Selective Recovery of Copper from Acid Leaching Solution through Slow Release Sulfide Precipitant
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Preparation of SRSP
2.3. Copper Selective Recovery Experiments
2.4. H2S Gas Release Analysis
2.5. Chemical Reaction Energy Calculation
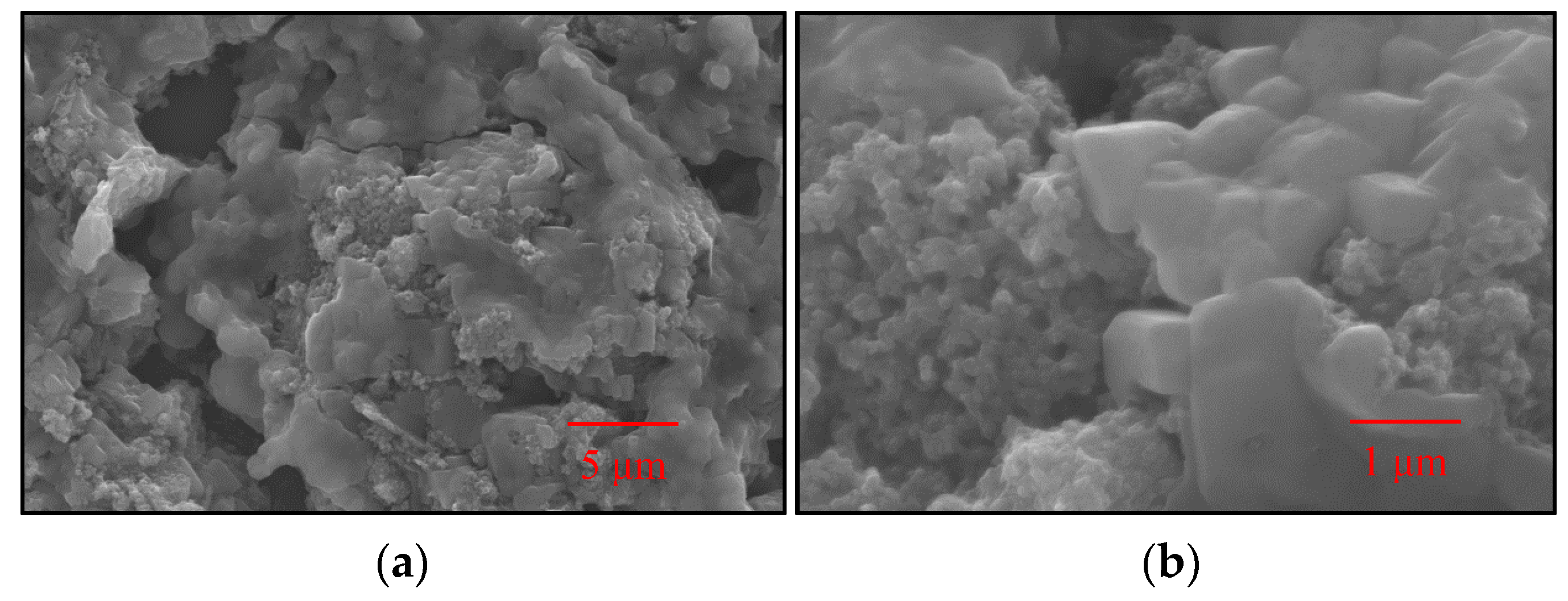
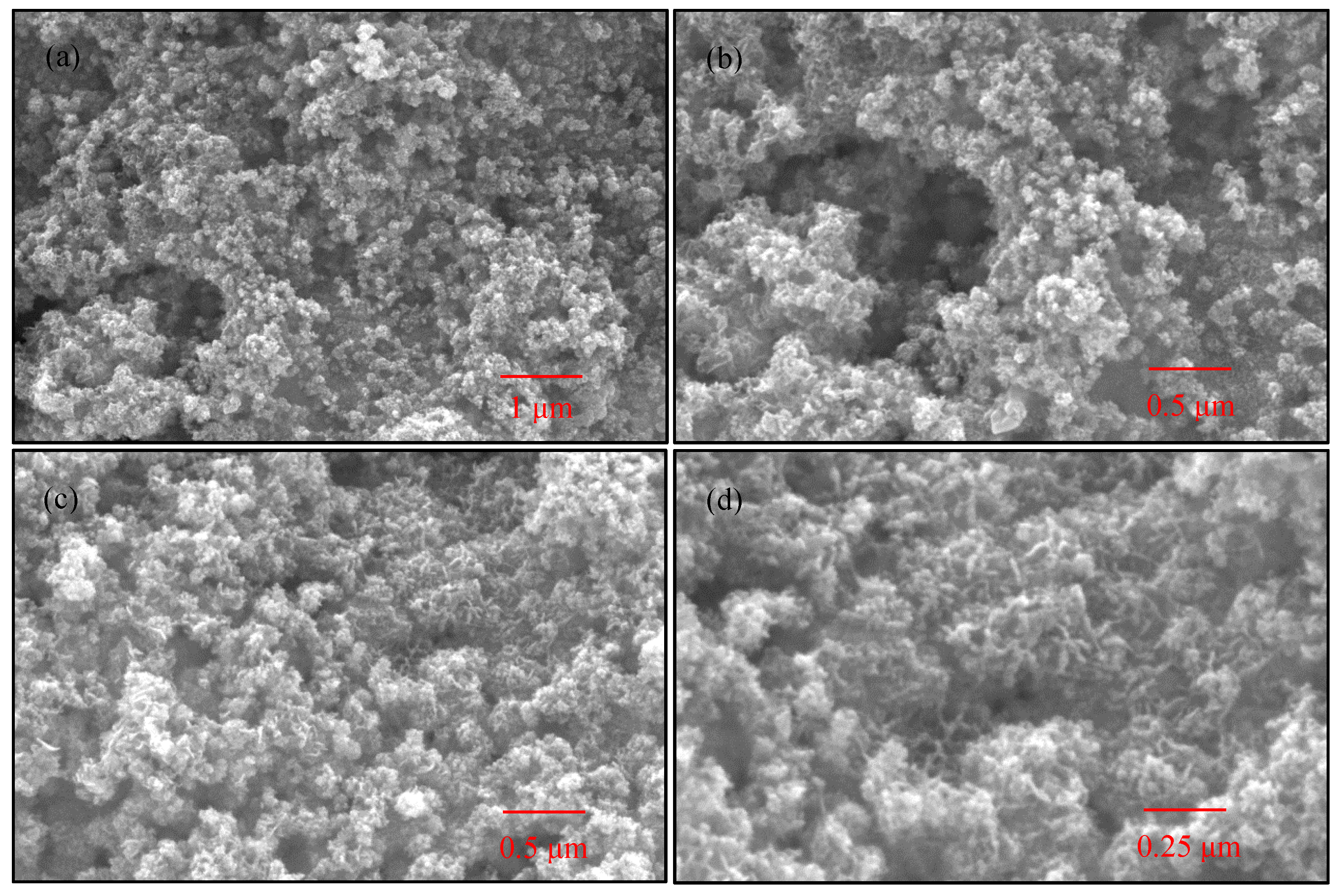
2.6. SEM Images Analysis
3. Results and Discussion
3.1. Copper Selective Recovery Results
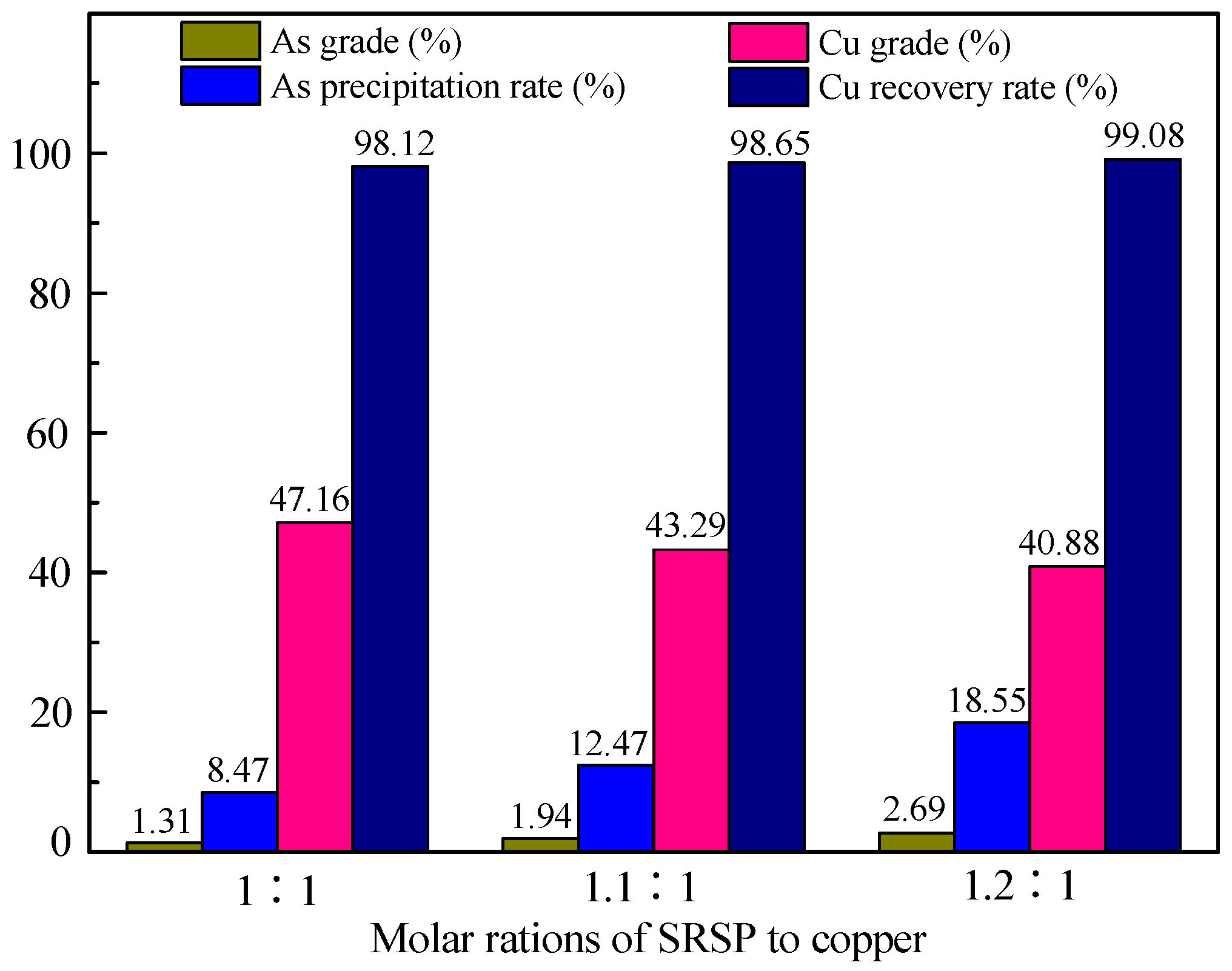
3.1.1. Effect of SRSP Dosage
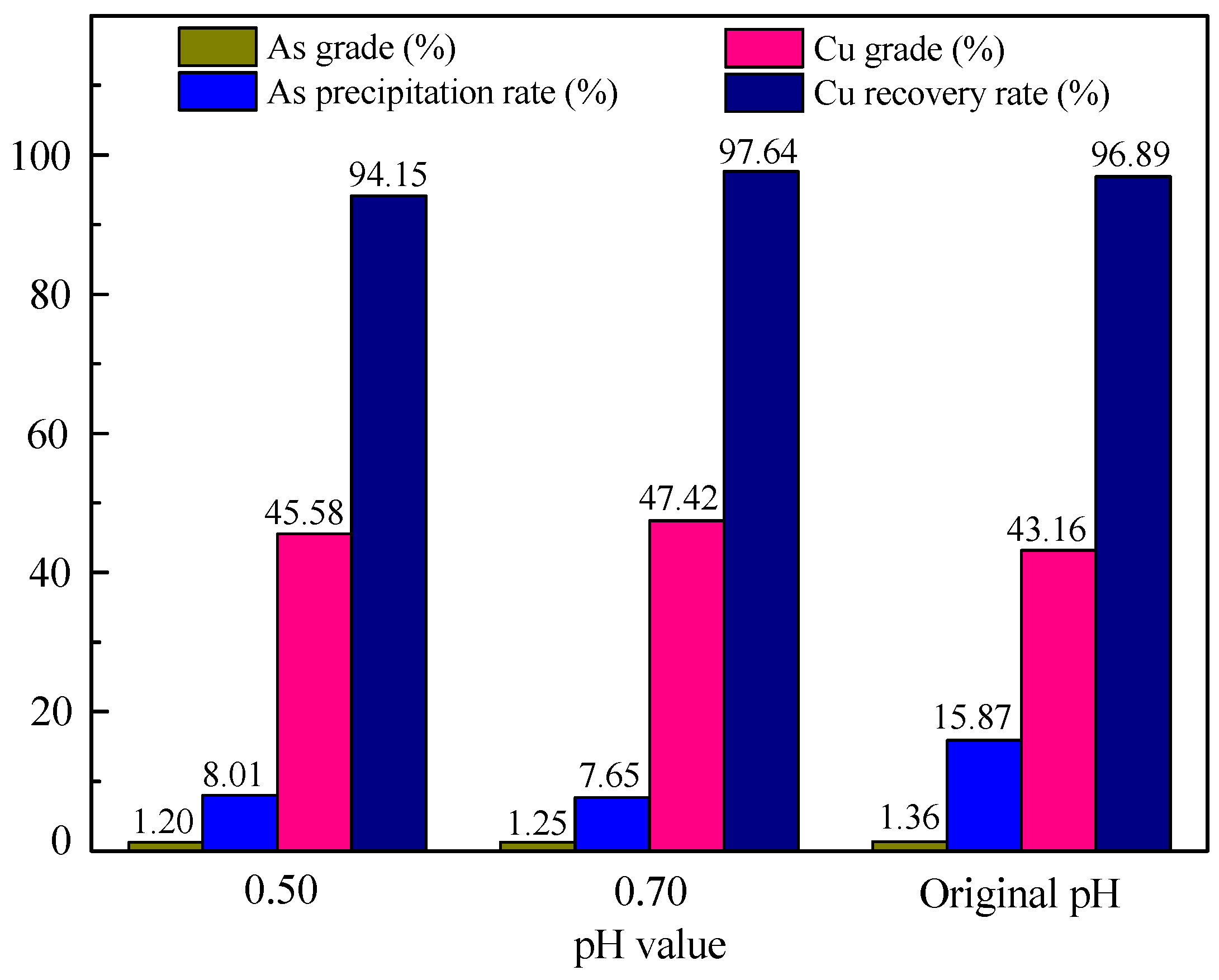
3.1.2. Effect of pH
