Cyanide Depression Mechanism for Sphalerite Flotation Separation Based on Density Functional Theory Calculations and Coordination Chemistry
Abstract
1. Introduction
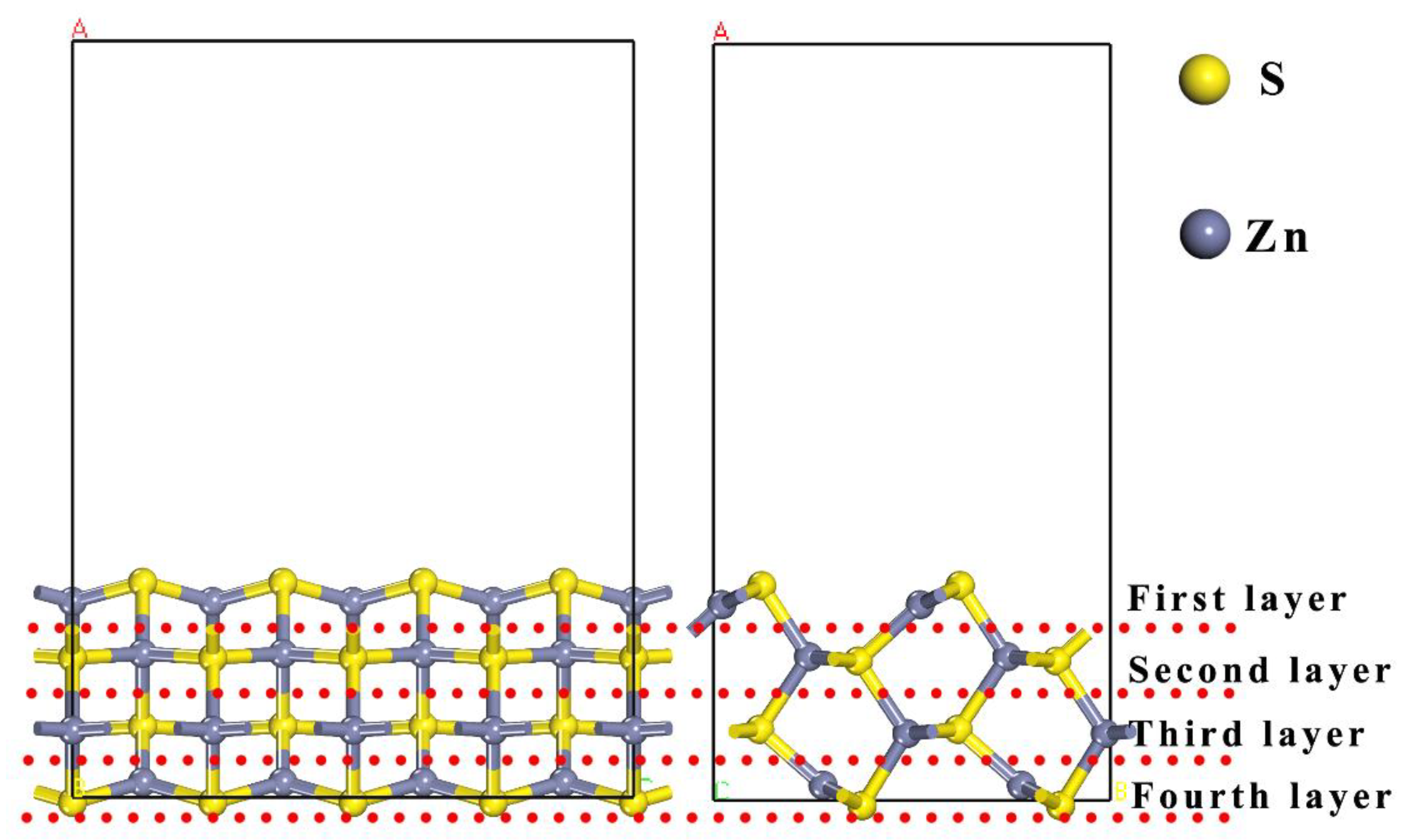
2. Computational Methods
3. Results and Discussion
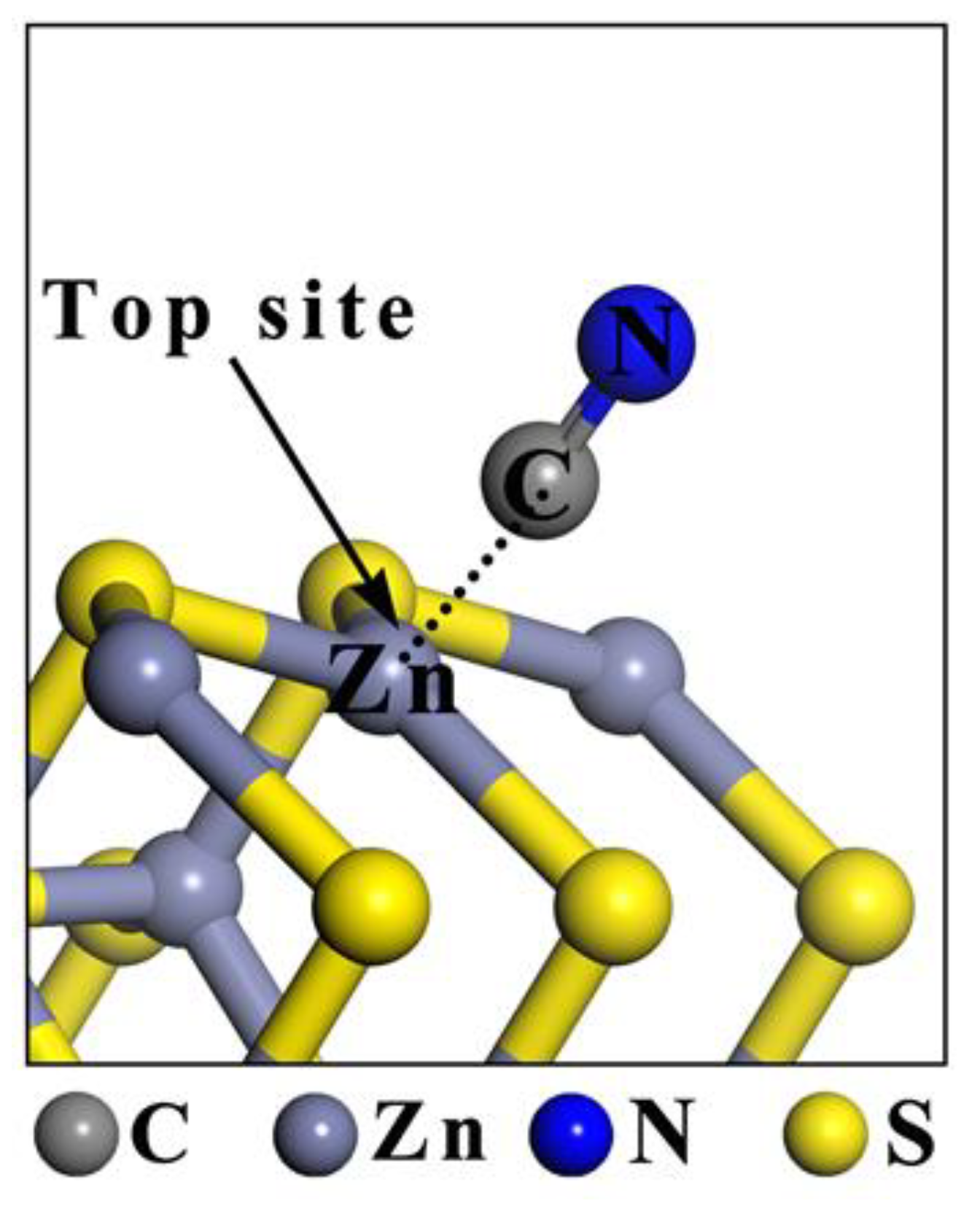
3.1. Adsorption Energy
3.2. Mulliken Population Analysis
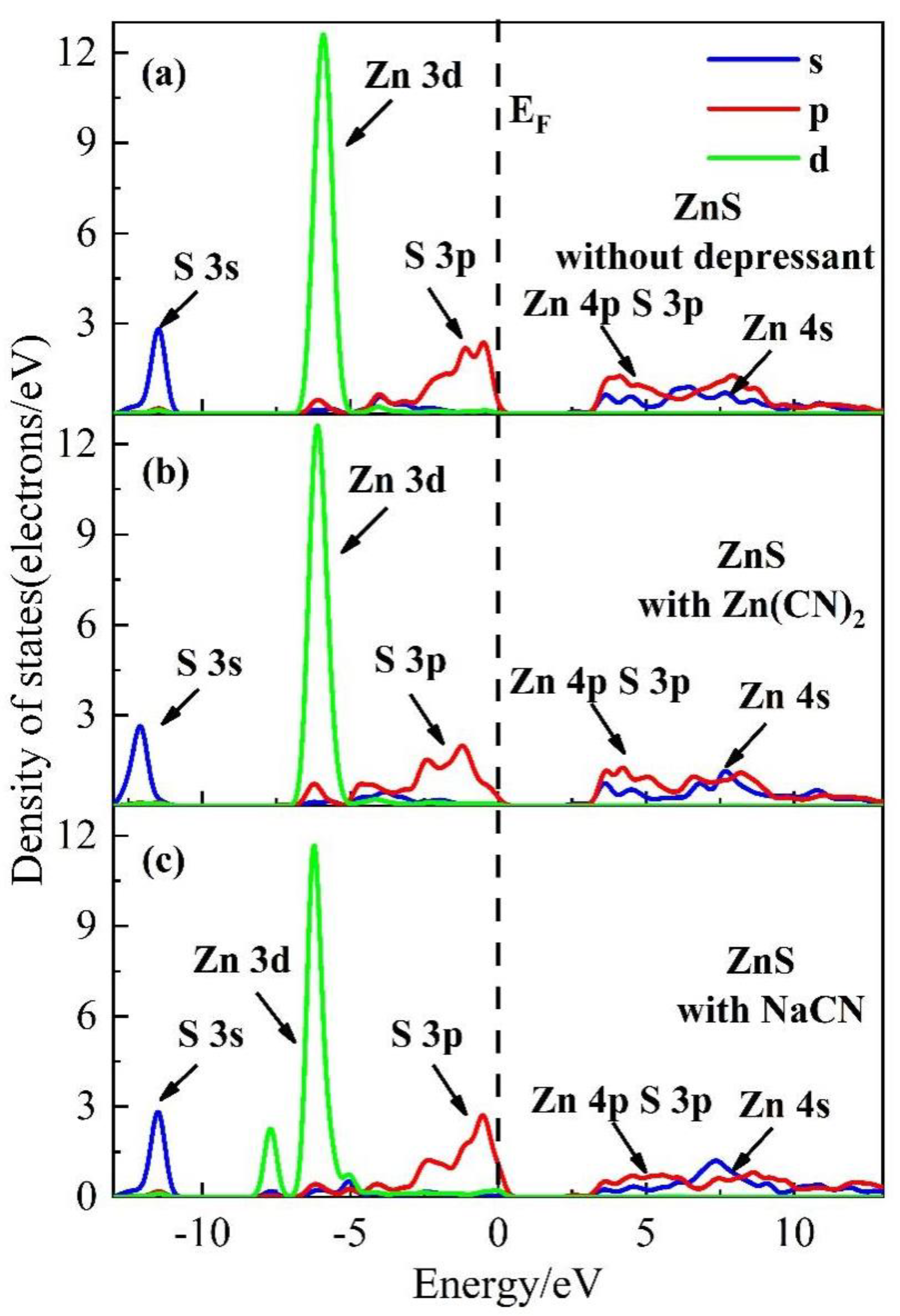
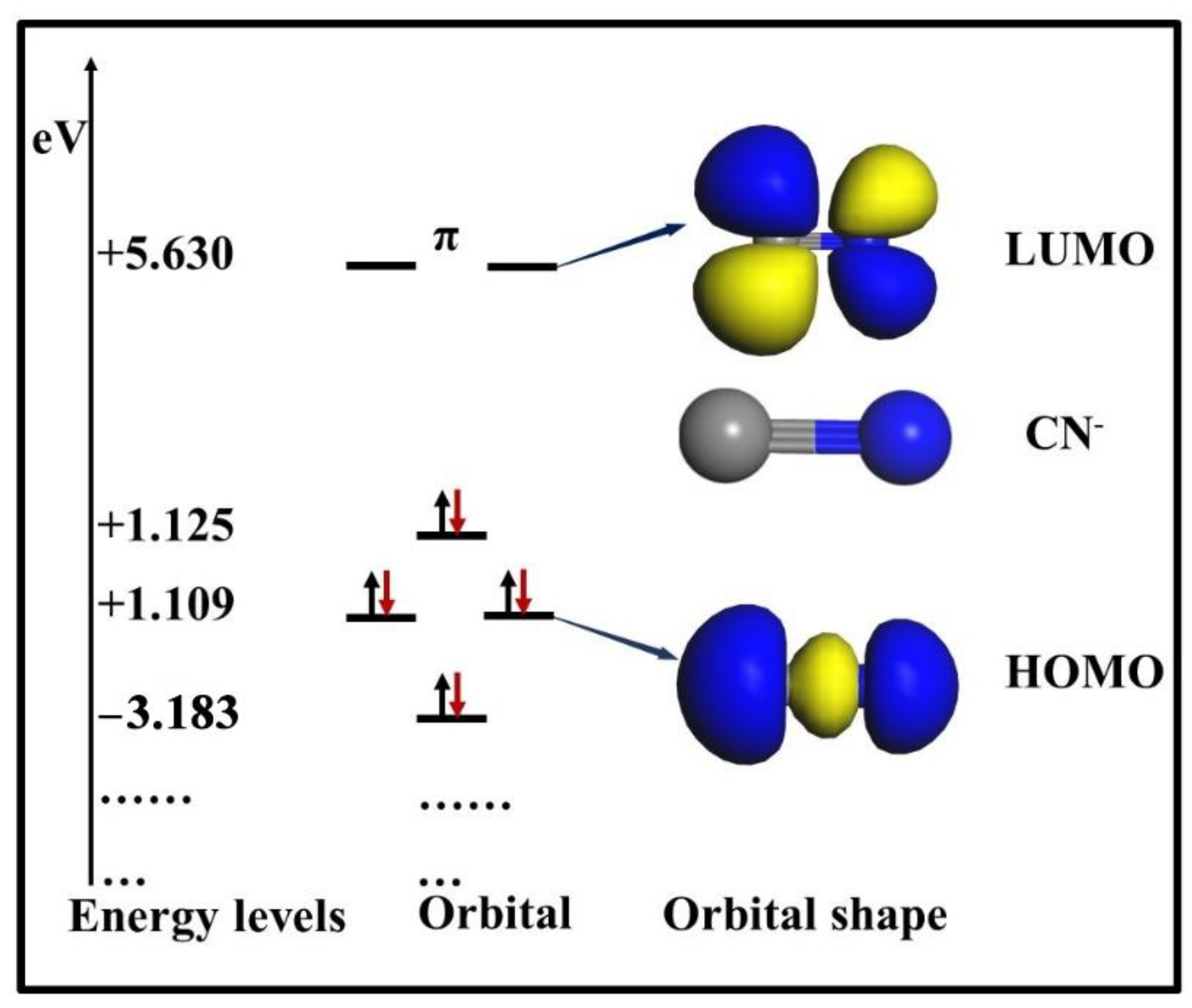
3.3. Density of States (DOS) Analysis
3.4. Coordination of CN− with Zn Ions on the Sphalerite Surface
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, H.; Wen, S.; Han, G.; Xu, L.; Feng, Q. Activation mechanism of lead ions in the flotation of sphalerite depressed with zinc sulfate. Miner. Eng. 2020, 146, 106132. [Google Scholar] [CrossRef]
- Hu, X.; Huang, H.; Chen, Y.; Mao, J. Theory and Technology of Flotation; Central South University of Technology Press: Changsha, China. (In Chinese)
- Botz, M.M.; Mudder, T.I.; Akcil, A.U. Cyanide treatment: Physical, chemical and biological processes. In Developments in Mineral Processing; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 672–702. [Google Scholar]
