The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications
Abstract
:1. Introduction
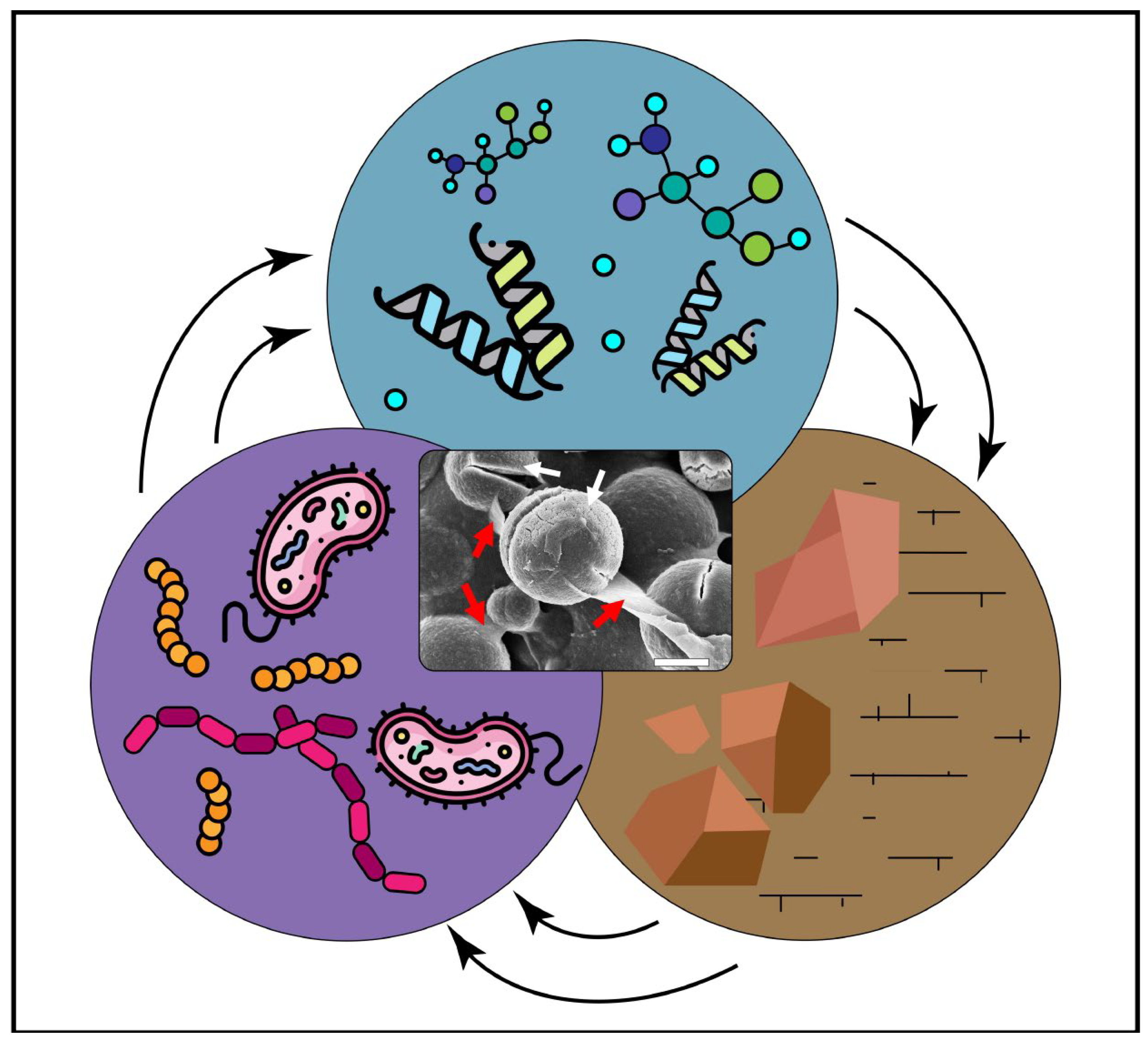
2. Biopolymers, Organic Compounds Relevant for Carbonate Precipitation
3. Types of Microbial Biomineralization
- Biologically influenced mineralization (organomineralization): Passive/indirect microbial biomineralization or biological mineralization. Minerals precipitate by the presence of microorganisms in the environment and the molecules and compounds they may have in their cell wall and/or secreted substances (e.g., EPS), acting as passive nucleation sites. An organic matrix/template enhances the crystal nucleation and precipitation. In this case, living (micro)organisms are not necessarily involved;
- Biologically induced mineralization (BIM): Active/direct microbial biomineralization or biological mineralization. Minerals precipitate as a consequence of the microbial metabolisms (i.e., sulfate reduction, aerobic respiration, methanogenesis, sulfur oxidation and photosynthesis). The metabolic activity of living microbial cells changes the physicochemical conditions (e.g., pH, Mg/Ca and alkalinity) of their surroundings, resulting in the inorganic mineral precipitation and mineral crystal growth. The microbial activity may also affect the mineral precipitate morphology, texture and chemical composition. The parameters modified may not offer any fitness advantage to the (micro)organisms involved but to the environment where they live;
- Biologically controlled mineralization (BCM): The genome of the (micro)organism(s) involved encodes for enzymes or metabolisms specifically evolved to act on the nucleation, precipitation and growth of the mineral crystals, and their synthesis is usually related to the survival of the producing organisms. The acting (micro)organisms are those benefiting from the biomineralization. This is an example of living organisms such as mollusks, which build shells for protection [24], or magnetotactic bacteria and their magnetosomes [11,91,92]. Not only isolated genes, but even entire gene clusters for biomineralization have been sequenced in microorganisms such as Bacillus subtilis [93].
4. Biopolymers Associated with Carbonates
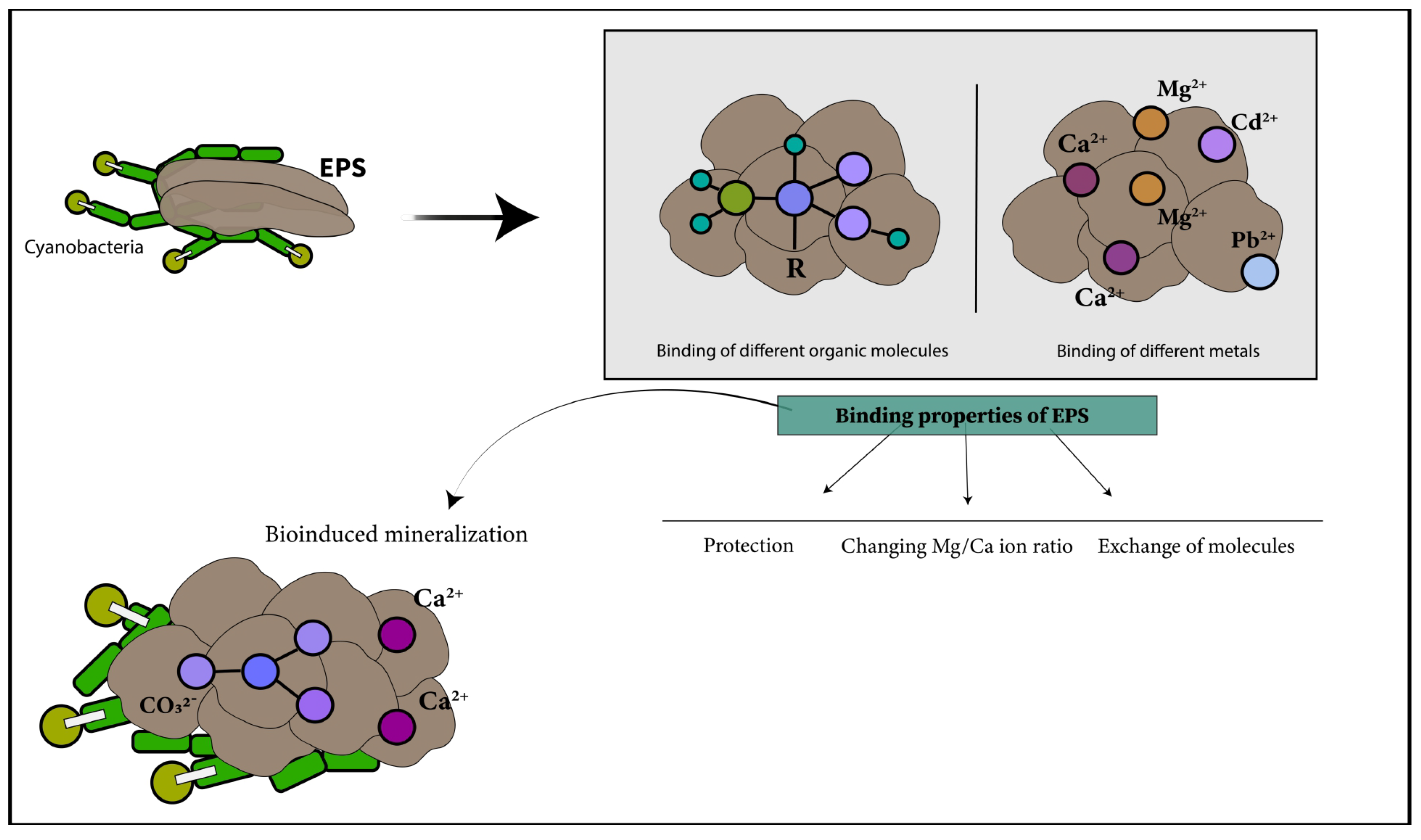
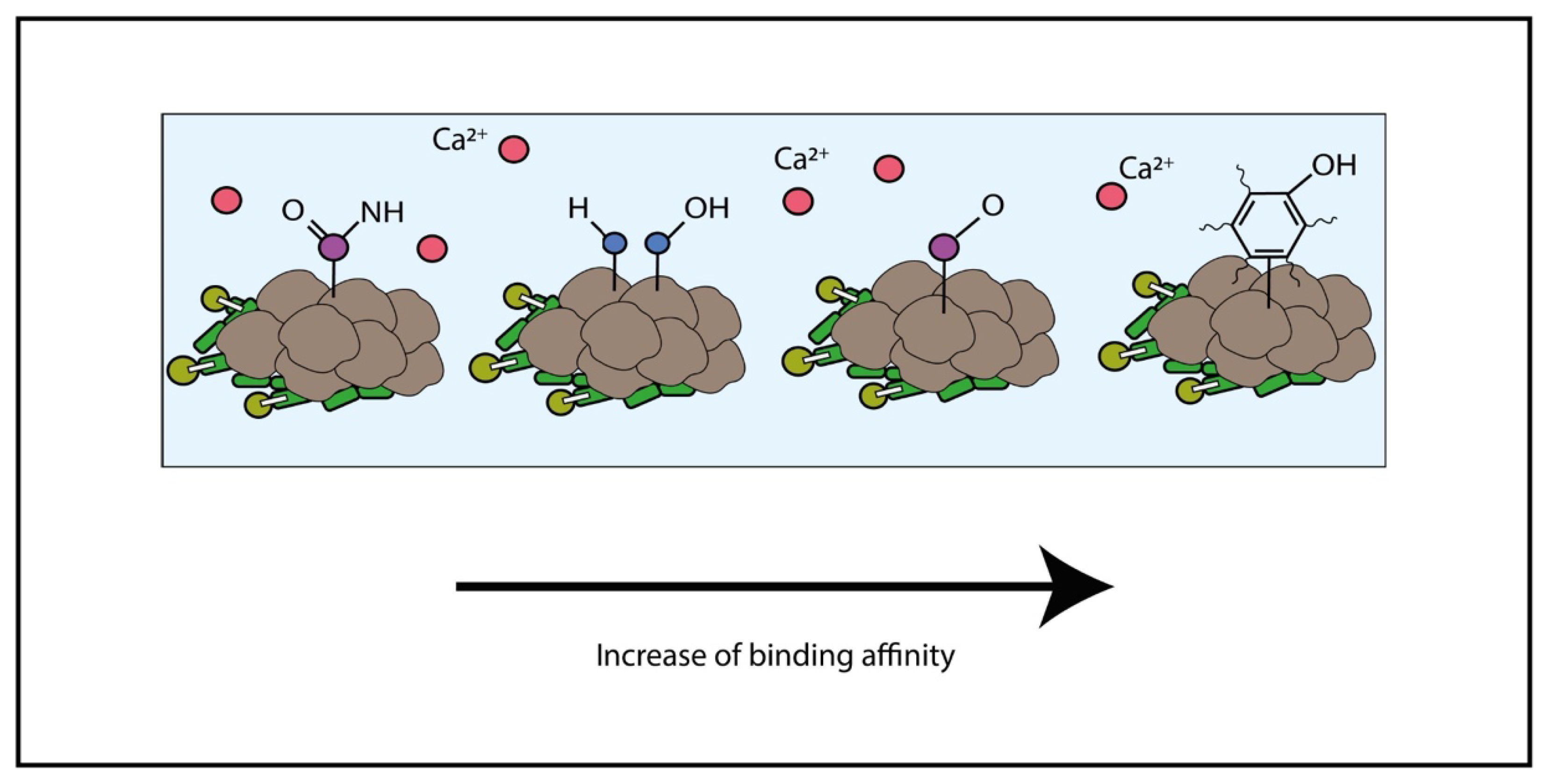
4.1. Adsorption and Linkage of Metal
4.2. Polymers Influencing Size, Morphology, Texture and Chemical Composition of Carbonate Minerals
4.3. Polymeric Substances Secreted by Microorganisms and Micritization, Lithification and Porosity Processes
5. Implications and Applications
5.1. Geology: Early Diagenesis, Burial Diagenesis, Carbonate Reservoirs and Ancient Carbonates
5.1.1. Diagenesis
5.1.2. Carbonate Reservoirs
- Dissolution of inorganic carbonates by various microbial metabolites.