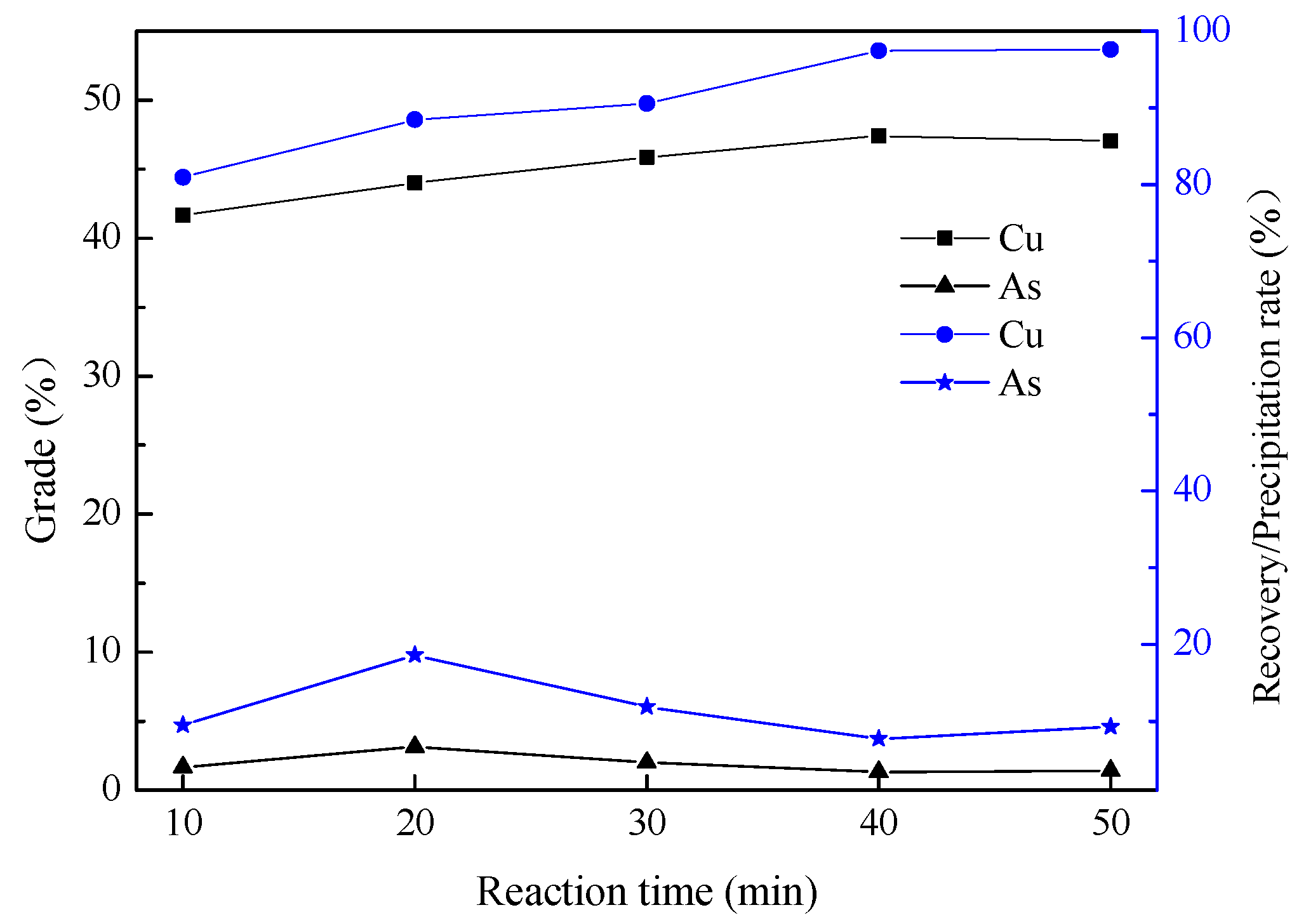
3.1.3. Effect of Reaction Time
3.2. Contrastive Results of Copper Selective Recovery by SRSP and Na2S
3.2.1. Contrastive Experiment
3.2.2. H2S Gas Release Analysis
3.3. Chemical Reaction Energy Calculation Analysis
3.4. SEM Images Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, X.; Chen, Y.; Wang, Q.; Wang, S.; Tian, Q. Copper and arsenic substance flow analysis of pyrometallurgical process for copper production. Trans. Nonferrous Met. Soc. 2022, 32, 364–376. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Wen, P.; Xia, L.; Ma, B.; Chen, J.; Wang, C. Co-treatment