. Titaniferous heavy mineral aggregates as a tool in exploration for pegmatitic and aplitic rare-metal deposits (SE Germany). Ore Geol. Rev. 2014, 57, 29–52. [Google Scholar] [CrossRef]
- Malkovets, V.G.; Rezvukhin, D.I.; Belousova, E.A.; Griffin, W.L.; Sharygin, L.S.; Tretiakova, L.G.; Gibsher, A.A.; O’Reilly, S.Y.; Kuzmin, D.V.; Litasov, K.D.; et al. Cr-rich rutile: A powerful tool for diamond exploration. Lithos 2016, 265, 304–311. [Google Scholar] [CrossRef]
- Kuşcu, M.; Cengiz, O.; Işık, K.; Gul, E.K. The origin and geochemical characteristics of rutile in eluvial and fluvial-alluvial placers and quartz veins of the Menderes Massif from the Neoproterozoic Pan-African Belt, Western Turkey. J. Afr. Earth Sci. 2018, 143, 10–27. [Google Scholar] [CrossRef]
- Plavsa, D.; Reddy, S.M.; Agangi, A.; Clark, C.; Kylander-Clark, A.; Tiddy, C.J. Microstructural, trace element and geochronological characterization of TiO2 polymorphs and implications for mineral exploration. Chem. Geol. 2018, 476, 130–149. [Google Scholar] [CrossRef]
- Agangi, A.; Reddy, S.M.; Plavsa, D.; Fougerouse, D.; Clark, C.; Roberts, M.; Johnson, T.E. Antimony in rutile as a pathfinder for orogenic gold deposits. Ore Geol. Rev. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Carocci, E.; Marignac, C.; Cathelineau, M.; Truche, L.; Lecomte, A.; Pinto, F. Rutile from Panasqueira (central Portugal): An excellent pathfinder for wolframite deposition. Minerals 2019, 9, 9. [Google Scholar] [CrossRef]
- Pereira, I.; Storey, C.; Darling, J.; Lana, C.; Alkmim, A.R. Two billion years of evolution enclosed in hydrothermal rutile: Recycling of the São Francisco Craton Crust and constraints on gold remobilisation processes. Gondwana Res. 2019, 68, 69–92. [Google Scholar] [CrossRef]
- Porter, J.K.; McNaughton, N.J.; Evans, N.J.; McDonald, B.J. Rutile as a pathfinder for metals exploration. Ore Geol. Rev. 2020, 120, 103406. [Google Scholar]
- Sciuba, M.; Beaudoin, G. Texture and trace element composition of rutile in orogenic gold deposits. Econ. Geol. 2021, 116, 1865–1892. [Google Scholar] [CrossRef]
- Inverno, C.M.C.; Oliveira, D.P.S.; Rodrigues, L. Inventariação e Prospecção de Terras Raras nas Regiões Fronteiriças da Beira Baixa e do Norte Alentejo; Unpublish INETI Report; INETI: Alfragide, Portugal, 2007; 2982p. [Google Scholar]
- Silva, A. A Litostratigrafia e Estrutura do Supergrupo Dúrico-Beirão (Complexo Xisto-Grauváquico), em Portugal, e sua correlação com as correspondentes sucessões em Espanha. Bolet. Minas 2013, 48, 97–142. [Google Scholar]
- Sousa, M. Considerações sobre a estratigrafia do Complexo Xisto-Grauváquico (CXG) e a sua relação com o Paleozóico Inferior. Quad. Geol. Ibérica 1984, 9, 9–36. [Google Scholar]
- Sequeira, A.J.D.; Cunha, P.P.; Ribeiro, M.L. Notícia Explicativa da Folha 25-B—Salvaterra do Extremo; LNEG: Lisboa, Portugal, 1999; 47p.
- Romão, J.; Cunha, P.P.; Pereira, A.; Dias, R.; Cabral, J.; Ribeiro, A. Notícia Explicativa das Folhas 25-C; 25-D; 29-A Rosmanhinhal Segura Retorta (Sector Norte); LNEG: Lisboa, Portugal, 2010; 54p.
