Trace Elements in Chromian Spinels from Four Siberian Kimberlites
Abstract
1. Introduction
2. Geological Background
3. Materials and Methods
4. Results
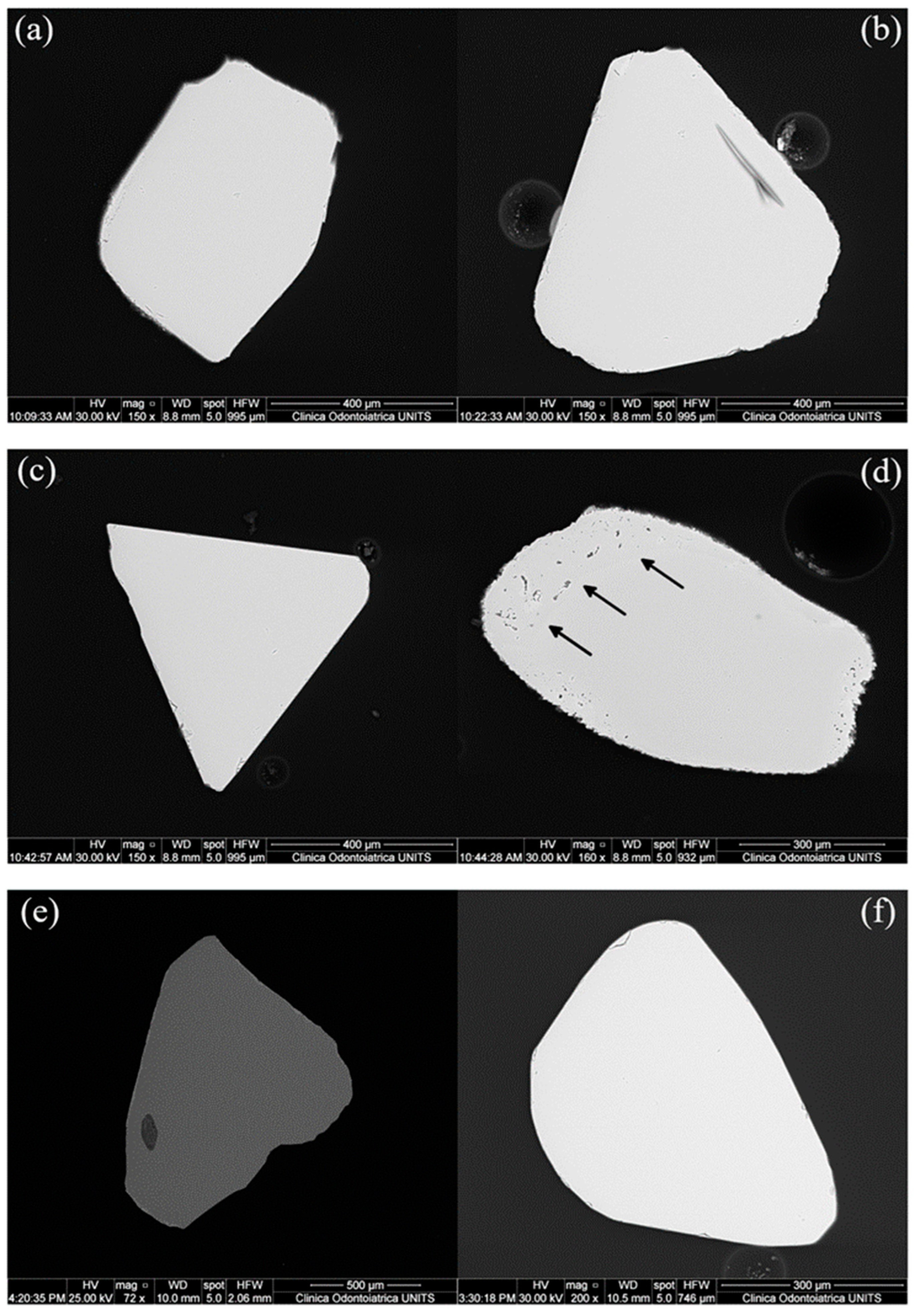
4.1. Textures
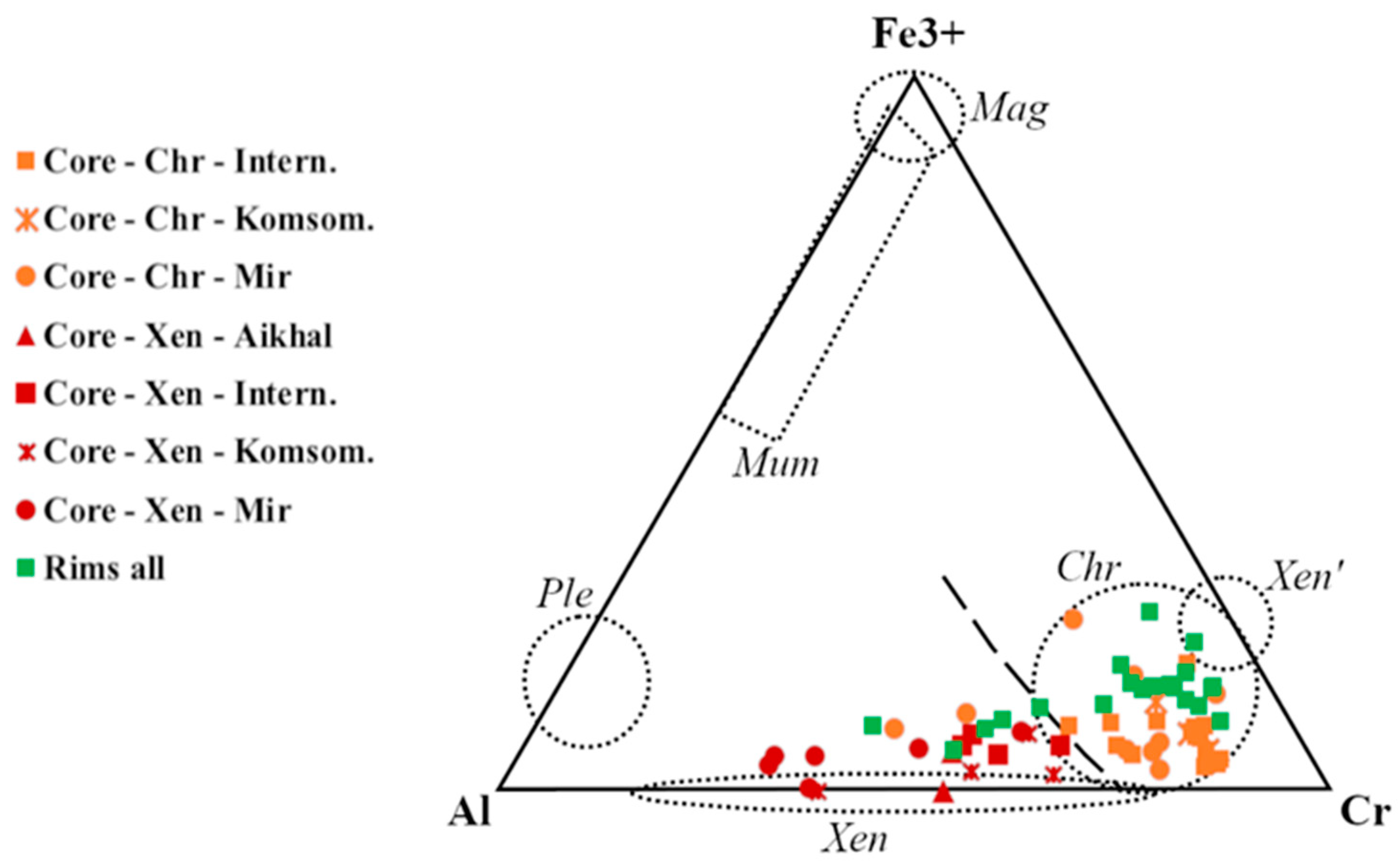
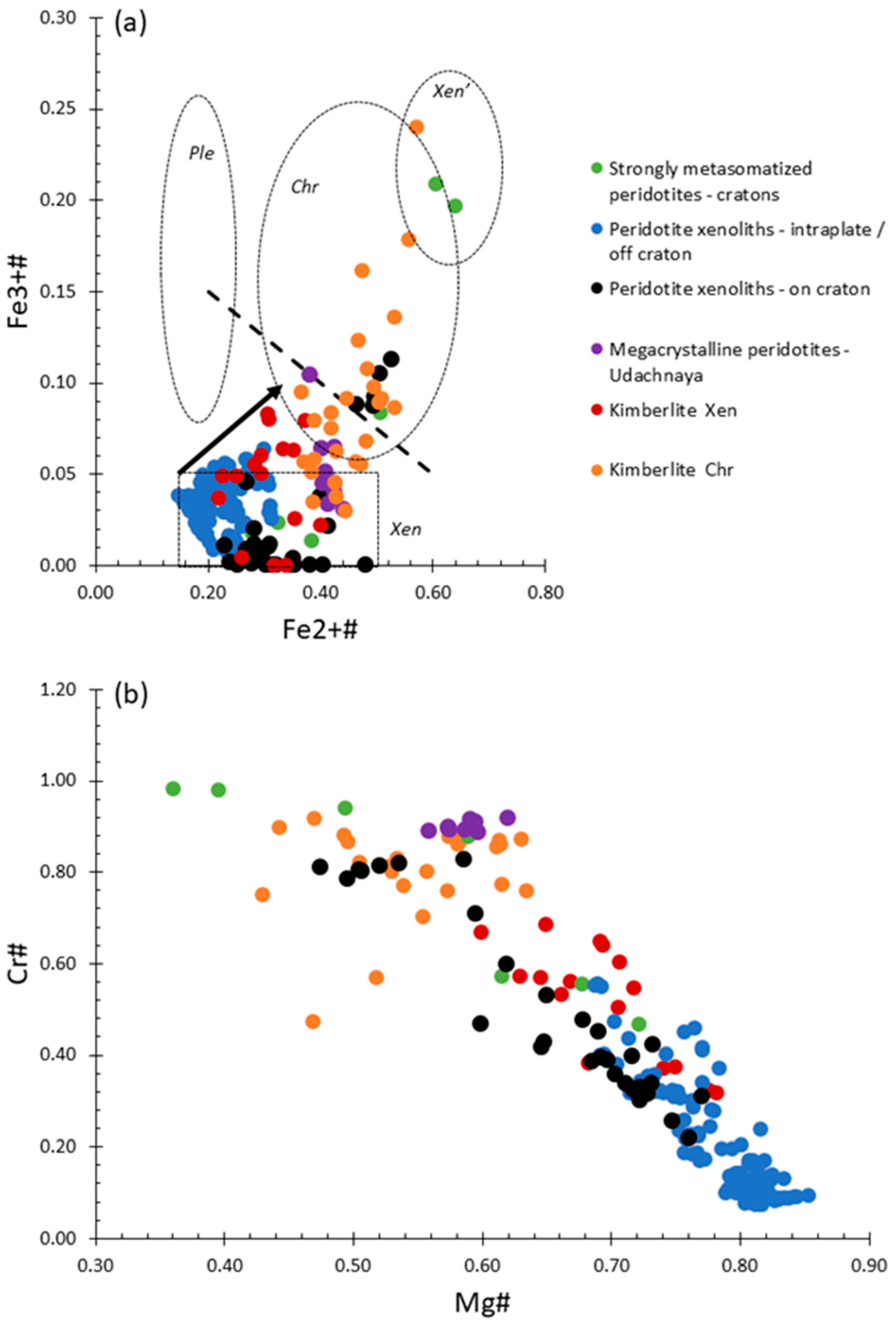
4.2. Major Element Chemistry and Classification
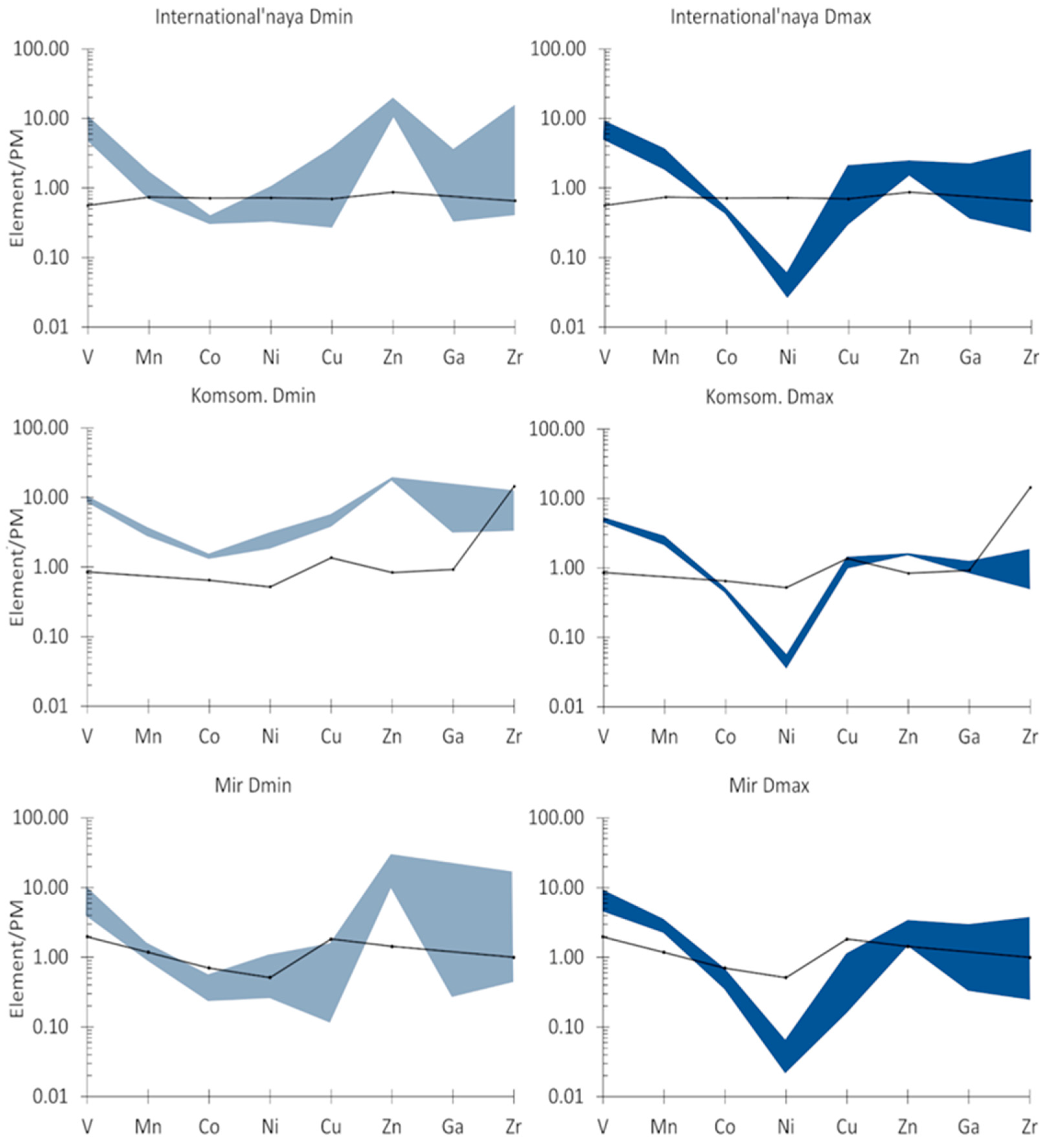
4.3. Trace Element Chemistry
5. Discussion
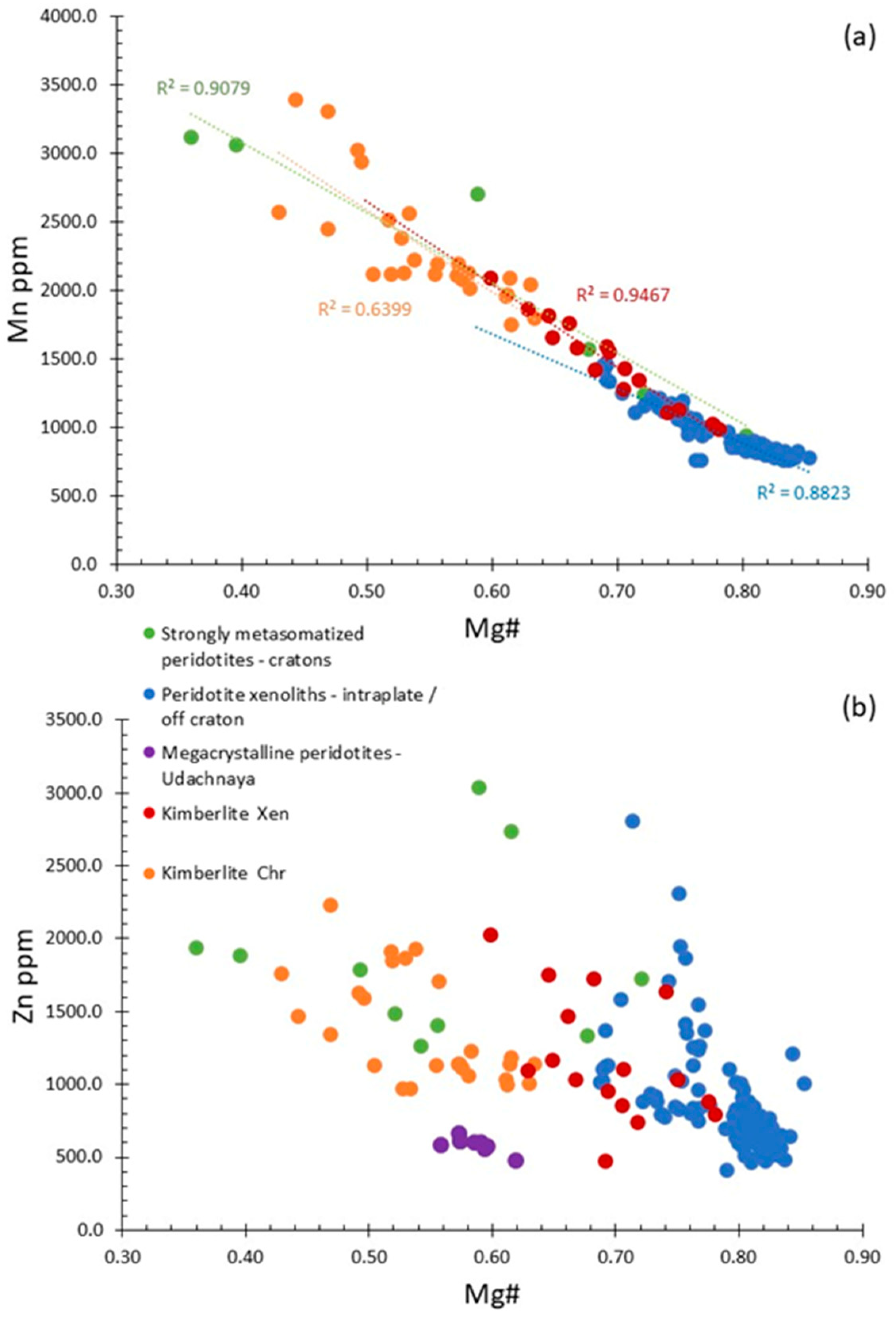
5.1. Manganese
5.2. Zinc
5.3. Nickel, Co, Sc, Ga, V
5.4. Trace Element Modelling for a Peridotitic Source
5.5. On the Partitioning of Some Trace Elements between Cr-Spinels and Kimberlitic Melts
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Irvine, T.N. Chromian spinel as a petrogenetic indicator. Part 1. Theory. Can. J. Earth Sci. 1965, 2, 648–672. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The Range of Spinel Compositions in Terrestrial Mafic and Ultramafic Rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M. Significance of Accessory Chrome Spinel in Identifying Serpentinite Paragenesis. Int. Geol. Rev. 2005, 47, 58–64. [Google Scholar] [CrossRef]
- Griffin, W.L.; Ryan, C.G.; Gurney, J.J.; Sobolev, N.V.; Win, T.T. Chromite macrocrysts in kimberlites and lamproites: Geochemistry and origin. In Proceedings of the 5th International Kimberlite Conference, Minas Gerais, Brazil; 1994; pp. 366–377. [Google Scholar]
- Lenaz, D.; Kamenetsky, V.S.; Crawford, A.J.; Princivalle, F. Melt inclusions in detrital spinel from the SE Alps Italy±Slovenia): A new approach to provenance studies of sedimentary basins. Contrib. Mineral. Petrol. 2000, 139, 748–758. [Google Scholar] [CrossRef]
- Kamenetsky, V.; Crawford, A.J.; Meffre, S. Factors controlling chemistry of magmatic spinel: An empirical study of associated olivine, Cr-spinel and melt inclusion from primitive rocks. J. Petrol. 2001, 42, 655–671. [Google Scholar] [CrossRef]