of copper smelting flue dust and arsenic sulfide residue by a pyrometallurgical approach for simultaneous removal and recovery of arsenic. J. Hazard. Mater. 2021, 416, 126149. [Google Scholar] [CrossRef] [PubMed]
- Gujre, N.; Rangan, L.; Mitra, S. Occurrence, geochemical fraction, ecological and health risk assessment of cadmium, copper and nickel in soils contaminated with municipal solid wastes. Chemosphere 2021, 271, 129573. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, D.; Li, B.; Wu, Y.; Wang, H.; Gu, Y.; Zuo, T. Patterns and challenges in the copper industry in China. Resour. Conserv. Recycl. 2017, 127, 1–7. [Google Scholar] [CrossRef]
- Northey, S.; Mohr, S.; Mudd, G.M.; Weng, Z.; Giurcob, D. Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycl. 2014, 83, 190–201. [Google Scholar] [CrossRef]
- Yang, Z.; Geng, L.; Zhou, H.; Liu, Z.; Xie, F.; Yang, S.; Luo, X. Improving the flotation separation of chalcopyrite from galena through high-temperature air oxidation pretreatment. Miner. Eng. 2022, 176, 107350. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Su, Z.; Lu, M.; Gu, F.; Liu, J.; Jiang, T. Recycling the domestic copper scrap to address the China’s copper sustainability. J. Mater. Res. Technol. 2020, 9, 2846–2855. [Google Scholar] [CrossRef]
- Mudd, G.M.; Jowitt, S.M. Growing global copper resources, reserves and production: Discovery is not the only control on supply. Econ. Geol. 2018, 113, 1235–1267. [Google Scholar] [CrossRef]
- Peng, Z.; Shen, Q.; Xie, W.; Zhang, Z.; Lei, J. Research status of arsenic and antimony removal in copper refining process. Nonferrous Met. Sci. Eng. 2021, 12, 28–33. Available online: http://www.xml-data.org/YSJSYKXGC/html/202101005.htm (accessed on 6 November 2022). (In Chinese).