- Ribeiro, A.; Antunes, M.T.; Ferreira, M.P.; Rocha, R.B.; Soares, A.F.; Zbyszewski, G.; Almeida, F.M.; Carvalho, D.; Monteiro, J.H. Introduction à la Géologie Générale du Portugal; Serviços Geológicos de Portugal: Lisboa, Portugal, 1979; pp. 1–114. [Google Scholar]
- Corretgé, L.G. Estudio Petrológico del Batolito de Cabeza de Araya (Cáceres). Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 1971. [Google Scholar]
- Antunes, I.M.H.R.; Neiva, A.M.; Silva, M.M.V. The mineralized veins and the impact of old mine workings on the environment at Segura, central Portugal. Chem. Geol. 2002, 190, 417–431. [Google Scholar] [CrossRef]
- Corretgé, L.G.; Suárez, O. A Garnet-Cordierite Granite Porphyry Containing Rapakivi Feldspars in the Cabeza de Araya Batholith (Extremadura, Spanish Hercynian Belt). Mineral. Petrol. 1994, 50, 97–111. [Google Scholar] [CrossRef]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Ramos, J.M.F.; Silva, P.B.; Silva, M.M.V.G.; Corfu, F. Petrogenetic links between lepidolite-subtype aplite-pegmatite, aplite veins and associated granites at Segura (central Portugal). Geochemistry 2013, 73, 323–341. [Google Scholar] [CrossRef]
- Martins, L.P. Mineral Resources of Portugal; DGEG: Lisbon, Portugal, 2012; 71p.
- Antunes, I.M.H.R.; Neiva, A.M.R.; Silva, M.M.V.G. Caracterização geoquímica de minerais de jazigos da região de Segura (Castelo Branco). Geol. Eng. Recur. Geol. 2003, 2, 157–168. [Google Scholar]
- Antunes, I.M.H.R.; Neiva, A.M.R.; Silva, M.M.V.G.; Silva, P.B. Mineralogia dos filões aplito-pegmatíticos litiníferos da região de Segura. In Ciências Geológicas—Ensino, Investigação e Sua História; Neiva, J.M.C., Ribeiro, A., Victor, L.M., Noronha, F., Ramalho, M.M., Eds.; Associação Portuguesa de Geólogos/Sociedade Geológica de Portugal: Lisboa, Portugal, 2010; Volume 1, pp. 3–14. [Google Scholar]
- Parfenoff, A.; Pomerol, C.; Tourenq, J. Les Minéraux en Grains: Méthodes d’étude et Determination; Masson et Cie, Éditeurs: Échandens, Switzerland, 1970; 578p. [Google Scholar]
