Vertebrate Taphonomy and Diagenesis: Implications of Structural and Compositional Alterations of Phosphate Biominerals
Abstract
:1. Introduction
2. Milestones: From Death to Discovery
2.1. Death
2.2. Post Mortem Alterations
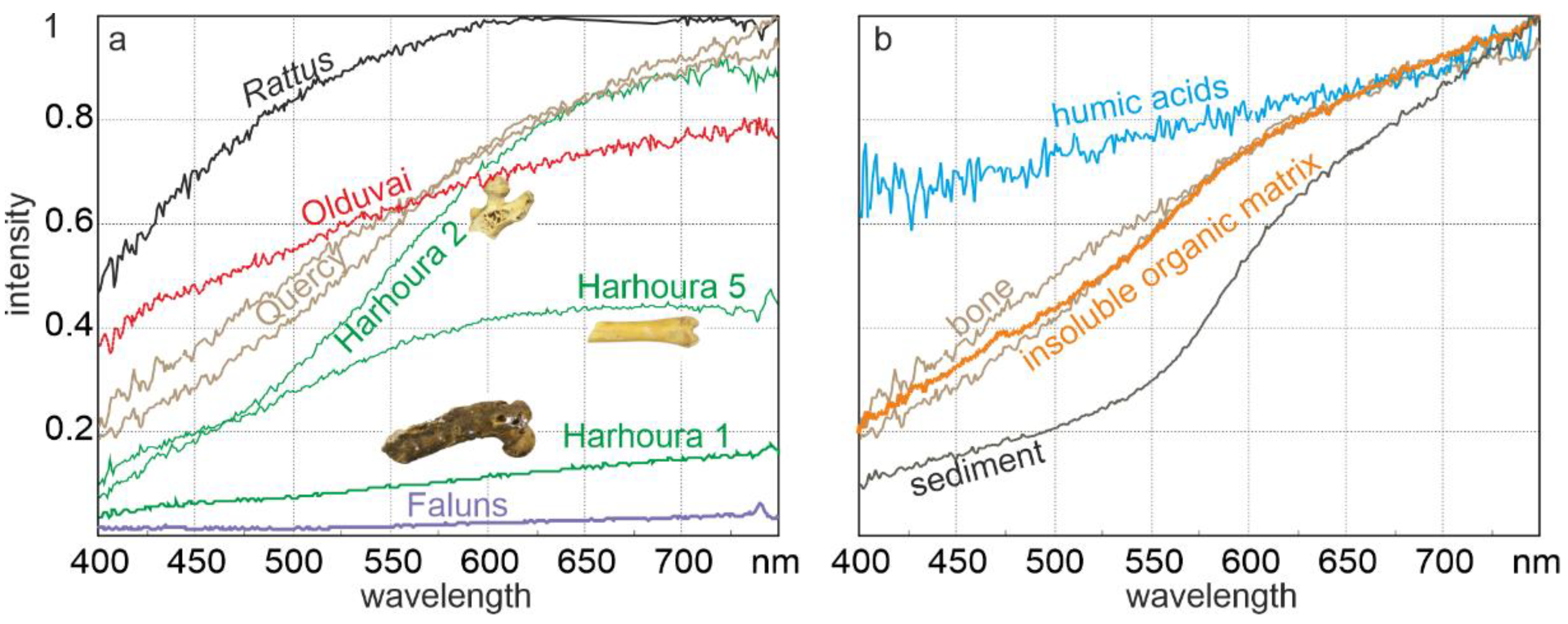
2.2.1. Surficial Alterations
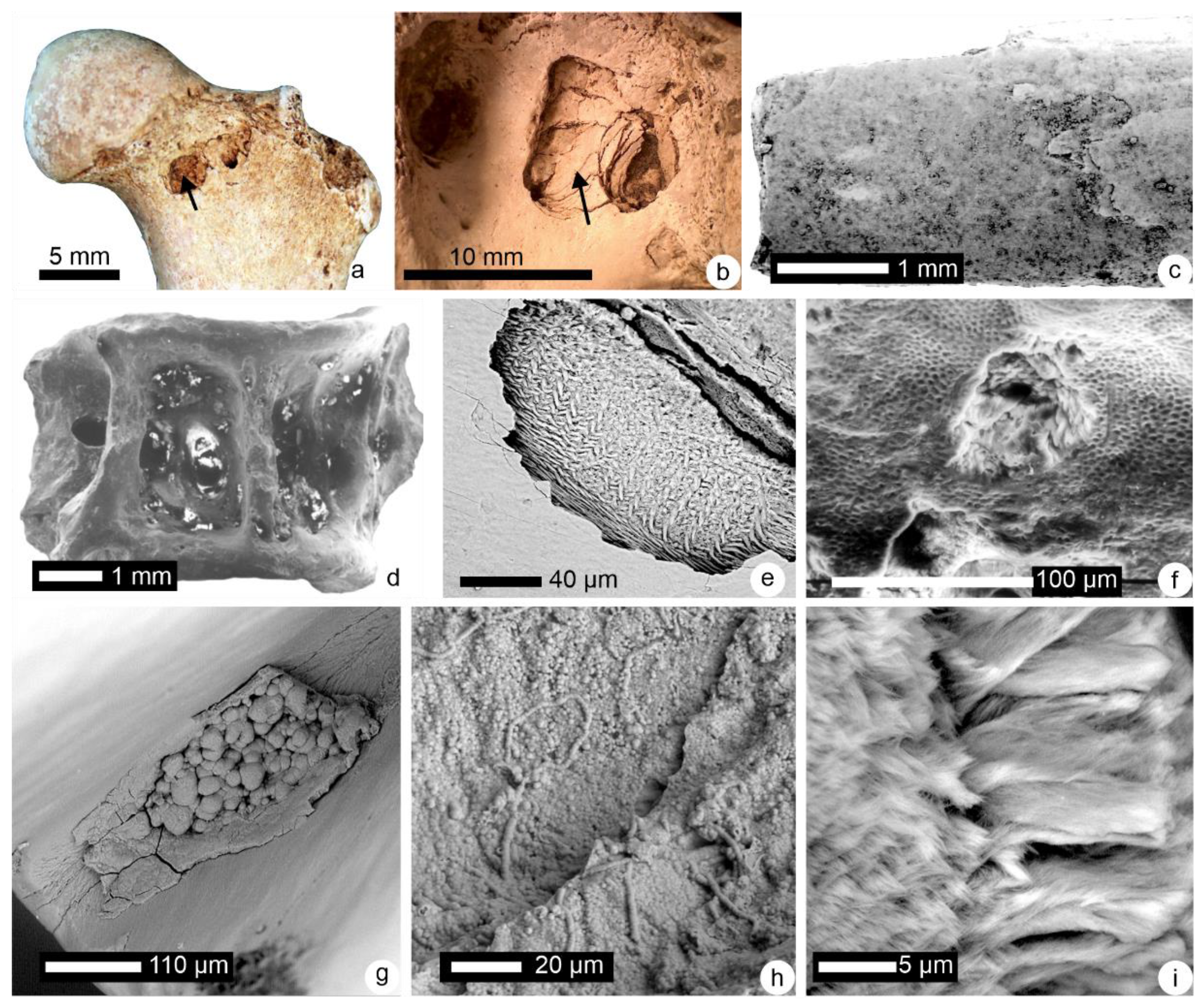
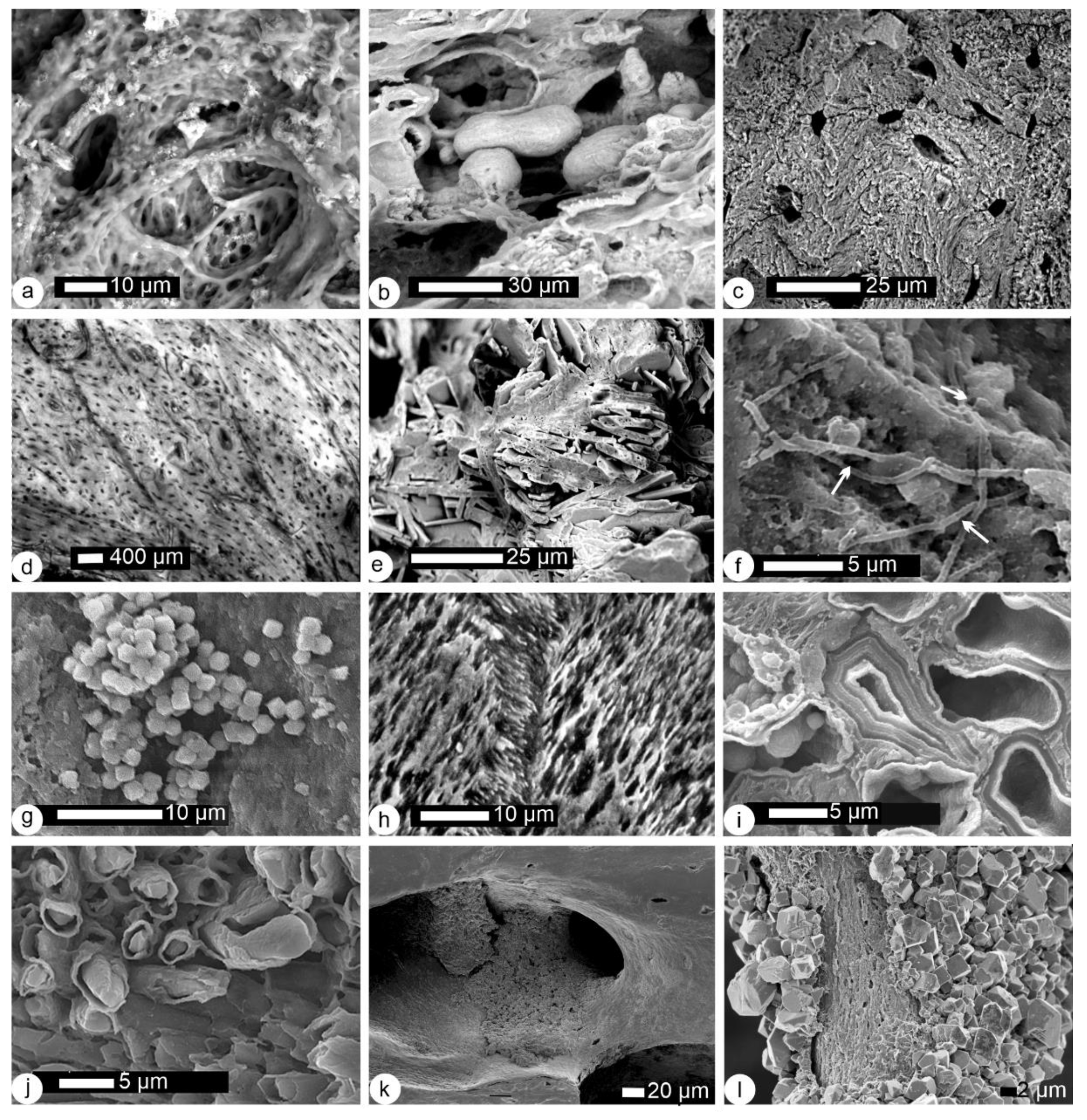
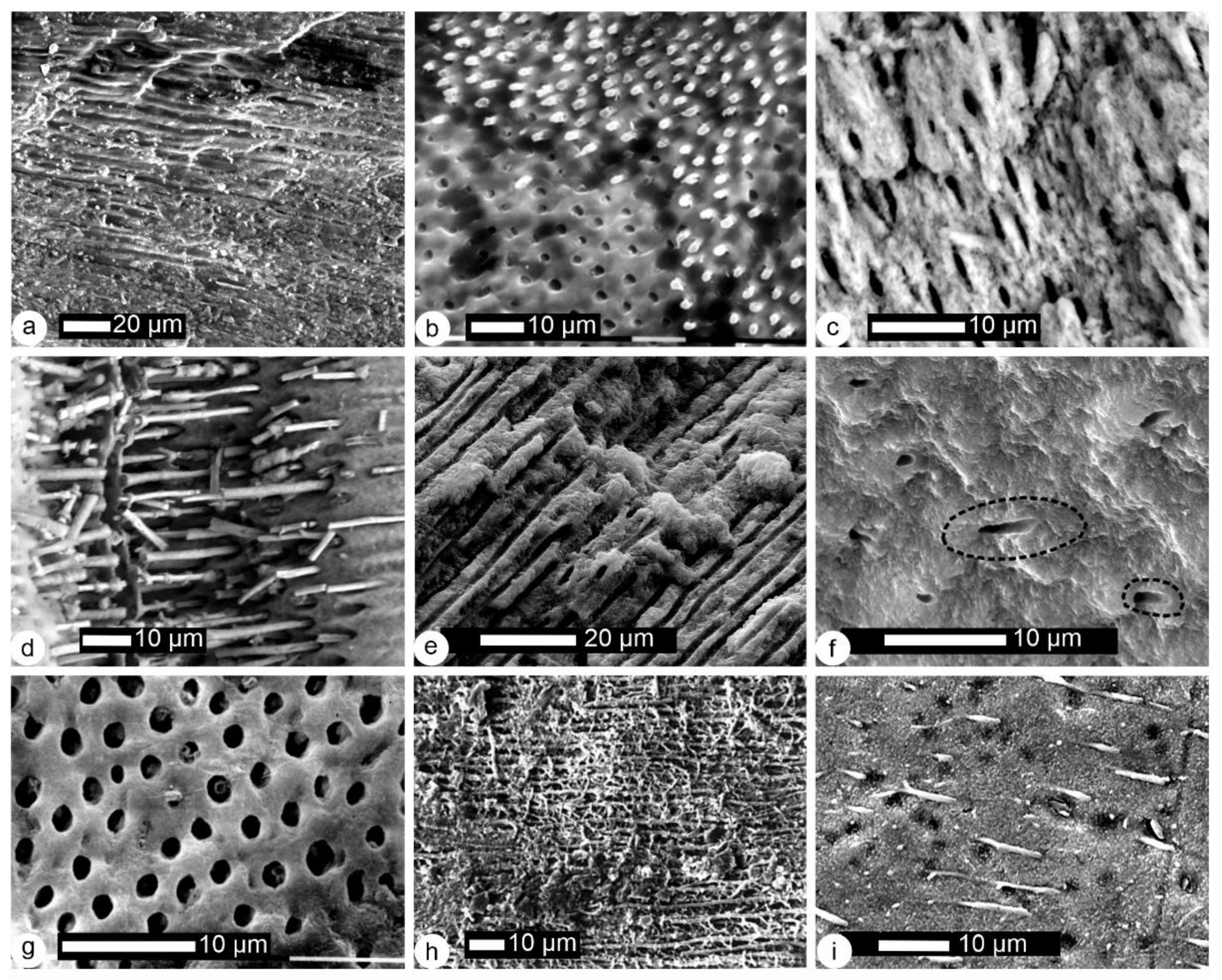
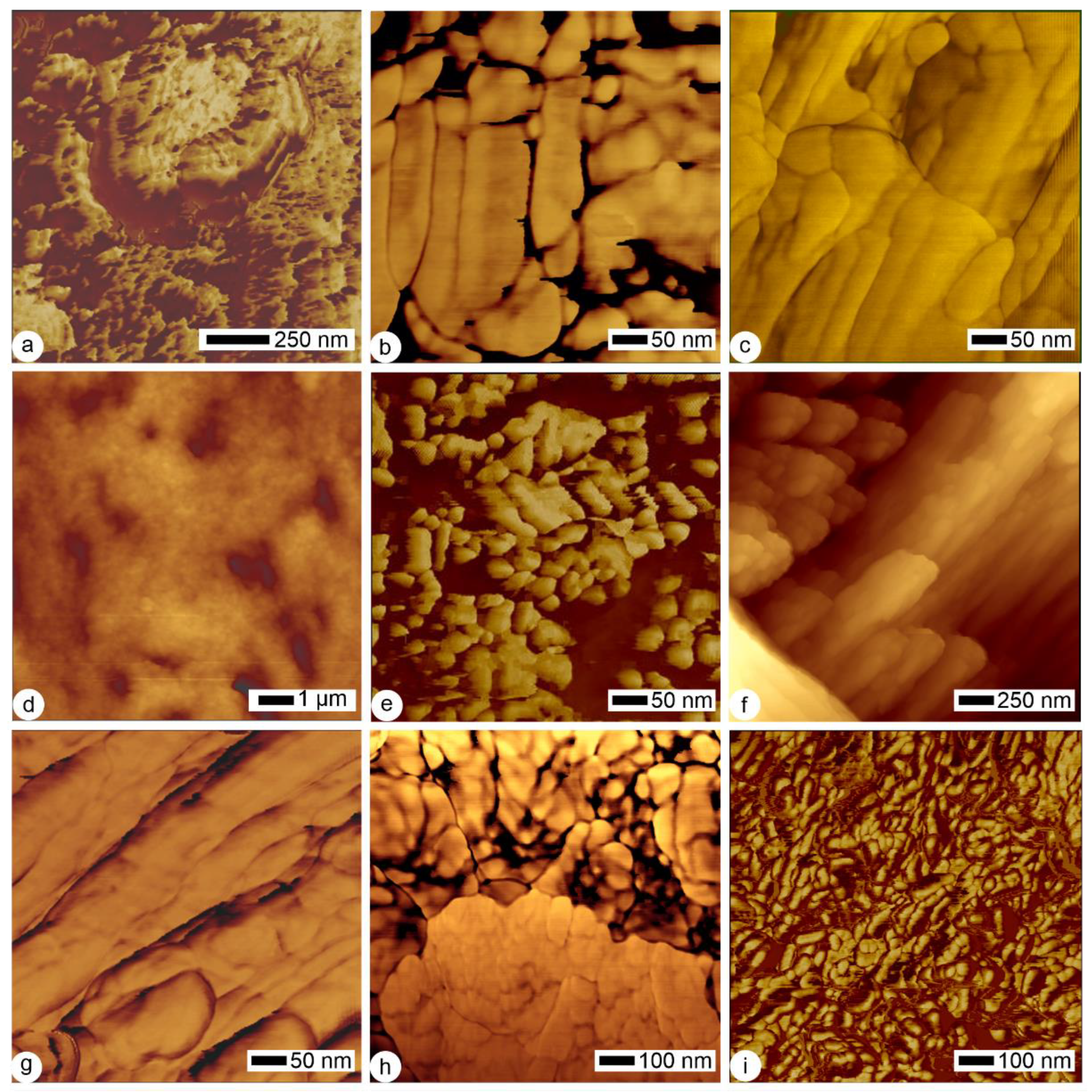
2.2.2. Histological–Structural Modifications
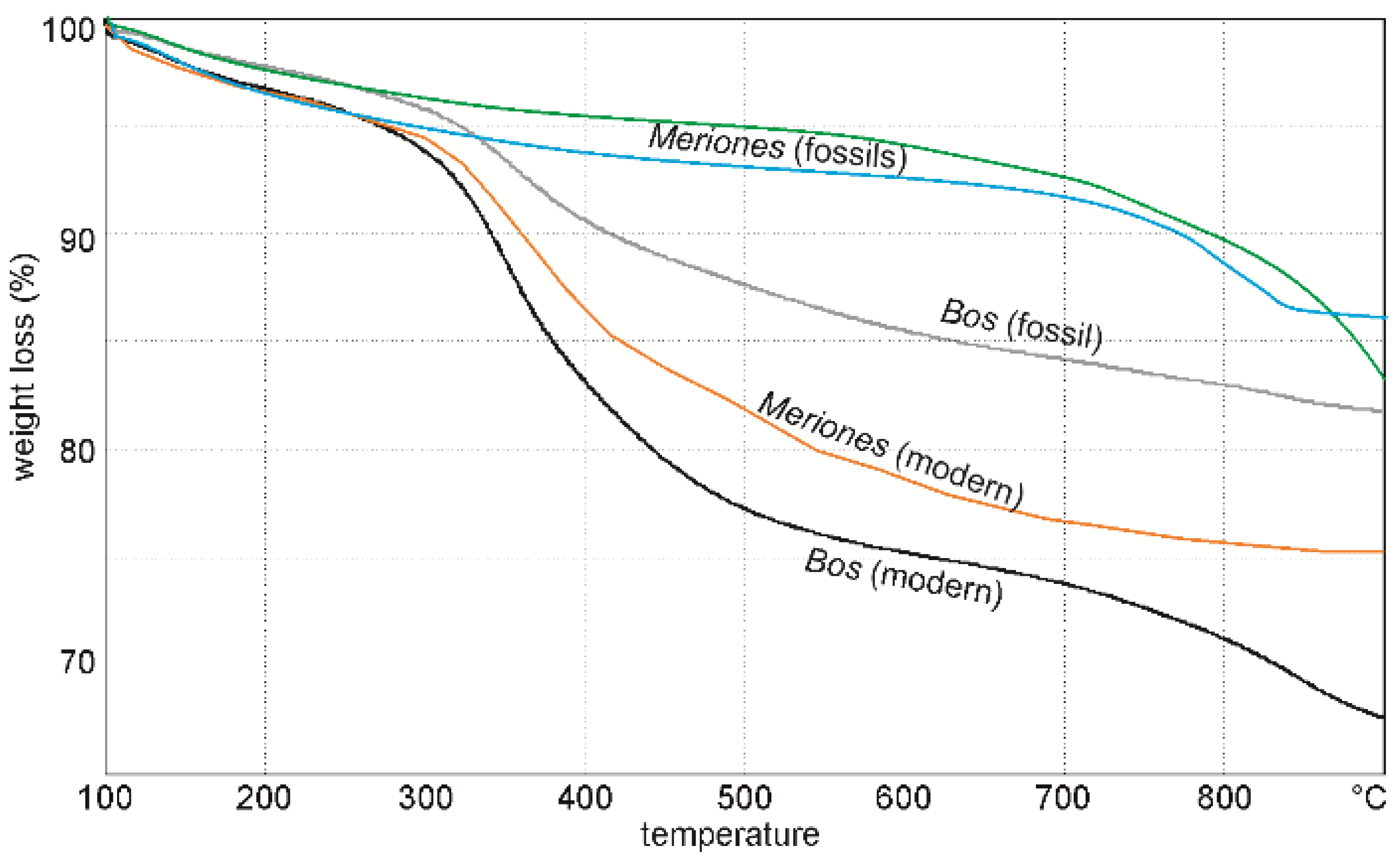
2.2.3. Bulk Composition
2.2.4. Mineralogical and Crystallographic Modifications
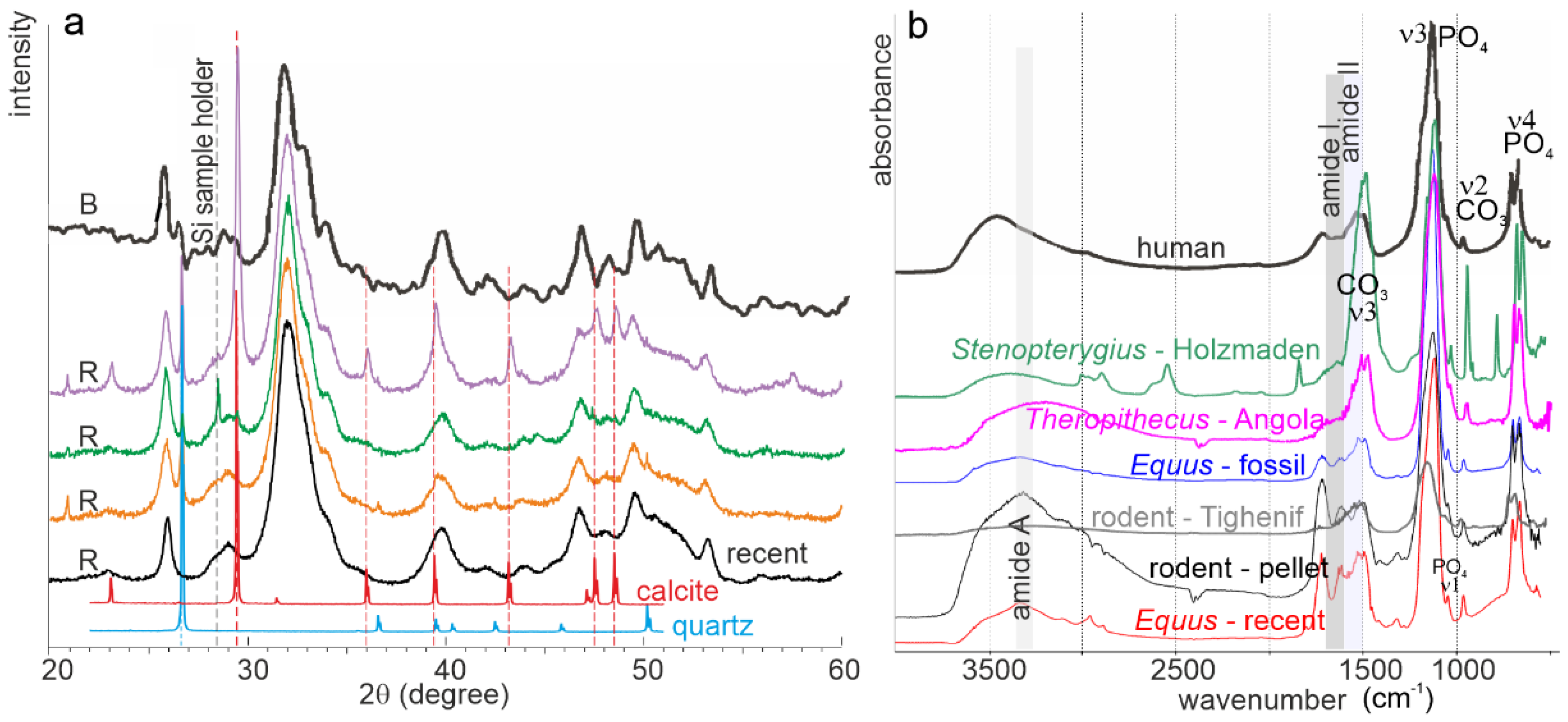
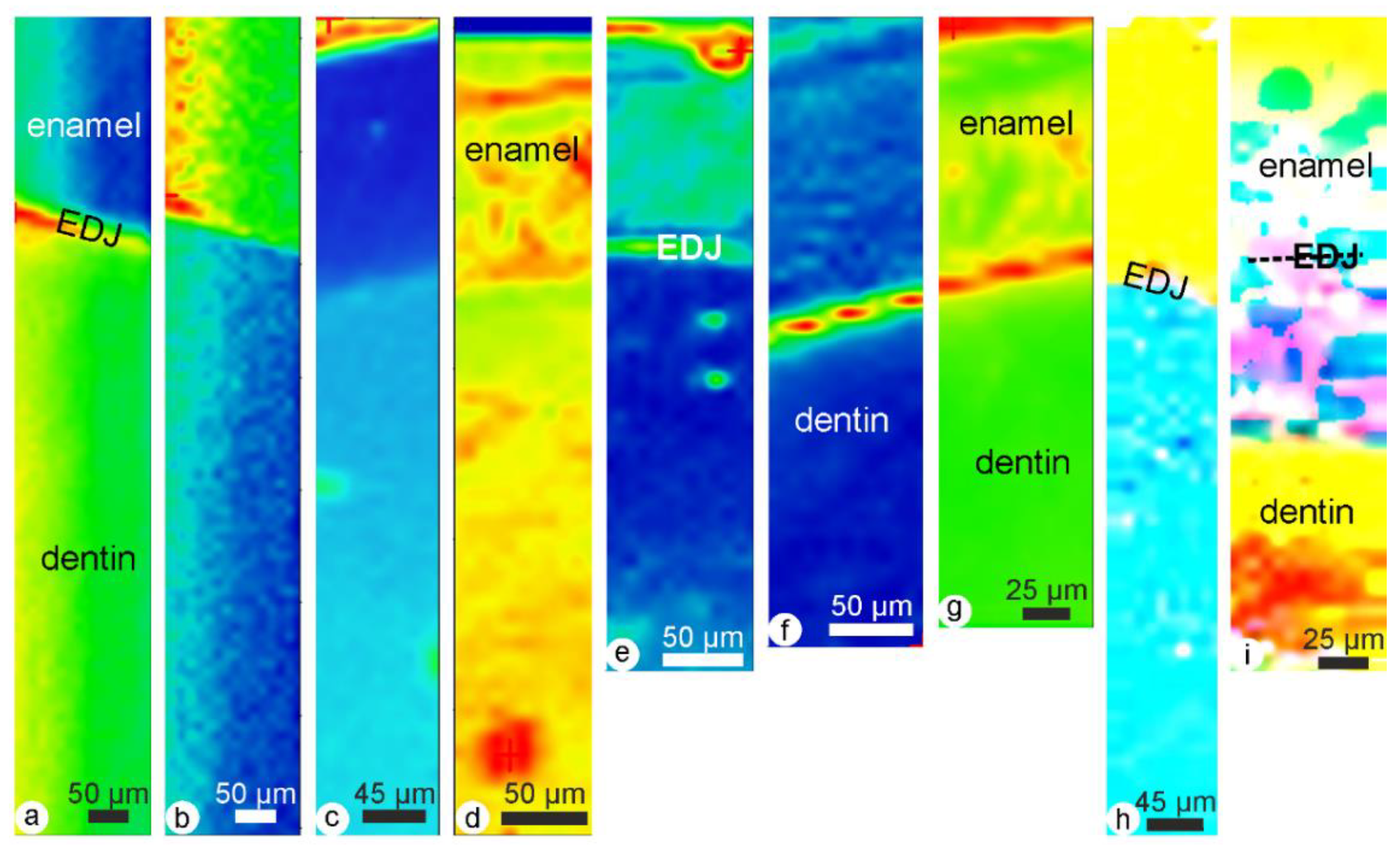
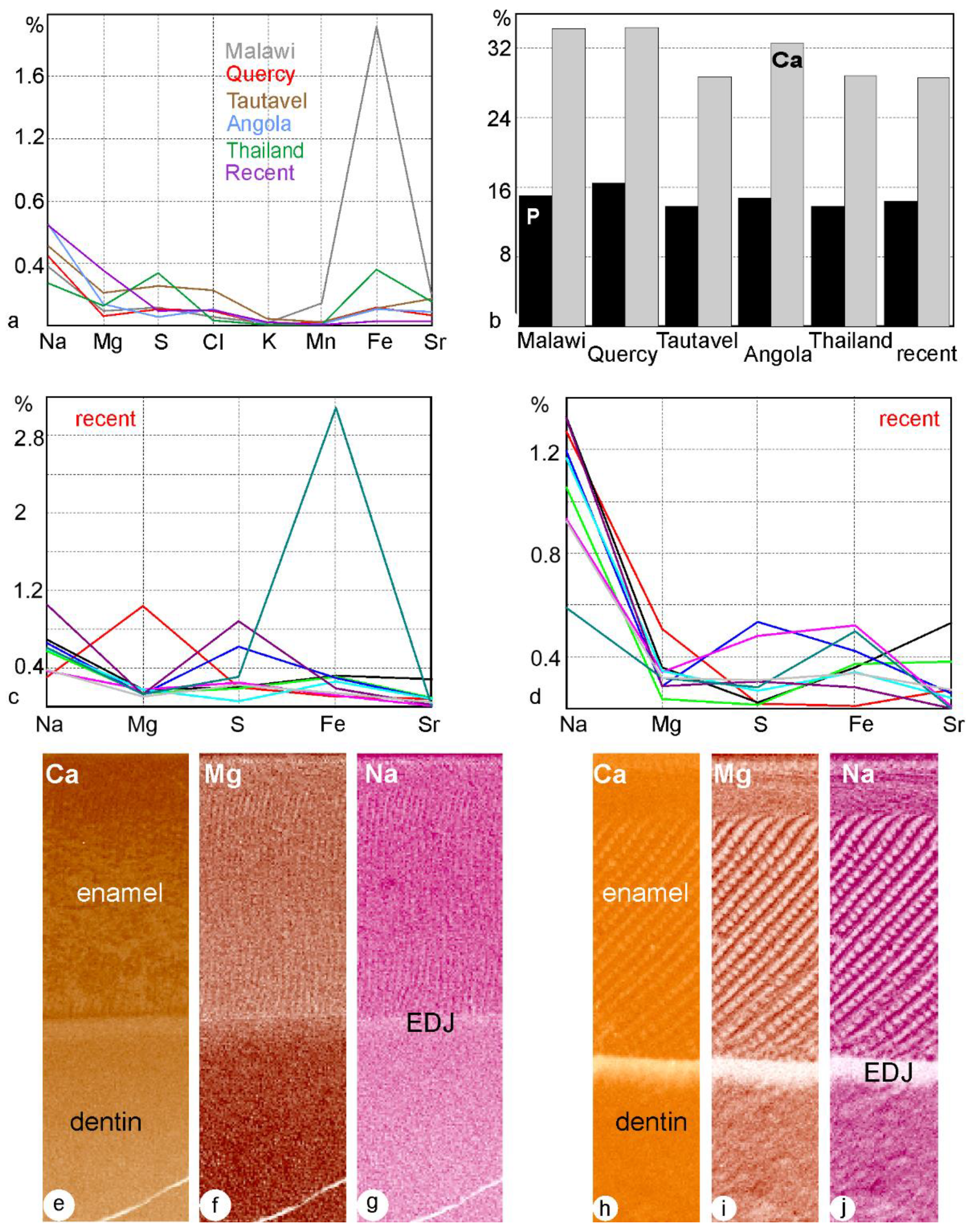
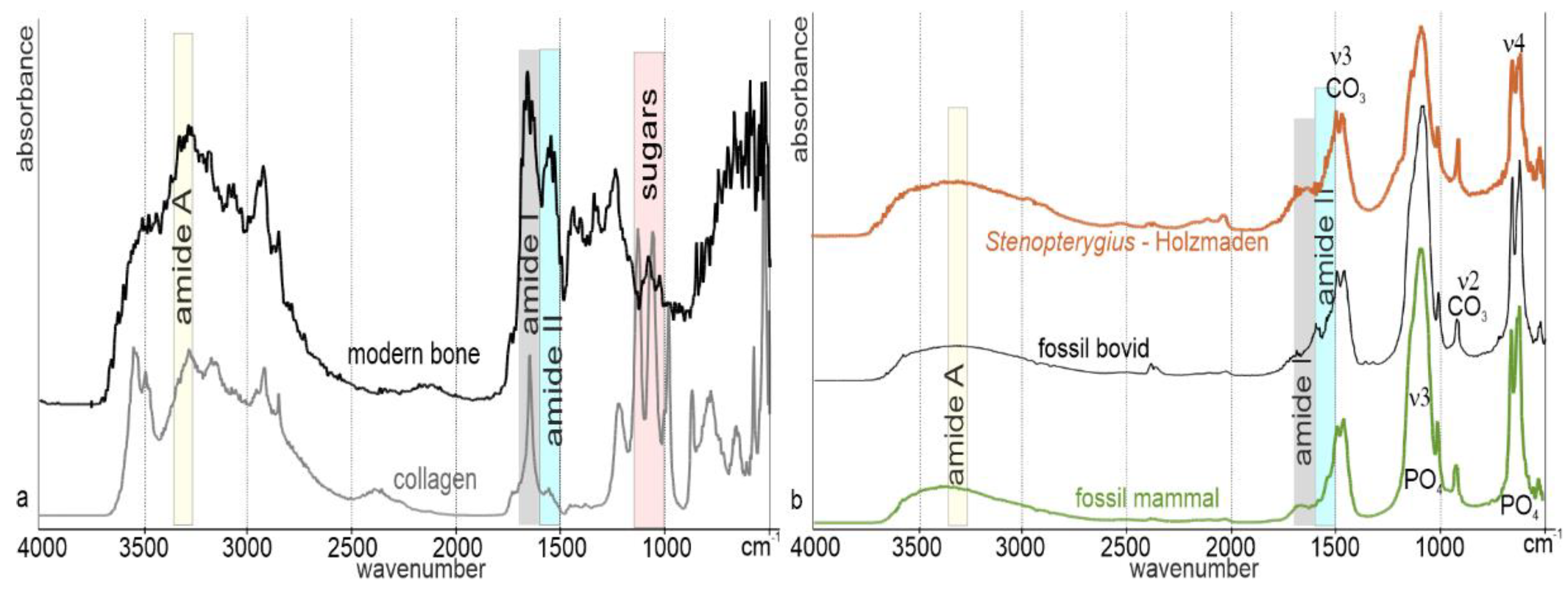
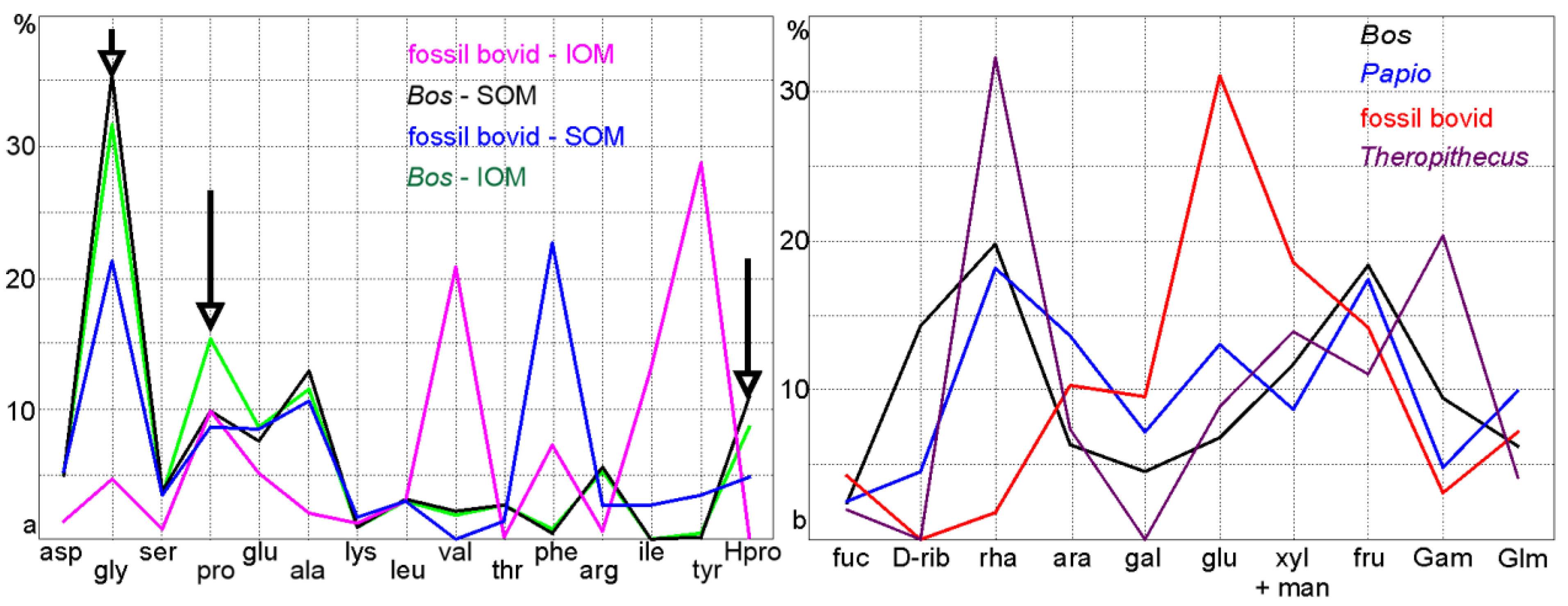
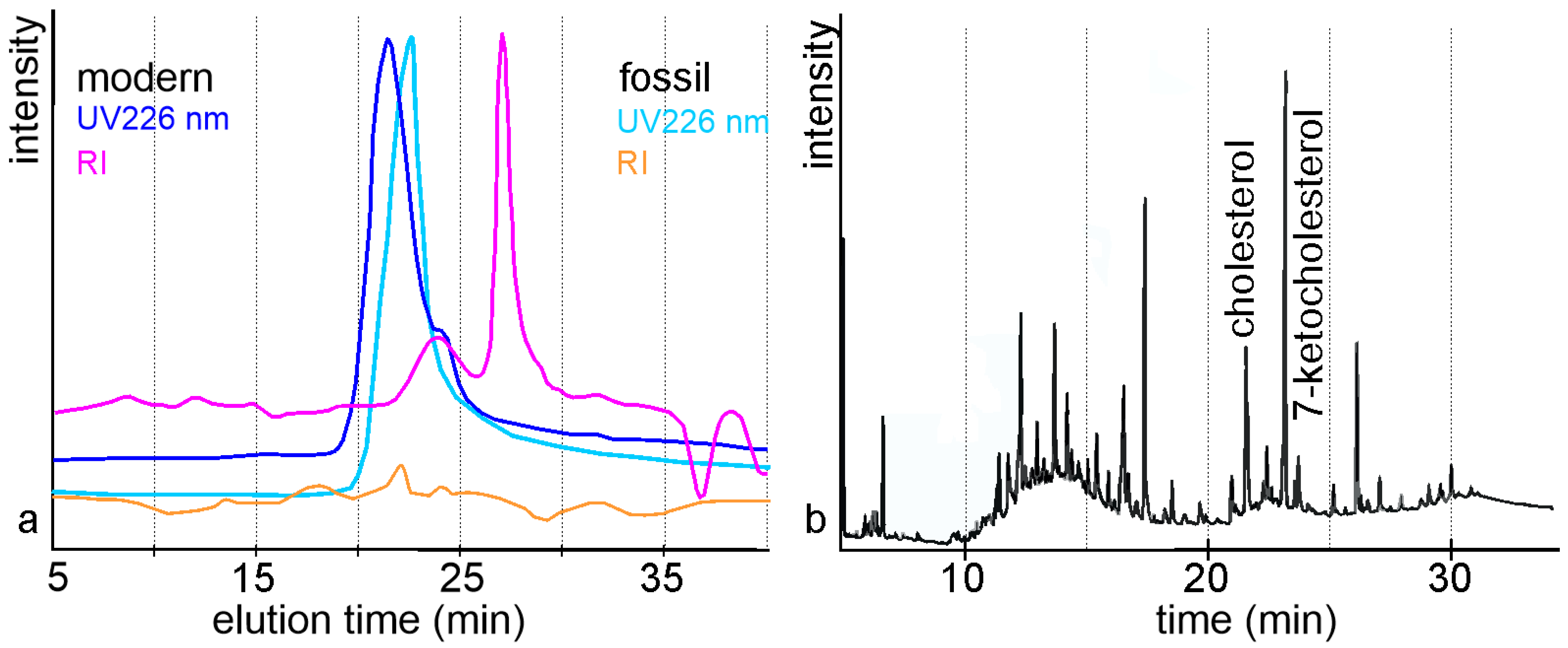
2.2.5. Chemical Modifications (Mineral and Organic Components)
3. Predation, Digestion or Diagenesis?
4. Some Pitfalls
4.1. The Perfect Preservation
4.2. Technical Pitfalls
4.3. Other Pitfalls
4.4. New Techniques
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengtson, S. Origins and early evolution of predation. Paleont. Soc. Pap. 2002, 8, 289–318. [Google Scholar] [CrossRef]
- Von Gumbel, C.W. Geognostische Besckreibung des Ostbayerischen Grenzgebirges oder des Bayerischen und Oberpfalzer Waldgebirges; Perthes: Gotha, Germany, 1868; 968p. [Google Scholar]
- Efremov, I.A. Taphonomy: A new branch of paleontology. Pan Am. Geol. 1940, 74, 81–93. [Google Scholar]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989; 324p. [Google Scholar]
- Anderson, P.S.L.; Westneat, M.W. A biomechanical model of feeding kinematics for Dunkleosteus terrelli (Arthrodira, Placodermi). Paleobiology 2009, 35, 251–269. [Google Scholar] [CrossRef]
- Fourvel, J.B. Hyénidés Modernes et Fossiles d’Europe et d’Afrique: Taphonomie Comparée de Leurs Assemblages Osseux. Ph.D. Thesis, Toulouse Le Mirail Université, Toulouse, France; 597p. Available online: https://tel.archives-ouvertes.fr/tel-00830276 (accessed on 10 December 2021).
- DeSantis, L.R.G. Dental microwear textures: Reconstructing diets of fossil mammals. Surf. Topogr. Metrol. Prop. 2016, 4, 023002. [Google Scholar] [CrossRef]
- Weissbrod, L.; Dayan, T.; Kaufman, D.; Weinstein-Evron, M. Micromammal taphonomy of el-Wad terrace, Mount Carmel, Israel: Distinguishing cultural from natural depositional agents in the Late Natufian. J. Archaeol. Sci. 2005, 32, 1–17. [Google Scholar] [CrossRef]
- Matthews, T. Taphonomic characteristics of micromammals predated by small mammalian carnivores in South Africa: Applications to fossil accumulations. J. Taphon. 2006, 4, 143–161. [Google Scholar]
- Matthews, T.; Rector, A.; Jacobs, Z.; Herries, A.I.R.; Marean, C.W. Environmental implications of micromammals accumulated close to MIS 6 to MIS5 transition at Pinnacle Point cave 9 (Mossel Bay, Wester, Cape Province, South Africa). Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 302, 213–229. [Google Scholar] [CrossRef]
- Fernández-Jalvo, Y.; Andrews, P. Atlas of Taphonomic Identifications: 1001+ Images of Fossil and Recent Mammal Bone Modification; Vertebrate Paleobiology and Paleoanthropology Series: Dordrecht, The Netherlands, 2016; pp. 1–359. [Google Scholar]
- Weber, K.; Winkler, D.E.; Schulz-Kornas, E.; Kaiser, Y.M.; Tütken, T. The good, the bad and the ugly—A visual guide for common post-mortem wear patterns in vertebrate teeth. Palaeogeogr. Palaeoclim. Palaeoecol. 2021, 578, 110577. [Google Scholar] [CrossRef]
- Epstein, A.G.; Epstein, J.B.; Harris, L.D. Conodont color alteration: An index to organic metamorphism. Geol. Surv. Prof. Pap. 1977, 995, 1–27. [Google Scholar]
- López-González, F.; Grandal-d’Anglade, A.; Vidal-Romaní, J.R. Deciphering bone depositional sequences in caves through the study of manganese coatings. J. Archaeol. Sci. 2006, 33, 707–717. [Google Scholar] [CrossRef]