- Schulze, D.J. Origins of chromian and aluminous spinel macrocrysts from kimberlites in southern Africa. Can. Mineral. 2001, 39, 361–376. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Zedgenizov, D.A.; Seryotkin, Y.V.; Yefimova, E.S.; Floss, C.; Taylor, L.A. Mineral inclusions in microdiamonds and macrodiamonds from kimberlites in Yacutia: A comparative study. Lithos 2004, 77, 225–242. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E.F. Petrogenesis of the Ophiolitic Giant Chromite Deposits of Kempirsai, Kazakhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 38, 1419–1458. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Arai, S.; Abdel-Aziz, Y.M.; Rahimi, A. Spinel composition as a petrogenetic indicator of the mantle section in the Neoproterozoic Bou Azzer ophiolite, Anti-Atlas, Morocco. Precambrian Res. 2005, 138, 225–234. [Google Scholar] [CrossRef]
- Ziberna, L.; Nimis, P.; Zanetti, A.; Marzoli, A.; Sobolev, N.V. Metasomatic processes in the Central Siberian Cratonic mantle: Evidence from garnet xenocrysts from the Zagadochnaya kimberlite. J. Petrol. 2013, 54, 2379–2409. [Google Scholar] [CrossRef]
- Ashchepkov, I.V.; Kuligin, S.S.; Vladykin, N.V.; Downes, H.; Vavilov, M.A.; Nigmatulina, E.N.; Babushkina, S.A.; Tychkov, N.S.; Khemelnikova, O.S. Comparison of mantle litosphere beneath early Triassic kimberlite fields in Siberian craton reconstructed from deep-seated xenocrysts. Geosci. Front. 2016, 7, 639–662. [Google Scholar] [CrossRef]
- Griffin, W.L.; Sobolev, N.V.; Ryan, C.G.; Pokhilenko, N.P.; Win, T.T.; Yefimova, E.S. Trace elements in garnets and chromites: Diamond formation in the Siberian lithosphere. Lithos 1993, 29, 235–256. [Google Scholar] [CrossRef]
- Paktunc, A.D.; Cabri, L.J. A proton-and electron-microprobe study of gallium, nickel and zinc distribution in chromian spinel. Lithos 1995, 35, 261–282. [Google Scholar] [CrossRef]
- Witt-Eickschen, G.; O’Neill, H.S.C. The effect of temperature on the equilibrium distribution of trace elements between clinopyroxene, orthopyroxene, olivine and spinel in upper mantle peridotite. Chem. Geol. 2005, 221, 65–101. [Google Scholar] [CrossRef]