- Klein, C.; Dutrow, B. Manual of Mineral Science (after J.D. Dana), 23rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; 716p. [Google Scholar]
- American Mineralogist Crystal Structure Database. Available online: http://rruff.geo.arizona.edu/AMS/amcsd.php (accessed on 1 April 2022).
- Viegas, L.; Rodrigues, L.; Romão, J.; Cardoso Fonseca, E. Estatística Univariada e Cartas de Médias Móveis em Prospecção Geoquímica: Aplicação à Área de Zebreira—Segura (Beira Baixa). Estud. Notas Trab. Serv. Fom. Min. 1988, 30, 29–36. [Google Scholar]
- Zack, T.; Kronz, A.; Foley, S.F.; Rivers, T. Trace element abundances in rutiles from eclogites and associated garnet mica schists. Chem. Geol. 2002, 184, 97–122. [Google Scholar] [CrossRef]
- Bromiley, G.D.; Hilairet, N. Hydrogen and minor element incorporation in synthetic rutile. Mineral. Mag. 2005, 69, 345–358. [Google Scholar] [CrossRef]
- Carruzzo, S.; Clarke, D.B.; Pelrine, K.M.; MacDonald, M.A. Texture, composition, and origin of rutile in the South Mountain Batholith, Nova Scotia. Can. Mineral. 2006, 44, 715–729. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Mineral. 1954, 39, 92–96. [Google Scholar]
- Myers, E.; Heine, V.; Dove, M. Thermodynamics of Al/Al avoidance in the ordering of Al/Si tetrahedral framework structures. Phys. Chem. Min. 1998, 25, 457–464. [Google Scholar] [CrossRef]
- Sarkar, A.; Khan, G.G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures. Nanoscale 2019, 11, 3414–3444. [Google Scholar] [CrossRef]
- Zack, T.; von Eynatten, H.; Kronz, A. Rutile geochemistry and its potential use in quantitative provenance studies. Sediment. Geol. 2004, 171, 37–58. [Google Scholar] [CrossRef]
- Luvizotto, G.L.; Zack, T. Nb and Zr behavior in rutile during high-grade metamorphism and retrogression: An example from the Ivrea-Verbano Zone. Chem. Geol. 2009, 261, 303–317. [Google Scholar] [CrossRef]