- Monge, G.; Carretero, M.I.; Pozo, M.; Barroso, C. Mineralogical changes in fossil bones from Cueva del Angel, Spain: Archaeological implications and occurrence of whitlokite. J. Archaeol. Sci. 2014, 46, 6–15. [Google Scholar] [CrossRef]
- Franchet, L. La coloration des os dans le sol. Le bouillage des cadavres au Moyen Age, l’incinération et ses phénomènes. Rev. Sci. 1933, 483–495, 520–532. [Google Scholar]
- Franchet, L. Coloration of fossil bones. Nature 1934, 33, 60. [Google Scholar]
- Dauphin, Y.; Nespoulet, R.; Stoetzel, E.; el Haijraoui, M.A.; Denys, C. Can colour be used as a proxy for paleoenvironmental reconstructions based on archaeological bones? El Harhoura 2 (Morocco) case study. J. Taphon. 2012, 10, 69–84. [Google Scholar]
- Dauphin, Y.; Lange-Badré, D. Evaluation de la conservation de l’os fossile: Intégration des différents niveaux d’observation. Paläont. Zeit. 2000, 74, 441–457. [Google Scholar] [CrossRef]
- Rensberger, J.M.; Krentz, H.B. Microscopic effects of predator digestion on the surfaces of bones and teeth. Scanning Microsc. 1988, 2, 30. [Google Scholar]
- Denys, C.; Kowalski, K.; Dauphin, Y. Mechanical and chemical alterations of skeletal tissues in a recent Saharian accumulation of faeces from Vulpes rueppelli (Carnivora, Mammalia). Acta Zool. Cracov. 1992, 35, 265–283. [Google Scholar]
- Denys, C.; Fernandez-Jalvo, Y.; Dauphin, Y. Experimental taphonomy: Preliminary results of the digestion of micromammal bones in the laboratory. C. R. Acad. Sci. 1995, 321, 803–809. [Google Scholar]
- Bell, L.S. Forensic Microscopy for Skeletal Tissues: Methods and Protocols; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 915, pp. 1–269. [Google Scholar]
- Turner-Walker, G.; Syversen, U. Quantifying histological changes in archaeological bones using BSE-SEM image analysis. Archaeometry 2002, 44, 461–468. [Google Scholar] [CrossRef]
- Crouzier, L.; Delvallée, A.; Ducourtieux, S.; Devoille, L.; Noircler, G.; Ulysse, C.; Taché, O.; Barruet, E.; Tromas, C.; Feltin, N. Development of a new hybrid approach combining AFM and SEM for the nanoparticle dimensional metrology. Beilstein J. Nanotechnol. 2019, 10, 1523–1536. [Google Scholar] [CrossRef] [Green Version]
- Kral, A.G.; Ziegler, A.; Tütken, T.; Geisler, T. Experimental aqueous alteration of cortical bone microarchitecture analyzed by quantitative micro-computed tomography. Front. Earth Sci. 2021, 9, 609496. [Google Scholar] [CrossRef]
- Andrews, P. Owls, Caves and Fossils; Natural History Museum Publications: London, UK, 1990; pp. 1–231. [Google Scholar]
- Leprince, P.; Dandrifosse, G.; Schoffeniels, E. The digestive enzymes and acidity of the pellets regurgitated by raptors. Biochem. Syst. Ecol. 1979, 7, 223–227. [Google Scholar] [CrossRef]
- Turner-Walker, G.; Jans, M. Reconstructing taphonomic histories using histological analysis. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 266, 227–235. [Google Scholar] [CrossRef]
- Weiner, S.; Traub, W. Bone structure: From angstroms to microns. FASEB 1992, 6, 879–885. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Vander Heiden, M.G.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, P.V.; Voegele, K.K.; Grandstaff, D.E.; Ash, R.D.; Zheng, W.; Schroeter, E.R.; Schweitzer, M.H.; Lacovara, K.J. Molecular tests support the viability of rare earth elements as proxies for fossil biomolecule preservation. Sci. Rep. 2020, 10, 15566. [Google Scholar] [CrossRef]
- Dauphin, Y.; Montuelle, S.; Quantin, C.; Massard, P. Estimating the preservation of tooth structures: Towards a new scale of observation. J. Taphon. 2007, 5, 43–56. [Google Scholar]
- Skinner, H.C.W. Biominerals. Mineral. Mag. 2005, 69, 621–641. [Google Scholar] [CrossRef]
- Dauphin, Y.; Massard, P. Diagenèse des os de rongeurs fossiles d’El Harhoura 2 (Maroc): Microstructure versus composition globale. Trav. Inst. Sci. 2015, 8, 31–42. [Google Scholar]
