Asphaltene Behavior during Thermal Recovery: A Molecular Study Based on Realistic Structures
Abstract
:1. Introduction
2. Methodology
2.1. Asphaltene Film
2.2. Viscosity Calculations
2.3. Water–Asphaltene Interactions and Interfacial Tension Calculations
2.4. Mechanical Stability of the Asphaltene Film
3. Results and Discussion
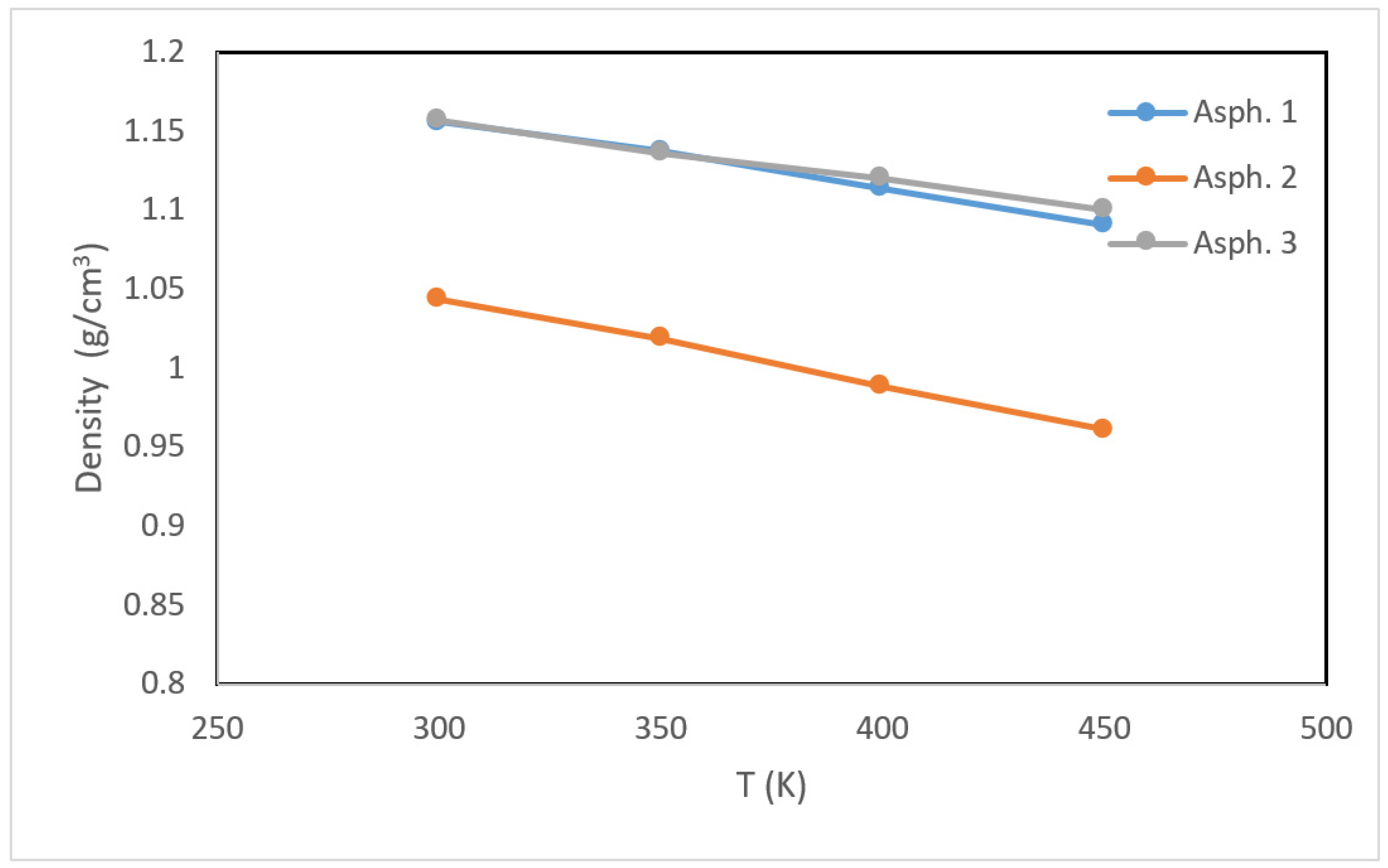
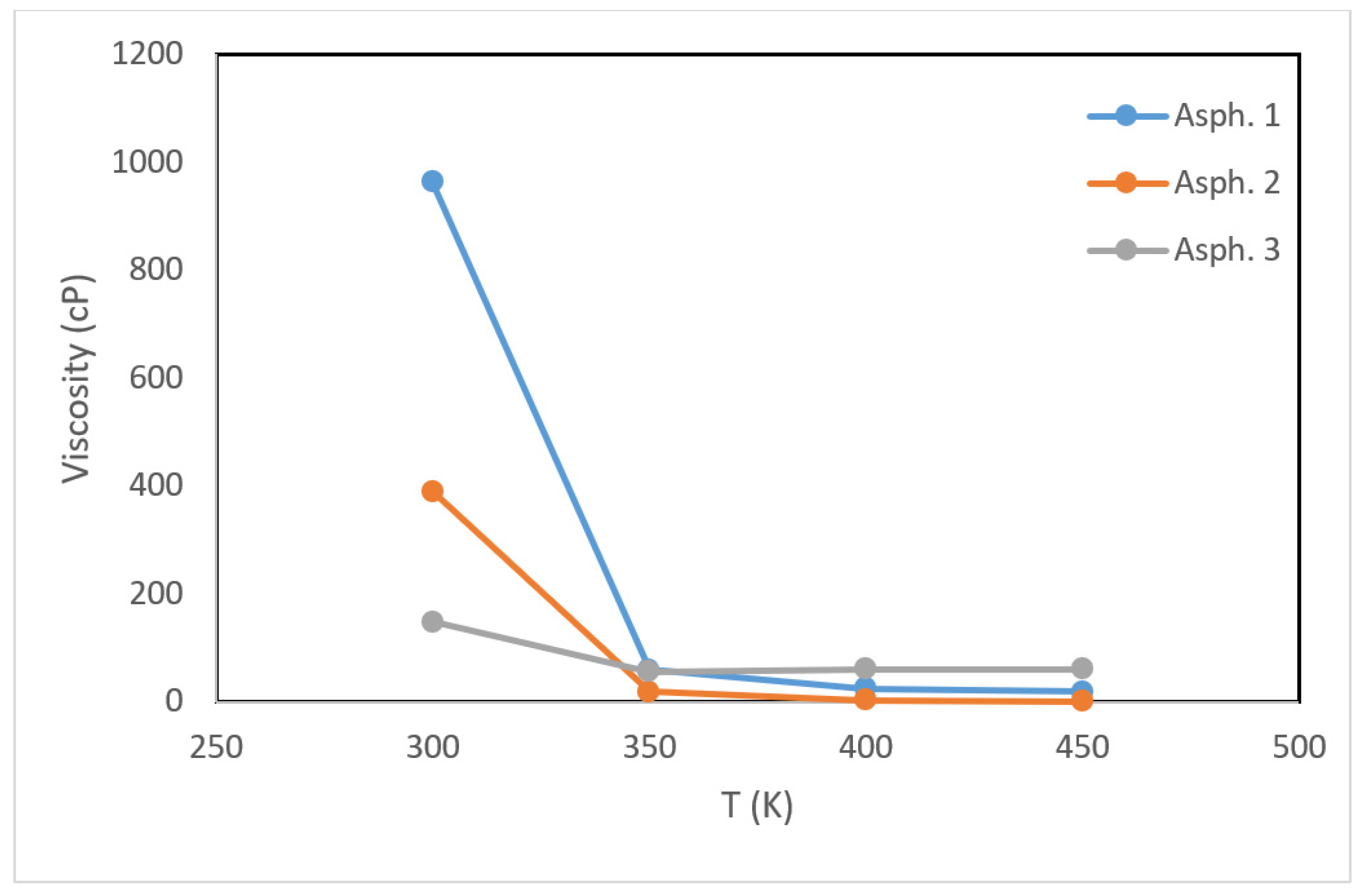
3.1. Asphaltene Physical Properties