- Maung, K.N.; Hashimoto, S.; Mizukami, M.; Morozumi, M.; Lwin, C.M. Assessment of the secondary copper reserves of nations. Environ. Sci. Technol. 2017, 51, 3824–3832. [Google Scholar] [CrossRef]
- Cao, H.; Fu, J.; Liu, Y.; Chen, S. Facile design of superhydrophobic and superoleophilic copper mesh assisted by candle soot for oil water separation. Colloids Surf. A 2018, 537, 294–302. [Google Scholar] [CrossRef]
- Yue, T.; Niu, Z.; Hu, Y.; Han, H.; Sun, W.; Tian, J.; Xu, Z.; Wang, L.; Yang, Y. Arsenic (V) adsorption on ferric oxyhydroxide gel at high alkalinity for securely recycling of arsenic-bearing copper slag. Appl. Surf. Sci. 2019, 478, 213–220. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Singh, T.A. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Hu, Z.; Du, Y.; Zhang, T.C.; Du, D. Mechanochemical activation on selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2021, 414, 125436. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Drahota, P.; Culka, A.; Racek, M. Characterization and pH-dependent environmental stability of arsenic trioxide-containing copper smelter flue dust. J. Environ. Manag. 2018, 209, 71–80. [Google Scholar] [CrossRef]
- Che, J.; Zhang, W.; Ma, B.; Chen, Y.; Wang, L.; Wang, C. A shortcut approach for cooperative disposal of flue dust and waste acid from copper smelting: Decontamination of arsenic-bearing waste and recovery of metals. Sci. Total Environ. 2022, 843, 157063. [Google Scholar] [CrossRef]
- Yang, B.; Huang, D.; Liu, D.; Zha, G.; Jiang, W.; Kong, X. Research and industrial application of a vacuum separation technique for recovering valuable metals from copper dross. Sep. Purif. Technol. 2020, 236, 116309. [Google Scholar] [CrossRef]
- Shahrashoub, M.; Bakhtiari, S. The efficiency of activated carbon/magnetite nanoparticles composites in copper removal: Industrial waste recovery, green synthesis, characterization, and adsorption-desorption studies. Microporous Mesoporous Mater. 2021, 311, 110692. [Google Scholar] [CrossRef]
- Khalid, M.K.; Hamuyuni, J.; Agarwal, V.; Pihlasalo, J.; Haapalainen, M.; Lundström, M. Sulfuric acid leaching for capturing value from copper rich converter slag. J. Clean. Prod. 2019, 215, 1005–1013. [Google Scholar] [CrossRef]
- Sirola, K.; Laatikainen, M.; Lahtinen, M.; Paatero, E. Removal of copper and nickel from concentrated ZnSO4 solutions with silica-supported chelating adsorbents. Sep. Purif. Technol. 2008, 64, 88–100. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ooi, B.S. A study on acid reclamation and copper recovery using low pressure nanofiltration membrane. Chem. Eng. J. 2010, 156, 257–263. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; He, Y.; Wang, Y.; Qian, X.; Jia, J. Evaluation of magnetic chitosan beads for adsorption of heavy metal ions. Sci. Total Environ. 2018, 627, 1396–1403. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Hu, Y.; Han, H.; Luo, X.; Sun, W.; Yue, T.; Wang, L.; Cao, X.; Zhou, H. Selective sulfide precipitation of copper ions from arsenic wastewater using monoclinic pyrrhotite. Sci. Total Environ. 2020, 705, 135816. [Google Scholar] [CrossRef]
- Jiang, G.; Peng, B.; Chai, L.; Wang, Q.; Shi, M.; Wang, Y.; Liu, H. Cascade sulfidation and separation of copper and arsenic from acidic wastewater via gas-liquid reaction. Trans. Nonferrous Met. Soc. 2017, 27, 925–931. [Google Scholar] [CrossRef]
- Veeken, A.H.M.; De Vries, S.; Van der Mark, A.; Rulkens, W.H. Selective precipitation of heavy metals as controlled by a sulfide-selective electrode. Sep. Sci. Technol. 2003, 38, 1–19. [Google Scholar] [CrossRef]
- Wu, J.; Chen, X.; Chen, S.; Wang, Y.; Yan, R. Recovery of copper from copper manganese residue by acid leaching and selective sulfide precipitation. Nonferrous Met. Sci. Eng. 2021, 12, 70–76. Available online: http://www.xml-data.org/YSJSYKXGC/html/202103009.htm (accessed on 6 November 2022). (In Chinese).