- Greiner, M.; Rodríguez-Navarro, A.; Heinig, M.F.; Mayer, K.; Kocsis, B.; Göhring, A.; Toncala, A.; Grupe, G.; Schmahl, W.W. Bone incineration: An experimental study on mineral structure, colour and crystalline state. J. Archaeol. Sci. Rep. 2019, 25, 507–518. [Google Scholar] [CrossRef]
- Mac Connell, D. Apatite—Its Crystal Chemistry, Mineralogy, Utilization and Biological Occurrence; Springer: Wien, Austria, 1973; pp. 1–111. [Google Scholar]
- Elliott, J.C. Infrared and Raman spectroscopy of calcified tissues. In Methods of Calcified Tissues Preparation; Dickson, G.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 413–434. [Google Scholar]
- Pan, Y.; Fleet, M.E. Compositions of the apatite-group minerals: Substitution mechanisms and controlling factors. Rev. Mineral. Geochem. 2002, 48, 13–49. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J. A mineralogical perspective on the apatite in bone. Mater. Sci. Engin. 2005, C25, 131–143. [Google Scholar] [CrossRef]
- Labs-Hochstein, J.; MacFadden, B.J. Quantification of diagenesis in cenozoic sharks: Elemental and mineralogical changes. Geochim. Cosmochim. Acta 2006, 70, 4921–4932. [Google Scholar] [CrossRef]
- Legeros, R.; Ben-Nissan, B. Introduction to synthetic and biological apatites. In Advances in Calcium Phosphate Biomaterials; Ben Nissan, B., Ed.; Springer Series in Biomaterials Science and Engineering: Heidelberg, Germany, 2014; Volume 2, Chapter 1. [Google Scholar] [CrossRef]
- Shemesh, A. Crystallinity and diagenesis of sedimentary apatites. Geochim. Cosmochim. Acta 1990, 54, 2422–2428. [Google Scholar] [CrossRef]
- Stiner, M.C.; Kuhn, S.L.; Surovell, T.A.; Goldberg, P.; Meignen, L.; Weiner, S.; Bar-Yosef, O. Bone preservation in Hayonim cave (Israel): A macroscopic and mineralogical study. J. Archaeol. Sci. 2001, 28, 643–659. [Google Scholar] [CrossRef] [Green Version]
- Farre, B.; Massard, P.; Nouet, J.; Dauphin, Y. Preservation of rodent bones from El Harhoura 2 cave (Morocco, Neolithic—Middle Palaeolithic): Microstructure, mineralogy, crystallinity and composition. J. Afr. Earth Sci. 2014, 92, 1–13. [Google Scholar] [CrossRef]
- Suzuki, M. Studies on the physicochemical nature of hard tissue. Infrared, N.M.R., X-ray diffraction investigation of hydroxylradical, crystalline water and carbonate substitution in biological apatites. In Physicochimie et Cristallographie des Apatites D’intérêt Biologique; CNRS: Paris, France; pp. 77–83.
- Stutman, J.M.; Termine, J.D.; Posner, A.S. Vibrational spectra and structure of the phosphate ion in some calcium phosphates. Trans. N.Y. Acad. Sci. 1965, 27, 669–675. [Google Scholar] [CrossRef]
- Adamiano, A.; Fabbri, D.; Falini, G.; Belcastro, G. A complementary approach using analytical pyrolysis to evaluate collagen degradation and mineral fossilization in archaeological bones: The case study of Vicenne-Campochiaro necropolis (Italy). J. Anal. Appl. Pyrolysis 2013, 100, 173–180. [Google Scholar] [CrossRef]
- Termine, J.D.; Posner, A.S. Infra-red determination of the percentage of crystallinity in apatitic calcium phosphates. Nature 1966, 211, 268–270. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Sillen, A. Biogenic and diagenetic Sr/Ca in Plio-Pleistocene fossils of the Omo Shungura formation. Paleobiology 1986, 12, 311–323. [Google Scholar] [CrossRef]
- Legeros, R.Z.; Trautz, O.R.; Klein, E.; Legeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Renugopalaskrishnan, V.; Collins, B.; Glimcher, M.J. Fourier Transform infrared spectroscopic study of the carbonate ions in bone mineral during aging. Calc. Tissue Intern. 1991, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Botha, J.; Lee-Thorp, J.; Sponheimer, M. An examination of triassic cynodont tooth enamel chemistry using Fourier Transform Infrared spectroscopy. Calc. Tissue Intern. 2004, 74, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Mineralization of bones and teeth. Elements 2007, 3, 385–391. [Google Scholar] [CrossRef]