- Norman, M.D. Melting and metasomatism in the continental lithosphere: Laser ablation ICPMS analysis of minerals in spinel lherzolites from eastern Australia. Contrib. Mineral. Petrol. 1998, 130, 240–255. [Google Scholar] [CrossRef]
- Glaser, S.M.; Foley, S.F.; Günther, D. Trace element compositions of minerals in garnet and spinel periodotite xenoliths from the Vitim volcanic field, Transbaikalia, eastern Siberia. In Developments in Geotectonics; Elsevier: Amsterdam, The Netherlands, 1999; Volume 24, pp. 263–285. [Google Scholar]
- Lenaz, D.; Musco, M.E.; Petrelli, M.; Caldeira, R.; De Min, A.; Marzoli, A.; Mata, J.; Perugini, D.; Princivalle, F.; Boumehdi, M.A.; et al. Restitic or not? Insight from trace element content and crystal—Structure of spinels in African mantle xenoliths. Lithos 2017, 278, 464–476. [Google Scholar] [CrossRef]
- Aulbach, S.; Giuliani, A.; Fiorentini, M.L.; Baumgartner, R.J.; Savard, D.; Kamenetsky, V.S.; Caruso, S.; Danyushevky, L.V.; Powell, W.; Griffin, W.L. Siderophile and chalcophile elements in spinels, sulphides and native Ni in strongly metasomatised xenoliths from the Bultfontein kimberlite (South Africa). Lithos 2021, 380, 105880. [Google Scholar] [CrossRef]
- Cherepanova, Y.; Artemieva, I.M.; Thybo, H.; Chemia, Z. Crustal structure of the Siberian craton and the West Siberian basin: An appraisal of existing seismic data. Tectonophysics 2013, 609, 154–183. [Google Scholar] [CrossRef]
- Davis, G.L.; Sobolev, N.V.; Kharkiv, A.D. New data on the age of Yakutian kimberlites obtained by the uranium-lead method on zircons. Dokl. Akad. Nauk SSSR 1980, 254, 175–179. [Google Scholar]
- Griffin, W.L.; Ryan, C.G.; Kaminsky, F.V.; O’Reilly, S.Y.; Natapov, L.M.; Win, T.T.; Kinny, P.D.; Ilupin, I.P. The Siberian lithosphere traverse: Mantle terranes and the assembly of the Siberian Craton. Tectonophysics 1999, 310, 1–35. [Google Scholar] [CrossRef]