- Generation of bacterial gases, influencing a decrease in the oil’s viscosity and, therefore, enhancing its flow through the pores.
- Production of surface-active compounds.
- High affinity of bacteria for solids, so the bacteria are able to replace the oil attached to the rock surface and to scroll the oil to the center of the pore to facilitate its flow and removal.
5.2. Engineering: Bioremediation, Plastics and Biomining
5.2.1. Bioremediation by Carbon Dioxide Fixation
- Cyanobacteria and microalgae assimilate large quantities of CO2 and tolerate high CO2 concentrations, being able to use CO2 sources such as fuel gas.
- Many species are unaffected by products such as the NOx and SOx present in industrial carbon sources such as flue gas, so they can be used in the treatment of that kind of product easily and in a cheap way.
- Halophilic cyanobacteria can be cultured in seawater, thus saving freshwater.
- Thermophilic cyanobacteria operate at high temperatures, saving fuel gas cooling.
- They can be genetically manipulated relatively easily for performance improvement.
- Nutrients for bacterial growth can be supplied through recycled wastewater.
5.2.2. Bioremediation by Removal of Heavy Metals from Contaminated Soils
5.2.3. Plastic Industry
5.2.4. Bioconstruction and Cementitious Materials
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, L.J.; Wilkinson, B.H.; Ivany, L.C. Continental Drift and Phanerozoic Carbonate Accumulation in Shallow-Shelf and Deep-Marine Settings. J. Geol. 2002, 110, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.S. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar]
- Swart, P.K.; Eberli, G.P.; McKenzie, J.A. Perspectives in Carbonate Geology: A Tribute to the Career of Robert Nathan Ginsburg; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-1-4443-1205-8. [Google Scholar]
- Levi, Y.; Albeck, S.; Brack, A.; Weiner, S.; Addadi, L. Control Over Aragonite Crystal Nucleation and Growth: An In Vitro Study of Biomineralization. Chem.-Eur. J. 1998, 4, 389–396. [Google Scholar] [CrossRef]
- Wang, X.; Kong, R.; Pan, X.; Xu, H.; Xia, D.; Shan, H.; Lu, J.R. Role of Ovalbumin in the Stabilization of Metastable Vaterite in Calcium Carbonate Biomineralization. J. Phys. Chem. B 2009, 113, 8975–8982. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, A.; Perri, E.; Mckenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Warthmann, R.; Rivadeneyra, M.; McKenzie, J.A. Microbial Dolomite Precipitation under Aerobic Conditions: Results from Brejo do Espinho Lagoon (Brazil) and Culture Experiments. In Perspectives in Carbonate Geology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 167–178. ISBN 978-1-4443-1206-5. [Google Scholar]
- Cai, Y.; Tang, R. Calcium phosphate nanoparticles in biomineralization and biomaterials. J. Mater. Chem. 2008, 18, 3775–3787. [Google Scholar] [CrossRef]
- Da Silva, S.; Bernet, N.; Delgenès, J.P.; Moletta, R. Effect of culture conditions on the formation of struvite by Myxococcus xanthus. Chemosphere 2000, 40, 1289–1296. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Pérez-García, I.; Ramos-Cormenzana, A. Struvite precipitation by soil and fresh water bacteria. Curr. Microbiol. 1992, 24, 343–347. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Zeth, K.; Hoiczyk, E.; Okuda, M. Ferroxidase-Mediated Iron Oxide Biomineralization: Novel Pathways to Multifunctional Nanoparticles. Trends Biochem. Sci. 2016, 41, 190–203. [Google Scholar] [CrossRef]
- Staicu, L.C.; Wojtowicz, P.J.; Pósfai, M.; Pekker, P.; Gorecki, A.; Jordan, F.L.; Barton, L.L. PbS biomineralization using cysteine: Bacillus cereus and the sulfur rush. FEMS Microbiol. Ecol. 2020, 96, fiaa151. [Google Scholar] [CrossRef] [PubMed]
- Sigel, A.; Sigel, H.; Sigel, R.K.O. Biomineralization: From Nature to Application, Volume 4; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-98631-8. [Google Scholar]
- Liu, B.; Cao, Y.; Huang, Z.; Duan, Y.; Che, S. Silica Biomineralization via the Self-Assembly of Helical Biomolecules. Adv. Mater. 2015, 27, 479–497. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology: Its significance for geology. Earth-Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Microbes as Geologic Agents: Their Role in Mineral Formation. Geomicrobiol. J. 1999, 16, 135–153. [Google Scholar] [CrossRef]
- Ehrlich, H.; Bailey, E.; Wysokowski, M.; Jesionowski, T. Forced Biomineralization: A Review. Biomimetics 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, W.E. Photolithotropic and chemoorganotrophic activity of bacteria and algae as related to beachrock formation and degradation (gulf of Aqaba, Sinai). Geomicrobiol. J. 1979, 1, 139–203. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Perthuisot, J.-P. Ca-carbonates precipitation and limestone genesis—the microbiogeologist point of view. Sediment. Geol. 1999, 126, 9–23. [Google Scholar] [CrossRef]
- Riding, R. Cyanophyte calcification and changes in ocean chemistry. Nature 1982, 299, 814–815. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Falini, G.; Albeck, S.; Weiner, S.; Addadi, L. Control of Aragonite or Calcite Polymorphism by Mollusk Shell Macromolecules. Science 1996, 271, 67–69. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.—A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Tambutté, S.; Holcomb, M.; Ferrier-Pagès, C.; Reynaud, S.; Tambutté, É.; Zoccola, D.; Allemand, D. Coral biomineralization: From the gene to the environment. J. Exp. Mar. Biol. Ecol. 2011, 408, 58–78. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.-Y.; Fang, Y.; Carlson, C.M.; Xu, H.; Ješovnik, A.; Sosa-Calvo, J.; Zarnowski, R.; Bechtel, H.A.; Fournelle, J.H.; et al. Biomineral armor in leaf-cutter ants. Nat. Commun. 2020, 11, 5792. [Google Scholar] [CrossRef] [PubMed]
- Hild, S.; Marti, O.; Ziegler, A. Spatial distribution of calcite and amorphous calcium carbonate in the cuticle of the terrestrial crustaceans Porcellio scaber and Armadillidium vulgare. J. Struct. Biol. 2008, 163, 100–108. [Google Scholar] [CrossRef]
- Wilt, F.H.; Killian, C.E.; Livingston, B.T. Development of calcareous skeletal elements in invertebrates. Differentiation 2003, 71, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Hoshi, K.; Amizuka, N. Current Concepts of Bone Biomineralization. J. Oral Biosci. 2008, 50, 1–14. [Google Scholar] [CrossRef]
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater. 2020, 1, 2. [Google Scholar] [CrossRef]
- Weiner, S. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Bolhuis, H.; Stal, L.J. Analysis of bacterial and archaeal diversity in coastal microbial mats using massive parallel 16S rRNA gene tag sequencing. ISME J. 2011, 5, 1701–1712. [Google Scholar] [CrossRef]
- Baumgartner, L.K.; Reid, R.P.; Dupraz, C.; Decho, A.W.; Buckley, D.H.; Spear, J.R.; Przekop, K.M.; Visscher, P.T. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries. Sediment. Geol. 2006, 185, 131–145. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.U.P.A.; Bergmann, K.D.; Boekelheide, N.; Tambutté, S.; Mass, T.; Marin, F.; Adkins, J.F.; Erez, J.; Gilbert, B.; Knutson, V.; et al. Biomineralization: Integrating mechanism and evolutionary history. Sci. Adv. 2022, 8, eabl9653. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.U.P.A.; Porter, S.M.; Sun, C.-Y.; Xiao, S.; Gibson, B.M.; Shenkar, N.; Knoll, A.H. Biomineralization by particle attachment in early animals. Proc. Natl. Acad. Sci. USA 2019, 116, 17659–17665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Miao, L.; Yuan, J.; Wang, H.; Wu, L. Application of enzymatic calcification for dust control and rainfall erosion resistance improvement. Sci. Total Environ. 2021, 759, 143468. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, N.; Cai, G.; Jin, Y.; Ding, N.; Shen, D. Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar. Georesources Geotechnol. 2017, 35, 1135–1146. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856. [Google Scholar] [CrossRef]
- Miftah, A.; Khodadadi Tirkolaei, H.; Bilsel, H. Biocementation of Calcareous Beach Sand Using Enzymatic Calcium Carbonate Precipitation. Crystals 2020, 10, 888. [Google Scholar] [CrossRef]
- Nakajima, D.; Kawashima, Y.; Shibata, H.; Yasumi, T.; Isa, M.; Izawa, K.; Nishikomori, R.; Heike, T.; Ohara, O. Simple and Sensitive Analysis for Dried Blood Spot Proteins by Sodium Carbonate Precipitation for Clinical Proteomics. J. Proteome Res. 2020, 19, 2821–2827. [Google Scholar] [CrossRef]
- Wilson, J.L. Limestone and dolomite reservoirs. Pet. Geol.(Engl. Transl.) 1980, 2. [Google Scholar]
- Norris, S.E. Characteristics of Limestone and Dolomite Aquifers in Western Ohio. J. AWWA 1957, 49, 464–468. [Google Scholar] [CrossRef]
- Burchette, T.P. Carbonate rocks and petroleum reservoirs: A geological perspective from the industry. Geol. Soc. Lond. Spec. Publ. 2012, 370, 17–37. [Google Scholar] [CrossRef]
- Smit, B.; Reimer, J.A.; Oldenburg, C.M.; Bourg, I.C. Introduction to Carbon Capture and Sequestration; World Scientific: Singapore, 2014; ISBN 978-1-78326-330-1. [Google Scholar]