- Boskey, A.; Camacho, N.P. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef] [Green Version]
- Kazanci, M.; Roschger, P.; Paschalis, E.P.; Klaushofer, K.; Fratzl, P. Bone osteonal tissues by Raman spectral imaging: Orientation—composition. J. Struct. Biol. 2006, 156, 489–496. [Google Scholar] [CrossRef]
- Gamsjaeger, S.; Masic, A.; Roschger, P.; Kazanci, M.; Dunlop, J.W.C.; Klaushofer, K.; Paschalis, E.P.; Fratzl, P. Cortical bone composition and orientation as a function of animal and tissue age in mice by Raman spectroscopy. Bone 2010, 47, 392–399. [Google Scholar] [CrossRef]
- Amarie, S.; Zaslansky, P.; Kajihara, Y.; Griesshaber, E.; Schmahl, W.W.; Keilmann, F. Nano-FTIR chemical mapping of minerals in biological materials. Beilstein J. Nanotech. 2012, 3, 312–323. [Google Scholar] [CrossRef]
- Dauphin, Y.; Castillo-Michel, H.; Farre, B.; Mataame, A.; Rbii, K.; Rihane, A.; Stoetzel, E.; Denys, C. Identifying predation on rodent teeth through structure and composition: A case from Morocco. Micron 2015, 75, 34–44. [Google Scholar] [CrossRef]
- Dauphin, Y.; Denis, A. Modifications de structure et de composition des biominéraux phosphatés des vertébrés. In TaphonomieS; Brugal, J.P., Ed.; Archives Contemporaines: Paris, France, 2017; pp. 24–43. [Google Scholar]
- Woess, C.; Unterberger, S.H.; Roider, C.; RitschMarte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisz, R.R.; Huang, T.D.; Roberts, E.M.; Peng, S.R.; Sullivan, C.; Stein, K.; LeBlanc, A.R.H.; Shieh, D.B.; Chang, R.S.; Chiang, C.C.; et al. Embryology of Early Jurassic dinosaur from China with evidence of preserved organic remains. Nature 2013, 496, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, R.; Paschalis, E.P.; Sherman, P.J.; Boskey, A.L. IR microscopic imaging of pathological states and fracture healing of bone. Appl. Spectr. 2000, 54, 1183–1191. [Google Scholar] [CrossRef]
- Parker, R.B.; Toots, H. Minor elements in fossil bone. Geol. Soc. Amer. Bull. 1970, 81, 925–932. [Google Scholar] [CrossRef]
- Dauphin, Y.; Williams, C.T.; Andrews, P.; Denys, C.; Fernandez-Jalvo, Y. Diagenetic alterations of micromammal fossil bones from Olduvai Bed I of the Lower Pleistocene sequence at Olduvai gorge, Tanzania. J. Sedim. Res. 1999, 69, 612–621. [Google Scholar] [CrossRef]
- Fowler, B.O. Infrared studies of apatites 1. vibrational assignments for calcium, strontium and barium hydroxyapatites utilizing isotopic substitution. Inorg. Chem. 1974, 13, 194–207. [Google Scholar] [CrossRef]
- Brophy, G.P.; Nash, J.T. Compositional, infrared, and X-ray analysis of fossil bone. Amer. Mineral. 1968, 53, 445–454. [Google Scholar]
- Sakae, T.; Mishima, H.; Kozawa, Y. Proboscidea fossil teeth suggest the evolution of enamel crystals. In Mechanisms and Phylogeny of Mineralization in Biological Systems; Suga, S.H., Nakahara, H., Eds.; Springer: Tokyo, Japan, 1991; pp. 477–481. [Google Scholar]
- El Feki, H.; Rey, C.; Vignoles, C.M. Carbonate ions in apatites: Infrared investigations in the n4 CO3 domain. Calc. Tissue Intern. 1991, 49, 269–274. [Google Scholar] [CrossRef]
- Dauphin, Y. Potential of the diffuse reflectance infrared Fourier transform (DRIFT) method in paleontological studies of bones. Appl. Spectr. 1993, 47, 52–55. [Google Scholar] [CrossRef]
- Dauphin, Y. Spectrométrie infrarouge (DRIFT) des os de rongeurs fossiles de Tighenif (Pleistocène, Algérie). Paläont. Z. 1993, 67, 377–395. [Google Scholar] [CrossRef]
- Thomas, D.B.; Fordyce, R.E.; Frew, R.D.; Gordon, K.C. A rapid, non-destructive method of detecting diagenetic alteration in fossil bone using Raman spectroscopy. J. Raman Spectr. 2007, 38, 1533–1537. [Google Scholar] [CrossRef]
- King, T.; Andrews, P.; Basak, B. Effect of taphonomic processes on dental microwear. Amer. J. Phys. Anthr. 1999, 108, 359–373. [Google Scholar] [CrossRef]