- Dachille, F.; Simons, P.Y.; Roy, R. Pressure-temperature studies of anatase, brookite, rutile and TiO2-II. Am. Mineral. 1968, 53, 1929–1939. [Google Scholar]
- Jamieson, J.C.; Olinger, B. Pressure-temperature studies of anatase, brookite, rutile, and TiO2(II): A discussion. Am. Mineral. 1968, 54, 1477–1481. [Google Scholar]
- Schmidt, A.; Weyer, S.; John, T.; Brey, G.P. HFSE systematics of rutile-bearing eclogites: New insights into subduction zone processes and implications for the earth’s HFSE budget. Geochim. Cosmochim. Acta 2009, 73, 455–468. [Google Scholar] [CrossRef]
- Triebold, S.; Luvizotto, G.L.; Tolosana-Delgado, R.; Zack, T.; von Eynat-ten, H. Discrimination of TiO2 polymorphs in sedimentary and metamorphic rocks. Contrib. Mineral. Petrol. 2011, 161, 581–596. [Google Scholar] [CrossRef]
- Van Baalen, M.R. Titanium mobility in metamorphic systems: A review. Chem. Geol. 1993, 110, 233–249. [Google Scholar] [CrossRef]
- Ciavatta, L.; Pirozzi, A. The formation of fluoride complexes of titanium(IV). Polyhedron 1983, 2, 769–774. [Google Scholar] [CrossRef]
- Manning, D.A.C.; Henderson, P. The behaviour of tungsten in granitic melt-vapour systems. Contrib. Mineral. Petrol. 1984, 86, 286–293. [Google Scholar] [CrossRef]
- Ciavatta, L.; Ferri, D.; Riccio, G. On the hydrolysis of the titanium(IV) ion in chloride media. Polyhedron 1985, 4, 15–22. [Google Scholar] [CrossRef]
- Aja, S.U.; Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of Zr and the solubility of some Zr-bearing minerals. Appl. Geochem. 1995, 10, 603–620. [Google Scholar] [CrossRef]
- Wu, Y.B.; Gao, S.; Zhang, H.F.; Yang, S.H.; Liu, X.C.; Jiao, W.F.; Liu, Y.S.; Yuan, H.L.; Gong, H.J.; He, M.C. U-Pb age, trace-element, and Hf-isotope compositions of zircon in a Quartz vein from eclogite in the western Dabie mountains: Constraints on fluid flow during early exhumation of ultrahigh-pressure rocks. Am. Mineral. 2009, 94, 303–312. [Google Scholar] [CrossRef]