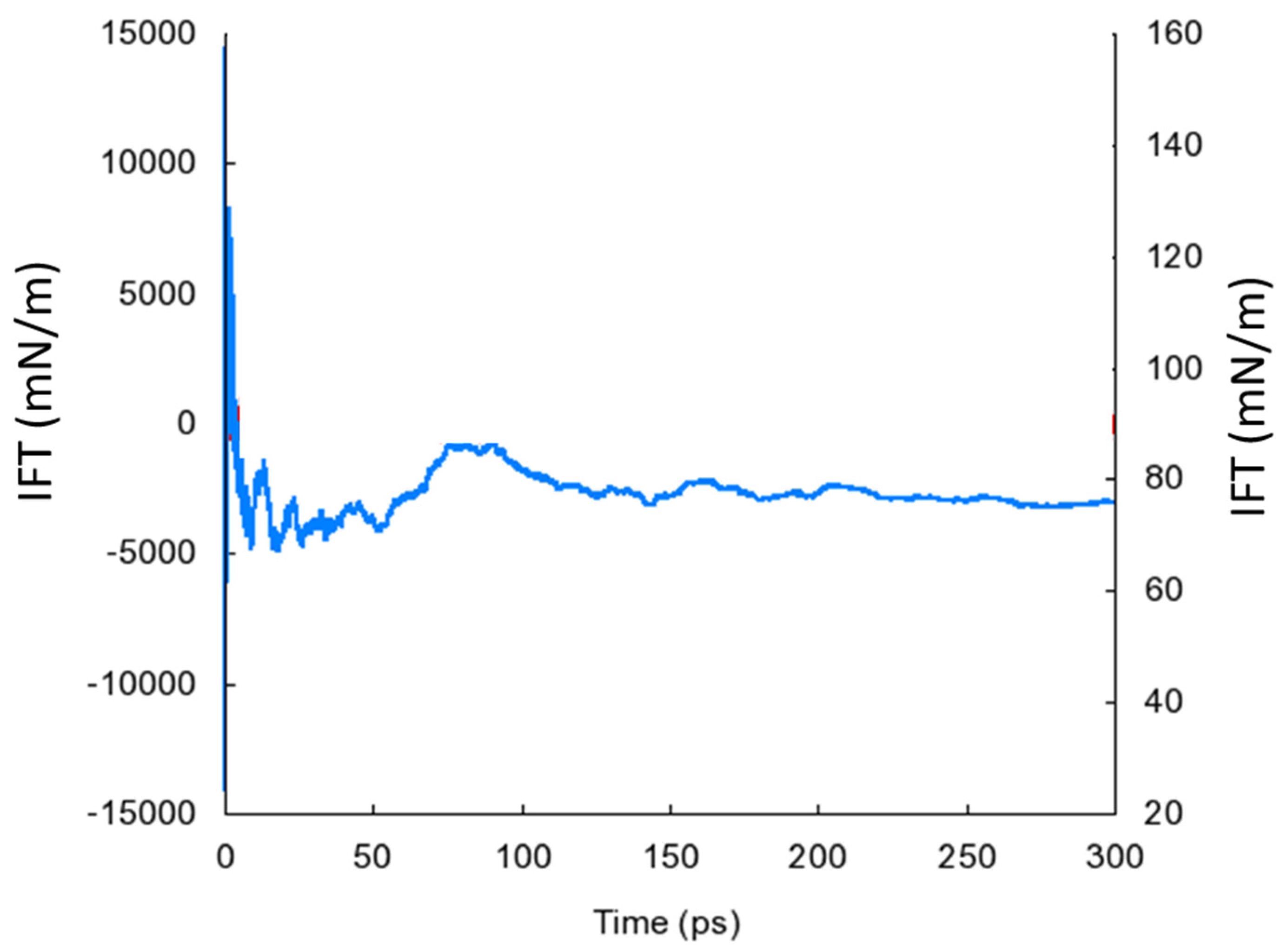
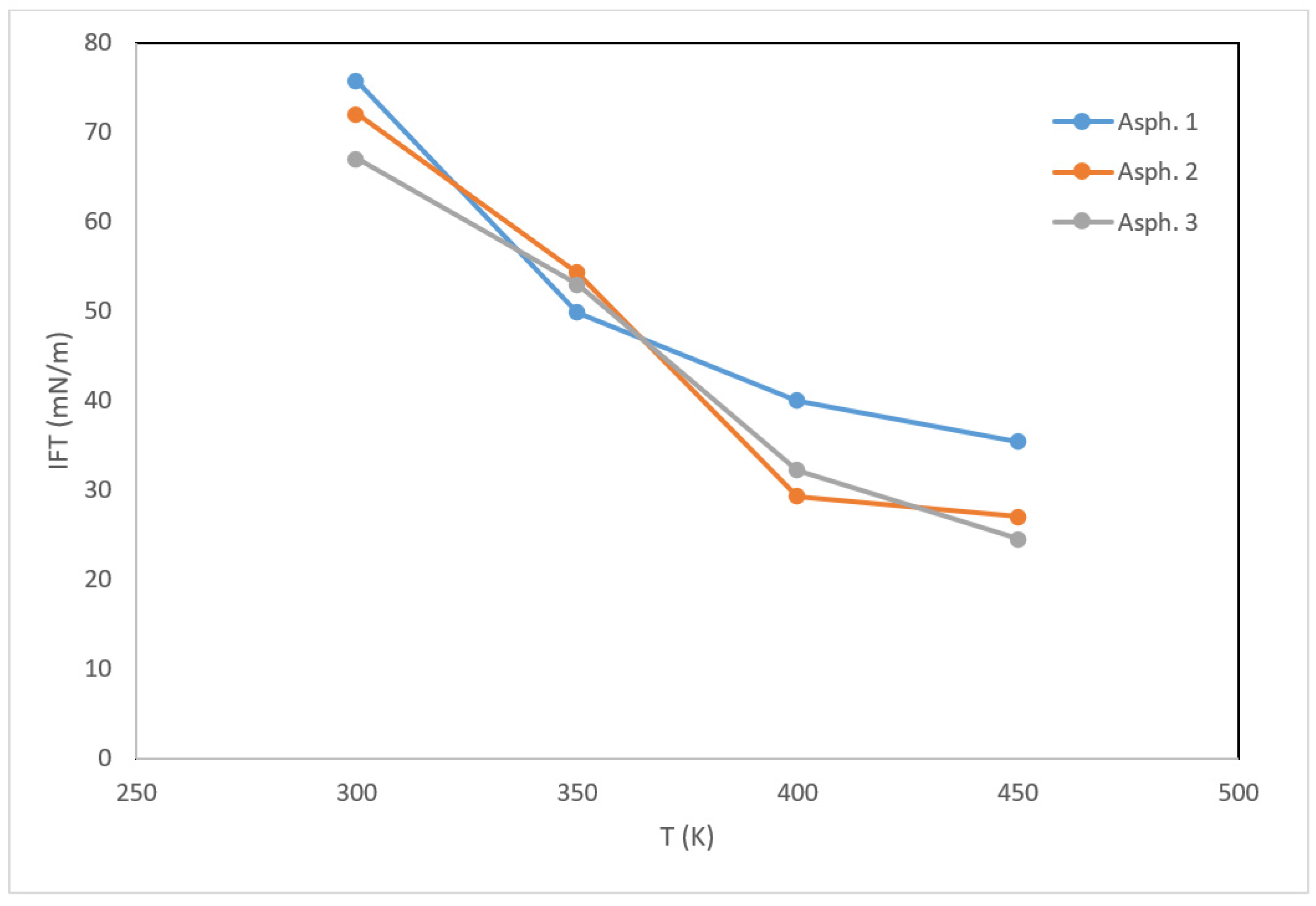
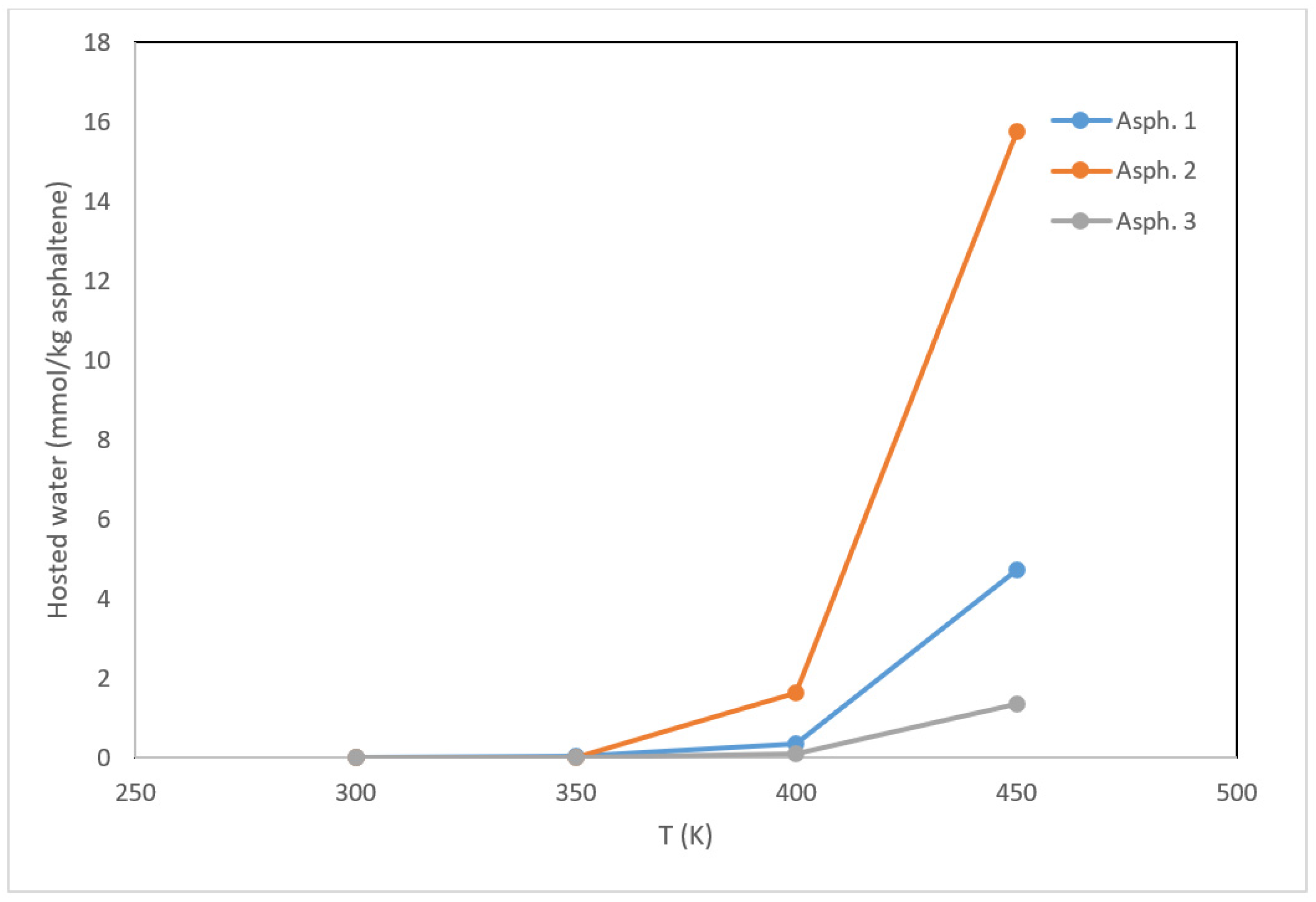
3.2. Interactions with Water and Interfacial Behavior
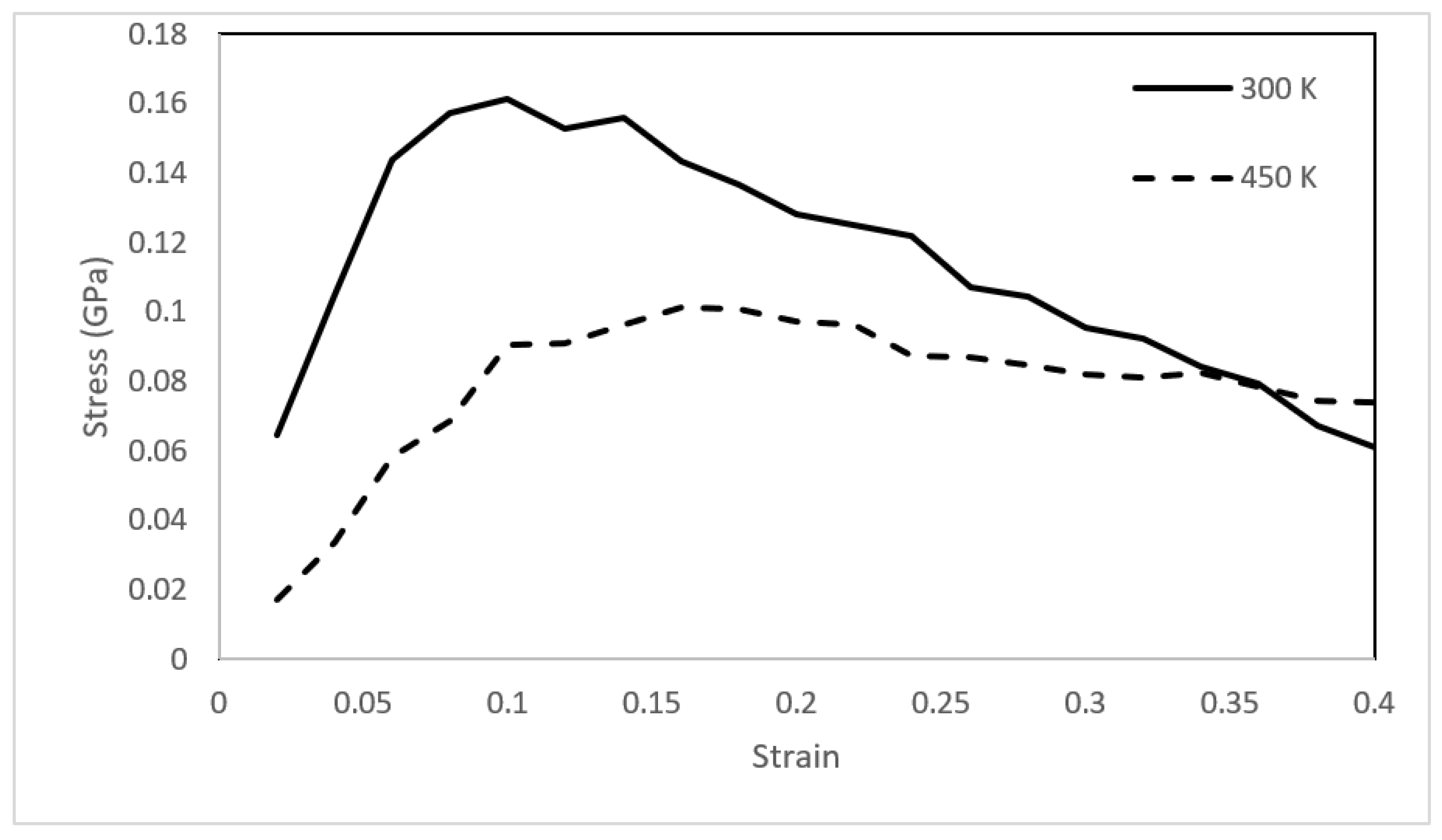
