Characterization of Discarded Lead–Zinc Sulfide Ore Tailings Based on Mineral Fragments
Abstract
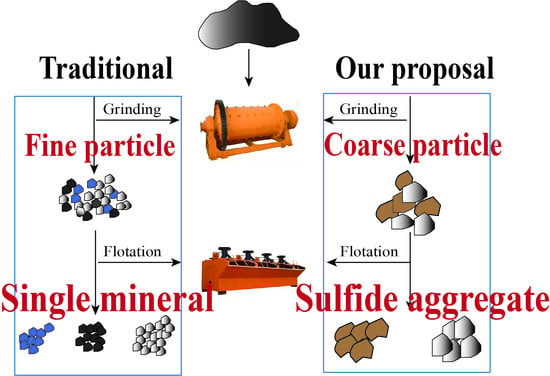
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Mineral Composition
2.2.2. Mineral Liberation
2.2.3. The Grade and Recovery of Concentrate
- ε, the recovery of Pb/Zn during separation, %;
- α, the grade of Pb/Zn in the ore, %;
- β, the grade of Pb/Zn in the concentrate, %;
- γ, the yield of concentrate during separation, %.
2.2.4. Grinding and Flotation Experiments
2.3. Technical Route
3. Results and Discussions
3.1. Ore’s Mineralogical Characteristics
3.1.1. Chemical Composition
3.1.2. Chemical Phase Analysis
3.1.3. Analysis of Ore Material’s Composition
3.1.4. Analysis of the Embedded Characteristics of Sulfide Minerals
3.2. Fragment’s Mineralogical Characteristics
3.2.1. Granularity Dissemination of Minerals after Primary Grinding
3.2.2. Particle Size and Liberation Degree of Sulfide Aggregates
3.3. Primary Flotation
3.3.1. Effects of Grinding Fineness and Carbon
3.3.2. Effects of Type and Dosage of Collector
3.3.3. Effect of #2 Oil Dosage
3.3.4. Effect of pH
3.4. Product Inspection
4. Conclusions
- (1)
- The MLA technology is used to measure two grinding products with a grinding fineness of −0.074 mm, accounting for 48% and 69%. With a grinding fineness of −0.074 mm accounting for 48%, it is more technically reasonable to use the sulfide aggregates as the recovery objects to carry out the rough particle flotation tailings;
- (2)
- Determine the characteristics of the aggregates fragments that can be re-covered after coarse grinding. The recovery object of coarse-grained mixed flotation is a sulfide mineral-rich conglomerate, which can be thrown after the liberation degree reaches 70%;
- (3)
- Galena and sphalerite are mostly lost to a −0.4 + 0.3 mm particle size, and the distribution rate of lead and zinc for the +0.4 mm particle size is higher, indicating that coarse-grained minerals are not easy to float;
- (4)
- The optimized process conditions: In the first step of flotation, the carbon in lead–zinc sulfide ore was removed, by adding #2 oil to the flotation tank at a dosage of 40 g/t. In the second flotation process, the pH was adjusted to 7.2, the dosage of isopropyl xanthate was 20 g/t, the dosage of #2 oil was 30 g/t; the flotation reagent in the third step was isopropyl xanthate, and the dosage was 7.5 g/t, with 15 g/t of #2 oil. At the selected conditions, the recoveries of Pb and Zn were 91.78% and 92.07%, respectively. Moreover, the grades of Pb and Zn were 1.6% and 5.71%, respectively, with the tailing discard rate reaching 50.6%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konieczny, A.; Pawlos, W.; Malgorzata, K. Evaluation of organic carbon separation from copper ore by pre-flotation. Physicochem. Probl. Miner. Process. 2013, 49, 189–201. [Google Scholar]
