Does Soil Drying in a Lab Affect Arsenic Speciation in Strongly Contaminated Soils?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
- -
- immediately, in fresh samples (F);
- -
- in oven-dried samples (O);
- -
- and in air-dried samples (A).
2.2. Soil Material
2.3. Manure
2.4. Single and Sequential Extractions
2.5. Statistics
3. Results and Discussion
3.1. Arsenic Released in Single Extractions from Nonamended Soils
3.2. Arsenic Released in Single Extractions from Manure-Amended Soils
3.3. The Effects of Soil Amendment with Manure and Drying on As Fractionation in SE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wenzel, W.W. Arsenic. In Heavy Metals in Soils. Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 241–282. [Google Scholar]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation-A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Karczewska, A.; Bogda, A.; Krysiak, A. Arsenic in soils in the areas of former mining and mineral processing in Lower Silesia, southwestern Poland. Trace Met. Other Contam. Environ. 2007, 9, 411–440. [Google Scholar]
- Karczewska, A.; Krysiak, A.; Mokrzycka, D.; Jezierski, P.; Szopka, K. Arsenic distribution in soils of former As mining area and processing. Pol. J. Environ. Stud. 2013, 22, 175–181. [Google Scholar]
- Dradrach, A.; Szopka, K.; Karczewska, A. Ecotoxicity of soil pore water on historical arsenic mine dumps—The effects of forest litter. Ecotoxicol. Environ. Saf. 2019, 181, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Dradrach, A.; Szopka, K.; Karczewska, A. Ecotoxicity of pore water in meadow soils affected by historical spills of arsenic-rich tailings. Minerals 2020, 10, 751. [Google Scholar] [CrossRef]
- Karczewska, A.; Kabała, C. Environmental risk assessment As a new basis for evaluation of soil contamination in Polish law. Soil Sci. Ann. 2017, 68, 67. [Google Scholar] [CrossRef] [Green Version]
- Rao, C.R.M.; Sahuquillo, A.; Sanchez, J.L. A review of the different methods applied in environmental geochemistry for single and sequential extraction of trace elements in soils and related materials. Water Air Soil Pollut. 2008, 189, 291–333. [Google Scholar] [CrossRef]
- Anawar, H.M.; Garcia-Sanchez, A.; Santa Regina, I. Evaluation of various chemical extraction methods to estimate plant-available arsenic in mine soils. Chemosphere 2008, 70, 1459–1467. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Manag. 2017, 186, 192–200. [Google Scholar] [CrossRef]
- ISO 19730: Soil Quality—Extraction of Trace Elements from Soil Using Ammonium Nitrate Solution; International Organization for Standardization: Geneva, Switzerland, 2008.
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. Tr. Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Liu, G.; Chen, M.; Li, W.; Gong, W. A critical review on the speciation and development of sequential extraction procedures for arsenic in soils. J. Agro-Environ. Sci. 2018, 37, 2629–2638. [Google Scholar]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Tan, K. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar]
- Hoenig, M. Preparation steps in environmental trace element analysis—Facts and traps. Talanta 2001, 54, 1021–1038. [Google Scholar] [CrossRef]
- Haynes, R.J.; Swift, R.S. Concentrations of extractable Cu, Zn, Fe, and Mn in a group of soils As influenced by air- and oven-drying and rewetting. Geoderma 1991, 49, 319–333. [Google Scholar] [CrossRef]