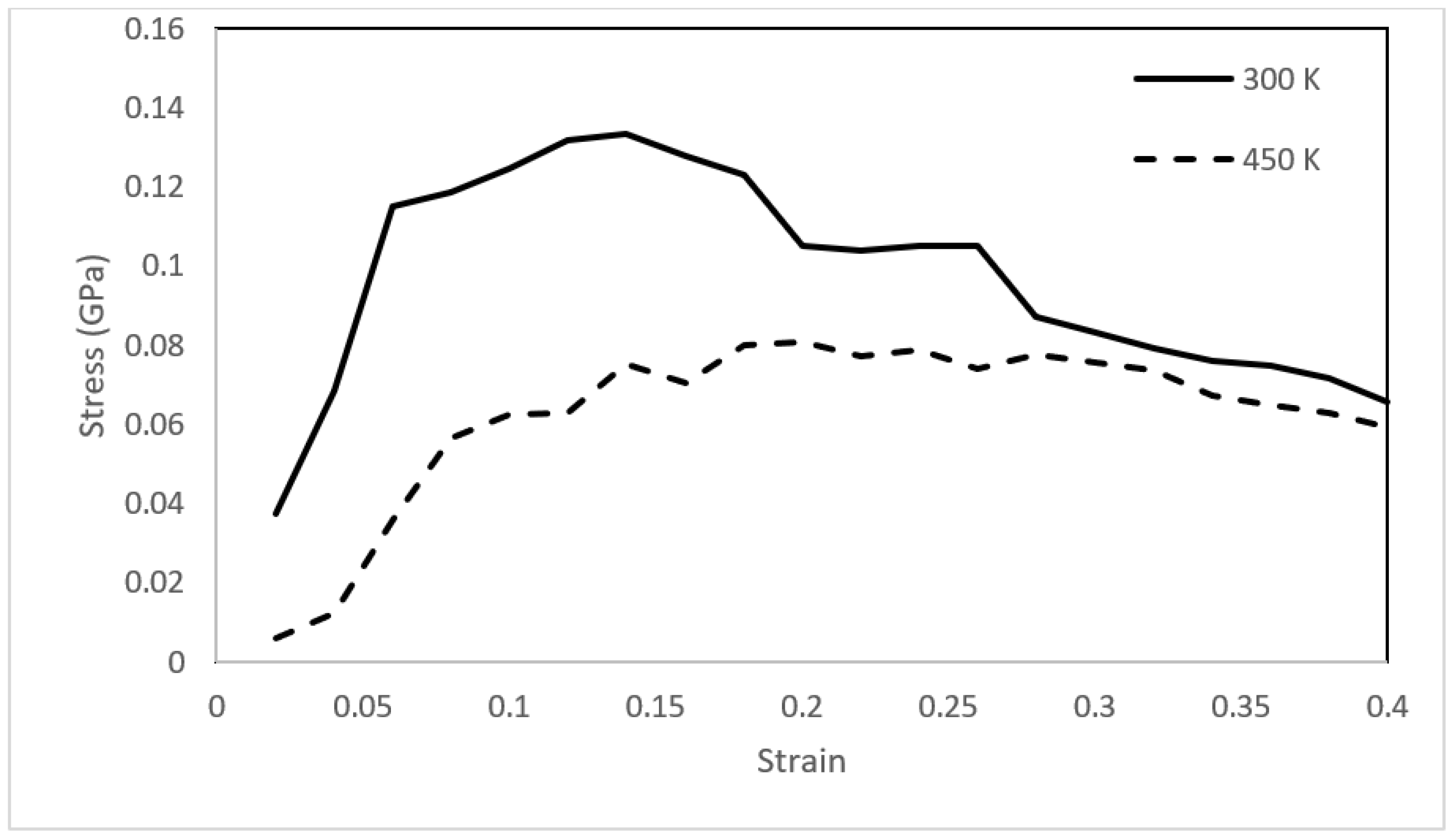
3.3. Mechanical Integrity
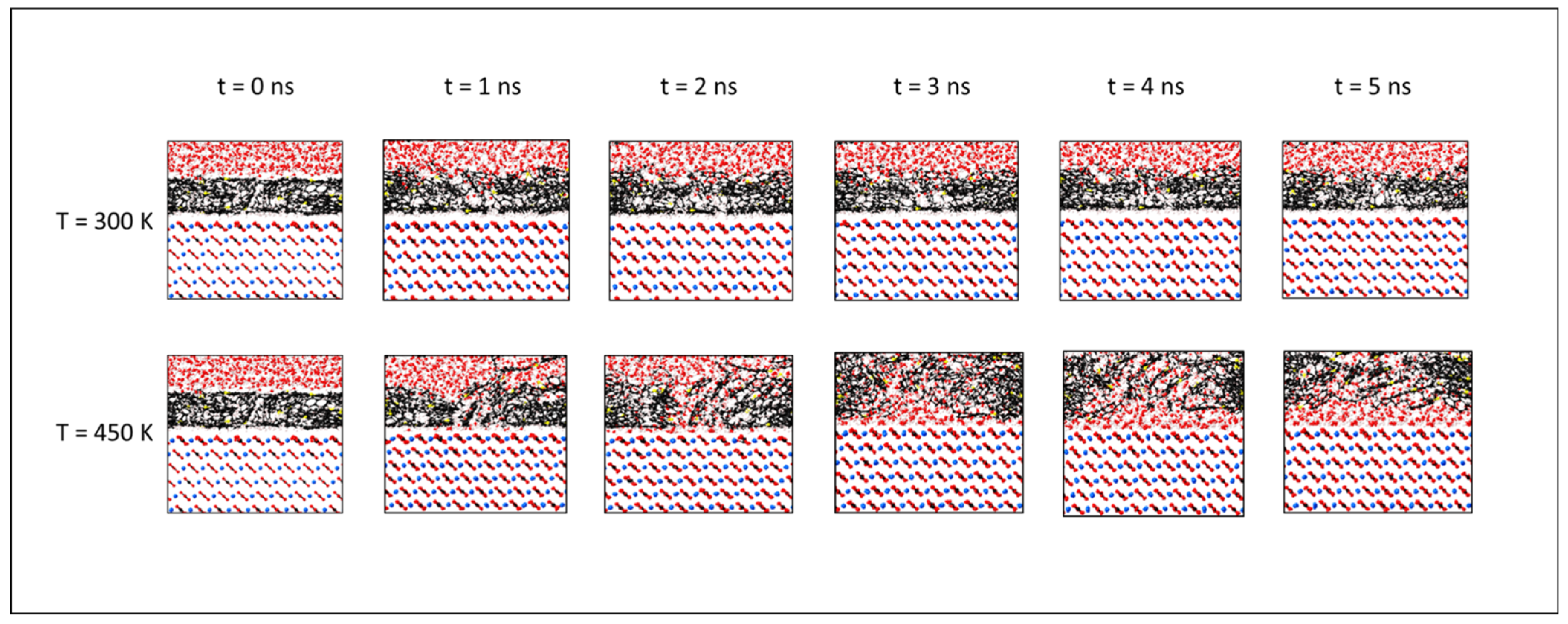
3.4. Wettability Alteration
3.5. Consistency with Experimental Observations
4. Conclusions
- The density of asphaltene was influenced by the chemical structure and temperature. Asphaltene containing a higher percentage of aromatic carbon had a higher density. The density was inversely correlated with temperature.