- Reddy, G.S.; Reddy, C.K.; Sekhar, D.M.R.; Ravindranath, K.; Chulet, M.R. Effect of formaldehyde and nickel sulphate solutions on the activation of cyanide-depressed sphalerite. Miner. Eng. 1991, 4, 151–160. [Google Scholar] [CrossRef]
- Sutherland, K.L.; Wark, I.W. (Australian Institute of Mining and Metallurgy, Melbourne, Australia). Personal communication, 1955. [Google Scholar]
- Guo, B.; Peng, Y.; Mai, Y. The effect of zinc cyanide on the flotation of gold from pyritic ore. Miner. Eng. 2016, 85, 106–111. [Google Scholar] [CrossRef]
- Seke, M.D.; Pistorius, P.C. Effect of cuprous cyanide, dry and wet milling on the selective flotation of galena and sphalerite. Miner. Eng. 2006, 19, 1–11. [Google Scholar] [CrossRef][Green Version]
- Wang, X.H.; Forssberg, K.S.E. The solution electrochemistry of sulfide-xanthate-cyanide systems in sulfide mineral flotation. Miner. Eng. 1996, 9, 527–546. [Google Scholar] [CrossRef]
- Guo, B.; Peng, Y.; Espinosa-Gomez, R. Cyanide chemistry and its effect on mineral flotation. Miner. Eng. 2014, 66–68, 25–32. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Depression of iron sulphide flotation in zinc roughers. Miner. Eng. 2001, 14, 1067–1079. [Google Scholar] [CrossRef]