- Klitzke, A.; Lang, F. Hydrophobicity of soil colloids and heavy metal mobilization: Effects of drying. J. Environ. Qual. 2007, 36, 1187–1193. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Groenenberg, J.E. Effects of soil oven-drying on concentrations and speciation of trace metals and dissolved organic matter in soil solution extracts of sandy soils. Geoderma 2001, 161, 147–158. [Google Scholar] [CrossRef]
- Supriatin, S.; Terrones, C.A.; Bussink, W.; Weng, L. Drying effects on selenium and copper in 0.01 M calcium chloride soil extractions. Geoderma 2015, 255, 104–114. [Google Scholar] [CrossRef]
- Kaiser, M.; Kleber, M.; Berhe, A.A. How air-drying and rewetting modify soil organic matter characteristics: An assessment to improve data interpretation and inference. Soil Biol. Biochem. 2015, 80, 324–340. [Google Scholar] [CrossRef]
- Hojdová, M.; Rohovec, J.; Chrastný, V.; Penížek, V.; Navrátil, T. The influence of sample drying procedures on mercury concentrations analyzed in soils. Bull. Environ. Contam. Toxicol. 2015, 94, 570–576. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Sun, J.; Liu, F.; Wang, J.; Zhang, Y. Effect of sample pretreatment on the fractionation of arsenic in anoxic soils. Environ. Sci. Pollut. Res. 2015, 22, 8367–8374. [Google Scholar] [CrossRef]
- Száková, J.; Tlustoš, P.; Goessler, W.; Frková, Z.; Najmanová, J. Mobility of arsenic and its compounds in soil and soil solution: The effect of soil pretreatment and extraction methods. J. Hazard. Mater. 2009, 172, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kosugi, T.; Riya, S.; Hashimoto, Y.; Hou, H.; Terada, A.; Hosomi, M. Potential for leaching of arsenic from excavated rock after different drying treatments. Chemosphere 2016, 154, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.D.; Postma, D.; Jakobsen, R. Release of arsenic associated with the reduction and transformation of iron oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Beesley, L.; Inneh, O.S.; Norton, G.J.; Moreno-Jimenez, E.; Pardo, T.; Clemente, R.; Dawson, J.J. assessing the influence of compost and biochar amendments on the mobility and toxicity of metals and arsenic in a naturally contaminated mine soil. Environ. Pollut. 2014, 186, 195–202. [Google Scholar] [CrossRef]
- Xie, H.; Han, D.; Cheng, J.; Zhou, P.; Wang, W. Fate and risk assessment of arsenic compounds in soil amended with poultry litter under aerobic and anaerobic circumstances. Water Air Soil Pollut. 2015, 226, 390. [Google Scholar] [CrossRef]
- Karczewska, A.; Gałk, B.; Dradrach, A.; Dradrach, A.; Lewińska, K.; Mołczan, M.; Cuske, M.; Gersztyn, L.; Litak, K. Solubility of arsenic and its uptake by ryegrass from polluted soils amended with organic matter. J. Geochem. Explor. 2017, 182, 193–200. [Google Scholar] [CrossRef]
- Karczewska, A.; Lewińska, K.; Siepak, M.; Gałka, B.; Dradrach, A.; Szopka, K. Transformation of beech forest litter As a factor that triggers arsenic solubility in soils developed on historical mine dumps. J. Soils Sed. 2018, 18, 2749–2758. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Jiménez, E.; Clemente, R.; Mestrot, A.; Meharg, A.A. Arsenic and selenium mobilisation from organic matter treated mine spoil with and without inorganic fertilization. Environ. Pollut. 2013, 173, 238–244. [Google Scholar] [CrossRef]
- McGeehan, S.L.; Fendorf, S.E.; Naylor, D.V. Alteration of arsenic sorption in flooded-dried soils. Soil Sci. Soc. Am. J. 1998, 62, 828–833. [Google Scholar] [CrossRef]