- Kostrovitsky, S.I.; Morikiyo, T.; Serov, I.V.; Yakovlev, D.A.; Amirzhanov, A.A. Isotope-geochemical systematics of kimberlites and related rocks from the Siberian Platform. Russ. Geol. Geophys. 2007, 48, 272–290. [Google Scholar] [CrossRef]
- Pearce, J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand. Newslett. 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Lenaz, D.; Logvinova, A.M.; Princivalle, F.; Sobolev, N.V. Structural parameters of chromite included in diamond and kimberlites from Siberia: A new tool for discriminating ultramafic source. Am. Mineral. 2009, 94, 1067–1070. [Google Scholar] [CrossRef]
- Lenaz, D.; Skogby, H.; Logvinova, A.M.; Sobolev, N.V.; Princivalle, F. A micro-Mössbauer study of chromites included in diamond and other mantle-related rocks. Phys. Chem. Miner. 2013, 40, 671–679. [Google Scholar] [CrossRef]
- Droop, G.T. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 1987, 51, 431–435. [Google Scholar] [CrossRef]
- Abersteiner, A.; Giuliani, A.; Kamenetsky, V.S.; Phillips, D. Petrographic and melt-inclusion constraints on the petrogenesis of a magmaclast from the Venetia kimberlite cluster, South Africa. Chem. Geol. 2017, 455, 331–341. [Google Scholar] [CrossRef]
- Kumar, S.P.; Shaikh, A.M.; Patel, S.C.; Sheikh, J.M.; Behera, D.; Pruseth, K.L.; Ravi, S.; Tappe, S. Multi-stage magmatic history of olivine–leucite lamproite dykes from Banganapalle, Dharwar craton, India: Evidence from compositional zoning of spinel. Mineral. Petrol. 2021, 115, 87–112. [Google Scholar] [CrossRef]
- Roeder, P.L.; Schulze, D.J. Cristallization of groundmass spinel in kimberlite. J. Petrol. 2008, 49, 1473–1495. [Google Scholar] [CrossRef]
- Doucet, L.S.; Ionov, D.A.; Golovin, A.V.; Pokhilenko, N.P. Depth, degrees and tectonic settings of mantle melting during craton formation: Inferences from major and trace element compositions of spinel harzburgite xenoliths from the Udachnaya kimberlite, central Siberia. Earth Planet. Sci. Lett. 2012, 359, 206–218. [Google Scholar] [CrossRef]
