The Crystal Structure and Crystal Chemistry of Mineral-like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclasite and Gatehouseite
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Raman Spectrometry
2.3. X-ray Diffractometry and Crystal Structure Solution
3. Results
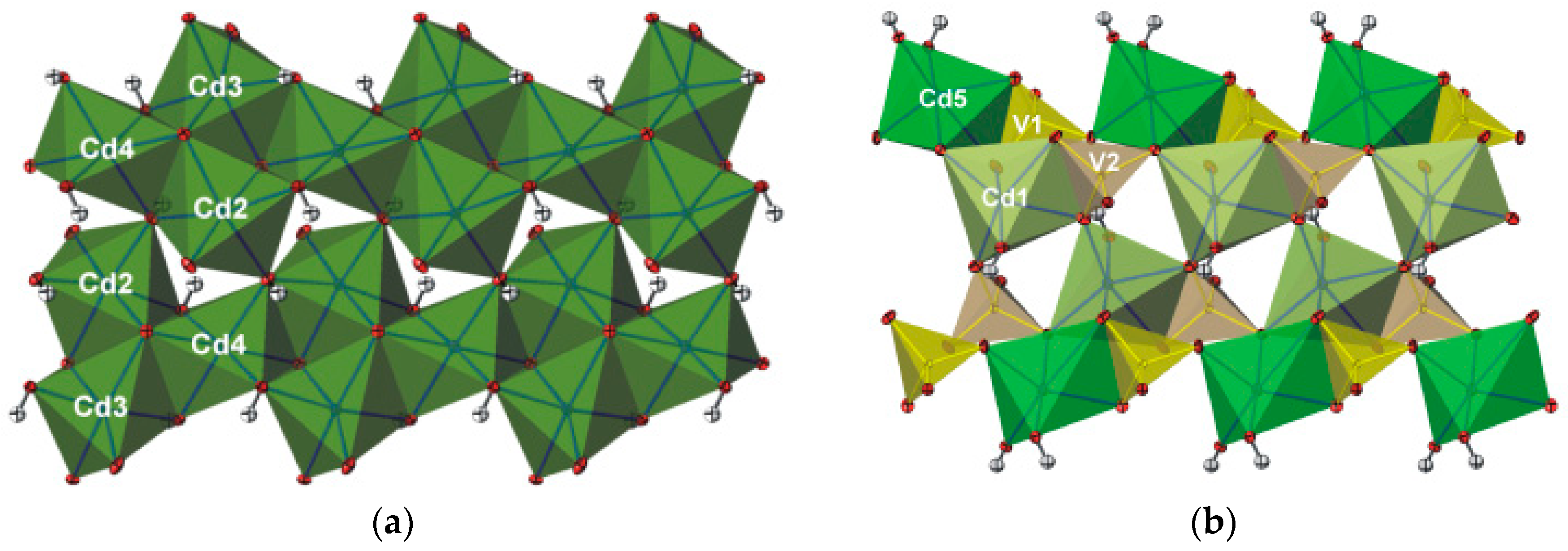
3.1. Crystal Structure
3.2. Raman Spectrometry
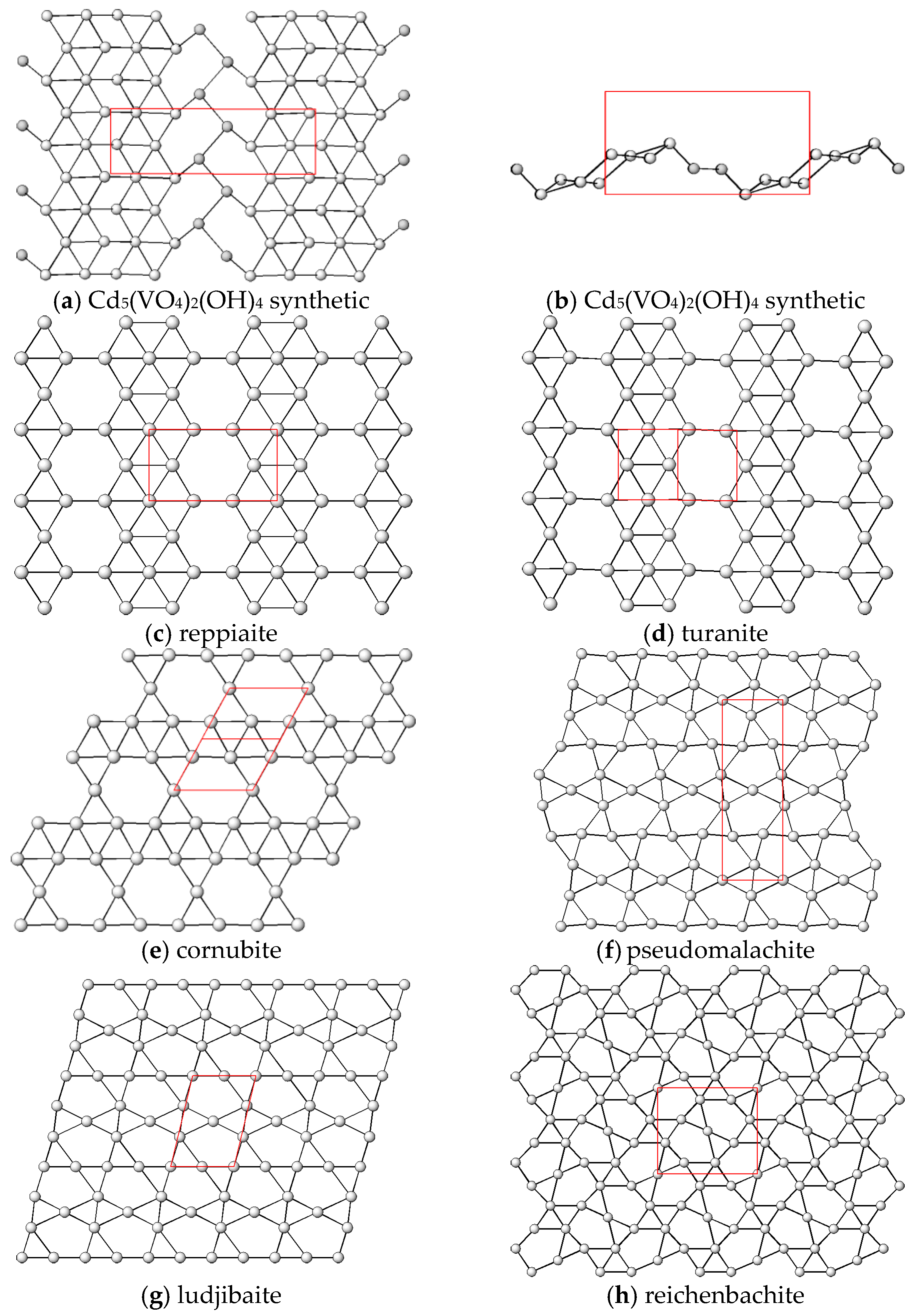
4. Discussion and Relationships to Similar Structures
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Puzio, B.; Manecki, M. The prediction method for standard enthalpies of apatites using the molar volume, lattice energy, and linear correlations from existing experimental data. Contrib. Mineral. Petrol. 2022, 177, 103–137. [Google Scholar] [CrossRef]
- Yakubovich, O.; Kiriukhina, G. A mero-plesiotype series of vanadates, arsenates and phosphates with blocks based on densely packed octahedral layers as repeating modules. Minerals 2021, 11, 273. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Guo, W.B.; Yang, M.; Tang, Y.Y.; Cui, M.Y.; Wang, N.N.; He, Z.Z. A frustrated ferrimagnet Cu5(VO4)2(OH)4 with a 1/5 magnetization plateau on a new spin-lattice of alternating triangular and honeycomb strips. Dalton Trans. 2015, 44, 20562–20567. [Google Scholar] [CrossRef]
- Machida, M.; Miyazaki, Y.; Matsunaga, Y.; Ikeue, K. Efficient catalyticdecomposition of sulfuric acid with copper vanadates as an oxygen-generating reaction for solar thermochemical water splitting cycles. Chem. Commun. 2011, 47, 9591–9593. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Bai, X.; Pan, C.; Zhu, Y. Investigations on the Phase Transition between CdV2O6 and Cd2V2O7 and their photocatalytic performances. Eur. J. Inorg. Chem. 2013, 2013, 3070–3075. [Google Scholar] [CrossRef]
- Oshikiri, M.; Boero, M.; Ye, J.; Zou, Z.; Kido, G. Electronic structures of promising photocatalysts InMO4, M = V, Nb, Ta... and BiVO4 for water decomposition in the visible wavelength region. J. Chem. Phys. 2002, 117, 7313–7318. [Google Scholar] [CrossRef]
- Wang, D.; Tang, J.; Zou, Z.; Ye, J. Photophysical and photocatalytic properties of a new series of visible-light-driven photocatalysts M3V2O8 (M = Mg, Ni, Zn). Chem. Mater. 2005, 17, 5177–5182. [Google Scholar] [CrossRef]