- Nayak, A.; Jena, M.S. Mineralogical characterization for selection of possible beneficiation route for low grade lead-zinc ore of Rampura Agucha, India. Trans. Indian Inst. Met. 2020, 73, 775–784. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Sun, G.; Xiao, S.; Lin, H. Oxygen-functionalized defect engineering of carbon additives enable lead-carbon batteries with high cycling stability. J. Energy Storage 2021, 43, 103205. [Google Scholar] [CrossRef]
- Zhang, L.M.; Gao, J.D.; Khoso, S.A.; Wang, L.; Liu, Y.; Ge, P.; Tian, M.; Sun, W. A reagent scheme for galena/sphalerite flotation separation: Insights from first-principles calculations. Miner. Eng. 2021, 167, 106885. [Google Scholar] [CrossRef]
- Nayak, A.; Jena, M.S.; Mandre, N.R. Beneficiation of Lead-Zinc ores—A review. Min. Proc. Ext. Met. Rev. 2022, 43, 564–583. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, D.Q.; Wang, D.H. A preliminary review of metallogenic regularity of silver deposits in China. Acta Geol. Sin. Engl. 2015, 89, 1002–1020. [Google Scholar]
- Mudd, G.M.; Jowitt, S.M.; Werner, T.T. The world’s lead-zinc mineral resources: Scarcity, data, issues and opportunities. Ore. Geol. Rev. 2017, 80, 1160–1190. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yang, X.L.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Pan, Z.; Xiong, J.; Cui, Y.; Wei, Q.; Jia, W.; Zhang, Z.; Jiao, F.; Qin, W. Effect mechanism of carbonaceous materials on the flotation separation of lead–zinc ore. Sep. Purif. Technol. 2022, 294, 121101. [Google Scholar] [CrossRef]
- Tromans, D. Mineral comminution: Energy efficiency considerations. Miner. Eng. 2008, 21, 613–620. [Google Scholar] [CrossRef]
- Godirilwe, L.L.; Magwaneng, R.S.; Sagami, R.; Haga, K.; Batnasan, A.; Aoki, S.; Kawasaki, T.; Matsuoka, H.; Mitsuhashi, K.; Kawata, M.; et al. Extraction of copper from complex carbonaceous sulfide ore by direct high-pressure leaching. Miner. Eng. 2021, 173, 107181. [Google Scholar] [CrossRef]
- Sun, W.; Su, J.F.; Zhang, G.; Hu, Y.H. Separation of sulfide lead-zinc-silver ore under low alkalinity condition. J. Cent. South Univ. 2012, 19, 2307–2315. [Google Scholar] [CrossRef]
- Oyelola, A.O.; Abdu, D.A.; Victor, A.D.; Igonwelundu, M.T.; Bosan, B.M.; Oyebode, A.B. Extraction of a low-grade zinc ore using gravity and froth flotation methods. J. Appl. Sci. Environ. Manag. 2017, 20, 903. [Google Scholar] [CrossRef] [Green Version]
- Sahu, P.; Jena, M.S.; Mandre, N.R.; Venugopal, R. Platinum group elements mineralogy, beneficiation, and extraction practices—An overview. Miner. Proc. Ext. Met. Rev. 2021, 42, 521–534. [Google Scholar] [CrossRef]
- Onal, G.; Bulut, G.; Gul, A.; Kangal, O.; Perek, K.T.; Arslan, F.J.M.E. Flotation of Aladag oxide lead-zinc ores. Miner. Eng. 2005, 18, 279–282. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Bao, L.Y.; Lu, J.F.; Pang, J.; Hu, P.J.; Huang, Y.S. Flotation response of cerussite after hydrothermal treatment with sulfur and the sulfidation mechanism. J. Mater. Res. Technol. 2021, 15, 2933–2942. [Google Scholar] [CrossRef]
- He, J.Y.; Zhang, H.L.; Yue, T.; Sun, W.; Hu, Y.H.; Zhang, C.Y. Effects of Hydration on the Adsorption of Benzohydroxamic Acid on the Lead-Ion-Activated Cassiterite Surface: A DFT Study. Langmuir 2021, 37, 2205–2212. [Google Scholar] [CrossRef]