- Grégoire, M.; Tinguely, C.; Bell, D.R.; Le Roex, A.P. Spinel lherzolite xenoliths from the Premier kimberlite (Kaapvaal craton, South Africa): Nature and evolution of the shallow upper mantle beneath the Bushveld complex. Lithos 2005, 84, 185–205. [Google Scholar] [CrossRef]
- Kopylova, M.G.; Russell, J.K.; Cookenboo, H. Petrology of peridotite and pyroxenite xenoliths from the Jericho kimberlite: Implications for the thermal state of the mantle beneath the Slave craton, northern Canada. J. Petrol. 1999, 40, 79–104. [Google Scholar] [CrossRef]
- Kostrovitsky, S.; Spetsius, Z.; Yakovlev, D.; Fon der Flaass, G.; Bogush, I.; Pokhilenko, N. Atlas of the Igneous Rocks and Diamond Deposits of the Yakut Kimberlite Province; Pokhilenko, N., Ed.; Migration Policy Group (MPG): Mirniy, Russia, 2015; p. 480. [Google Scholar]
- Navrotsky, A.; Kleppa, O.J. The thermodynamics of cation distributions in simple spinels. J. Inorg. Nucl. Chem. 1967, 29, 2701–2714. [Google Scholar] [CrossRef]
- Wijbrans, C.H.; Klemme, S.; Berndt, J.; Vollmer, C. Experimental determination of trace element partition coefficients between spinel and silicate melt: The influence of chemical composition and oxygen fugacity. Contrib. Mineral. Petrol. 2015, 169, 45. [Google Scholar] [CrossRef]
- Griffin, W.L.; Ryan, C.G. Trace elements in indicator minerals: Area selection and target evaluation in diamond exploration. J. Geochem. Explor. 1995, 53, 311–337. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. (A) 1976, 32, 751–767. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Navrotsky, A. Simple spinels: Crystallographic parameters, cation radii, lattice energies and cation distributions. Am. Mineral. 1983, 68, 191–194. [Google Scholar]