- Shi, R.; Wang, Y.; Zhou, F.; Zhu, Y. Zn3V2O7(OH)2(H2O)2 and Zn3V2O8 nanostructures: Controlled fabrication and photocatalytic performance. J. Mater. Chem. 2011, 21, 6313–6320. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, Y.M.; Tsuboi, T.; Seo, H.J. Novel yellow-emitting phosphors of Ca5M4(VO4)6 (M = Mg, Zn) with isolated VO4 tetrahedra. Opt. Express 2012, 20, 4360–4368. [Google Scholar] [CrossRef]
- Djemal, A.; Louati, B.; Guidara, K. Synthesis and characterization of orthovanadates compounds Li(1–x)NaxCdVO4 (x = 0, 0.25). J. Alloys Compd. 2016, 683, 610–618. [Google Scholar] [CrossRef]
- Đorđević, T.; Karanović, L.; Tillmanns, E. Structural and spectroscopic study of Mg13.4(OH)6(HVO4)2(H0.2VO4)6. Cryst. Res. Technol. 2008, 43, 1202–1209. [Google Scholar] [CrossRef]
- Đorđević, T.; Stojanović, J.; Karanović, L. Zn1.86Cd0.14(OH)VO4. Acta Cryst. 2010, E66, i79. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.; Karanović, L. Ba[Co3(VO4)2(OH)2] with a regular Kagome lattice. Acta Cryst. 2013, C69, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.; Karanović, L. A new anion-deficient fluorite-related superstructure of Bi28V8O62 . J. Solid State Chem. 2014, 220, 259–269. [Google Scholar] [CrossRef]
- Đorđević, T.; Kolitsch, U.; Nasdala, L. A single-crystal X-ray and Raman spectroscopic study of hydrothermally synthesized arsenates and vanadates with the descloizite and adelite structure types. Am. Mineral. 2016, 101, 1135–1149. [Google Scholar] [CrossRef]
- Ulická, Ľ. Crystal structure of Cd(VO3)2·4H2O and its comparison with the structure of Ca(VO3)2·4H2O and α-Cd(VO3). Chem. Pap. 1988, 42, 11–19. [Google Scholar]
- Nonius. COLLECT Data Collection Software, 2005–2007; Nonius BV: Delft, The Netherlands, 2002. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. In Methods in Enzymology; Carter, J.C.W., Sweet, R.M., Eds.; Academic Press: New York, NY, USA, 1997; Volume 276, pp. 307–326. [Google Scholar]
- Otwinowski, Z.; Borek, D.; Majewski, W.; Minor, W. Multiparametric scaling of diffraction intensities. Acta Cryst. 2003, A59, 228–234. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Elliott, P.; Pring, A. The crystal structure of gatehouseite. Mineral. Mag. 2011, 75, 2823–2832. [Google Scholar] [CrossRef]
- Dowty, E. ATOMS for Windows; Version 5.1; Shape Software: Kingsport, TN, USA, 2000. [Google Scholar]