- Similar to the density, the viscosity of asphaltene increased with increasing temperature. The asphaltene structure containing the largest share of fused benzene rings had the highest viscosity at lower temperatures. At higher temperatures, however, the presence of sulfur seemed to restrict further reduction in the viscosity. The pour points of the asphaltene models were between 300 and 350 K.
- The temperature affected the interfacial tension, which decreased from more than 70 mN/m at 300 K to approximately 24 mN/m at 450 K. This can be explained by the enhanced ability of asphaltene to disperse the water phase under increasing temperature.
- Mechanical analysis revealed the solid-like behavior of asphaltene with both elastic and plastic deformation at 300 K. The mechanical behavior changed drastically upon heating, and the toughness of the asphaltene films decreased by approximately 60%.
- The findings provide in-depth insights into asphaltene behavior as a function of temperature. These insights can be used to optimize the design of thermal recovery processes. The findings of this study should be experimentally validated and repeated by extending this study to other asphaltene models.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Temperature, K | |
| Presssure, psi, MPa, or Pa | |
| Pore volume, m3 | |
| Young’s modulus, GPa | |
| Bulk modulus, GPa | |
| Shear modulus, GPa | |
| Poisson’s ratio, dimensionless parameter | |
| Separation distance between two center forces | |
| Atomic charge | |
| Dielectric constant | |
| Distance at which the interaction potential is zero | |
| Maximum amplitude of the potential well | |
| E0 | Energy of the structure, J |
| Etot | Energy after deformation, J |
References
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in asphaltene science and the Yen-Mullins model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Farooq, U.; Patil, A.; Panjwani, B.; Simonsen, G. Review on application of nanotechnology for asphaltene adsorption, crude oil demulsification, and produced water treatment. Energy Fuels 2021, 35, 19191–19210. [Google Scholar] [CrossRef]
- Adebiyi, F.M. An insight into asphalene precipitation, deposition and management stratagems in petroleum industry. J. Pipeline Sci. Eng. 2021, 1, 419–427. [Google Scholar] [CrossRef]
- Mukhametshina, A.; Hascakir, B. Bitumen extraction by expanding solvent-steam assisted gravity drainage (ES-SAGD) with asphaltene solvents and non-solvents. In Proceedings of the SPE Heavy Oil Conference Canada, Calgary, AB, Canada, 10–12 June 2014. [Google Scholar]
- Alqam, M.H.; Abu-Khamsin, S.A.; Sultan, A.S.; Al-Afnan, S.F.; Alawani, N.A. An investigation of factors influencing carbonate rock wettability. Energy Rep. 2021, 7, 1125–1132. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the molecular structures of asphaltenes by atomic force microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef]
- Hasanvand, M.Z.; Montazeri, M.; Salehzadeh, M.; Amiri, M.; Fathinasab, M. A literature review of asphaltene entity, precipitation, and deposition: Introducing recent models of deposition in the well column. J. Oil Gas Petrochem. Sci. 2018, 1, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Mullins, O. Review of the molecular structure and aggregation of asphaltenes and petroleomics. SPE J. 2008, 13, 48–57. [Google Scholar] [CrossRef]
- Andersen, S.I.; Birdi, K.S. Aggregation of asphaltenes as determined by calorimetry. J. Colloid Interface Sci. 1991, 142, 497–502. [Google Scholar] [CrossRef]
- Alizadeh, A.; Nakhli, H.; Kharrat, R.; Ghazanfari, M.H. An experimental investigation of asphaltene precipitation during natural production of heavy and light oil reservoirs: The role of pressure and temperature. Pet. Sci. Technol. 2011, 29, 1054–1065. [Google Scholar] [CrossRef]
- Mansoori, G.A. Modeling of asphaltene and other heavy organic depositions. J. Pet. Sci. Eng. 1997, 17, 101–111. [Google Scholar] [CrossRef]
- Speight, J.G. Petroleum asphaltenes—Part 1: Asphaltenes, resins and the structure of petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.G. Wettability literature survey—Part 6: The effects of wettability on waterflooding. J. Pet. Technol. 1987, 39, 1605–1622. [Google Scholar] [CrossRef]
- Berezin, V.M.; Yarygina, V.S.; Dubrovina, N.A. Adsorption of asphaltenes and tar from petroleum by sandstone. Neftepromysl. Delo 1982, 5, 15–17. [Google Scholar]
- Buckley, J.S.; Liu, Y.; Monsterleet, S. Mechanisms of wetting alteration by crude oils. SPE J. 1998, 3, 54–61. [Google Scholar] [CrossRef]
- Collins, S.H.; Melrose, J.C. Adsorption of asphaltenes and water on reservoir rock minerals. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Denver, CO, USA, 1–3 June 1983. [Google Scholar]
- Basu, S.; Sharma, M.M. Investigating the role of crude oil components on wettability alteration using atomic force microscopy. SPE J. 1999, 4, 235–241. [Google Scholar] [CrossRef]
- Crocker, M.E.; Marchin, L.M. Wettability and adsorption characteristics of crude-oil. J. Pet. Technol. 1998, 40, 470–479. [Google Scholar] [CrossRef]
- Amin, J.S.; Nikooee, E.; Ghatee, M.H.; Ayatollahi, S.; Alamdari, A.; Sedghamiz, T. Investigating the effect of different asphaltene structures on surface topography and wettability alteration. Appl. Surf. Sci. 2011, 257, 8341–8349. [Google Scholar] [CrossRef]
- Standal, S.; Haavik, J.; Blokhus, A.M.; Skauge, A. Effect of polar organic components on wettability as studied by adsorption and contact angles. J. Pet. Sci. Eng. 1999, 24, 131–144. [Google Scholar] [CrossRef]