- Lavina, B.; Salviulo, G.; Della Giusta, A. Cation distribution and structure modelling of spinel solid solutions. Phys. Chem. Miner. 2002, 29, 10–18. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; O’Driscoll, B.; Lenaz, D.; Gertisser, R.; Kronz, A. Compositionally heterogeneous podiform chromitite in the Shetland Ophiolite Complex (Scotland): Implications for chromitite petrogenesis and late-stage alteration in the upper mantle portion of a supra-subduction zone ophiolite. Lithos 2013, 162–163, 279–300. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; O’Driscoll, B.; Lenaz, D.; Zanetti, A.; Gertisser, R. Chromitite petrogenesis in the mantle section of the Ballantrae Ophiolite Complex (Scotland). Lithos 2019, 344–345, 51–67. [Google Scholar] [CrossRef]
- Colás, V.; Padròn-Navarta, J.A.; González-Jiménez, J.M.; Griffin, W.L.; Fanlo, I.; O’Reilly, S.Y.; Gervilla, F.; Proenza, J.A.; Pearson, N.J.; Escayola, M.P. Compositional effects of the solubility of minor and trace elements in oxide spinel minerals: Insight from crystal-crystal partition coefficient in chromite exsolution. Am. Mineral. 2016, 101, 1360–1372. [Google Scholar] [CrossRef]
- Burns, R.G. The partitioning of trace transition elements in crystal structures: A provocative review with applications to mantle geochemistry. Geochem. Cosmochim. Acta 1973, 37, 2395–2403. [Google Scholar] [CrossRef]
- Stosch, H.G. Sc, Cr, Co and Ni partitioning between minerals from spinel peridotite xenoliths. Contrib. Mineral. Petrol. 1981, 78, 166–174. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Speich, L.; Smith, C.B.; Gaillou, E.; Kohn, S.C.; Wibberley, E.; Chapman, J.G.; Howell, D.; Davy, A.T. The Unique Nature of Argyle Fancy Diamonds: Internal Structure, Paragenesis, and Reasons for Color. In Geoscience and Exploration of the Argyle, Bunder, Diavik, and Murowa Diamond Deposits; Society of Economic Geologists: Littleton, CO, USA, 2018. [Google Scholar]
- Dare, S.A.; Pearce, J.A.; McDonald, I.; Styles, M.T. Tectonic discrimination of peridotites using fO2–Cr# and Ga–Ti–FeIII systematics in chrome–spinel. Chem. Geol. 2009, 261, 199–216. [Google Scholar]