- Gagné, O.C.; Hawthorne, F.C. Bond-length distributions for ions bonded to oxygen: Results for the transition metals and qu antification of the factors underlying bond-length variation in inorganic solids. IUCrJ 2020, 7, 581–629. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-Valence Parameters Obtained from a Systematic Analysis of the Inorganic Crystal Structure Database. Acta Cryst. 1985, B41, 244–247. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O–H stretching frequencies and O–H∙∙∙O hydrogen bond lengths in minerals. Mon. Chem. 1999, 130, 1047–1059. [Google Scholar] [CrossRef]
- Moore, P.B.; Molin-Case, J. Crystal chemistry of the basic manganese arsenates: V. Mixed manganese coordination in the atomic arrangement of arsenoclasite. Am. Mineral. 1971, 56, 1539–1552. [Google Scholar]
- Pring, A.; Birch, W.D. Gatehouseite, a new manganese hydroxy phosphate from Iron Monarch, South Australia. Mineral. Mag. 1993, 57, 309–313. [Google Scholar] [CrossRef]
- Ruszala, F.A.; Anderson, J.B.; Kostiner, E. Crystal structures of two isomorphs of arsenoclasite: Co5(PO4)2(OH)4 and Mn5(PO4)2(OH)4. Inorg. Chem. 1977, 16, 2417–2422. [Google Scholar] [CrossRef]
- Basso, R.; Lucchetti, G.; Zefiro, L.; Palenzona, A. Reppiaite, Mn5(OH)4(VO4)2, a new mineral from Val Graveglia (Northern Apennines, Italy). Z. Kristallogr. 1992, 201, 223–234. [Google Scholar] [CrossRef]
- Barbier, J. Crystal structure of Ni5(AsO4)2(OH)4 and its comparison to other M5(XO4)2(OH)4 compounds. Eur. J. Mineral. 1996, 8, 77–84. [Google Scholar] [CrossRef]
- Sieber, N.H.W.; Hofmeister, W.; Tillmanns, E.; Abraham, K. Neue mineraldaten fur kupferphosphate und-arsenate von Reichenbach/Odw. Fortschr. Mineral. 1984, 62, 231–232. [Google Scholar]
- Tillmanns, E.; Hofmeister, W.; Petitjean, K. Cornubite, Cu5(AsO4)2(OH)4, first occurence of single crystals, mineralogical description and crystal structure. Bull. Geol. Soc. Finland 1985, 57, 119–127. [Google Scholar] [CrossRef]
- Janeczek, J.; Ciesielczuk, J.; Dulski, M.; Krzykawski, T. Chemical composition and Raman spectroscopy of cornubite and its relation to cornwallite in Miedzianka, the Sudety Mts, Poland. N. Jb. Miner. Abh. 2016, 193, 265–274. [Google Scholar] [CrossRef]
- Sokolova, E.V.; Hawthorne, F.C.; Karpenko, V.Y.; Agakhanov, A.A.; Pautov, L.A. Turanite, Cu5(VO4)2(OH)4, from the Tyuya-Muyun radium-uranium deposit, Osh Region, Kyrgyzstan: A new structure for an old mineral Locality: Osh Region, Kyrgyzstan. Can. Mineral. 2004, 42, 731–739. [Google Scholar] [CrossRef][Green Version]