- Zhang, L.; Greenfield, M.L. Analyzing properties of model asphalts using molecular simulation. Energy Fuels 2007, 21, 1712–1716. [Google Scholar] [CrossRef]
- Fenistein, D.; Barré, L.; Broseta, D.; Espinat, D.; Livet, A.; Roux, J.N.; Scarsella, M. Viscosimetric and neutron scattering study of asphaltene aggregates in mixed toluene/heptane solvents. Langmuir 1998, 14, 1013–1020. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liu, G.; Yuan, S. Molecular dynamics simulation: The behavior of asphaltene in crude oil and at the oil/water interface. Energy Fuels 2014, 28, 7368–7376. [Google Scholar] [CrossRef]
- Headen, T.F.; Boek, E.S.; Skipper, N.T. Evidence for asphaltene nanoaggregation in toluene and heptane from molecular dynamics simulations. Energy Fuels 2009, 23, 1220–1229. [Google Scholar] [CrossRef]
- Hu, C.; Garcia, N.C.; Xu, R.; Cao, T.; Yen, A.; Garner, S.A.; Macias, J.M.; Joshi, N.; Hartman, R.L. Interfacial properties of asphaltenes at the heptol–brine interface. Energy Fuels 2016, 30, 80–87. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T. Molecular dynamics simulations reveal inhomogeneity-enhanced stacking of violanthrone-78-based polyaromatic compounds in n-heptane−toluene mixtures. J. Phys. Chem. B 2015, 119, 8660–8668. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T. One-dimensional self-assembly of polyaromatic compounds revealed by molecular dynamics simulations. J. Phys. Chem. 2014, 118, 12772–12780. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T.; Bhattacharjee, S. Molecular dynamics investigation on the aggregation of Violanthrone78-based model asphaltenes in toluene. Energy Fuels 2014, 28, 3604–3613. [Google Scholar] [CrossRef]
- Jian, C.; Tang, T.; Bhattacharjee, S. Probing the effect of side-chain length on the aggregation of a model asphaltene using molecular dynamics simulations. Energy Fuels 2017, 27, 2057–2067. [Google Scholar] [CrossRef]
- Kuznicki, T.; Masliyah, J.H.; Bhattacharjee, S. Aggregation and partitioning of model asphaltenes at toluene—water interfaces: Molecular dynamics simulations. Energy Fuels 2009, 23, 5027–5035. [Google Scholar] [CrossRef]
- Sedghi, M.; Goual, L.; Welch, W.; Kubelka, J. Effect of asphaltene structure on association and aggregation using molecular dynamics. J. Phys. Chem. 2013, 117, 5765–5776. [Google Scholar] [CrossRef]
- Ungerer, P.; Rigby, D.; Leblanc, B.; Yiannourakou, M. Sensitivity of the aggregation behaviour of asphaltenes to molecular weight and structure using molecular dynamics. Mol. Simul. 2013, 40, 115–122. [Google Scholar] [CrossRef]
- Teklebrhan, R.B.; Ge, L.; Bhattacharjee, S.; Xu, Z.; Sjöblom, J. Probing structure–nanoaggregation relations of polyaromatic surfactants: A molecular dynamics simulation and dynamic light scattering study. J. Phys. Chem. B 2012, 116, 5907–5918. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, F.; Molinari, D. A multiscale approach to the simulation of asphaltenes. Comput. Theor. Chem. 2011, 975, 76–82. [Google Scholar] [CrossRef]
- Stukan, M.R.; Ligneul, P.; Boek, E.S. Molecular dynamics simulation of spontaneous imbibition in nanopores and recovery of asphaltenic crude oils using surfactants for EOR applications. Oil Gas Sci. Technol. 2012, 67, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Porte, G.; Zhou, H.; Lazzeri, V. Reversible description of asphaltene colloidal association and precipitation. Langmuir 2003, 19, 40–47. [Google Scholar] [CrossRef]
- Kuznicki, T.; Masliyah, J.H.; Bhattacharjee, S. Molecular dynamics study of model molecules resembling asphaltene-like structures in aqueous organic solvent systems. Energy Fuels 2008, 22, 2379–2389. [Google Scholar] [CrossRef]
- Mikami, Y.; Liang, Y.; Matsuoka, T.; Boek, E.S. Molecular dynamics simulations of asphaltenes at the oil–water interface: From nanoaggregation to thin-film formation. Energy Fuels 2013, 27, 1838–1845. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Ren, S. Molecular dynamics simulation of self-aggregation of asphaltenes at an oil/water interface: Formation and destruction of the asphaltene protective film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, S.; Mansoori, G.A. Effect of CO2 on the interfacial and transport properties of water/binary and asphaltenic oils: Insights from molecular dynamics. Energy Fuels 2018, 32, 5409–5417. [Google Scholar] [CrossRef]
- Green, D.W.; Willhite, G.P. Enhanced Oil Recovery (6); Henry L. Doherty Memorial Fund of AIME, Society of Petroleum Engineers: Richardson, TX, USA, 1998. [Google Scholar]
- Aljawad, M.S.; Alafnan, S.; Abu-Khamsin, S. Artificial lift and mobility enhancement of heavy oil reservoirs utilizing a renewable energy-powered heating element. ACS Omega 2019, 4, 20048–20058. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, K.; Hammami, A.; Kharrat, A.; Zhang, D.; Allenson, S.; Creek, J.; Kabir, S.; Jamaluddin, A.; Marshall, A.G.; Rodgers, R.P.; et al. Asphaltenes—Problematic but rich in potential. Oilfield Rev. 2007, 19, 24–43. [Google Scholar]
- Gray, M.R.; Assenheimer, G.; Boddez, L.; McCaffrey, W.C. Melting and fluid behavior of asphaltene films at 200−500 °C. Energy Fuels 2004, 18, 1419–1423. [Google Scholar] [CrossRef]
- Alafnan, S. Utilization of depleted heavy oil reservoirs for carbon dioxide storage and sequestration: A molecular level assessment. Int. J. Greenh. Gas Control 2022, 119, 103741. [Google Scholar] [CrossRef]
- Waldmann, M.; Hagler, A.T. New combining rules for rare gas van der Waals parameters. J. Comput. Chem. 1993, 14, 1077–1084. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Afagwu, C.; Al-Afnan, S.; Patil, S.; Aljaberi, J.; Mahmoud, M.A.; Li, J. The impact of pore structure and adsorption behavior on kerogen tortuosity. Fuel 2021, 303, 121261. [Google Scholar] [CrossRef]