- Ionov, D.A.; Doucet, L.S.; Ashchepkov, I.V. Composition of the lithospheric mantle in the Siberian craton: New constraints from fresh peridotites in the Udachnaya-East kimberlite. J. Petrol. 2010, 51, 2177–2210. [Google Scholar] [CrossRef]
- Mallmann, G.; O’Neill, H.S.C. The crystal/melt partitioning of V during mantle melting as a function of oxygen fugacity compared with some other elements (Al, P, Ca, Sc, Ti, Cr, Fe, Ga, Y, Zr and Nb). J. Petrol. 2009, 50, 1765–1794. [Google Scholar] [CrossRef]
- Rielli, A.; Tomkins, A.G.; Nebel, O.; Brugger, J.; Etschmann, B.; Paterson, D. Garnet peridotites reveal spatial and temporal changes in the oxidation potential of subduction. Sci. Rep. 2018, 8, 16411. [Google Scholar] [CrossRef]
- Frost, D.J.; McCammon, C.A. The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci. 2008, 36, 389–420. [Google Scholar] [CrossRef]
- Horn, I.; Foley, S.F.; Jackson, S.E.; Jenner, G.A. Experimentally determined partitioning of high field strength-and selected transition elements between spinel and basaltic melt. Chem. Geol. 1994, 117, 193–218. [Google Scholar] [CrossRef]
- Sattari, P.; Brenan, J.M.; Horn, I.; McDonough, W.F. Experimental constraints on the sulfide-and chromite-silicate melt partitioning behavior of rhenium and platinum-group elements. Econ. Geol. 2002, 97, 385–398. [Google Scholar] [CrossRef]
- Righter, K.; Leeman, W.P.; Hervig, R.L. Partitioning of Ni, Co and V between spinel-structured oxides and silicate melts: Importance of spinel composition. Chem. Geol. 2006, 227, 1–25. [Google Scholar] [CrossRef]
- Brenan, J.M.; Finnigan, C.F.; McDonough, W.F.; Homolova, V. Experimental constraints on the partitioning of Ru, Rh, Ir, Pt and Pd between chromite and silicate melt: The importance of ferric iron. Chem. Geol. 2012, 302, 16–32. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Y.; Yan, W.; Liu, X. Trace element partitioning between MgAl2O4-spinel and carbonatitic silicate melt from 3 to 6 GPa, with emphasis on the role of cation order-disorder. Solid Earth Sci. 2019, 4, 43–65. [Google Scholar] [CrossRef]