- Martens, W.N.; Frost, R.L.; Williams, P.A. The basic copper phosphate minerals pseudomalachite, ludjibaite and reichenbachite: An infrared emission and Raman spectroscopic study. N. Jb. Miner. Mh. 2003, 2003, 337–362. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Zolotarev, A.A.; Popova, V.I. Hydrogen bonding and structural complexity in the Cu5(PO4)2(OH)4 polymorphs (pseudomalachite, ludjibaite, reichenbachite): Combined experimental and theoretical study. Struct. Chem. 2016, 27, 1716–1723. [Google Scholar] [CrossRef]
- Makovicky, E. The pseudomalachite-ludjibaite-reichenbachite conundrum: OD description. Can. Mineral. 2019, 57, 571–581. [Google Scholar] [CrossRef]
- Shoemaker, G.L.; Anderson, J.B.; Kostiner, E. Refinement of the crystal structure of pseudomalachite. Am. Mineral. 1977, 62, 1042–1048. [Google Scholar]
- Anderson, J.B.; Shoemaker, G.L.; Kostiner, E.; Ruszala, F.A. The crystal structure of synthetic Cu5(PO4)2(OH)4, a polymorph of pseudomalachite. Am. Mineral. 1977, 62, 115–121. [Google Scholar]
- Sieber, N.H.W.; Tillmanns, E.; Medenbach, O. Hentschelite, CuFe2(PO4)2(OH)2, a new member of the lazulite group, and reichenbachite, Cu5(PO4)2(OH)4, a polymorph of pseudomalachite, two new copper phosphate minerals from Reichenbach, Germany. Am. Mineral. 1987, 72, 404–408. [Google Scholar]
- Braithwaite, R.S.W.; Ryback, G. Reichenbachite from Cornwall and Portugal. Mineral. Mag. 1994, 58, 449–451. [Google Scholar] [CrossRef][Green Version]
- Piret, P.; Deliens, M. Description de la ludjibaïte, un polymorphe de la pseudomalachite, Cu5(PO4)2(OH)4. Bull. Mineral. 1988, 111, 167–171. [Google Scholar] [CrossRef]
- Arlt, T.; Armbruster, T. Single-crystal X-ray structure refinement of cornwallite, Cu5(AsO4)2(OH)4: A comparison with its polymorph cornubite and the (PO4)-analogue pseudomalachite. Neues Jahrb. Mineral. Monatsh. 1999, 10, 468–480. [Google Scholar]
- Shoemaker, G.L.; Anderson, J.B.; Kostiner, E. The crystal structure of a third polymorph of Cu5(PO4)2(OH)4. Am. Mineral. 1981, 66, 169–181. [Google Scholar]
- Monfort, O.; Petrisková, P. Binary and ternary vanadium oxides: General overview, physical properties, and photochemical processes for environmental applications. Processes 2021, 9, 214. [Google Scholar] [CrossRef]



| Chemical Formula | Cd5H4O12V2 |
|---|---|
| Temperature | 293 |
| Formula weight, Mr (g/mol) | 859.91 |