- Alafnan, S. Petrophysics of kerogens based on realistic structures. ACS Omega 2021, 6, 9549–9558. [Google Scholar] [CrossRef]
- Alafnan, S.; Solling, T.; Mahmoud, M. Effect of kerogen thermal maturity on methane adsorption capacity: A molecular modeling approach. Molecules 2020, 25, 3764. [Google Scholar] [CrossRef]
- Alafnan, S.; Sultan, A.S.; Aljaberi, J. Molecular fractionation in the organic materials of source rocks. ACS Omega 2020, 5, 18968–18974. [Google Scholar] [CrossRef]
- Alafnan, S.; Falola, Y.; Al Mansour, O.; AlSamadony, K.; Awotunde, A.; Aljawad, M. Enhanced recovery from organic-rich shales through carbon dioxide injection: Molecular-level investigation. Energy Fuels 2020, 34, 16089–16098. [Google Scholar] [CrossRef]
- Aljaberi, J.; Alafnan, S.; Glatz, G.; Sultan, A.S.; Afagwu, C. The impact of kerogen tortuosity on shale permeability. SPE J. 2020, 26, 765–779. [Google Scholar] [CrossRef]
- Alqam, M.H.; Abu-Khamsin, S.A.; Alafnan, S.F.; Sultan, A.S.; Al-Majed, A.; Okasha, T. The impact of carbonated water on wettability: Combined experimental and molecular simulation approach. SPE J. 2021, 27, 945–957. [Google Scholar] [CrossRef]
- Alafnan, S. Utilization of supercritical carbon dioxide for mechanical degradation of organic matters contained in shales. Fuel 2022, 319, 123427. [Google Scholar] [CrossRef]
- Barber, C.B.; Dobkin, D.P.; Huhdanpaa, H. The quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996, 22, 469–483. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Greenfield, M.L. Relaxation time, diffusion, and viscosity analysis of model asphalt systems using molecular simulation. J. Chem. Phys. 2007, 127, 194502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dysthe, D.K.; Fuchs, A.H.; Rousseau, B. Fluid transport properties by equilibrium molecular dynamics. I. Methodology at extreme fluid states. J. Chem. Phys. 1999, 110, 4047–4059. [Google Scholar] [CrossRef] [Green Version]
- Alafnan, S. The impact of pore structure on kerogen geomechanics. Geofluids 2021, 2021, 4093895. [Google Scholar] [CrossRef]
- Yang, M.; Rodger, P.M.; Harding, J.H.; Stipp, S.L.S. Molecular dynamics simulations of peptides on calcite surface. Mol. Simul. 2009, 35, 547–553. [Google Scholar] [CrossRef]
- Mutisya, S.M.; Kirch, A.; de Almeida, J.M.; Sánchez, V.M.; Miranda, C.R. Molecular Dynamics Simulations of Water Confined in Calcite Slit Pores: An NMR Spin Relaxation and Hydrogen Bond Analysis. J. Phys. Chem. C 2017, 121, 6674–6684. [Google Scholar] [CrossRef]
- Rao, D.N. Wettability effects in thermal recovery operations. SPE Reserv. Eval. Eng. 1999, 2, 420–430. [Google Scholar] [CrossRef]
- Tang, G.-Q.; Lowry, D.; Lee, V. Recovery mechanism of steam injection in heavy oil carbonate reservoir. In Proceedings of the SPE Western North American Region Meeting, Anchorage, Alaska, USA, 7–11 May 2011. [Google Scholar]
- Al-Hadhrami, H.S.; Blunt, M.J. Thermally induced wettability alteration to improve oil recovery in fractured reservoirs. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2001; p. SPE 71866. [Google Scholar]
- Roosta, A.B.; Escrochi, M.F.; Khatibi, V.J.; Ayatollahi, V.J.S.; Schafiee, M. Investigating the mechanism of thermally induced wettability alteration. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 15–18 March 2009. [Google Scholar]
- Bardon, C.; Longeron, D.G. Influence of very low interfacial tensions on relative permeability. Soc. Pet. Eng. J. 1980, 20, 391–401. [Google Scholar] [CrossRef]





| Asphaltene | Molecular Structure | Molecular Formula | Percentage of Aromatic Carbon |
|---|---|---|---|
| 1 | 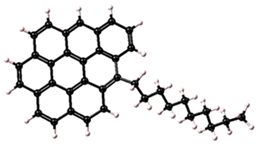 | C37H33 | 73% |
| 2 | 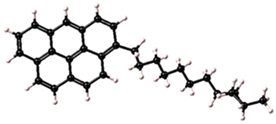 | C31H35 | 58% |
| 3 | 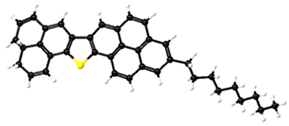 | C38H35S | 73% |
| T (K) | Fugacity (MPa) |
|---|---|
| 300 | 0.004 |
| 350 | 0.052 |
| 375 | 0.137 |
| 400 | 0.311 |
| 450 | 1.149 |
| Reference | Type of Formation | Property under Investigation | Observation |
|---|---|---|---|
| Rao [62] | Carbonate | Wettability | Upon hot water injection, the wettability shifted from oil to water wet |
| Tang et al. [63] | Carbonate | Viscosity | The viscosity of the oil reduced to 2 cP as the temperature reached 240 °C |
| Al-Hadrami and Blunt [64] | Carbonate | Interfacial tension | The interfacial tension reduced significantly as the temperature increased which resulted in reduced capillary forces |
| Roosta et al. [65] | Carbonate/Quartz | Wettability | The wettability shifted toward water wet system as the temperature increased |
| Bardon and Longeron [66] | NA | Interfacial tension | The interfacial tension reduced which influenced the relative permeability positively |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafnan, S. Asphaltene Behavior during Thermal Recovery: A Molecular Study Based on Realistic Structures. Minerals 2022, 12, 1315. https://doi.org/10.3390/min12101315
Alafnan S. Asphaltene Behavior during Thermal Recovery: A Molecular Study Based on Realistic Structures. Minerals. 2022; 12(10):1315. https://doi.org/10.3390/min12101315
Chicago/Turabian StyleAlafnan, Saad. 2022. "Asphaltene Behavior during Thermal Recovery: A Molecular Study Based on Realistic Structures" Minerals 12, no. 10: 1315. https://doi.org/10.3390/min12101315
APA StyleAlafnan, S. (2022). Asphaltene Behavior during Thermal Recovery: A Molecular Study Based on Realistic Structures. Minerals, 12(10), 1315. https://doi.org/10.3390/min12101315