- van Sijl, J.; Allan, N.L.; Davies, G.R.; van Westrenen, W. Titanium in subduction zone fluids: First insights from ab initio molecular metadynamics simulations. Geochim. Cosmochim. Acta 2010, 74, 2797–2810. [Google Scholar] [CrossRef]
- Milnes, A.R.; Fitzpatrick, R.W. Titanium and zirconium minerals. In Minerals in Soil Environments, 2nd ed.; SSSA Book Series 1; Dixon, J.B., Weed, S.B., Eds.; SSSA: Madison, WI, USA, 1989; pp. 1131–1205. [Google Scholar]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Haggerty, J.E.S.; Schelhas, L.T.; Kitchaev, D.A.; Mangum, J.S.; Garten, L.M.; Sun, W.; Stone, K.H.; Perkins, J.D.; Toney, M.F.; Ceder, G.; et al. High-fraction brookite films from amorphous precursors. Sci. Rep. 2017, 7, 15232. [Google Scholar] [CrossRef]
- Liu, Z.R.R.; Zhou, M.F.; Williams-Jones, A.E.; Wang, W.; Gao, J.F. Diagenetic mobilization of Ti and formation of brookite/anatase in early Cambrian black shales, South China. Chem. Geol. 2019, 506, 79–96. [Google Scholar] [CrossRef]
- Green, T.H.; Pearson, N.J. An experimental study of Nb and Ta partitioning between Ti-rich minerals and silicate liquids at high pressure and temperature. Geochim. Cosmochim. Acta 1987, 51, 55–62. [Google Scholar] [CrossRef]
- Brenan, J.M.; Shaw, H.F.; Phinney, D.L.; Ryerson, F.J. Rutile-aqueous fluid partitioning of Nb, Ta, Hf, Zr, U and Th: Implications for high field strength element depletions in island-arc basalts. Earth Planet. Sci. Lett. 1994, 128, 327–339. [Google Scholar] [CrossRef]
- Adam, J.; Green, T.H.; Sie, S.H.; Ryan, C.G. Trace element partitioning between aqueous fluids, silicate melts and minerals. Eur. J. Mineral. 1997, 9, 569–584. [Google Scholar] [CrossRef]
- Stalder, R.; Foley, S.F.; Brey, G.P.; Horn, I. Mineral-aqueous fluid partitioning of trace elements at 900–1200 degrees C and 3.0–5.7 GPa: New experimental data for garnet, clinopyroxene, and rutile, and implications for mantle metasomatism. Geochim. Cosmochim. Acta 1998, 62, 1781–1801. [Google Scholar] [CrossRef]