- Williams, R.J.P.; Miller, A.; Phillips, D.C.; Williams, R.J.P. An introduction to biominerals and the role of organic molecules in their formation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1984, 304, 411–424. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The Shape and Structure of Proteins. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Murphy, K.P.; Freire, E. Thermodynamics of Structural Stability and Cooperative Folding Behavior in Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Richards, F.M., Edsall, J.T., Eisenberg, D.S., Eds.; Academic Press: Cambridge, MA, USA, 1992; Volume 43, pp. 313–361. [Google Scholar]
- Agarwal, P.K. Enzymes: An integrated view of structure, dynamics and function. Microb. Cell Factories 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouzounis, C.A.; Coulson, R.M.R.; Enright, A.J.; Kunin, V.; Pereira-Leal, J.B. Classification schemes for protein structure and function. Nat. Rev. Genet. 2003, 4, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.M.; Rahman, S.A.; Furnham, N.; Thornton, J.M. The Classification and Evolution of Enzyme Function. Biophys. J. 2015, 109, 1082–1086. [Google Scholar] [CrossRef] [Green Version]
- Yee, N.; Fein, J. Cd adsorption onto bacterial surfaces: A universal adsorption edge? Geochim. Cosmochim. Acta 2001, 65, 2037–2042. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Jahnz, M.; Schwille, P. A New Embedded Process for Compartmentalized Cell-Free Protein Expression and On-line Detection in Microfluidic Devices. ChemBioChem 2005, 6, 811–814. [Google Scholar] [CrossRef]
- Song, E.-H.; Shang, J.; Ratner, D.M. 9.08—Polysaccharides. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. ISBN 978-0-08-087862-1. [Google Scholar]
- Kawano, M.; Hwang, J. Roles of microbial acidic polysaccharides in precipitation rate and polymorph of calcium carbonate minerals. Appl. Clay Sci. 2011, 51, 484–490. [Google Scholar] [CrossRef]
- Mann, K.; Siedler, F.; Treccani, L.; Heinemann, F.; Fritz, M. Perlinhibin, a Cysteine-, Histidine-, and Arginine-Rich Miniprotein from Abalone (Haliotis laevigata) Nacre, Inhibits In Vitro Calcium Carbonate Crystallization. Biophys. J. 2007, 93, 1246–1254. [Google Scholar] [CrossRef] [Green Version]
- Addadi, L.; Weiner, S. Interactions between acidic proteins and crystals: Stereochemical requirements in biomineralization. Proc. Natl. Acad. Sci. USA 1985, 82, 4110–4114. [Google Scholar] [CrossRef] [Green Version]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989; ISBN 978-0-19-504977-0. [Google Scholar]
- Pipich, V.; Balz, M.; Wolf, S.E.; Tremel, W.; Schwahn, D. Nucleation and Growth of CaCO3 Mediated by the Egg-White Protein Ovalbumin: A Time-Resolved in situ Study Using Small-Angle Neutron Scattering. J. Am. Chem. Soc. 2008, 130, 6879–6892. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.S. Polysaccharides and Proteoglycans in Calcium Carbonate-based Biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Henriques, I.D.S.; Love, N.G. The role of extracellular polymeric substances in the toxicity response of activated sludge bacteria to chemical toxins. Water Res. 2007, 41, 4177–4185. [Google Scholar] [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284. [Google Scholar] [CrossRef]
- Romanek, C.S.; Jiménez-López, C.; Navarro, A.R.; Sánchez-Román, M.; Sahai, N.; Coleman, M. Inorganic synthesis of Fe–Ca–Mg carbonates at low temperature. Geochim. Cosmochim. Acta 2009, 73, 5361–5376. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Teng, F.-Z.; Sanchez, W.R.; Romanek, C.S.; Sanchez-Navas, A.; Sánchez-Román, M. Experimental constraints on magnesium isotope fractionation during abiogenic calcite precipitation at room temperature. Geochim. Cosmochim. Acta 2020, 281, 102–117. [Google Scholar] [CrossRef]
- Wen, Y.; Sánchez-Román, M.; Li, Y.; Wang, C.; Han, Z.; Zhang, L.; Gao, Y. Nucleation and stabilization of Eocene dolomite in evaporative lacustrine deposits from central Tibetan plateau. Sedimentology 2020, 67, 3333–3354. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kenward, P.A.; Fowle, D.A.; Goldstein, R.H.; González, L.A.; Moore, D.S. Surface chemistry allows for abiotic precipitation of dolomite at low temperature. Proc. Natl. Acad. Sci. USA 2013, 110, 14540–14545. [Google Scholar] [CrossRef]
- Sanz-Montero, M.E.; Cabestrero, Ó.; Sánchez-Román, M. Microbial Mg-rich Carbonates in an Extreme Alkaline Lake (Las Eras, Central Spain). Front. Microbiol. 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, I.M.; Kenward, P.A.; Dipple, G.M.; Raudsepp, M. Room Temperature Magnesite Precipitation. Cryst. Growth Des. 2017, 17, 5652–5659. [Google Scholar] [CrossRef]
- Power, I.M.; Wilson, S.A.; Thom, J.M.; Dipple, G.M.; Gabites, J.E.; Southam, G. The hydromagnesite playas of Atlin, British Columbia, Canada: A biogeochemical model for CO2 sequestration. Chem. Geol. 2009, 260, 286–300. [Google Scholar] [CrossRef]
- Sajjad, M.; Kim, K. Studies on the interactions of Ca2+ and Mg2+ with EPS and their role in determining the physicochemical characteristics of granular sludge in SBR system. Process Biochem. 2015, 50, 966–972. [Google Scholar] [CrossRef]
- Gutierrez, T.; Biller, D.V.; Shimmield, T.; Green, D.H. Metal binding properties of the EPS produced by Halomonas sp. TG39 and its potential in enhancing trace element bioavailability to eukaryotic phytoplankton. BioMetals 2012, 25, 1185–1194. [Google Scholar] [CrossRef]
- d’Abzac, P.; Bordas, F.; Joussein, E.; van Hullebusch, E.D.; Lens, P.N.L.; Guibaud, G. Metal binding properties of extracellular polymeric substances extracted from anaerobic granular sludges. Environ. Sci. Pollut. Res. 2013, 20, 4509–4519. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, J.; Yang, R.; Wu, L. Distribution, characteristics of extracellular polymeric substances of Phanerochaete chrysosporium under lead ion stress and the influence on Pb removal. Sci. Rep. 2020, 10, 17633. [Google Scholar] [CrossRef]
- Hao, L.; Guo, Y.; Byrne, J.M.; Zeitvogel, F.; Schmid, G.; Ingino, P.; Li, J.; Neu, T.R.; Swanner, E.D.; Kappler, A.; et al. Binding of heavy metal ions in aggregates of microbial cells, EPS and biogenic iron minerals measured in-situ using metal- and glycoconjugates-specific fluorophores. Geochim. Cosmochim. Acta 2016, 180, 66–96. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Weitzel, F.; Jiménez-López, C.; Griesshaber, E.; Fernández-Díaz, L.; Rodríguez-Navarro, A.; Ziegler, A.; Schmahl, W.W. Directing Effect of Bacterial Extracellular Polymeric Substances (EPS) on Calcite Organization and EPS–Carbonate Composite Aggregate Formation. Cryst. Growth Des. 2020, 20, 1467–1484. [Google Scholar] [CrossRef]
- Demain, A.L.; Adrio, J.L. Contributions of Microorganisms to Industrial Biology. Mol. Biotechnol. 2008, 38, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N.; Nikaido, H. Microbial Biotechnology: Fundamentals of Applied Microbiology; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-1-139-46563-2. [Google Scholar]
- Nigam, P.S. Microbial Enzymes with Special Characteristics for Biotechnological Applications. Biomolecules 2013, 3, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Cueva, M.E.; Horsfall, L.E. The contribution of microbially produced nanoparticles to sustainable development goals. Microb. Biotechnol. 2017, 10, 1212–1215. [Google Scholar] [CrossRef]
- Luyts, K.; Napierska, D.; Nemery, B.; Hoet, P.H.M. How physico-chemical characteristics of nanoparticles cause their toxicity: Complex and unresolved interrelations. Environ. Sci. Process. Impacts 2013, 15, 23–38. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef]
- Thompson, J.B.; Ferris, F.G.; Smith, D.A. Geomicrobiology and sedimentology of the mixolimnion and chemocline in Fayetteville Green Lake, New York. PALAIOS 1990, 5, 52–75. [Google Scholar] [CrossRef]
- Westbroek, P. Life as a Geologic Force: New Opportunities for Paleontology? Paleobiology 1983, 9, 91–96. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Buczynski, C. Bacterially Induced Lithification of Microbial Mats. PALAIOS 1992, 7, 277–293. [Google Scholar] [CrossRef]
- Morita, R.Y. Calcite precipitation by marine bacteria. Geomicrobiol. J. 1980, 2, 63–82. [Google Scholar] [CrossRef]
- Chafetz, H.S. Marine peloids; a product of bacterially induced precipitation of calcite. J. Sediment. Res. 1986, 56, 812–817. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Schübbe, S. Controlled Biomineralization by and Applications of Magnetotactic Bacteria. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2007; Volume 62, pp. 21–62. [Google Scholar]
- Bazylinski, D.A.; Frankel, R.B. Biologically Controlled Mineralization in Prokaryotes. Rev. Miner. Geochem. 2003, 54, 217–247. [Google Scholar] [CrossRef]