- Li, Y.; Zhu, X.; Qi, X.; Shu, B.; Zhang, X.; Li, K.; Wei, Y.; Hao, F.; Wang, H. Efficient removal of arsenic from copper smelting wastewater in form of scorodite using copper slag. J. Clean. Prod. 2020, 270, 122428. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, X.; Qian, L.; Luan, J.; Jin, B. Separation of arsenic and extraction of zinc and copper from high-arsenic copper smelting dusts by alkali leaching followed by sulfuric acid leaching. J. Environ. Chem. Eng. 2021, 9, 105997. [Google Scholar] [CrossRef]
- Liu, Z.; Sheng, M.; He, Y.; Zhou, H.; Huang, J.; Luo, X.; Zhang, Y. Coordination mechanism of aluminum with oxalate and fluoride in aluminum crystallization from vanadium extraction wastewater. J. Mol. Liq. 2022, 347, 117992. [Google Scholar] [CrossRef]
- Zhang, W.; Che, J.; Xia, L.; Wen, P.; Chen, J.; Ma, B.; Wang, C. Efficient removal and recovery of arsenic from copper smelting flue dust by a roasting method: Process optimization, phase transformation and mechanism investigation. J. Hazard. Mater. 2021, 412, 125232. [Google Scholar] [CrossRef] [PubMed]


| Type of Sulfide Precipitant | Cu Grade (%) | As Grade (%) | Cu Recovery Rate (%) | As Precipitation Rate (%) |
|---|---|---|---|---|
| Na2S | 48.16 | 3.01 | 86.04 | 17.19 |
| SRSP | 47.22 | 1.25 | 98.06 | 7.31 |
| Number | Chemical Reaction Equations | ∆G (kJ/mol) |
|---|---|---|
| 1 | FeS + 2H+ = H2S + Fe2+ | −598.22 |
| 2 | MnS + 2H+ = H2S + Mn2+ | −753.38 |
| 3 | ZnS + 2H+ = H2S + Zn2+ | −1054.26 |
| 4 | S2− + Cu2+ = CuS | −2720.36 |
| 5 | 2HS− + Cu2+ = CuS + H2S | −2105.98 |
| 6 | ZnS + Cu2+ = CuS + Zn2+ | −283.13 |
| 7 | S2− + 2H+ = H2S | −3491.49 |
| 8 | HS− + H+ = H2S | −1438.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Yang, Z.; Zhou, H.; Zhang, Y.; Sun, W.; Han, H. Selective Recovery of Copper from Acid Leaching Solution through Slow Release Sulfide Precipitant. Minerals 2022, 12, 1571. https://doi.org/10.3390/min12121571
Luo X, Yang Z, Zhou H, Zhang Y, Sun W, Han H. Selective Recovery of Copper from Acid Leaching Solution through Slow Release Sulfide Precipitant. Minerals. 2022; 12(12):1571. https://doi.org/10.3390/min12121571
Chicago/Turabian StyleLuo, Xianping, Zhizhao Yang, Hepeng Zhou, Yongbing Zhang, Wei Sun, and Haisheng Han. 2022. "Selective Recovery of Copper from Acid Leaching Solution through Slow Release Sulfide Precipitant" Minerals 12, no. 12: 1571. https://doi.org/10.3390/min12121571
APA StyleLuo, X., Yang, Z., Zhou, H., Zhang, Y., Sun, W., & Han, H. (2022). Selective Recovery of Copper from Acid Leaching Solution through Slow Release Sulfide Precipitant. Minerals, 12(12), 1571. https://doi.org/10.3390/min12121571