| Space group (No.), Z | Orthorhombic, P212121, 4 |
| a (Å) | 19.011 (4) |
| b (Å) | 6.0133 (12) |
| c (Å) | 9.5411 (19) |
| V (Å3) | 1090.7 (4) |
| F (000), ρcalc (g/cm3) | 1544, 5.237 |
| Absorption coefficient, μ (mm−1) | 11.26 |
| Tmin/Tmax | 0.399/0.552 |
| Crystal size (mm3) | 0.10 × 0.08 × 0.06 |
| Crystal detector distance (mm) | 40 |
| Frame rotation width (°) | 1 |
| Total no. of frames | 1032 |
| Collection time per frame (s) | 150 |
| h, k, l ranges | ±26, ±8, ±13 |
| Absorption correction | Multi-scan |
| Reflections collected/unique | 12,585/3180 |
| Observed reflections [I > 2 σ(I)] | 3079 |
| Rint | 0.026 |
| 2θmax (°) | 30 |
| Extinction coefficient, k [a] | 0.00064 (8) |
| Refined parameters | 185 |
| R indices [I > 4 σ(I)] | R1 = 0.015 |
| wR2 = 0.036 | |
| R indices (all data) | R1 = 0.016 |
| wR2 = 0.036 | |
| Goodness of fit, S | 1.08 |
| (Δ/σ)max | 0.001 |
| (Δρ)max, (Δρ)min (e−Å−3) | 0.74; −1.00 |
| a, b[b] | 0.0159, 1.4781 |
| Atom | x | y | z | Uiso */Ueq (Å2) |
|---|---|---|---|---|
| Cd1 | 0.43166(2) | 0.27295(6) | 0.75333(4) | 0.01236(8) |
| Cd2 | 0.47036(2) | −0.05464(6) | 0.39132(4) | 0.01098(7) |
| Cd3 | 0.28220(2) | −0.06980(6) | 0.35388(3) | 0.01123(8) |
| Cd4 | 0.37741(3) | 0.44179(6) | 0.37772(4) | 0.01280(8) |
| Cd5 | 0.31528(3) | 0.57571(6) | 0.99733(4) | 0.01303(8) |
| V1 | 0.37572(4) | 0.11180(12) | 1.06892(6) | 0.00862(11) |
| V2 | 0.37360(4) | −0.19731(12) | 0.68339(8) | 0.00850(15) |
| O1 | 0.45222(18) | 0.2654(6) | 0.5217(4) | 0.0137(7) |
| H1 | 0.4911(15) | 0.321(10) | 0.523(7) | 0.021 * |
| O2 | 0.29638(18) | 0.6100(6) | 1.2347(4) | 0.0129(7) |
| H2 | 0.2569(15) | 0.554(10) | 1.240(7) | 0.019 * |
| O3 | 0.45626(17) | 0.6368(6) | 0.2526(4) | 0.0141(7) |
| H3 | 0.427(3) | 0.683(10) | 0.196(5) | 0.021 * |
| O4 | 0.21431(18) | 0.7472(6) | 0.9713(3) | 0.0124(7) |
| H4 | 0.203(3) | 0.789(10) | 1.050(3) | 0.019 * |
| O5 | 0.35026(17) | 0.5523(6) | 0.7643(4) | 0.0132(6) |
| O6 | 0.37237(19) | −0.2267(6) | 0.5019(4) | 0.0126(6) |
| O7 | 0.3196(2) | 0.0164(6) | 0.7275(4) | 0.0170(8) |
| O8 | 0.45553(18) | −0.1139(7) | 0.7297(4) | 0.0166(8) |
| O9 | 0.37949(18) | 0.1117(5) | 0.2501(4) | 0.0117(6) |
| O10 | 0.29428(19) | 0.2058(6) | 1.0171(4) | 0.0151(7) |
| O11 | 0.43564(19) | 0.2935(6) | 0.9938(4) | 0.0158(7) |
| O12 | 0.39246(19) | 0.8495(6) | 1.0155(4) | 0.0156(7) |
| Distance <Mean> | Bond Lengths (Å) | Distance <Mean> | Bond Lengths (Å) |