- Wang, H.; Wen, S.; Han, G.; Feng, Q. Effect of copper ions on surface properties of ZnSO4-depressed sphalerite and its response to flotation. Sep. Purif. Technol. 2019, 228, 115756. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Chen, Y.; Guo, J. A DFT study of the effect of natural impurities on the electronic structure of galena. Int. J. Miner. Process. 2011, 98, 132–136. [Google Scholar] [CrossRef]
- de Oliveira, C.; Duarte, H.A. Disulphide and metal sulphide formation on the reconstructed (001) surface of chalcopyrite: A DFT study. Appl. Surf. Sci. 2010, 257, 1319–1324. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite—Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J. The first-principle study of the effect of lattice impurity on adsorption of CN on sphalerite surface. Miner. Eng. 2010, 23, 676–684. [Google Scholar] [CrossRef]
- Nie, Q.; Wang, M.; Qiu, T.; Qiu, X. Density Functional Theory and XPS Studies of the Adsorption of Cyanide on Chalcopyrite Surfaces. ACS Omega 2020, 5, 22778–22785. [Google Scholar] [CrossRef]
- Chen, J. The interaction of flotation reagents with metal ions in mineral surfaces: A perspective from coordination chemistry. Miner. Eng. 2021, 171, 107067. [Google Scholar] [CrossRef]