- Lan, Z.Y.; Lai, Z.N.; Zheng, Y.X.; Lv, J.F.; Pang, J.; Ning, J.L. Thermochemical modification for the surface of smithsonite with sulfur and its flotation response. Miner. Eng. 2020, 150, 106271. [Google Scholar] [CrossRef]
- Kursun, H.; Ulusoy, U. Zinc recovery from a Lead-Zinc-Copper ore by ultrasonically assisted column flotation. Particul. Sci. Technol. 2015, 33, 349–356. [Google Scholar] [CrossRef]
- Lai, H.; Deng, J.; Liu, Q.; Wen, S.; Song, Q. Surface chemistry investigation of froth flotation products of lead-zinc sulfide ore using ToF-SIMS and multivariate analysis. Sep. Purif. Technol. 2020, 254, 117655. [Google Scholar] [CrossRef]
- Li, S.; Guo, N.; Wu, H.; Qiu, G.; Liu, X. High efficient mixed culture screening and selected microbial community shift for bioleaching process. Trans. Nonferrous Met. Soc. China 2011, 21, 1383–1387. [Google Scholar] [CrossRef]
- Lan, Z.; Lai, Z.; Zheng, Y.; Lv, J.; Pang, J.; Ning, J. Recovery of Zn, Pb, Fe and Si from a low-grade mining ore by sulfidation roasting-beneficiation-leaching processes. J. Cent. South Univ. 2020, 27, 37–51. [Google Scholar] [CrossRef]
- Jian, S.; Sun, W.; Zheng, Y.X. Application of a biconical dense medium cyclone to pre-treat a low-grade Pb-Zn sulfide ore. Physicochem. Probl. Miner. Process. 2019, 55, 981–990. [Google Scholar]
- Souza, A.; Krüger, F.L.V.; Araújo, F.G.D.S.; Mendes, J.J. Mineralogical Characterization Applied to Iron Ore Tailings from the Desliming Stage with Emphasis on Quantitative Electron Microscopy (QEM). Mater. Res. 2021, 24, e20190677. [Google Scholar] [CrossRef]
- Sun, Y.S.; Cao, Y.; Han, Y.X.; Li, Y.J.; Liu, J. Evaluation system of producing high purified iron concentrate based on mineralogical genetic characteristics of raw material. Met. Mine. 2018, 47, 76–79. [Google Scholar]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ge, Q. Estimation of lead and zinc emissions from mineral exploitation based on characteristics of lead-zinc deposits in China. Trans. Nonferrous Met. Soc. China 2011, 21, 2513–2519. [Google Scholar] [CrossRef]
- Hinsberg, V.J.V. Wills’ mineral processing technology: An introduction to the practical aspects of ore treatment and mineral recovery. Am. Miner. 1988, 93, 259–260. [Google Scholar]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]












| Composition | Pb | Zn | Cu | K | Fe | TS | TC | Al | Mg | Ca | Mn | Ti |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents (%) | 0.86 | 3.09 | 0.06 | 1.81 | 17.10 | 11.56 | 7.50 | 3.16 | 2.42 | 1.88 | 0.49 | 0.18 |
| Elements | Phases | Contents/% | Distribution Rate/% |
|---|---|---|---|
| Lead | Sulfide | 0.74 | 86.04 |
| Oxide | 0.06 | 6.98 | |
| Others | 0.06 | 6.98 | |
| Sum | 0.86 | 100.00 | |
| Zinc | Sulfide | 2.72 | 88.03 |
| Oxide | 0.32 | 10.36 | |
| Others | 0.05 | 1.61 | |
| Sum | 3.09 | 100.00 | |
| Carbon | Graphite | 5.01 | 66.80 |
| Carbonate | 2.49 | 33.20 | |
| Sum | 7.50 | 100.00 |