- Mishima, H.; Kakei, M.; Sasagawa, I.; Miake, Y. Nature of apatite crystals in the tooth of Eusthenopteron from Devonian. J. Hard Tissue Biol. 2017, 26, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Açil, Y.; Mobasseri, A.E.; Warnke, P.H.; Terheyden, H.; Wiltfang, J.; Springer, I. Detection of mature collagen in human dental enamel. Calc.Tissue Intern. 2005, 76, 121–126. [Google Scholar] [CrossRef]
- Van der Rest, M.; Garrone, R. Collagen family of proteins. FASEB J. 1991, 5, 2814–2823. [Google Scholar] [CrossRef] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Easteo, J.E. The amino acid composition of Mammalia, collagen and gelatin. Biochem J. 1955, 61, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Pawlicki, R. Topochemical localization of lipids in dinosaur bone by means of Sudan B black. Acta Histochem. 1977, 59, 40–46. [Google Scholar] [CrossRef]
- Pawlicki, R. Histochemical reactions for mucopolysaccharides in the dinosaur bone studies on Epon- and methacrylate-embedded semithin sections as well as on isolated osteocytes and ground sections of bone. Acta Histochem. 1977, 58, 75–78. [Google Scholar] [CrossRef]
- Ulrich, M.M.W.; Perizonius, W.R.K.; Spoor, C.F.; Sandberg, P.; Vermeer, C. Extraction of osteocalcin from fossil bones and teeth. Biochem. Biophys. Res. Com. 1987, 149, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Muyzer, G.; Sandberg, P.; Knapen, M.H.J.; Vermeer, C.; Collins, M.; Westbroek, P. Preservation of the bone protein osteocalcin in dinosaurs. Geology 1992, 20, 871–874. [Google Scholar] [CrossRef]
- Evershed, R.P.; Turner-Walker, G.; Hedges, R.E.M.; Tuross, N.; Leyden, A. Preliminary results for the analysis of lipids in ancient bone. J. Archaeol. Sci. 1995, 22, 277–290. [Google Scholar] [CrossRef] [Green Version]
- Poinar, H.N.; Stankiewicz, B.A. Protein preservation and DNA retrieval from ancient tissues. Proc. Natl. Acad. Sci. USA 1999, 96, 8426–8431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobberstein, R.C.; Collins, M.J.; Craig, O.E.; Taylor, G.; Penkman, K.E.H.; Ritz-Timme, S.R. Archaeological collagen: Why worry about collagen diagenesis? Archaeol. Anthropol. Sci. 2009, 1, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.J.; Kuczynski, C.A.; Townsend, T.; Reeder, T.W.; Mulcahy, D.G.; Sites, J.W., Jr. Combining phylogenomics and fossils in higher-level squamate reptile phylogeny: Molecular data change the placement of fossil taxa. System. Biol. 2010, 59, 674–688. [Google Scholar] [CrossRef] [Green Version]
- Woodward, S.R.; Weyand, N.J.; Bunnell, M. DNA sequence from Cretaceous period bone fragments. Science 1994, 266, 1229–1232. [Google Scholar] [CrossRef]
- Zischler, H.; Höss, M.; Handt, O.; von Haeseler, A.; van der Kuyl, A.C.; Goudsmit, J. Detecting dinosaur DNA. Science 1995, 268, 1192–1193. [Google Scholar] [CrossRef] [Green Version]
- Haouchar, D.; Haile, J.; McDowell, M.C.; Murray, D.C.; White, N.E.; Allcock, R.J.N.; Phillips, M.J.; Prideaux, G.J.; Bunce, M. Thorough assessment of DNA preservation from fossil bone and sediments excavated from a late Pleistocene—Holocene cave deposit on Kangaroo Island, South Australia. Quater. Sci. Rev. 2014, 84, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Ferrari., G.; Cuevas, A.; Gondek-Wyrozemsha, A.T.; Ballantyne, R.; Kersten, O.; Palsdottir, A.H.; van der Jagt, I.; Hufthammer, A.K.; Ystgaard, I.; Wickler, S.; et al. The preservation of ancient DNA in archaeological fish bone. J. Archaeol. Sci. 2021, 126, 105317. [Google Scholar] [CrossRef]
- Korvenkontio, V.A. Mikroskopische Untersuchungen an Nagerincisiven unter Hinweis auf die Schmelzstruktur der Backenzähne. Ann. Zool. Soc. Zool. Bot. Fenn. Vanamo 1934, 2, 1–274. [Google Scholar]
- Peyer, B. Comparative Odontology; Zangerl, T., Translator; University Chicago Press: Chicago, IL, USA, 1968; pp. 1–458. [Google Scholar]