- Foley, S.F.; Barth, M.G.; Jenner, G.A. Rutile/melt partition coefficients for trace elements and an assessment of the influence of rutile on the trace element characteristics of subduction zone magmas. Geochim. Et Cosmochim. Acta 2000, 64, 933–938. [Google Scholar] [CrossRef]
- Klemme, S.; Prowatke, S.; Hametner, K.; Günther, D. Partitioning of trace elements between rutile and silicate melts: Implications for subduction zones. Geochim. Cosmochim. Acta 2005, 69, 2361–2371. [Google Scholar] [CrossRef]
- Tanis, E.A.; Simon, A.; Tschauner, O.; Chow, P.; Xiao, Y.; Burnley, P.C.; Cline, C.J., II; Hanchar, J.M.; Pettke, T.; Shen, G.; et al. The mobility of Nb in rutile-saturated NaCl- and NaF-bearing aqueous fluids from 1–6.5 GPa and 300–800 degrees C. Am. Mineral. 2015, 100, 1600–1609. [Google Scholar] [CrossRef]
- Mallmann, G.; Fonseca, R.O.C.; Silva, A.B. An experimental study of the partitioning of trace elements between rutile and silicate melt as a function of oxygen fugacity. An. Acad. Bras. Cienc. 2014, 86, 1609–1629. [Google Scholar] [CrossRef] [PubMed]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can. Mineral. 1996, 34, 1111–1126. [Google Scholar]



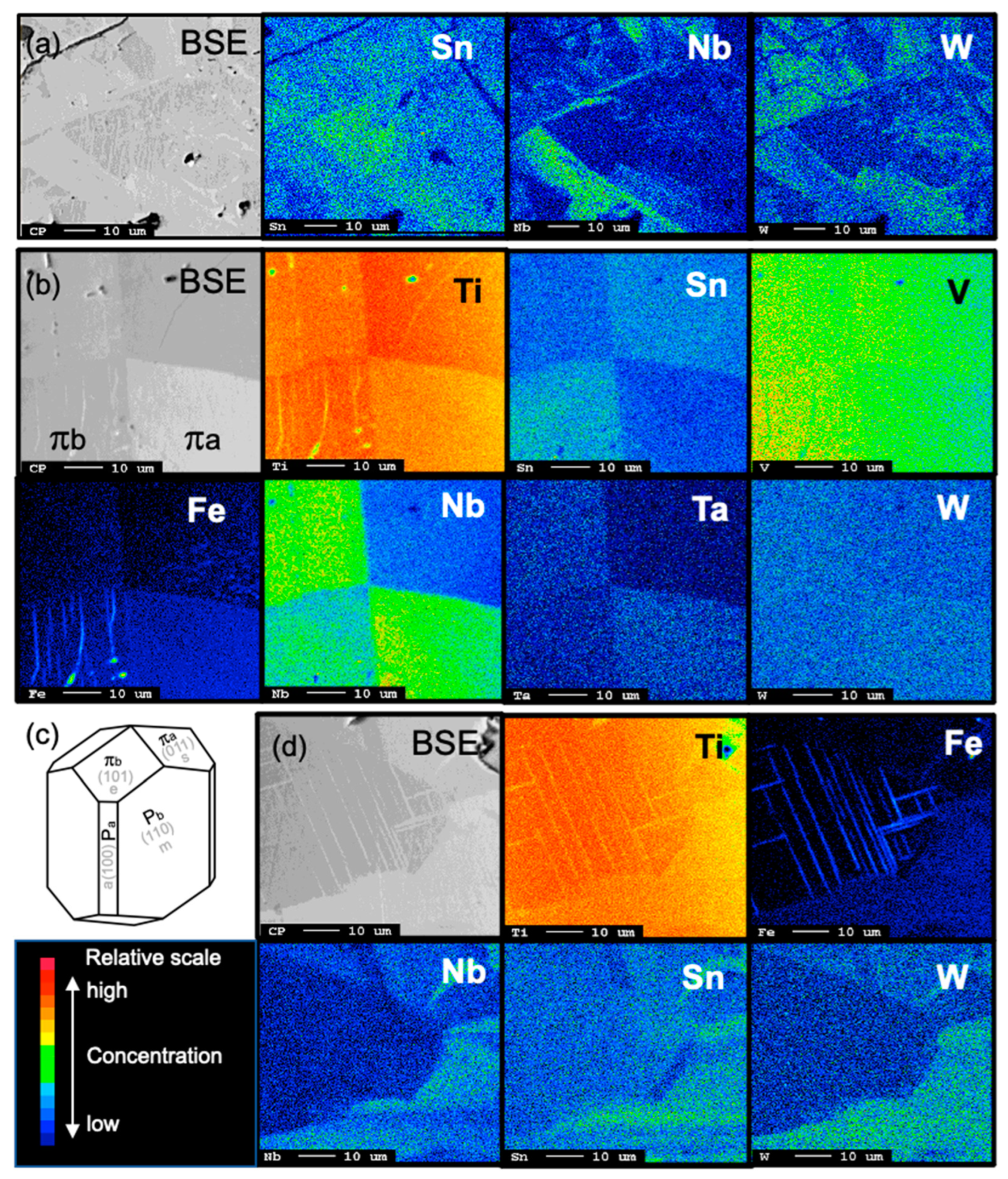
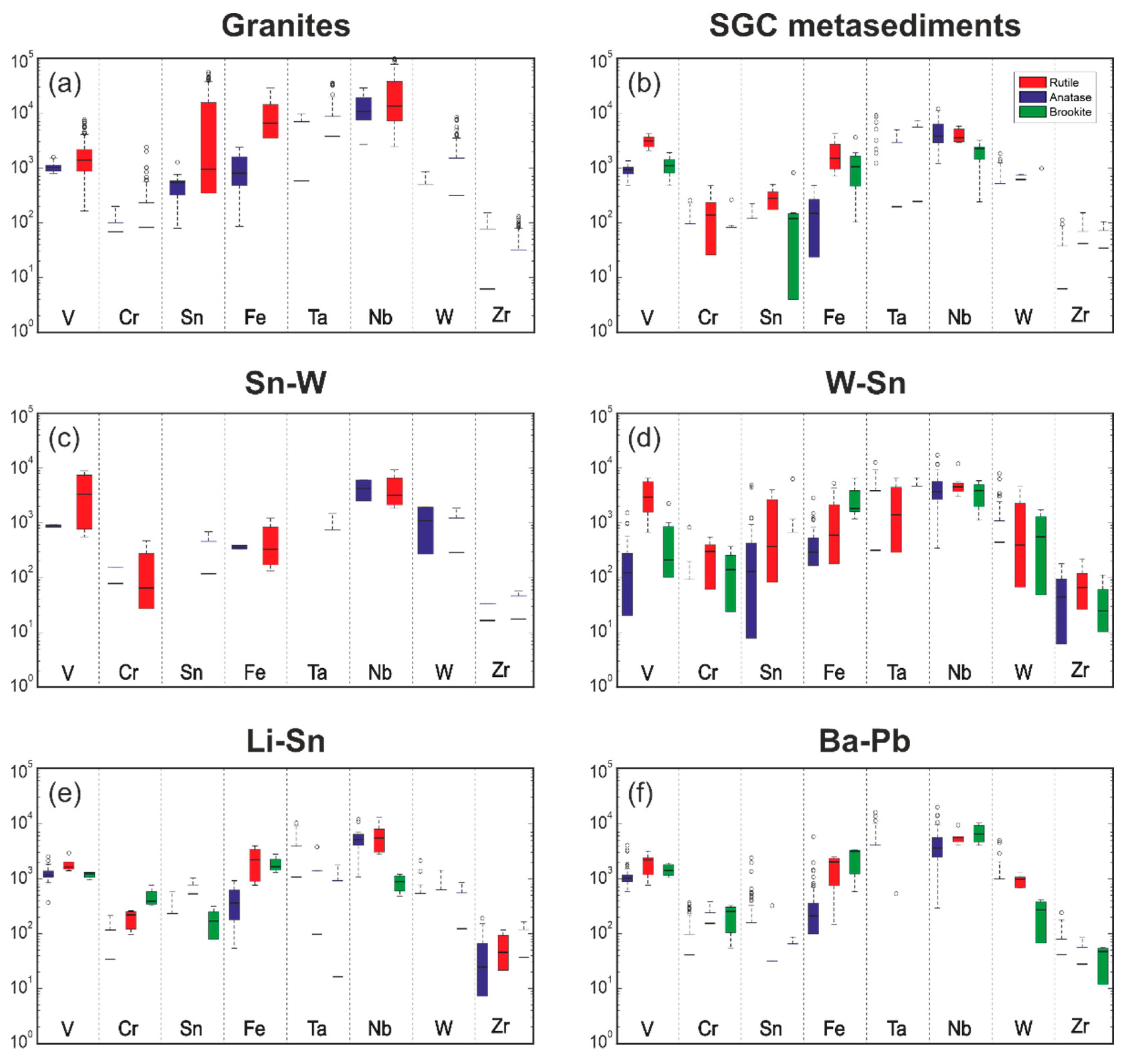
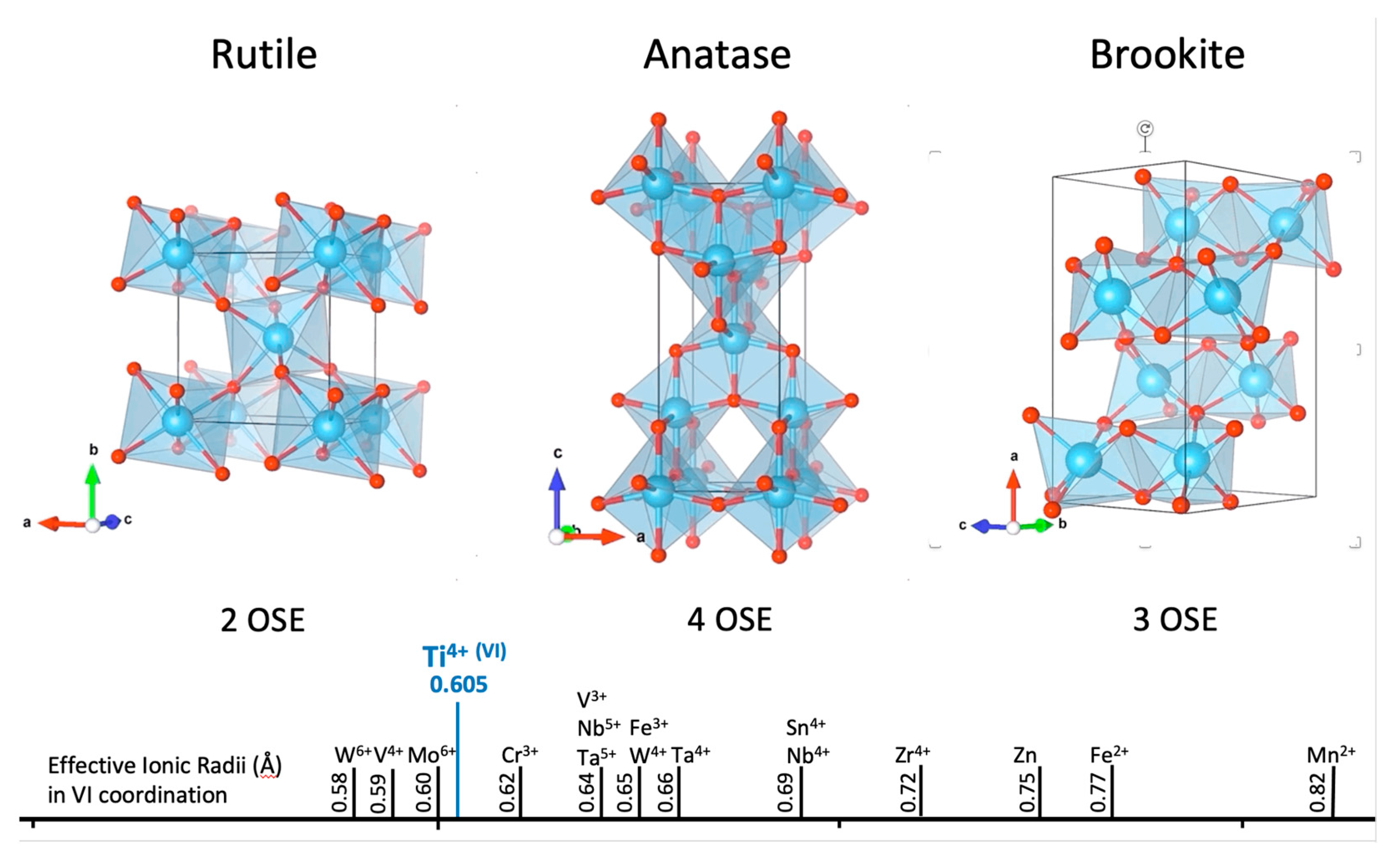
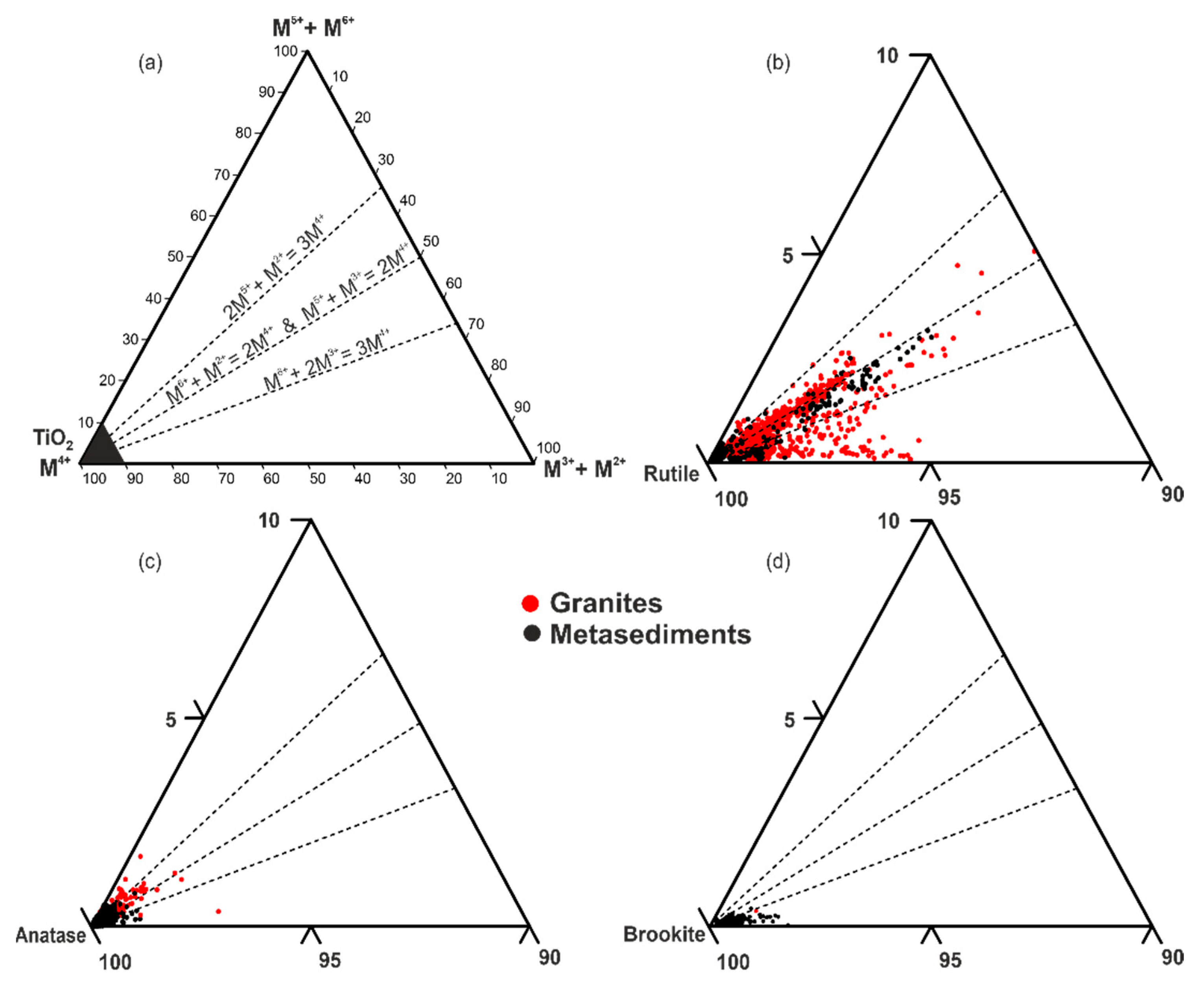
| Mineral | % | Mineral | % | Mineral | % |
|---|---|---|---|---|---|
| Iron oxyhydroxide | 43.18 | Andalusite | 0.78 | Corundum | 0.03 |
| Tourmaline | 11.52 | Biotite | 0.74 | Classic monazite | 0.03 |
| Magnetite | 11.03 | Brookite | 0.72 | Epidote | 0.01 |
| Ilmenite | 5.61 | Zoisite | 0.42 | Chlorite | 0.01 |
| Cassiterite | 4.57 | Zircon | 0.41 | Sillimanite | 0.01 |
| Nodular monazite | 3.41 | Scheelite | 0.38 | Xenotime | 0.01 |
| Anatase | 2.73 | Altered pyrite | 0.34 | Topaz | <0.01 |
| Wolframite | 2.20 | Garnet | 0.30 | Magnetic leucoxene | <0.01 |
| Baryte | 2.19 | Gold | 0.12 | Nonmagnetic leucoxene | <0.01 |
| Apatite | 2.03 | Muscovite | 0.07 | ||