- Barabesi, C.; Galizzi, A.; Mastromei, G.; Rossi, M.; Tamburini, E.; Perito, B. Bacillus subtilis Gene Cluster Involved in Calcium Carbonate Biomineralization. J. Bacteriol. 2007, 189, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, K.L.; Kading, T.J.; Braissant, O.; Dupraz, C.; Visscher, P.T. Inside the alkalinity engine: The role of electron donors in the organomineralization potential of sulfate-reducing bacteria. Geobiology 2012, 10, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef] [Green Version]
- Dhami, N.K.; Quirin, M.E.C.; Mukherjee, A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol. Eng. 2017, 103, 106–117. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of Calcium Carbonate Polymorphs by the Bacterial Strains Isolated from Calcareous Sites. J. Microbiol. Biotechnol. 2013, 23, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Dittrich, M.; Sibler, S. Cell surface groups of two picocyanobacteria strains studied by zeta potential investigations, potentiometric titration, and infrared spectroscopy. J. Colloid Interface Sci. 2005, 286, 487–495. [Google Scholar] [CrossRef]
- Li, Q.; Csetenyi, L.; Gadd, G.M. Biomineralization of Metal Carbonates by Neurospora crassa. Environ. Sci. Technol. 2014, 48, 14409–14416. [Google Scholar] [CrossRef]
- Liu, R.; Huang, S.; Zhang, X.; Song, Y.; He, G.; Wang, Z.; Lian, B. Bio-mineralisation, characterization, and stability of calcium carbonate containing organic matter. RSC Adv. 2021, 11, 14415–14425. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Dominguez-Vera, J.M.; Garcia-Ruiz, J.M. Influence of lysozyme on the precipitation of calcium carbonate: A kinetic and morphologic study. Geochim. Cosmochim. Acta 2003, 67, 1667–1676. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, H.; Shelobolina, E.S.; Konishi, H.; Roden, E.E. Precipitation of low-temperature disordered dolomite induced by extracellular polymeric substances of methanogenic Archaea Methanosarcina barkeri: Implications for sedimentary dolomite formation. Am. Miner. 2021, 106, 69–81. [Google Scholar] [CrossRef]
- Trump, B.F.; Berezesky, I.K.; Sato, T.; Laiho, K.U.; Phelps, P.C.; DeClaris, N. Cell calcium, cell injury and cell death. Environ. Health Perspect. 1984, 57, 281–287. [Google Scholar] [CrossRef] [PubMed]
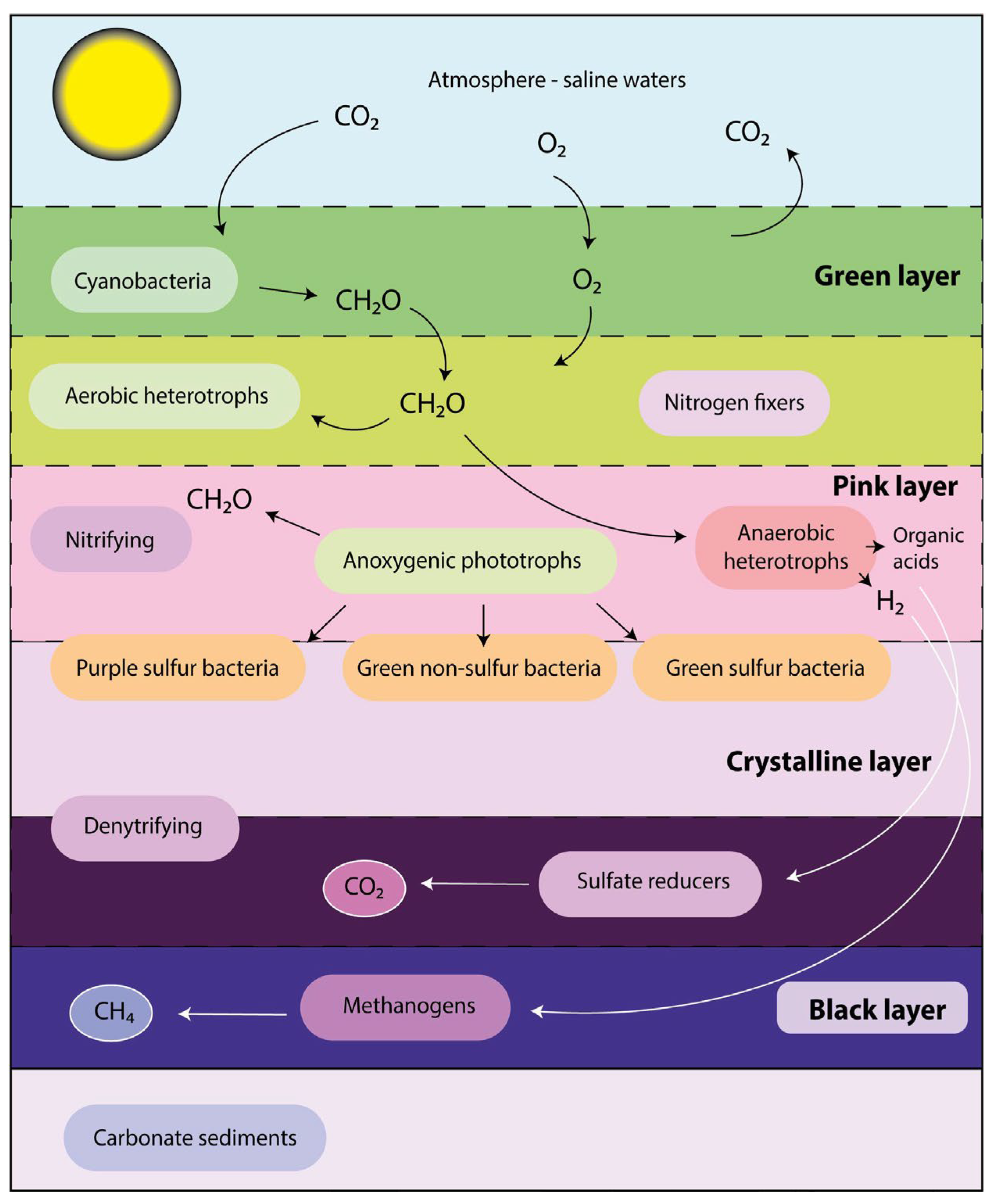
- Prieto-Barajas, C.M.; Valencia-Cantero, E.; Santoyo, G. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electron. J. Biotechnol. 2018, 31, 48–56. [Google Scholar] [CrossRef]
- Bolhuis, H.; Cretoiu, M.S.; Stal, L.J. Molecular ecology of microbial mats. FEMS Microbiol. Ecol. 2014, 90, 335–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 1–13. ISBN 978-94-007-3855-3. [Google Scholar]
- Jacob-Lopes, E.; Zepka, L.Q.; Queiroz, M.I. Cyanobacteria and carbon sequestration. In Cyanobacteria; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 65–71. ISBN 978-1-118-40223-8. [Google Scholar]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Barton, L.L.; Fauque, G.D. Chapter 2 Biochemistry, Physiology and Biotechnology of Sulfate-Reducing Bacteria. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2009; Volume 68, pp. 41–98. [Google Scholar]
- Jørgensen, B.B. The sulfur cycle of a coastal marine sediment (Limfjorden, Denmark)1. Limnol. Oceanogr. 1977, 22, 814–832. [Google Scholar] [CrossRef]
- Visscher, P.T.; Reid, R.P.; Bebout, B.M. Microscale observations of sulfate reduction: Correlation of microbial activity with lithified micritic laminae in modern marine stromatolites. Geology 2000, 28, 919–922. [Google Scholar] [CrossRef]
- Plée, K.; Pacton, M.; Ariztegui, D. Discriminating the Role of Photosynthetic and Heterotrophic Microbes Triggering Low-Mg Calcite Precipitation in Freshwater Biofilms (Lake Geneva, Switzerland). Geomicrobiol. J. 2010, 27, 391–399. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Gat, D.; Tsesarsky, M.; Wahanon, A.; Ronen, Z. Ureolysis and MICP with Model and Native Bacteria: Implications for Treatment Strategies. In Geo-Congress 2014: Geo-Characterization and Modeling for Sustainability; American Society of Civil Engineers: Reston, VA, USA, 2014; pp. 1713–1720. [Google Scholar] [CrossRef]
- Lesley, A.; Warren, P.A.M.; Parmar, F.N. Grant Ferris Microbially Mediated Calcium Carbonate Precipitation: Implications for Interpreting Calcite Precipitation and for Solid-Phase Capture of Inorganic Contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar] [CrossRef]
- Burt, C.D.; Cabrera, M.L.; Rothrock, M.J., Jr.; Kissel, D.E. Urea Hydrolysis and Calcium Carbonate Precipitation in Gypsum-Amended Broiler Litter. J. Environ. Qual. 2018, 47, 162–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Andersson, M.; Laukkanen, M.; Nurmiaho-Lassila, E.-L.; Rainey, F.A.; Niemelä, S.I.; Salkinoja-Salonen, M. Bacillus thermosphaericus sp. nov. a New Thermophilic Ureolytic: Bacillus Isolated from Air. Syst. Appl. Microbiol. 1995, 18, 203–220. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The Influence of Bacillus pasteurii on the Nucleation and Growth of Calcium Carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
- Helmi, F.M.; Elmitwalli, H.R.; Elnagdy, S.M.; El-Hagrassy, A.F. Calcium carbonate precipitation induced by ureolytic bacteria Bacillus licheniformis. Ecol. Eng. 2016, 90, 367–371. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J. Ind. Microbiol. Biotechnol. 2009, 36, 981–988. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Khoshdelnezamiha, G.; Senian, N.; Ong, D.E.L.; Nissom, P.M. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol. Eng. 2017, 109, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Ciurli, S.; Marzadori, C.; Benini, S.; Deiana, S.; Gessa, C. Urease from the soil bacterium Bacillus pasteurii: Immobilization on Ca-polygalacturonate. Soil Biol. Biochem. 1996, 28, 811–817. [Google Scholar] [CrossRef]
- Bachmeier, K.L.; Williams, A.E.; Warmington, J.R.; Bang, S.S. Urease activity in microbiologically-induced calcite precipitation. J. Biotechnol. 2002, 93, 171–181. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S.; Saint, C. A Review of Enzyme Induced Carbonate Precipitation (EICP): The Role of Enzyme Kinetics. Sustain. Chem. 2021, 2, 92–114. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-Art Review of the Applicability and Challenges of Microbial-Induced Calcite Precipitation (MICP) and Enzyme-Induced Calcite Precipitation (EICP) Techniques for Geotechnical and Geoenvironmental Applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Chekroun, K.B.; Rodríguez-Navarro, C.; González-Muñoz, M.T.; Arias, J.M.; Cultrone, G.; Rodríguez-Gallego, M. Precipitation and Growth Morphology of Calcium Carbonate Induced by Myxococcus Xanthus: Implications for Recognition of Bacterial Carbonates. J. Sediment. Res. 2004, 74, 868–876. [Google Scholar] [CrossRef]
- Karatas, I. Microbiological Improvement of the Physical Properties of Soils. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2008. [Google Scholar]
- Martin, D.; Dodds, K.; Butler, I.B.; Ngwenya, B.T. Carbonate Precipitation under Pressure for Bioengineering in the Anaerobic Subsurface via Denitrification. Environ. Sci. Technol. 2013, 47, 8692–8699. [Google Scholar] [CrossRef]
- Torres-Aravena, Á.E.; Duarte-Nass, C.; Azócar, L.; Mella-Herrera, R.; Rivas, M.; Jeison, D. Can Microbially Induced Calcite Precipitation (MICP) through a Ureolytic Pathway Be Successfully Applied for Removing Heavy Metals from Wastewaters? Crystals 2018, 8, 438. [Google Scholar] [CrossRef] [Green Version]
- Nayanthara, P.G.N.; Dassanayake, A.B.N.; Nakashima, K.; Kawasaki, S. Microbial Induced Carbonate Precipitation Using a Native Inland Bacterium for Beach Sand Stabilization in Nearshore Areas. Appl. Sci. 2019, 9, 3201. [Google Scholar] [CrossRef] [Green Version]
- Zamarreňo, D.V.; May, E.; Inkpen, R. Influence of Environmental Temperature on Biocalcification by Non-sporing Freshwater Bacteria. Geomicrobiol. J. 2009, 26, 298–309. [Google Scholar] [CrossRef]
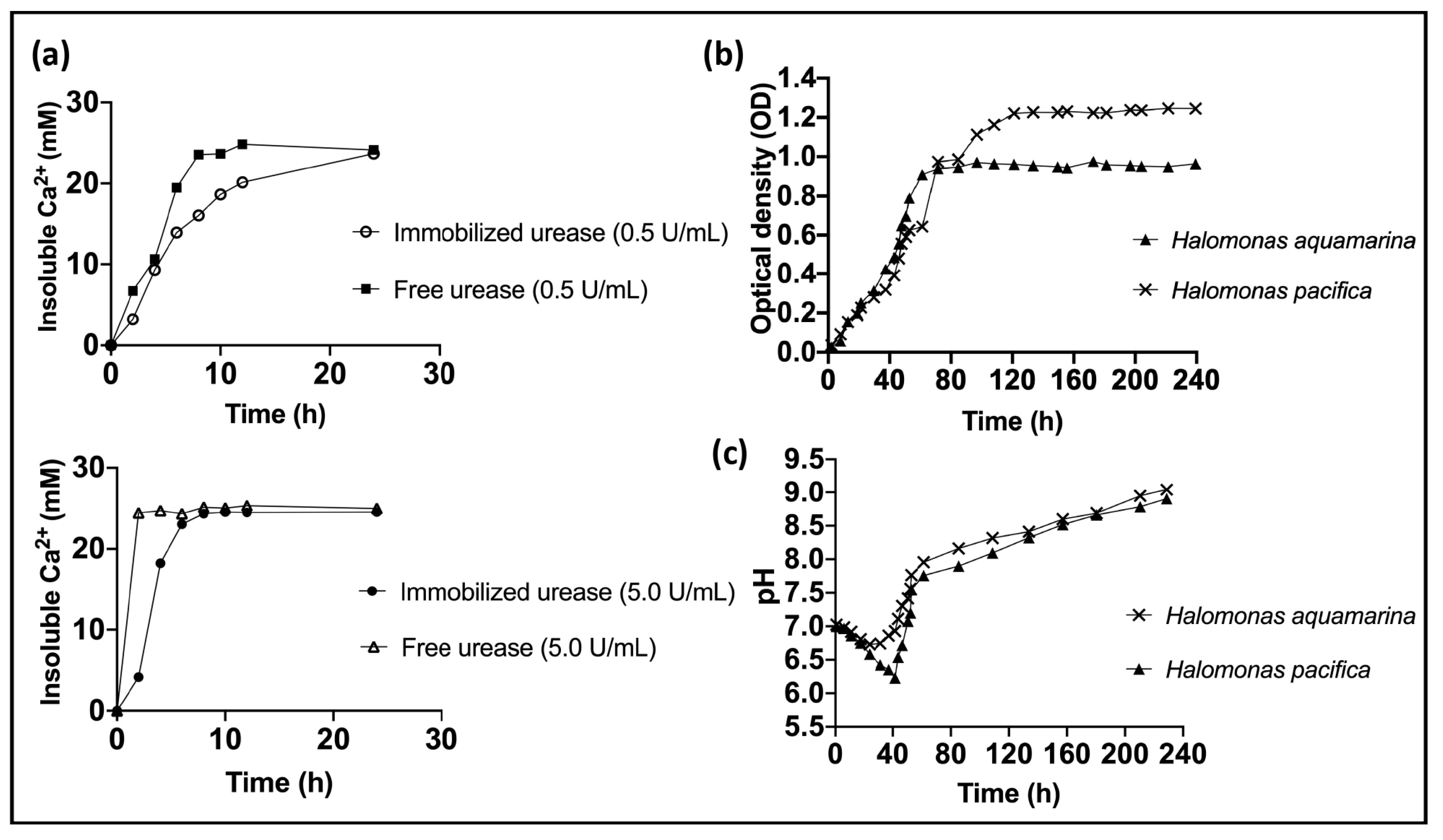
- Krajewska, B. Urease-aided calcium carbonate mineralization for engineering applications: A review. J. Adv. Res. 2018, 13, 59–67. [Google Scholar] [CrossRef]
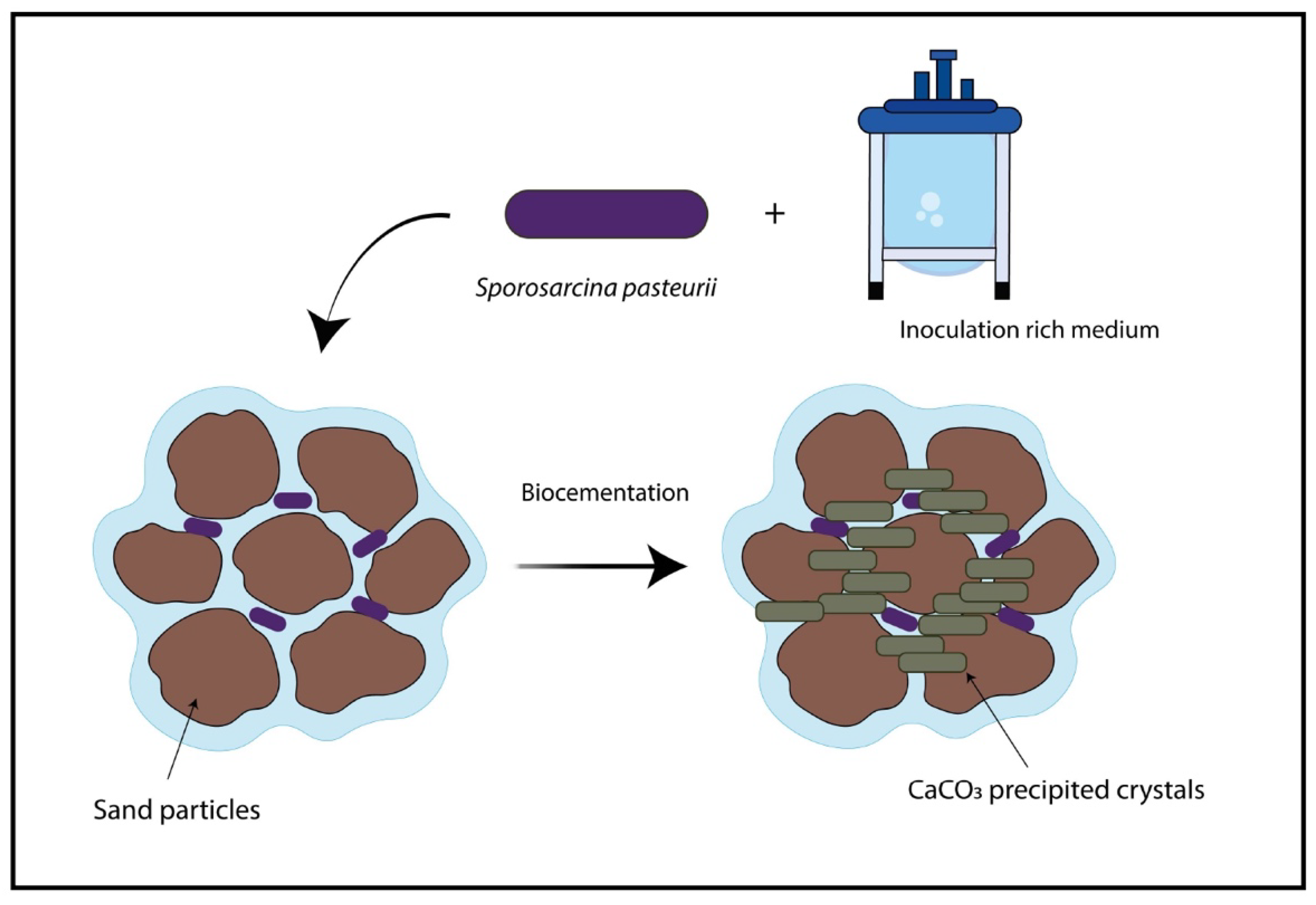
- Castro-Alonso, M.J.; Montañez-Hernandez, L.E.; Sanchez-Muñoz, M.A.; Franco, M.R.M.; Narayanasamy, R.; Balagurusamy, N. Microbially Induced Calcium Carbonate Precipitation (MICP) and Its Potential in Bioconcrete: Microbiological and Molecular Concepts. Front. Mater. 2019, 6, 126. [Google Scholar] [CrossRef]
- Henze, J.; Randall, D.G. Microbial induced calcium carbonate precipitation at elevated pH values (>11) using Sporosarcina pasteurii. J. Environ. Chem. Eng. 2018, 6, 5008–5013. [Google Scholar] [CrossRef]
- McCoy, D.D.; Cetin, A.; Hausinger, R.P. Characterization of urease from Sporosarcina ureae. Arch. Microbiol. 1992, 157, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Cosmidis, J.; Benzerara, K. Why do microbes make minerals? Comptes Rendus Géosci. 2022, 354, 1–39. [Google Scholar] [CrossRef]
- Barnes, H. Oceanography And Marine Biology; CRC Press: Boca Raton, FL, USA, 1990; ISBN 978-1-4822-6728-0. [Google Scholar]