- Loroch, D.; Klemme, S.; Berndt, J.; Rohrbach, A. Experimentally determined trace element partition coefficients between hibonite, melilite, spinel, and silicate melts. Data Brief 2018, 21, 2447–2463. [Google Scholar] [CrossRef]

| Element | Dsp/melt | Source | Melt |
|---|---|---|---|
| V | 1.62 | [53] | Alkali basalt |
| 3.22 | [55] | Picrite | |
| Mn | 0.70 | [57] | CMAS |
| 0.90 | [36] | FCMAST | |
| Co | 2.00 | [55] | Ankaramite |
| 6.00 | [53] | Alkali basalt | |
| Ni | 0.24 | [55] | Ankaramite |
| 12.86 | [55] | Ankaramite | |
| Cu | 0.20 | [57] | Komatiite |
| 0.80 | [57] | MORB | |
| Zn | 1.00 | [53] | Alkali basalt |
| 12.00 | [58] | CMAST | |
| Ga | 3.10 | [53] | Alkali basalt |
| 11.30 | [36] | FCMAST | |
| Zr | 0.02 | [36] | FCMAST |
| 0.14 | [53] | Alkali basalt |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venier, M.; Ziberna, L.; Princivalle, F.; Petrelli, M.; Lughi, V.; Logvinova, A.; Sobolev, N.V.; Turco, G.; Lenaz, D. Trace Elements in Chromian Spinels from Four Siberian Kimberlites. Minerals 2022, 12, 1439. https://doi.org/10.3390/min12111439
Venier M, Ziberna L, Princivalle F, Petrelli M, Lughi V, Logvinova A, Sobolev NV, Turco G, Lenaz D. Trace Elements in Chromian Spinels from Four Siberian Kimberlites. Minerals. 2022; 12(11):1439. https://doi.org/10.3390/min12111439
Chicago/Turabian StyleVenier, Marco, Luca Ziberna, Francesco Princivalle, Maurizio Petrelli, Vanni Lughi, Alla Logvinova, Nikolay V. Sobolev, Gianluca Turco, and Davide Lenaz. 2022. "Trace Elements in Chromian Spinels from Four Siberian Kimberlites" Minerals 12, no. 11: 1439. https://doi.org/10.3390/min12111439
APA StyleVenier, M., Ziberna, L., Princivalle, F., Petrelli, M., Lughi, V., Logvinova, A., Sobolev, N. V., Turco, G., & Lenaz, D. (2022). Trace Elements in Chromian Spinels from Four Siberian Kimberlites. Minerals, 12(11), 1439. https://doi.org/10.3390/min12111439