| Rutile | 1.18 | Cinnabar | 0.05 | Und. minerals | 6.33 |
| Total | 100% |
| Granites | SGC Metasediments | Sn-W | W-Sn | Li-Sn | Ba-Pb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral | % | Mineral | % | Mineral | % | Mineral | % | Mineral | % | Mineral | % |
| Tourmaline | 57.71 | Fe O-OH. | 52.93 | Fe O-OH. | 42.60 | Fe O-OH. | 37.24 | Ilmenite | 49.92 | Fe O-OH. | 35.50 |
| Biotite | 13.86 | Magnetite | 31.75 | Cassiterite | 35.50 | Wolframite | 37.24 | Magnetite | 19.97 | Baryte | 22.19 |
| Apatite | 7.69 | Anatase | 6.61 | Wolframite | 17.04 | Cassiterite | 10.63 | Cassiterite | 12.48 | Magnetite | 14.20 |
| Rutile | 4.61 | Altered Pyrite | 2.54 | Apatite | 1.70 | Anatase | 4.25 | Scheelite | 4.99 | Anatase | 5.32 |
| Altered pyrite | 2.77 | Ilmenite | 2.54 | Epidote | 0.56 | Und. minerals | 4.25 | Altered pyrite | 3.99 | Cassiterite | 5.32 |
| Garnet | 2.77 | Rutile | 1.59 | Nod. Monazite | 0.56 | Magnetite | 2.97 | Tourmaline | 3.99 | Rutile | 5.32 |
| Fe Ox-hyd. | 2.77 | Und. minerals | 1.59 | Anatase | 0.29 | Brookite | 0.86 | Anatase | 1.00 | Ilmenite | 2.84 |
| Zoisite | 2.77 | Brookite | 0.31 | Brookite | 0.29 | Scheelite | 0.86 | Rutile | 1.00 | Tourmaline | 2.84 |
| Anatase | 1.84 | Gold | 0.06 | Gold | 0.29 | Ilmenite | 0.50 | Zircon | 1.00 | Zoisite | 2.84 |
| Zircon | 1.84 | Scheelite | 0.06 | Rutile | 0.29 | Tourmaline | 0.50 | Nod. Monazite | 0.67 | Andalusite | 1.07 |
| Ilmenite | 0.46 | Topaz | 0.29 | Baryte | 0.14 | Zoisite | 0.67 | Brookite | 1.07 | ||
| Magnetite | 0.46 | Scheelite | 0.29 | Gold | 0.14 | Brookite | 0.17 | Altered pyrite | 0.47 | ||
| Andalusite | 0.37 | Und. minerals | 0.29 | Muscovite | 0.14 | Gold | 0.17 | Wolframite | 0.47 | ||
| Muscovite | 0.06 | Rutile | 0.14 | Gold | 0.18 | ||||||
| Zircon | 0.14 | Scheelite | 0.18 | ||||||||
| Zircon | 0.18 | ||||||||||
| Rutile (ppm) | V | Cr | Fe | Sn | Nb | Ta | W | Zr |
|---|---|---|---|---|---|---|---|---|