- Quigley, M.S.; Santschi, P.H.; Hung, C.-C.; Guo, L.; Honeyman, B.D. Importance of acid polysaccharides for 234Th complexation to marine organic matter. Limnol. Oceanogr. 2002, 47, 367–377. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, J.; Yuan, Z.; Wang, T.; Zhang, Y.; Wang, F. Bioremediation of Cd by strain GZ-22 isolated from mine soil based on biosorption and microbially induced carbonate precipitation. Environ. Sci. Pollut. Res. 2017, 24, 372–380. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. Effect of Strontium Contaminants upon the Size and Solubility of Calcite Crystals Precipitated by the Bacterial Hydrolysis of Urea. Environ. Sci. Technol. 2006, 40, 1008–1014. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Cosmochim. Acta 2005, 69, 4199–4210. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Rivadeneyra, M.A.; Vasconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth Planet. Sci. Lett. 2009, 285, 131–139. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; McKenzie, J.A.; de Luca Rebello Wagener, A.; Romanek, C.S.; Sánchez-Navas, A.; Vasconcelos, C. Experimentally determined biomediated Sr partition coefficient for dolomite: Significance and implication for natural dolomite. Geochim. Cosmochim. Acta 2011, 75, 887–904. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Fernández-Remolar, D.; Amils, R.; Sánchez-Navas, A.; Schmid, T.; Martin-Uriz, P.S.; Rodríguez, N.; McKenzie, J.A.; Vasconcelos, C. Microbial mediated formation of Fe-carbonate minerals under extreme acidic conditions. Sci. Rep. 2014, 4, 4767. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Román, M.; Puente-Sánchez, F.; Parro, V.; Amils, R. Nucleation of Fe-rich phosphates and carbonates on microbial cells and exopolymeric substances. Front. Microbiol. 2015, 6, 1024. [Google Scholar] [CrossRef]
- Geesey, G.G.; Jang, L.; Jolley, J.G.; Hankins, M.R.; Iwaoka, T.; Griffiths, P.R. Binding of Metal Ions by Extracellular Polymers of Biofilm Bacteria. Water Sci. Technol. 1988, 20, 161–165. [Google Scholar] [CrossRef]
- Liu, Y.; Lam, M.C.; Fang, H.H. Adsorption of heavy metals by EPS of activated sludge. Water Sci. Technol. 2001, 43, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.-P.; Xu, J.; Luo, H.-W.; Li, W.-W.; Li, W.-H.; Yu, H.-Q.; Xie, Z.; Wei, S.-Q.; Hu, F.-C. Thermodynamic analysis on the binding of heavy metals onto extracellular polymeric substances (EPS) of activated sludge. Water Res. 2013, 47, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.; Kim, S.M.; Kim, S.Y.; Lee, S.G.; Kim, I.S. Effect of heavy metals (Cu, Pb, and Ni) on the compositions of EPS in biofilms. Water Sci. Technol. 2001, 43, 41–48. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Vasconcelos, C.; Schmid, T.; Dittrich, M.; McKenzie, J.A.; Zenobi, R.; Rivadeneyra, M.A. Aerobic microbial dolomite at the nanometer scale: Implications for the geologic record. Geology 2008, 36, 879–882. [Google Scholar] [CrossRef]
- Popall, R.M.; Bolhuis, H.; Muyzer, G.; Sánchez-Román, M. Stromatolites as Biosignatures of Atmospheric Oxygenation: Carbonate Biomineralization and UV-C Resilience in a Geitlerinema sp.—Dominated Culture. Front. Microbiol. 2020, 11, 948. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Song, X.; Li, Y.; Zhang, W.; Zhang, H.; Niu, L. Cultivation substrata differentiate the properties of river biofilm EPS and their binding of heavy metals: A spectroscopic insight. Environ. Res. 2020, 182, 109052. [Google Scholar] [CrossRef]
- Beveridge, T.J. Ultrastructure, Chemistry, and Function of the Bacterial Wall. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 1981; Volume 72, pp. 229–317. [Google Scholar]
- Douglas, S.; Beveridge, T.J. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef]
- Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 12096–12101. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Wang, F.; Yue, L. Phase and morphology evolution of vaterite crystals in water/ethanol binary solvent. Cryst. Res. Technol. 2011, 46, 140–144. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water–Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, F.; Farfan, G.A.; Xu, H. Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications. ACS Omega 2022, 7, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Cailleau, G.; Dupraz, C.; Verrecchia, E.P. Bacterially Induced Mineralization of Calcium Carbonate in Terrestrial Environments: The Role of Exopolysaccharides and Amino Acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Kadan, Y.; Tollervey, F.; Varsano, N.; Mahamid, J.; Gal, A. Intracellular nanoscale architecture as a master regulator of calcium carbonate crystallization in marine microalgae. Proc. Natl. Acad. Sci. USA 2021, 118, e2025670118. [Google Scholar] [CrossRef]
- Aizenberg, J.; Addadi, L.; Weiner, S.; Lambert, G. Stabilization of amorphous calcium carbonate by specialized macromolecules in biological and synthetic precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Aizenberg, J.; Hanson, J.; Ilan, M.; Leiserowitz, L.; Koetzle, T.F.; Addadi, L.; Weiner, S. Morphogenesis of calcitic sponge spicules: A role for specialized proteins interacting with growing crystals. FASEB J. 1995, 9, 262–268. [Google Scholar] [CrossRef] [Green Version]
- Belcher, A.M.; Wu, X.H.; Christensen, R.J.; Hansma, P.K.; Stucky, G.D.; Morse, D.E. Control of crystal phase switching and orientation by soluble mollusc-shell proteins. Nature 1996, 381, 56–58. [Google Scholar] [CrossRef]
- Butler, M.F.; Glaser, N.; Weaver, A.C.; Kirkland, M.; Heppenstall-Butler, M. Calcium Carbonate Crystallization in the Presence of Biopolymers. Cryst. Growth Des. 2006, 6, 781–794. [Google Scholar] [CrossRef]
- Broughton, P.L. Microbial EPS-mediated amorphous calcium carbonate–monohydrocalcite–calcite transformations during early tufa deposition. Depos. Rec. 2022, 1–28. [Google Scholar] [CrossRef]
- Wang, X.; Sun, H.; Xia, Y.; Chen, C.; Xu, H.; Shan, H.; Lu, J.R. Lysozyme mediated calcium carbonate mineralization. J. Colloid Interface Sci. 2009, 332, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, A.; Rodríguez-Navarro, A.B.; Gómez-Morales, J.; Jiménez-Lopez, C.; Nys, Y.; García-Ruiz, J.M. Influence of Model Globular Proteins with Different Isoelectric Points on the Precipitation of Calcium Carbonate. Cryst. Growth Des. 2008, 8, 1495–1502. [Google Scholar] [CrossRef]
- Connolly, J.; Kaufman, M.; Rothman, A.; Gupta, R.; Redden, G.; Schuster, M.; Colwell, F.; Gerlach, R. Construction of two ureolytic model organisms for the study of microbially induced calcium carbonate precipitation. J. Microbiol. Methods 2013, 94, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heveran, C.M.; Liang, L.; Nagarajan, A.; Hubler, M.H.; Gill, R.; Cameron, J.C.; Cook, S.M.; Srubar, W.V. Engineered Ureolytic Microorganisms Can Tailor the Morphology and Nanomechanical Properties of Microbial-Precipitated Calcium Carbonate. Sci. Rep. 2019, 9, 14721. [Google Scholar] [CrossRef]
- Gadd, G.-M.; Burford, E.-P.; Fomina, M. Biogeochemical Activities of Microorganisms in Mineral Transformations: Consequences for Metal and Nutrient Mobility. J. Microbiol. Biotechnol. 2003, 13, 323–331. [Google Scholar]
- Violante, A.; Huang, P.M.; Gadd, G.M. Biophysico-Chemical Processes of Heavy Metals and Metalloids in Soil Environments; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 978-0-470-17547-7. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 6730305. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Metals and microorganism: A problem of definition. FEMS Microbiol. Lett. 1992, 100, 197–203. [Google Scholar] [CrossRef]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef]
- Reid, R.P.; Macintyre, I.G. Microboring Versus Recrystallization: Further Insight into the Micritization Process. J. Sediment. Res. 2000, 70, 24–28. [Google Scholar] [CrossRef]
- Kobluk, D.R.; Risk, M.J. Micritization and Carbonate-Grain Binding by Endolithic Algae1. AAPG Bull. 1977, 61, 1069–1082. [Google Scholar] [CrossRef]
- Boulos, R.A.; Zhang, F.; Tjandra, E.S.; Martin, A.D.; Spagnoli, D.; Raston, C.L. Spinning up the polymorphs of calcium carbonate. Sci. Rep. 2014, 4, 3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politi, Y.; Levi-Kalisman, Y.; Raz, S.; Wilt, F.; Addadi, L.; Weiner, S.; Sagi, I. Structural Characterization of the Transient Amorphous Calcium Carbonate Precursor Phase in Sea Urchin Embryos. Adv. Funct. Mater. 2006, 16, 1289–1298. [Google Scholar] [CrossRef]
- Loste, E.; Meldrum, F.C. Control of calcium carbonate morphology by transformation of an amorphous precursor in a constrained volume. Chem. Commun. 2001, 10, 901–902. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, E7670–E7678. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Golubic, S.; Perkins, R.D.; Lukas, K.J. Boring Microorganisms and Microborings in Carbonate Substrates. In The Study of Trace Fossils: A Synthesis of Principles, Problems, and Procedures in Ichnology; Frey, R.W., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 229–259. ISBN 978-3-642-65923-2. [Google Scholar]