- Hounfodji, J.W.; Kanhounnon, W.G.; Kpotin, G.; Atohoun, G.S.; Lainé, J.; Foucaud, Y.; Badawi, M. Molecular insights on the adsorption of some pharmaceutical residues from wastewater on kaolinite surfaces. Chem. Eng. J. 2021, 407, 127176. [Google Scholar] [CrossRef]
- Dai, P.; Chen, H.; Chen, L.; Liu, Y.; Wei, Z. Depression mechanism of peracetic acid for flotation separation of chalcopyrite from arsenopyrite based on coordination chemistry. Miner. Eng. 2022, 186, 107757. [Google Scholar] [CrossRef]
- Dai, P.; Wei, Z.; Chen, L.; Liu, Y. Adsorption of butyl xanthate on arsenopyrite (001) and Cu2+-activated arsenopyrite (001) surfaces: A DFT study. Chem. Phys. 2022, 562, 111668. [Google Scholar] [CrossRef]
- Harmer, S.L.; Goncharova, L.V.; Kolarova, R. Surface structure of sphalerite studied by medium energy ion scattering and XPS. Surf. Sci. 2007, 601, 352–361. [Google Scholar] [CrossRef]
- Qiu, T.; He, Y.; Qiu, X.; Yang, X. Density functional theory and experimental studies of Cu2+ activation on a cyanide-leached sphalerite surface. J. Ind. Eng. Chem. 2017, 45, 307–315. [Google Scholar] [CrossRef]