| Minimum | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l |
| Maximum | 10,937 | 4201 | 29,258 | 55,665 | 85,347 | 19,139 | 57,173 | 1577 |
| Average | 1712 | 168 | 5882 | 4462 | 5723 | 890 | 5126 | 68 |
| Median | 1271 | <b.d.l | 4508 | 882 | 3845 | <b.d.l | 1491 | <b.d.l |
| Std. Dev. | 1388 | 370 | 5196 | 7900 | 6269 | 1990 | 8898 | 163 |
| Anatase (ppm) | ||||||||
| Minimum | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l |
| Maximum | 5289 | 1006 | 17,699 | 4892 | 12,296 | 16,895 | 10,523 | 992 |
| Average | 754 | 23 | 472 | 85 | 1575 | 261 | 694 | 99 |
| Median | 877 | <b.d.l | 894 | <b.d.l | 1139 | <b.d.l | <b.d.l | <b.d.l |
| Std. Dev. | 545 | 88 | 280 | 387 | 1510 | 833 | 1566 | 193 |
| Brookite (ppm) | ||||||||
| Minimum | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l | <b.d.l |
| Maximum | 2855 | 1526 | 9553 | 6333 | 4649 | 2334 | 3457 | 429 |
| Average | 762 | 141 | 2268 | 123 | 1082 | 401 | 373 | 42 |
| Median | 714 | <b.d.l | 1780 | <b.d.l | 811 | <b.d.l | <b.d.l | <b.d.l |
| Std. Dev. | 622 | 236 | 1748 | 640 | 862 | 575 | 633 | 115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaspar, M.; Grácio, N.; Salgueiro, R.; Costa, M. Trace Element Geochemistry of Alluvial TiO2 Polymorphs as a Proxy for Sn and W Deposits. Minerals 2022, 12, 1248. https://doi.org/10.3390/min12101248
Gaspar M, Grácio N, Salgueiro R, Costa M. Trace Element Geochemistry of Alluvial TiO2 Polymorphs as a Proxy for Sn and W Deposits. Minerals. 2022; 12(10):1248. https://doi.org/10.3390/min12101248
Chicago/Turabian StyleGaspar, Miguel, Nuno Grácio, Rute Salgueiro, and Mafalda Costa. 2022. "Trace Element Geochemistry of Alluvial TiO2 Polymorphs as a Proxy for Sn and W Deposits" Minerals 12, no. 10: 1248. https://doi.org/10.3390/min12101248
APA StyleGaspar, M., Grácio, N., Salgueiro, R., & Costa, M. (2022). Trace Element Geochemistry of Alluvial TiO2 Polymorphs as a Proxy for Sn and W Deposits. Minerals, 12(10), 1248. https://doi.org/10.3390/min12101248