- von Koenigswald, W.; Sander, P.M. Tooth Enamel Microstructure; Balkema, A.A., Ed.; CRC Press: Rotterdam, The Netherlands, 1997; pp. 1–280. [Google Scholar]
- Stahl, P.W. The recovery and interpretation of microvertebrate bone assemblages from archaeological contexts. J. Archaeol. Meth. Theory 1996, 3, 31–75. [Google Scholar] [CrossRef]
- Dodson, P.; Wexlar, D. Taphonomic investigations of owl pellets. Paleobiology 1979, 5, 275–284. [Google Scholar] [CrossRef]
- Voorhies, M. Taphonomy and population dynamics of an early Pleistocene vertebrate fauna Knox County Nebraska. In Contributions to Geology; University Wyoming Press: Laramie, WY, USA, Special Paper 1.
- Domínguez-Rodrigo, M. Critical review of the MNI (minimum number of individuals) as a zooarchaeological unit of quantification. Archaeol. Anthropol. Sci. 2012, 4, 47–59. [Google Scholar] [CrossRef]
- Bockenski, Z.M.; Tomek, T. Preservation of bird bones: Erosion versus digestion by owls. Intern. J. Osteoarch. 1997, 7, 372–387. [Google Scholar] [CrossRef]
- Fernández-Jalvo, Y.; Andrews, P.; Sevilla, P.; Requejo, V. Digestion vs. abrasion features in rodent bones. Lethaia 2014, 47, 323–336. [Google Scholar] [CrossRef]
- Denys, C. Le gisement Pliocène de Laetoli (Tanzanie, Afrique de l’Est): Analyse taphonomique des assemblages de microvertébrés. Palaeontographica 1986, A194, 69–98. [Google Scholar]
- Czaplewski, N.J. An owl-pellet accumulation of small Pliocene vertebrates from the Verde Formation, Arizona, USA. Palaeont. Electr. 2011, 14, 1–33. [Google Scholar]
- Arriaza, M.C.; Domínguez-Rodrigo, M.; Yravedra, J.; Baquedano, E. Lions as bone accumulators? Paleontological and ecological implications of a modern bone assemblage from Olduvai Gorge. PLoS ONE 2016, 11, e0153797. [Google Scholar] [CrossRef]
- Andrews, P.; Nesbit Evans, E.M. Small mammal bone accumulations produced by mammalian carnivores. Paleobiology 1983, 9, 289–307. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Weiner, S. Chemical, enzymatic and spectroscopic characterization of “collagen” and other organic fractions from prehistoric bones. Geochim. Cosmochim. Acta 1988, 52, 2197–2206. [Google Scholar] [CrossRef]
- Collins, M.J.; Nielsen-Marsh, C.M.; Hiller, J.; Smith, C.I.; Roberts, J.P. The survival of organic matter in bone: A review. Archaeometry 2002, 44, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Turner-Walker, G. The chemical and microbial degradation of bones and teeth. In Advances in Human Palaeopathology; Pinhasi, R., Mays, S., Eds.; Wiley & Sons: Chichester, UK, 2008; pp. 3–30. [Google Scholar]
- Trueman, C.; Behrensmeyer, A.; Tuross, N.; Weiner, S. Mineralogical and compositional changes in bones exposed on soil surfaces in Amboseli National Park, Kenya: Diagenetic mechanisms and the role of sediment pore fluids. J. Archaeol. Sci. 2004, 31, 721–739. [Google Scholar] [CrossRef]
- Janssens, K.; Vincze, L.; Vekemans, B.; Williams, C.T.; Radtke, M.; Haller, M.; Knöchel, A. The non-destructive determination of REE in fossilized bone using synchrotron radiation induced K-line X-ray microfluorescence analysis. Fresenius J. Anal. Chem. 1999, 363, 413–420. [Google Scholar] [CrossRef]
- Denys, C.; Williams, C.T.; Dauphin, Y.; Andrews, P.; Fernandez-Jalvo, Y. Diagenetical changes in Pleistocene small mammal bones from Olduvai Bed I. Palaeogeogr. Palaeoclim. Palaeoecol. 1996, 126, 121–134. [Google Scholar] [CrossRef]
- Williams, C.T. Alteration of chemical composition of fossil bones by soil processes and groundwater. In Trace Elements in Environmental History; Grupe, G., Herrmann, B., Eds.; Springer: Berlin, Germany, 1988; pp. 27–40. [Google Scholar]
- Rosling, A.; Suttle, K.B.; Johnasson, E.; Van Hees, P.A.W.; Banfield, J.F. Phosphorous availability influences the dissolution of apatite by soil fungi. Geobiology 2007, 5, 265–280. [Google Scholar] [CrossRef]