- Qiu, T.; Nie, Q.; He, Y.; Yuan, Q. Density functional theory study of cyanide adsorption on the sphalerite (1 1 0) surface. Appl. Surf. Sci. 2019, 465, 678–685. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Chen, J. Coordination Principle of Minerals Flotation; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]


| Adsorption Type | Adsorption Energy (kJ/mol) |
|---|---|
| BX + ZnS (110) | −66.12 |
| CN− + ZnS (110) | −233.88 |
| Zn(CN)2 + ZnS (110) | −99.76 |
| Zn(OH)2 + ZnS (110) | −33.42 |
| Model | Atom | s | p | d | Total | Charge |
|---|---|---|---|---|---|---|
| ZnS | Zn | 0.89 | 0.69 | 9.98 | 11.56 | 0.44 |
| S | 1.86 | 4.65 | 0.00 | 6.51 | −0.51 | |
| ZnS + CN− | Zn | 0.75 | 0.89 | 9.91 | 11.54 | 0.46 |
| S | 1.87 | 4.58 | 0.00 | 6.44 | −0.44 | |
| ZnS + Zn(CN)2 | Zn | 0.84 | 0.68 | 9.98 | 11.50 | 0.50 |
| S | 1.83 | 4.71 | 0.00 | 6.54 | −0.54 | |
| ZnS + Zn(OH)2 | Zn | 0.84 | 0.71 | 9.97 | 11.52 | 0.48 |
| S | 1.85 | 4.67 | 0.00 | 6.51 | −0.51 |
| Adsorption Mode | Chemical Bond | Population | Bond Length (Å) |
|---|---|---|---|
| CN− + ZnS | Zn-C | 0.66 | 1.74 |
| Zn(CN)2 + ZnS | Zn-S | 0.42 | 2.37 |