- Garcia-Pichel, F.; Ramírez-Reinat, E.; Gao, Q. Microbial excavation of solid carbonates powered by P-type ATPase-mediated transcellular Ca2+ transport. Proc. Natl. Acad. Sci. USA 2010, 107, 21749–21754. [Google Scholar] [CrossRef] [Green Version]
- Cockell, C.S.; Herrera, A. Why are some microorganisms boring? Trends Microbiol. 2008, 16, 101–106. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microbiol. Spectr. 2015, 3, 201–222. [Google Scholar] [CrossRef] [Green Version]
- Roca, C.; Alves, V.D.; Freitas, F.; Reis, M.A.M. Exopolysaccharides enriched in rare sugars: Bacterial sources, production, and applications. Front. Microbiol. 2015, 6, 288. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary Deposits of Benthic Microbial Communities. PALAIOS 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Suarez-Gonzalez, P.; Benito, M.I.; Quijada, I.E.; Mas, R.; Campos-Soto, S. ‘Trapping and binding’: A review of the factors controlling the development of fossil agglutinated microbialites and their distribution in space and time. Earth-Sci. Rev. 2019, 194, 182–215. [Google Scholar] [CrossRef] [Green Version]
- Suarez-Gonzalez, P.; Quijada, I.E.; Benito, M.I.; Mas, R. Sedimentology of Ancient Coastal Wetlands: Insights From A Cretaceous Multifaceted Depositional System. J. Sediment. Res. 2015, 85, 95–117. [Google Scholar] [CrossRef]
- Arp, G.; Reimer, A.; Reitner, J. Photosynthesis-Induced Biofilm Calcification and Calcium Concentrations in Phanerozoic Oceans. Science 2001, 292, 1701–1704. [Google Scholar] [CrossRef] [Green Version]
- Campos-Soto, S.; Benito, M.I.; Mas, R.; Caus, E.; Cobos, A.; Suárez-González, P.; Quijada, I.E. Revisiting the Late Jurassic-Early Cretaceous of the NW South Iberian Basin: New ages and sedimentary environments. J. Iber. Geol. 2016, 42, 69–94. [Google Scholar] [CrossRef] [Green Version]
- Lanés, S.; Palma, R.M. Environmental implications of oncoids and associated sediments from the Remoredo Formation (Lower Jurassic) Mendoza, Argentina. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 140, 357–366. [Google Scholar] [CrossRef]
- Reid, R.P.; Visscher, P.T.; Decho, A.W.; Stolz, J.F.; Bebout, B.M.; Dupraz, C.; Macintyre, I.G.; Paerl, H.W.; Pinckney, J.L.; Prufert-Bebout, L.; et al. The role of microbes in accretion, lamination and early lithification of modern marine stromatolites. Nature 2000, 406, 989–992. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Knoll, A.H. Stromatolites in Precambrian Carbonates: Evolutionary Mileposts or Environmental Dipsticks? Annu. Rev. Earth Planet. Sci. 1999, 27, 313–358. [Google Scholar] [CrossRef]
- Eymard, I.; Alvarez, M. del P.; Bilmes, A.; Vasconcelos, C.; Ariztegui, D. Tracking Organomineralization Processes from Living Microbial Mats to Fossil Microbialites. Minerals 2020, 10, 605. [Google Scholar] [CrossRef]
- Margulis, L.; Barghoorn, E.S.; Ashendorf, D.; Banerjee, S.; Chase, D.; Francis, S.; Giovannoni, S.; Stolz, J. The microbial community in the layered sediments at Laguna Figueroa, Baja California, Mexico: Does it have Precambrian analogues? Precambrian Res. 1980, 11, 93–123. [Google Scholar] [CrossRef]
- Oremland, R.S.; Saltikov, C.W.; Wolfe-Simon, F.; Stolz, J.F. Arsenic in the Evolution of Earth and Extraterrestrial Ecosystems. Geomicrobiol. J. 2009, 26, 522–536. [Google Scholar] [CrossRef]
- Parro, V.; de Diego-Castilla, G.; Moreno-Paz, M.; Blanco, Y.; Cruz-Gil, P.; Rodríguez-Manfredi, J.A.; Fernández-Remolar, D.; Gómez, F.; Gómez, M.J.; Rivas, L.A.; et al. A Microbial Oasis in the Hypersaline Atacama Subsurface Discovered by a Life Detector Chip: Implications for the Search for Life on Mars. Astrobiology 2011, 11, 969–996. [Google Scholar] [CrossRef]
- Demarchi, B. Amino Acids and Proteins in Fossil Biominerals: An Introduction for Archaeologists and Palaeontologists; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-119-08951-3. [Google Scholar]
- Saitta, E.T.; Vinther, J.; Crisp, M.K.; Abbott, G.D.; Kaye, T.G.; Pittman, M.; Bull, I.; Fletcher, I.; Chen, X.; Collins, M.J.; et al. Non-Avian Dinosaur Eggshell Calcite Contains Ancient, Endogenous Amino Acids. bioRxiv 2020. bioRxiv:2020.06.02.129999. [Google Scholar] [CrossRef]
- Hendy, J.; Welker, F.; Demarchi, B.; Speller, C.; Warinner, C.; Collins, M.J. A guide to ancient protein studies. Nat. Ecol. Evol. 2018, 2, 791–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benzerara, K.; Menguy, N.; López-García, P.; Yoon, T.-H.; Kazmierczak, J.; Tyliszczak, T.; Guyot, F.; Brown, G.E. Nanoscale detection of organic signatures in carbonate microbialites. Proc. Natl. Acad. Sci. USA 2006, 103, 9440–9445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piggot, A. Microbial Influences on Sedimentation and Early Diagenesis in Carbonate Environments. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, 2014. [Google Scholar]
- Machel, H. Investigations of Burial Diagenesis in Carbonate Hydrocarbon Reservoir Rocks. Geosci. Can. 2005, 32, 103–128. [Google Scholar]
- Reid, R.P.; Macintyre, I.G. Carbonate recrystallization in shallow marine environments: A widespread diagenetic process forming micritized grains. J. Sediment. Res. 1998, 68, 928–946. [Google Scholar] [CrossRef]
- Budd, D.A.; Perkins, R.D. Bathymetric zonation and paleoecological significance of microborings in Puerto Rican shelf and slope sediments. J. Sediment. Res. 1980, 50, 881–903. [Google Scholar] [CrossRef]
- Swinchatt, J.P. Algal Boring: A Possible Depth Indicator in Carbonate Rocks and Sediments. GSA Bull. 1969, 80, 1391–1396. [Google Scholar] [CrossRef]
- Wacey, D.; Kilburn, M.; Stoakes, C.; Aggleton, H.; Brasier, M. Ambient Inclusion Trails: Their Recognition, Age Range and Applicability to Early Life on Earth. In Links Between Geological Processes, Microbial Activities&Evolution of Life: Microbes and Geology; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Modern Approaches in Solid Earth Sciences; Springer: Dordrecht, The Netherlands, 2008; pp. 113–134. ISBN 978-1-4020-8306-8. [Google Scholar]
- Yang, X.; Han, J.; Wang, X.; Schiffbauer, J.D.; Uesugi, K.; Sasaki, O.; Komiya, T. Euendoliths versus ambient inclusion trails from Early Cambrian Kuanchuanpu Formation, South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 476, 147–157. [Google Scholar] [CrossRef]
- Cosse, R. Oil and Gas Field Development Techniques. Basics of Reservoir Engineering; Editions Technips: Paris, France, 1993. [Google Scholar]
- Lazar, I.; Petrisor, I.G.; Yen, T.F. Microbial Enhanced Oil Recovery (MEOR). Pet. Sci. Technol. 2007, 25, 1353–1366. [Google Scholar] [CrossRef]
- Roehl, P.O.; Choquette, P.W. Carbonate Petroleum Reservoirs; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4612-5040-1. [Google Scholar]
- Tanner, R.S.; Udegbunam, E.O.; McInerney, M.J.; Knapp, R.M. Microbially enhanced oil recovery from carbonate reservoirs. Geomicrobiol. J. 1991, 9, 169–195. [Google Scholar] [CrossRef]
- Tang, C.-S.; Yin, L.; Jiang, N.; Zhu, C.; Zeng, H.; Li, H.; Shi, B. Factors affecting the performance of microbial-induced carbonate precipitation (MICP) treated soil: A review. Environ. Earth Sci. 2020, 79, 94. [Google Scholar] [CrossRef]
- Zobell, C.E. Bacteriological Reviews; Scripps Institution of Oceanography, University of California: La Jolla, CA, USA, 1946; pp. 1–49. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Vardhan, K.H.; Jeevanantham, S.; Karishma, S.B.; Yaashikaa, P.R.; Vellaichamy, P. A review on systematic approach for microbial enhanced oil recovery technologies: Opportunities and challenges. J. Clean. Prod. 2020, 258, 120777. [Google Scholar] [CrossRef]
- Kryachko, Y. Novel approaches to microbial enhancement of oil recovery. J. Biotechnol. 2018, 266, 118–123. [Google Scholar] [CrossRef]
- Gong, H.; Li, Y.; Dong, M.; Ma, S.; Liu, W. Effect of wettability alteration on enhanced heavy oil recovery by alkaline flooding. Colloids Surf. 2016, 488, 28–35. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Q.; Li, A. Displacement mechanisms of enhanced heavy oil recovery by alkaline flooding in a micromodel. Particuology 2012, 10, 298–305. [Google Scholar] [CrossRef]
- Armstrong, R.T.; Wildenschild, D. Investigating the pore-scale mechanisms of microbial enhanced oil recovery. J. Pet. Sci. Eng. 2012, 94–95, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Hao, B.; Cao, G.; Wang, J.; Feng, Y.; Tan, X.; Wang, W. A study on the microbial community structure in oil reservoirs developed by water flooding. J. Pet. Sci. Eng. 2014, 122, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Head, I.M.; Gray, N.D.; Larter, S.R. Life in the slow lane; biogeochemistry of biodegraded petroleum containing reservoirs and implications for energy recovery and carbon management. Front. Microbiol. 2014, 5, 566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cusack, F.; Singh, S.; Mccarthy, C.; Grieco, J.; De Rocco, M.; Nguyen, D.; Lappin-Scott, H.; Costerton, J.W. Enhanced oil recovery—three-dimensional sandpack simulation of ultramicrobacteria resuscitation in reservoir formation. Microbiology 1992, 138, 647–655. [Google Scholar] [CrossRef]