- Smits, M.M.; Bonneville, S.; Benning, L.G.; Banwart, S.A.; Leake, J.R. Plant-driven weathering of apatite—The role of an ectomycorrhizal fungus. Geobiology 2013, 10, 445–456. [Google Scholar] [CrossRef]
- Bada, J.L.; Wang, X.S.; Hamilton, H. Preservation of key biomolecules in the fossil record: Current knowledge and future challenges. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1999, 354, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Holmes, K.M.; Robson Brown, K.A.; Oates, W.P.; Collins, M.J. Assessing the distribution of African Palaeolithic sites: A predictive model of collagen degradation. J. Archaeol. Sci. 2005, 32, 157–166. [Google Scholar] [CrossRef]
- Bodzioch, A. Idealized model of mineral infillings in bones of fossil freshwater animals, on the example of Late Triassic Metoposaurs from Krasiejów (Poland). Austin J. Earth Sci. 2015, 2, 1–6. [Google Scholar]
- Hinz, E.A.; Kohn, M.J. The effect of tissue structure and soil chemistry on trace element uptake in fossils. Geochim. Cosmochim. Acta 2010, 74, 3213–3231. [Google Scholar] [CrossRef]
- Dauphin, Y.; Castillo-Michel, H.; Denys, C.; El Hajiraoui, M.A.; Nespoulet, R.; Stoetzel, E. Diagenetic alterations of Meriones incisors (Rodentia) of El Harhoura 2 cave, Morocco (Late Pleistocene—Middle Hococene). PalZ 2018, 92, 163–177. [Google Scholar] [CrossRef]
- Rosin, Z.M.; Kwiecinski, Z. Digestibility of prey by the White Strok (Ciconia ciconia) under experimental conditions. Ornis Fenn. 2011, 88, 40–50. [Google Scholar]
- Cummings, J.H.; Duke, G.E.; Jegers, A.A. Corrosion of bone by solutions simulating raptor gastric juice. Raptor Res. 1976, 10, 55–57. [Google Scholar]
- Keenan, S.W.; Engel, A.S. Early diagenesis and recrystallization of bone. Geochim. Cosmochim. Acta 2017, 196, 209–233. [Google Scholar] [CrossRef]
- Massard, P.; Dauphin, Y. Sols, sédiments et biominéraux phosphatés: Expériences in vitro. In TaphonomieS; Brugal, J.P., Ed.; Archives Contemporaines: Paris, France, 2017; pp. 89–106. [Google Scholar]
- Lev, M.; Weinstein-Evron, M.; Yeshurun, R. Squamate bone taphonomy: A new experimental framework and its application to the Natufian zooarchaeological record. Scient. Rep. 2020, 10, 9373. [Google Scholar] [CrossRef] [PubMed]
- Schulmerich, M.V.; Cole, J.H.; Dooley, K.A.; Morris, M.D.; Kreider, J.M.; Goldstein, S.A.; Srinivasan, S.; Pogue, B.W. Noninvasive Raman tomographic imaging of canine bone tissue. J. Biomed. Opt. 2008, 13, 020506. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.D.; Jabbour, R.S.; Horton, C.F.; Ibarra, C.L.; MacDowell, A.A. Color Changes in modern and fossil teeth induced by synchrotron microtomography. Amer. J. Phys. Anthr. 2012, 149, 172–180. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Sur la composition de quelques protéines dinosauriennes. C. R. Hebd. Séances Acad. Sci. 1969, 269, 1489–1491. [Google Scholar]
- Whelton, H.L.; Hammann, S.; Cramp, L.J.E.; Dunne, J.; Roffet-Salque, M.; Evershed, R.P. A call for cautíon in the analysis of lipids and other small biomolecules from archaeological contexts. J. Archaeol. Sci. 2021, 132, 105397. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Avci, R.; Collier, T.; Goodwind, M.B. Microscopic, chemical and molecular methods for examining fossil preservation. C. R. Palevol. 2008, 7, 159–184. [Google Scholar] [CrossRef]
- Carnot, A. Recherche du fluor dans les os modernes et les os fossiles. C. R. Hebd. Séances Acad. Sci. 1982, 114, 1189. [Google Scholar]
- Henderson, P.; Marlow, C.A.; Molleson, T.I.; Williams, C.T. Patterns of chemical change during bone fossilization. Nature 1983, 306, 358–360. [Google Scholar] [CrossRef]
- Molleson, T.I.; Williams, C.T.; Cressey, G.; Din, V.K. Radiographically opaque bones from lead-lined coffins at Christ Church, Spitalfields, London; an extreme example of bone diagenesis. Bull. Soc. Geol. Fr. 1998, 169, 425–432. [Google Scholar]