|---|---|---|---|
| Cd1—O1 | 2.245(4) | Cd2—O3 ii | 2.271(4) |
| Cd1—O8 i | 2.256(4) | Cd2—O11 iii | 2.287(3) |
| Cd1—O5 | 2.287(3) | Cd2—O3 iv | 2.295(4) |
| Cd1—O11 | 2.299(4) | Cd2—O1 | 2.317(4) |
| Cd1—O8 | 2.381(4) | Cd2—O6 | 2.378(4) |
| Cd1—O7 | 2.642(4) | Cd2—O9 | 2.408(3) |
| <Cd1—O> | 2.352 | <Cd2—O> | 2.326 |
| Cd3—O4 v | 2.241(4) | Cd4—O1 | 2.244(4) |
| Cd3—O2 vi | 2.252(3) | Cd4—O3 | 2.246(4) |
| Cd3—O10 vii | 2.282(3) | Cd4—O4 v | 2.265(4) |
| Cd3—O7 vii | 2.302(4) | Cd4—O2 viii | 2.293(4) |
| Cd3—O9 | 2.365(3) | Cd4—O6 ix | 2.321(3) |
| Cd3—O6 | 2.413(4) | Cd4—O9 | 2.329(3) |
| <Cd3—O> | 2.309 | <Cd4—O> | 2.283 |
| Cd5—O4 | 2.193(3) | ||
| Cd5—O12 | 2.212(3) | ||
| Cd5—O10 | 2.268(4) | ||
| Cd5—O2 | 2.302(4) | ||
| Cd5—O5 | 2.325(3) | ||
| Cd5—O11 | 2.849(4) | ||
| <Cd5—O> | 2.358 | ||
| V1—O12 iv | 1.688(3) | V2—O8 | 1.695(4) |
| V1—O10 | 1.721(4) | V2—O7 | 1.698(3) |
| V1—O9 x | 1.731(4) | V2—O6 | 1.741(4) |
| V1—O11 | 1.733(4) | V2—O5 iv | 1.749(3) |
| <V1—O> | 1.718 | <V2—O> | 1.721 |
| Site | Cd1 | Cd2 | Cd3 | Cd4 | Cd5 | V1 | V2 | Σνij ** |
|---|---|---|---|---|---|---|---|---|
| O1 | 0.398 | 0.328 | 0.399 | 1.125 | ||||
| O2 | 0.389 | 0.349 | 0.341 | 1.079 | ||||
| O3 | 0.3710.348 | 0.397 | 1.116 | |||||
| O4 | 0.402 | 0.377 | 0.458 | 1.237 | ||||
| O5 | 0.355 | 0.321 | 1.157 | 1.834 | ||||
| O6 | 0.278 | 0.253 | 0.324 | 1.182 | 2.037 | |||
| O7 | 0.136 | 0.341 | 1.328 | 1.805 | ||||
| O8 | 0.3860.275 | 1.339 | 2.001 | |||||
| O9 | 0.256 | 0.288 | 0.317 | 1.215 | 2.076 | |||
| O10 | 0.360 | 0.374 | 1.248 | 1.982 | ||||
| O11 | 0.344 | 0.355 | 0.078 | 1.208 | 1.985 | |||
| O12 | 0.435 | 1.365 | 1.800 | |||||
| Σνij | 1.894 | 1.936 | 2.033 | 2.163 | 2.007 | 5.036 | 5.006 |
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| O1—H1···O12 iii | 0.81(1) | 2.25(3) | 3.017(5) | 157(6) |
| O2—H2···O5 xiii | 0.82(1) | 2.15(2) | 2.967(5) | 173(6) |
| O3—H3···O12 viii | 0.82(1) | 2.10(3) | 2.869(5) | 156(6) |
| O4—H4···O7 xiii | 0.82(1) | 2.10(2) | 2.900(5) | 164(6) |
| Compound Mineral Name | a(Å), b(Å), c(Å) | α(o), β(o), γ(o) | V(Å3) | Space Group | Reference |
|---|---|---|---|---|---|
| Mn5(AsO4)2(OH)4 arsenoclasite | 18.290(20) 5.75(1) 9.31(2) | 90, 90, 90 | 979.11 | P212121 | [31] |
| Mn5(P0.88Si0.09As0.03O4)2(OH)4 gatehouseite | 17.9733(18) 5.6916(11) 9.130(4) | 90, 90, 90 | 933.9 (3) | P212121 | [23] |