- Romero-Zerón, L. Chemical Enhanced Oil Recovery (cEOR): A Practical Overview; BoD—Books on Demand: Norderstedt, Germany, 2016; ISBN 978-953-51-2700-0. [Google Scholar]
- Nikolova, C.; Gutierrez, T. Use of Microorganisms in the Recovery of Oil from Recalcitrant Oil Reservoirs: Current State of Knowledge, Technological Advances and Future Perspectives. Front. Microbiol. 2020, 10, 2996. [Google Scholar] [CrossRef] [Green Version]
- Kowalewski, E.; Rueslåtten, I.; Steen, K.H.; Bødtker, G.; Torsæter, O. Microbial improved oil recovery—Bacterial induced wettability and interfacial tension effects on oil production. J. Pet. Sci. Eng. 2006, 52, 275–286. [Google Scholar] [CrossRef]
- Folmsbee, M.; Duncan, K.; Han, S.O.; Nagle, D.; Jennings, E.; McInerney, M. Re-identification of the halotolerant, biosurfactant-producing Bacillus licheniformis strain JF-2 as Bacillus mojavensis strain JF-2. Syst. Appl. Microbiol. 2006, 29, 645–649. [Google Scholar] [CrossRef]
- Al-Wahaibi, Y.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef]
- Daryasafar, A.; Jamialahmadi, M.; Moghaddam, M.B.; Moslemi, B. Using biosurfactant producing bacteria isolated from an Iranian oil field for application in microbial enhanced oil recovery. Pet. Sci. Technol. 2016, 34, 739–746. [Google Scholar] [CrossRef]
- Suri, N.; Gassara, F.; Stanislav, P.; Voordouw, G. Microbially Enhanced Oil Recovery by Alkylbenzene-Oxidizing Nitrate-Reducing Bacteria. Front. Microbiol. 2019, 10, 1243. [Google Scholar] [CrossRef]
- Chen, C.; Shen, Y.; An, D.; Voordouw, G. Use of Acetate, Propionate, and Butyrate for Reduction of Nitrate and Sulfate and Methanogenesis in Microcosms and Bioreactors Simulating an Oil Reservoir. Appl. Environ. Microbiol. 2017, 83, e02983-16. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.; Wang, M.; Xing, Y.; Lan, G.; Ge, T.; Yan, X.; Gu, T. Characterization and optimization of biosurfactants produced by Acinetobacter baylyi ZJ2 isolated from crude oil-contaminated soil sample toward microbial enhanced oil recovery applications. Biochem. Eng. J. 2014, 90, 49–58. [Google Scholar] [CrossRef]
- Gray, M.R.; Yeung, A.; Foght, J.M.; Yarranton, H.W. Potential Microbial Enhanced Oil Recovery Processes: A Critical Analysis; OnePetro: Richardson, TX, USA, 2008. [Google Scholar]
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef] [Green Version]
- Lichtschlag, A.; Pearce, C.R.; Suominen, M.; Blackford, J.; Borisov, S.M.; Bull, J.M.; de Beer, D.; Dean, M.; Esposito, M.; Flohr, A.; et al. Suitability analysis and revised strategies for marine environmental carbon capture and storage (CCS) monitoring. Int. J. Greenh. Gas Control 2021, 112, 103510. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.; Mehra, A. A review on ex situ mineral carbonation. Environ. Sci. Pollut. Res. 2021, 28, 12202–12231. [Google Scholar] [CrossRef]
- Pratama, G.B.S.; Yasuhara, H.; Kinoshita, N.; Putra, H. Application of soybean powder as urease enzyme replacement on EICP method for soil improvement technique. IOP Conf. Ser. Earth Environ. Sci. 2021, 622, 012035. [Google Scholar] [CrossRef]
- Rohy, H.; Arab, M.; Zeiada, W.; Omar, M.; Almajed, A.; Tahmaz, A. One Phase Soil Bio-Cementation with EICP-Soil Mixing. In Proceedings of the 4th World Congress on Civil, Structural, and Environmental Engineering (CSEE’19), Rome, Italy, April 2019. [Google Scholar]
- Maren, T.H. Carbonic anhydrase: Chemistry, physiology, and inhibition. Physiol. Rev. 1967, 47, 595–781. [Google Scholar] [CrossRef] [PubMed]
- Kupriyanova, E.; Villarejo, A.; Markelova, A.; Gerasimenko, L.; Zavarzin, G.; Samuelsson, G.; Los, D.A.; Pronina, N. Extracellular carbonic anhydrases of the stromatolite-forming cyanobacterium Microcoleus chthonoplastes. Microbiology 2007, 153, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.; Labhsetwar, N.; Kotwal, S.; Rayalu, S. Single enzyme nanoparticle for biomimetic CO2 sequestration. J. Nanopart. Res. 2011, 13, 263–271. [Google Scholar] [CrossRef]
- Wanjari, S.; Prabhu, C.; Yadav, R.; Satyanarayana, T.; Labhsetwar, N.; Rayalu, S. Immobilization of carbonic anhydrase on chitosan beads for enhanced carbonation reaction. Process Biochem. 2011, 46, 1010–1018. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Sivanesan, S.D.; Mudliar, S.; Kaur, S.; Tripathi, A.K.; Chakrabarti, T. Bio-sequestration of carbon dioxide using carbonic anhydrase enzyme purified from Citrobacter freundii. World J. Microbiol. Biotechnol. 2009, 25, 981–987. [Google Scholar] [CrossRef]
- Deocampo, D.M. Chapter 1 The Geochemistry of Continental Carbonates. In Developments in Sedimentology; Alonso-Zarza, A.M., Tanner, L.H., Eds.; Carbonates in Continental Settings: Geochemistry, Diagenesis and Applications; Elsevier: Amsterdam, The Netherlands, 2010; Volume 62, pp. 1–59. [Google Scholar]
- Okyay, T.O.; Rodrigues, D.F. Biotic and abiotic effects on CO2 sequestration during microbially-induced calcium carbonate precipitation. FEMS Microbiol. Ecol. 2015, 91, fiv017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, K.; Al-Muraikhi, M.; Alghasal, G.S.H.S.; Saadaoui, I.; Bounnit, T.; Rasheed, R.; Dalgamouni, T.; Al Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Potential of novel desert microalgae and cyanobacteria for commercial applications and CO2 sequestration. J. Appl. Phycol. 2019, 31, 2231–2243. [Google Scholar] [CrossRef]
- Jansson, C.; Northen, T. Calcifying cyanobacteria—The potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 2010, 21, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, M.U.E. The biology of carbonate precipitation by cyanobacteria. Facies 1992, 26, 81–101. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Ajo-Franklin, C.M.; Northen, T.; Jansson, C. Cyanobacteria as Biocatalysts for Carbonate Mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gérard, E.; Estève, I.; Moreira, D.; Tavera, R.; López-García, P. Cyanobacterial calcification in modern microbialites at the submicrometer scale. Biogeosciences 2013, 10, 5255–5266. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Oliver, I.M.; Cam, N.; Boudier, T.; Blondeau, M.; Leroy, E.; Cosmidis, J.; Skouri-Panet, F.; Guigner, J.-M.; Férard, C.; et al. Biomineralization Patterns of Intracellular Carbonatogenesis in Cyanobacteria: Molecular Hypotheses. Minerals 2016, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- McCutcheon, J.; Power, I.M.; Shuster, J.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon Sequestration in Biogenic Magnesite and Other Magnesium Carbonate Minerals. Environ. Sci. Technol. 2019, 53, 3225–3237. [Google Scholar] [CrossRef]
- Okyay, T.O.; Nguyen, H.N.; Castro, S.L.; Rodrigues, D.F. CO2 sequestration by ureolytic microbial consortia through microbially-induced calcite precipitation. Sci. Total Environ. 2016, 572, 671–680. [Google Scholar] [CrossRef]
- Rani, S.; Prusty, B.K.; Pal, S.K. Characterization of shales from Damodar valley coalfields for CH4 recovery and CO2 sequestration. Environ. Technol. Innov. 2020, 18, 100739. [Google Scholar] [CrossRef]
- Landa-Marbán, D.; Tveit, S.; Kumar, K.; Gasda, S.E. Practical approaches to study microbially induced calcite precipitation at the field scale. Int. J. Greenh. Gas Control 2021, 106, 103256. [Google Scholar] [CrossRef]
- Tveit, S.; Gasda, S.E.; Hægland, H.; Bødtker, G.; Elenius, M. Numerical Study Of Microbially Induced Calcite Precipitation As A Leakage Mitigation Solution For CO2 Storage; European Association of Geoscientists & Engineers: Houten, The Netherlands, 2018; Volume 2018, pp. 1–5. [Google Scholar]
- Fujita, Y.; Taylor, J.L.; Wendt, L.M.; Reed, D.W.; Smith, R.W. Evaluating the Potential of Native Ureolytic Microbes To Remediate a 90Sr Contaminated Environment. Environ. Sci. Technol. 2010, 44, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, Z.; Ghorbanzadeh, N.; Farhangi, M.B.; Khalili Rad, M. Isolating and Using Bacteria, Producing Urease and L-asparaginase, and Effective on Calcium Carbonate Bioproduction to Remove Zinc from Contaminated Solutions. Iran. J. Soil Water Res. 2021, 52, 549–562. [Google Scholar] [CrossRef]
- Vithanage, M.; Bandara, T.; Al-Wabel, M.I.; Abduljabbar, A.; Usman, A.R.A.; Ahmad, M.; Ok, Y.S. Soil Enzyme Activities in Waste Biochar Amended Multi-Metal Contaminated Soil; Effect of Different Pyrolysis Temperatures and Application Rates. Commun. Soil Sci. Plant Anal. 2018, 49, 635–643. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K.S. MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: A review. Environ. Sci. Ecotechnol. 2021, 6, 100096. [Google Scholar] [CrossRef]
- Li, M.; Fu, Q.-L.; Zhang, Q.; Achal, V.; Kawasaki, S. Bio-grout based on microbially induced sand solidification by means of asparaginase activity. Sci. Rep. 2015, 5, 16128. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.-H.; Han, S.-H.; Shin, Y.; Oh, S.J.; So, J.-S. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl. Biochem. Biotechnol. 2014, 172, 2907–2915. [Google Scholar] [CrossRef]