- Garcia-Alix, A.; Minwer-Barakat, R.; Martin Suarez, E.; Freudenthal, M.; Delgado Huertas, A. Cinnabar mineralization in fossil small mammal remains as a consequence of diagenetic processes. Lethaia 2013, 46, 1–6. [Google Scholar] [CrossRef]
- Asaka, T.; Akiyama, M.; Domon, T.; Nishie, W.; Natsuga, K.; Fujita, Y.; Abe, R.; Kitagawa, Y.; Shimizu, H. Type XVII Collagen is a key player in tooth enamel formation. Amer. J. Pathol. 2009, 174, 91–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duverger, O.; Beniash, E.; Morasso, M.I. Keratins as components of the enamel organic matrix. Matrix Biol. 2016, 52–54, 260–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conn, K.M.; Termine, J.D. Matrix protein profiles in calf bone development. Bone 1985, 6, 33–36. [Google Scholar] [CrossRef]
- Homburg, J.A. Archeology in relation to soils. In Encyclopedia of Soils in the Environment; Hillel, D., Rosenweig, C., Powlson, D., Scow, K., Singer, M., Sparks, D., Eds.; Elsevier: Oxford, UK, 2005; pp. 95–102. [Google Scholar]
- Retallack, G.J. Soils of the Past—An Introduction to Paleopedology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2018; Volume 212, p. 520. [Google Scholar]
- Berna, F.; Matthews, A.; Weiner, S. Solubilities of bone mineral from archaeological sites: The recrystallization window. J. Archaeol. Sci. 2004, 31, 867–882. [Google Scholar] [CrossRef]
- Montgelard, C.; Buchy, M.C.; Gautret, P.; Dauphin, Y. Biogeochemical characterization of ichthyosaur bones from Holzmaden (Germany, Lias). Bull. Soc. Géol. Fr. 1997, 168, 759–766. [Google Scholar]
- Colleary, C.; Lamadrid, H.M.; O’Reilly, S.S.; Dolocan, A.; Nesbitt, S.J. Molecular preservation in mammoth bone and variation based on burial environment. Scient. Rep. 2021, 11, 2662. [Google Scholar] [CrossRef]
- Aramendi, J.; Maté-González, M.A.; Yravedra, J.; Cruz Ortega, M.; Arriaza, M.C.; González-Aguilera, D.; Baquedano, E.; Domínguez-Rodrigo, M. Discerning carnivore agency through the three-dimensional study of tooth pits: Revisiting crocodile feeding behaviour at FLK- Zinj and FLK NN3 (Olduvai Gorge, Tanzania). Palaeogeogr. Palaeoclim. Palaeoecol. 2017, 488, 93–102. [Google Scholar] [CrossRef]
- Georgiadis, M.; Guizar-Sicairos, M.; Gschwend, O.; Hangartner, P.; Bunk, O.; Müller, R.; Schneider, P. Ultrastructure organization of human trabeculae assessed by 3D sSAXS and relation to bone microarchitecture. PLoS ONE 2016, 11, e0159838. [Google Scholar] [CrossRef] [PubMed]
- Lacel, A.; Wojtkow, M.; Tomanis, M.; Spiak, Z.; Graczyk, S.; Pezowicz, C. The influence of a standardized experimental environment on early diagenetic changes to animal bone. Geoarchaeology 2021, 36, 755–767. [Google Scholar] [CrossRef]
- Raguin, E.; Rechav, K.; Brumfeld, V.; Shahar, R.; Weiner, S. Unique three-dimensional structure of a fish pharyngeal jaw subjected to unusually high mechanical loads. J. Struct. Biol. 2020, 211, 107530. [Google Scholar] [CrossRef] [PubMed]
- Raguin, E.; Rechav, K.; Shahar, R.; Weiner, S. Focused ion beam-SEM 3D analysis of mineralized osteonal bone: Lamellae and cement sheath structures. Acta Biomat. 2021, 121, 497–513. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauphin, Y. Vertebrate Taphonomy and Diagenesis: Implications of Structural and Compositional Alterations of Phosphate Biominerals. Minerals 2022, 12, 180. https://doi.org/10.3390/min12020180
Dauphin Y. Vertebrate Taphonomy and Diagenesis: Implications of Structural and Compositional Alterations of Phosphate Biominerals. Minerals. 2022; 12(2):180. https://doi.org/10.3390/min12020180
Chicago/Turabian StyleDauphin, Yannicke. 2022. "Vertebrate Taphonomy and Diagenesis: Implications of Structural and Compositional Alterations of Phosphate Biominerals" Minerals 12, no. 2: 180. https://doi.org/10.3390/min12020180
APA StyleDauphin, Y. (2022). Vertebrate Taphonomy and Diagenesis: Implications of Structural and Compositional Alterations of Phosphate Biominerals. Minerals, 12(2), 180. https://doi.org/10.3390/min12020180