| Mn5.09Fe0.01Al0.01(OH)4 (P0.90As0.09V0.01O4)2(OH)4 gatehouseite | 9.097(2) 5.693(2) 18.002(10) | 90, 90, 90 | 932.4 (8) | P212121 | [32] |
| Mn5(PO4)2(OH)4 gatehouseite synthetic | 9.110(1) 18.032(4) 5.6923(6) | 90, 90, 90 | 935.08 | P212121 | [33] |
| Co5(PO4)2(OH)4 gatehouseite type synthetic | 8.903(2) 17.397(2) 5.5154(4) | 90, 90, 90 | 854.26 | P212121 | [33] |
| Cd5(VO4)2(OH)4 gatehouseite type synthetic | 19.011(4) 6.0133(12) 9.5411(19) | 90, 90, 90 | 1090.7 (4) | P212121 | This work |
| Mn5(V0.89As0.11O4)2(OH)4 reppiaite | 9.604(2) 9.558(2) 5.393(1) | 90, 98.45(1), 90 | 489.68 | C2/m | [34] |
| Ni5(AsO4)2(OH)4 reppiaite type synthetic | 9.291(2) 9.008(2) 5.149(1) | 90, 98.70(3), 90 | 425.98 | C2/m | [35] |
| Cu5(VO4)2(OH)4 turanite | 5.3834(2) 6.2736(3) 6.8454(3) | 86.169(1), 91.681(1),92.425(1) | 230.38 (2) | P | [39] |
| Cu5(AsO4)2(OH)4 cornubite | 6.121(1) 6.251(1) 6.790(1) | 92.93(1), 111.30(1),107.47(1) | 227.11 | P | [36] |
| Cu5(PO4)2(OH)4 pseudomalachite | 4.4728(4) 5.7469(5) 17.032(3) | 90, 91.043(7), 90 | 437.73 | P21/c | [43] |
| Cu5(AsO4)2(OH)4 cornwallite | 4.600(2) 5.757(3) 17.380(6) | 90, 91.87 (3), 90 | 460.02 | P21/c | [48] |
| Cu5(PO4)2(OH)4 reichenbachite synthetic | 9.186(2) 10.684(2) 4.461(1) | 90, 92.31(1), 90 | 437.46 | P21/a | [44] |
| Cu5(PO4)2(OH)4 ludjibaite | 4.445(1) 5.873(1) 8.668(3) | 103.62(2), 90.35(2), 93.02(1) | 219.57 | P | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanović, L.; Ðorđević, T. The Crystal Structure and Crystal Chemistry of Mineral-like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclasite and Gatehouseite. Minerals 2022, 12, 1601. https://doi.org/10.3390/min12121601
Karanović L, Ðorđević T. The Crystal Structure and Crystal Chemistry of Mineral-like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclasite and Gatehouseite. Minerals. 2022; 12(12):1601. https://doi.org/10.3390/min12121601
Chicago/Turabian StyleKaranović, Ljiljana, and Tamara Ðorđević. 2022. "The Crystal Structure and Crystal Chemistry of Mineral-like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclasite and Gatehouseite" Minerals 12, no. 12: 1601. https://doi.org/10.3390/min12121601
APA StyleKaranović, L., & Ðorđević, T. (2022). The Crystal Structure and Crystal Chemistry of Mineral-like Cd5(VO4)2(OH)4, a Novel Isomorph of Arsenoclasite and Gatehouseite. Minerals, 12(12), 1601. https://doi.org/10.3390/min12121601