| Zn(OH)2 + ZnS | Zn-S | 0.10 | 2.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Tong, X.; Xie, X.; Xie, R.; Song, Q.; Cui, Y.; Xiao, Y.; Dai, P. Cyanide Depression Mechanism for Sphalerite Flotation Separation Based on Density Functional Theory Calculations and Coordination Chemistry. Minerals 2022, 12, 1271. https://doi.org/10.3390/min12101271
Chen H, Tong X, Xie X, Xie R, Song Q, Cui Y, Xiao Y, Dai P. Cyanide Depression Mechanism for Sphalerite Flotation Separation Based on Density Functional Theory Calculations and Coordination Chemistry. Minerals. 2022; 12(10):1271. https://doi.org/10.3390/min12101271
Chicago/Turabian StyleChen, Hang, Xiong Tong, Xian Xie, Ruiqi Xie, Qiang Song, Yiqi Cui, Youming Xiao, and Pulin Dai. 2022. "Cyanide Depression Mechanism for Sphalerite Flotation Separation Based on Density Functional Theory Calculations and Coordination Chemistry" Minerals 12, no. 10: 1271. https://doi.org/10.3390/min12101271
APA StyleChen, H., Tong, X., Xie, X., Xie, R., Song, Q., Cui, Y., Xiao, Y., & Dai, P. (2022). Cyanide Depression Mechanism for Sphalerite Flotation Separation Based on Density Functional Theory Calculations and Coordination Chemistry. Minerals, 12(10), 1271. https://doi.org/10.3390/min12101271