- Sepúlveda, S.; Duarte-Nass, C.; Rivas, M.; Azócar, L.; Ramírez, A.; Toledo-Alarcón, J.; Gutiérrez, L.; Jeison, D.; Torres-Aravena, Á. Testing the Capacity of Staphylococcus equorum for Calcium and Copper Removal through MICP Process. Minerals 2021, 11, 905. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Zhang, D. Remediation of copper-contaminated soil by Kocuria flava CR1, based on microbially induced calcite precipitation. Ecol. Eng. 2011, 37, 1601–1605. [Google Scholar] [CrossRef]
- Achal, V.; Pan, X.; Fu, Q.; Zhang, D. Biomineralization based remediation of As(III) contaminated soil by Sporosarcina ginsengisoli. J. Hazard. Mater. 2012, 201–202, 178–184. [Google Scholar] [CrossRef]
- Puyen, Z.M.; Villagrasa, E.; Maldonado, J.; Diestra, E.; Esteve, I.; Solé, A. Biosorption of lead and copper by heavy-metal tolerant Micrococcus luteus DE2008. Bioresour. Technol. 2012, 126, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Deng, B.; Thornton, E.C.; Yang, J.; Amonette, J.E. Incorporation of Chromate into Calcium Carbonate Structure During Coprecipitation. Water Air Soil Pollut. 2007, 179, 381–390. [Google Scholar] [CrossRef]
- Bamforth, S.M.; Manning, D.A.C.; Singleton, I.; Younger, P.L.; Johnson, K.L. Manganese removal from mine waters—Investigating the occurrence and importance of manganese carbonates. Appl. Geochem. 2006, 21, 1274–1287. [Google Scholar] [CrossRef]
- Marsili, E.; Beyenal, H.; Di Palma, L.; Merli, C.; Dohnalkova, A.; Amonette, J.E.; Lewandowski, Z. Uranium removal by sulfate reducing biofilms in the presence of carbonates. Water Sci. Technol. 2005, 52, 49–55. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Liu, R.; Du, Y.-J.; Bi, Y.-Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Sun, S.; Mao, L.-B.; Lei, Z.; Yu, S.-H.; Cölfen, H. Hydrogels from Amorphous Calcium Carbonate and Polyacrylic Acid: Bio-Inspired Materials for “Mineral Plastics”. Angew. Chem. Int. Ed. 2016, 55, 11765–11769. [Google Scholar] [CrossRef] [Green Version]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.-J.; Schmidt, G. Highly Extensible, Tough, and Elastomeric Nanocomposite Hydrogels from Poly(ethylene glycol) and Hydroxyapatite Nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef]
- Xia, Y.; Gu, Y.; Zhou, X.; Xu, H.; Zhao, X.; Yaseen, M.; Lu, J.R. Controllable Stabilization of Poly(N-isopropylacrylamide)-Based Microgel Films through Biomimetic Mineralization of Calcium Carbonate. Biomacromolecules 2012, 13, 2299–2308. [Google Scholar] [CrossRef]
- Gebauer, D.; Oliynyk, V.; Salajkova, M.; Sort, J.; Zhou, Q.; Bergström, L.; Salazar-Alvarez, G. A transparent hybrid of nanocrystalline cellulose and amorphous calcium carbonate nanoparticles. Nanoscale 2011, 3, 3563–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissinen, T.; Li, M.; Davis, S.A.; Mann, S. In situ precipitation of amorphous and crystalline calcium sulphates in cellulose thin films. CrystEngComm 2014, 16, 3843–3847. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Engelsen, S.B.; Hansen, H.C.B.; Larsen, O.; Skibsted, L.H. Calcium carbonate crystallization in the α-chitin matrix of the shell of pink shrimp, Pandalus borealis, during frozen storage. J. Cryst. Growth 1997, 177, 125–134. [Google Scholar] [CrossRef]
- Saito, T.; Oaki, Y.; Nishimura, T.; Isogai, A.; Kato, T. Bioinspired stiff and flexible composites of nanocellulose-reinforced amorphous CaCO 3. Mater. Horiz. 2014, 1, 321–325. [Google Scholar] [CrossRef]
- Yoshida, N.; Higashimura, E.; Saeki, Y. Catalytic Biomineralization of Fluorescent Calcite by the Thermophilic Bacterium Geobacillus thermoglucosidasius. Appl. Environ. Microbiol. 2010, 76, 7322–7327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacki, J.J.; Bullard, J.W.; Sant, G.; Brown, K.; Glasser, F.P.; Jones, S.; Ley, T.; Livingston, R.; Nicoleau, L.; Olek, J.; et al. Cements in the 21st century: Challenges, perspectives, and opportunities. J. Am. Ceram. Soc. 2017, 100, 2746–2773. [Google Scholar] [CrossRef]
- Shen, L.; Yu, W.; Li, L.; Zhang, T.; Abshir, I.Y.; Luo, P.; Liu, Z. Microorganism, Carriers, and Immobilization Methods of the Microbial Self-Healing Cement-Based Composites: A Review. Materials 2021, 14, 5116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, H.X.; Cheng, X.H. Role of calcium sources in the strength and microstructure of microbial mortar. Constr. Build. Mater. 2015, 77, 160–167. [Google Scholar] [CrossRef]
- Murugan, R.; Suraishkumar, G.K.; Mukherjee, A.; Dhami, N.K. Influence of native ureolytic microbial community on biocementation potential of Sporosarcina pasteurii. Sci. Rep. 2021, 11, 20856. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, A.I.; Palombo, E.A.; Ong, D.E.L.; Nissom, P.M. Biocementation of sand by Sporosarcina pasteurii strain and technical-grade cementation reagents through surface percolation treatment method. Constr. Build. Mater. 2019, 228, 116828. [Google Scholar] [CrossRef]
- Abo-El-Enein, S.A.; Ali, A.H.; Talkhan, F.N.; Abdel-Gawwad, H.A. Application of microbial biocementation to improve the physico-mechanical properties of cement mortar. HBRC J. 2013, 9, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.G.; Anderson, C.M.; Graddy, C.M.R.; DeJong, J.T.; Nelson, D.C.; Ginn, T.R. Large-Scale Comparison of Bioaugmentation and Biostimulation Approaches for Biocementation of Sands. J. Geotech. Geoenviron. Eng. 2017, 143, 04016124. [Google Scholar] [CrossRef]
- Gomez, M.G.; Martinez, B.C.; DeJong, J.T.; Hunt, C.E.; de Vlaming, L.A.; Major, D.W.; Dworatzek, S.M. Field-scale bio-cementation tests to improve sands. Proc. Inst. Civ. Eng.—Ground Improv. 2015, 168, 206–216. [Google Scholar] [CrossRef]
- Kantzas, A.; Stehmeier, L.; Marentette, D.F.; Ferris, F.G.; Jha, K.N.; Maurits, F.M. A Novel Method of Sand Consolidation Through Bacteriogenic Mineral Plugging; OnePetro: Richardson, TX, USA, 1992. [Google Scholar]
- Bang, S.S.; Ramakrishnan, V. Microbiologically—Enhanced Crack Remediation (MECR). Proc. Microbiol. Soc. Korea Conf. 2001, 11, 26–36. [Google Scholar]
- De Belie, N.; De Muynck, W. Crack repair in concrete using biodeposition. In Concrete Repair, Rehabilitation and Retrofitting II; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-10359-9. [Google Scholar]
- Achal, V.; Mukerjee, A.; Reddy, M.S. Biogenic treatment improves the durability and remediates the cracks of concrete structures. Constr. Build. Mater. 2013, 48, 1–5. [Google Scholar] [CrossRef]
- Tiano, P.; Biagiotti, L.; Mastromei, G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: Methods of evaluation. J. Microbiol. Methods 1999, 36, 139–145. [Google Scholar] [CrossRef]
- Dick, J.; De Windt, W.; De Graef, B.; Saveyn, H.; Van der Meeren, P.; De Belie, N.; Verstraete, W. Bio-deposition of a calcium carbonate layer on degraded limestone by Bacillus species. Biodegradation 2006, 17, 357–367. [Google Scholar] [CrossRef]
- Singh, J. Fungal Problems in Historic Buildings. J. Archit. Conserv. 2000, 6, 17–37. [Google Scholar] [CrossRef]
- González, J.M.; Sáiz-Jiménez, C. Application of molecular nucleic acid-based techniques for the study of microbial communities in monuments and artworks. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2005, 8, 189–194. [Google Scholar]
- Webster, A.; May, E. Bioremediation of weathered-building stone surfaces. Trends Biotechnol. 2006, 24, 255–260. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Teasdale, P.R. Optimization of Enzyme Induced Carbonate Precipitation (EICP) as a Ground Improvement Technique. Geo-Congress 2020, 2020, 552–561. [Google Scholar] [CrossRef]
- Meng, H.; Shu, S.; Gao, Y.; Yan, B.; He, J. Multiple-phase enzyme-induced carbonate precipitation (EICP) method for soil improvement. Eng. Geol. 2021, 294, 106374. [Google Scholar] [CrossRef]
- Sun, X.; Miao, L.; Wang, H.; Yuan, J.; Fan, G. Enhanced rainfall erosion durability of enzymatically induced carbonate precipitation for dust control. Sci. Total Environ. 2021, 791, 148369. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robles-Fernández, A.; Areias, C.; Daffonchio, D.; Vahrenkamp, V.C.; Sánchez-Román, M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals 2022, 12, 1562. https://doi.org/10.3390/min12121562
Robles-Fernández A, Areias C, Daffonchio D, Vahrenkamp VC, Sánchez-Román M. The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals. 2022; 12(12):1562. https://doi.org/10.3390/min12121562
Chicago/Turabian StyleRobles-Fernández, Ana, Camila Areias, Daniele Daffonchio, Volker C. Vahrenkamp, and Mónica Sánchez-Román. 2022. "The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications" Minerals 12, no. 12: 1562. https://doi.org/10.3390/min12121562
APA StyleRobles-Fernández, A., Areias, C., Daffonchio, D., Vahrenkamp, V. C., & Sánchez-Román, M. (2022). The Role of Microorganisms in the Nucleation of Carbonates, Environmental Implications and Applications. Minerals, 12(12), 1562. https://doi.org/10.3390/min12121562