| Minerals | Metallic Minerals | Gangue Minerals |
|---|---|---|
| Major minerals | Pyrite + Marcasite: 14%± | Quartz: 40%± |
| Pyrrhotite: 8%± | Dolomite: 12%± | |
| Sphalerite: 5%± | Muscovite: 8% | |
| Minor minerals | Galena: 1%± | Graphite: 5%± |
| Chalcopyrite: 0.1%± | Apatite: 2%± | |
| Trace minerals | Bornite | Tourmaline, Calcite, Chlorite, etc. |
| Mineral | −0.074 mm Accounts for 48% | −0.074 mm Accounts for 69% | ||||
|---|---|---|---|---|---|---|
| D50/μm | Maximum Particle Size/μm | Monomer Liberation Degree/% | D50/μm | Maximum Particle Size/μm | Monomer Liberation Degree/% | |
| Sphalerite | 88 | 500 | 23.92 | 50 | 180 | 46.04 |
| Galena | 53 | 250 | 32.52 | 36 | 106 | 61.15 |
| Pyrite | 88 | 600 | 59.62 | 75 | 210 | 73.63 |
| Pyrrhotite | 103 | 475 | 69.91 | 67 | 250 | 81.85 |
| Sulfide aggregates | 136 | 600 | 69.88 | 68 | 242 | 83.56 |
| Product Name | Yield/% | Grade/% | Recovery Rate/% | ||
|---|---|---|---|---|---|
| Pb | Zn | Pb | Zn | ||
| Carbon Coarse Concentrate | 19.50 | 0.95 | 2.92 | 21.50 | 18.60 |
| Rough concentrate | 29.90 | 2.03 | 8.40 | 70.28 | 82.04 |
| Mixed rough concentrate | 49.40 | 1.60 | 5.71 | 91.78 | 92.07 |
| Tailings | 50.60 | 0.14 | 0.47 | 8.22 | 7.93 |
| Raw ore | 100.00 | 0.86 | 3.06 | 100.00 | 100.00 |
| Grain Grade/mm | Yield/% | Grade | Distribution Rate | Negative Accumulation | |||
|---|---|---|---|---|---|---|---|
| Pb | Zn | Pb | Zn | Pb | Zn | ||
| +0.6 | 0.37 | 0.22 | 0.66 | 0.56 | 0.51 | 100.00 | 100.00 |
| 0.6 + 0.45 | 1.50 | 0.17 | 0.67 | 1.78 | 2.11 | 99.44 | 99.49 |
| −0.45 + 0.4 | 6.11 | 0.20 | 0.70 | 8.54 | 9.02 | 97.66 | 97.37 |
| −0.4 + 0.3 | 27.10 | 0.16 | 0.75 | 30.32 | 42.86 | 89.11 | 88.36 |
| −0.3 + 0.2 | 14.05 | 0.18 | 0.74 | 17.69 | 21.93 | 58.80 | 45.50 |
| −0.2 + 0.15 | 9.78 | 0.13 | 0.36 | 8.89 | 7.42 | 41.11 | 23.57 |
| −0.15 + 0.1 | 8.55 | 0.10 | 0.17 | 5.98 | 3.07 | 32.22 | 16.15 |
| −0.1 + 0.074 | 6.11 | 0.10 | 0.15 | 4.06 | 1.93 | 26.24 | 13.08 |
| −0.074 | 26.44 | 0.12 | 0.20 | 22.18 | 11.15 | 22.18 | 11.15 |
| Total | 100.00 | 0.14 | 0.47 | 100.00 | 100.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Hu, W.; Xiao, F.; Liu, X.; Yu, H.; Yuan, H.; Wang, H. Characterization of Discarded Lead–Zinc Sulfide Ore Tailings Based on Mineral Fragments. Minerals 2022, 12, 1279. https://doi.org/10.3390/min12101279
Zhao J, Hu W, Xiao F, Liu X, Yu H, Yuan H, Wang H. Characterization of Discarded Lead–Zinc Sulfide Ore Tailings Based on Mineral Fragments. Minerals. 2022; 12(10):1279. https://doi.org/10.3390/min12101279
Chicago/Turabian StyleZhao, Jianqi, Wentao Hu, Fusheng Xiao, Xinwei Liu, Hongdong Yu, Huan Yuan, and Huajun Wang. 2022. "Characterization of Discarded Lead–Zinc Sulfide Ore Tailings Based on Mineral Fragments" Minerals 12, no. 10: 1279. https://doi.org/10.3390/min12101279
APA StyleZhao, J., Hu, W., Xiao, F., Liu, X., Yu, H., Yuan, H., & Wang, H. (2022). Characterization of Discarded Lead–Zinc Sulfide Ore Tailings Based on Mineral Fragments. Minerals, 12(10), 1279. https://doi.org/10.3390/min12101279