- Carrijo, D.R.; Akbar, N.; Reis, A.F.; Li, C.; Gaudin, A.C.; Parikh, S.J.; Linquist, B.A. Impacts of variable soil drying in alternate wetting and drying rice systems on yields, grain arsenic concentration and soil moisture dynamics. Field Crops Res. 2018, 222, 101–110. [Google Scholar] [CrossRef]
- Li, C.; Carrijo, D.R.; Nakayama, Y.; Linquist, B.A.; Green, P.G.; Parikh, S.J. Impact of alternate wetting and drying irrigation on arsenic uptake and speciation in flooded rice systems. Agric. Ecosyst. Environ. 2019, 272, 188–198. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, H.; Fu, Q.; Zhu, J.; Huang, G. Water management of alternate wetting and drying reduces the accumulation of arsenic in brown rice-as dynamic study from rhizosphere soil to rice. Ecotox. Environ. Saf. 2019, 185, 109711. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Beare, M.H.; Stoklas, U.; St-Georges, P. Biodegradability of soluble organic matter in maize-cropped soils. Geoderma 2003, 113, 237–252. [Google Scholar] [CrossRef]
- Szopka, K.; Gruss, I.; Gruszka, D.; Karczewska, A.; Gediga, K.; Gałka, B.; Dradrach, A. The Effects of Forest Litter and Waterlogging on the Ecotoxicity of Soils Strongly Enriched in Arsenic in a Historical Mining Site. Forests 2021, 12, 355. [Google Scholar] [CrossRef]
- Zhang, H.; Selim, H. Colloid mobilization and arsenite transport in soil columns: Effect of ionic strength. J. Environ. Qual. 2007, 36, 1273–1280. [Google Scholar] [CrossRef]
- Lescure, T.; Moreau, J.; Charles, C.; Saanda, T.B.A.; Thouin, H.; Pillas, N.; Battaglia-Brunet, F. Influence of organic matters on asIII oxidation by the microflora of polluted soils. Environ. Geochem. Health 2016, 38, 911–925. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Qiao, J.; Li, F.; Zhang, X.; Du, Y.; Hu, M.; Sun, W. Community dynamics of As (V)-reducing and As (III)-oxidizing genes during a wet–dry cycle in paddy soil amended with organic matter, gypsum, or iron oxide. J. Hazard. Mater. 2020, 393, 122485. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Wang, J.; Hou, Q.; Zhang, Y. Impact of temperature on the aging mechanisms of arsenic in soils: Fractionation and bioaccessibility. Environ. Sci. Pollut. Res. 2016, 23, 4594–4601. [Google Scholar] [CrossRef]
- Pigna, M.; Caporale, A.G.; Cavalca, L.; Sommella, A.; Violante, A. Arsenic in the soil environment: Mobility and phytoavailability. Environ. Eng. Sci. 2015, 32, 551–563. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, K.; Duczmal-Czernikiewicz, A.; Karczewska, A.; Dradrach, A.; Iqbal, M. Arsenic Forms in Soils of Various Settings in the Historical Ore Mining and Processing Site of Radzimowice, Western Sudetes. Minerals 2021, 11, 491. [Google Scholar] [CrossRef]
- Srithongkul, C.; Wongsaipun, S.; Krongchai, C.; Santasup, C.; Kittiwachana, S. Investigation of mobility and bioavailability of arsenic in agricultural soil after treatment by various soil amendments using sequential extraction procedure and multivariate analysis. Catena 2019, 181, 104084. [Google Scholar] [CrossRef]
- Dybowska, A.; Farago, M.; Valsami-Jones, E.; Thornton, I. Operationally defined associations of arsenic and copper from soil and mine waste in south-west England. Chem. Speciat. Bioavailab. 2005, 17, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Larios, R.; Fernández-Martínez, R.; Rucandio, I. Comparison of three sequential extraction procedures for fractionation of arsenic from highly polluted mining sediments. Anal. Bioanal. Chem. 2012, 40, 2909–2921. [Google Scholar] [CrossRef]
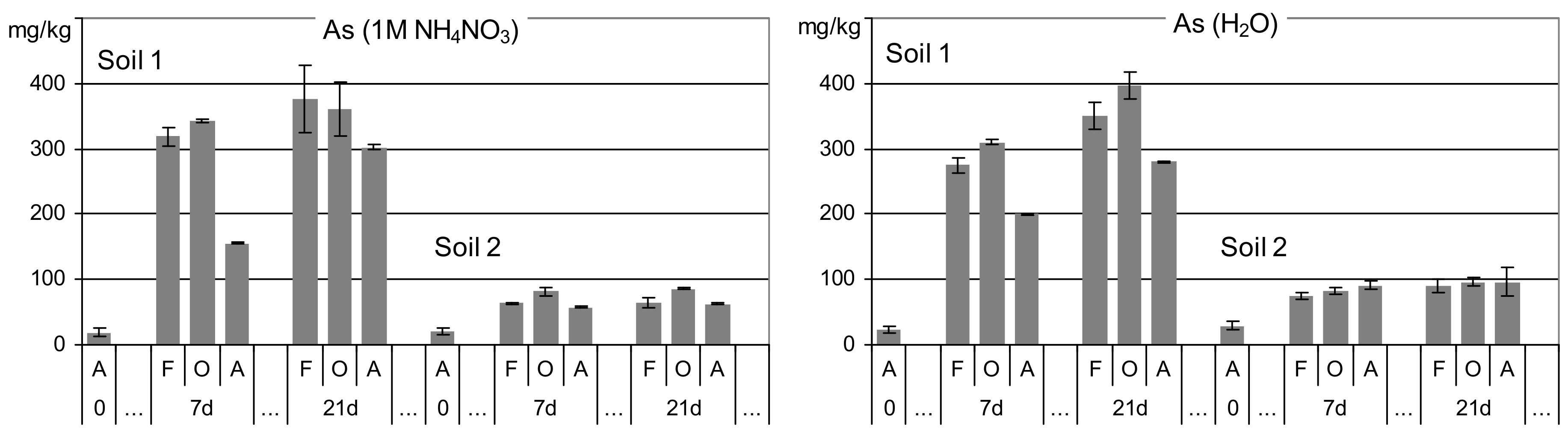
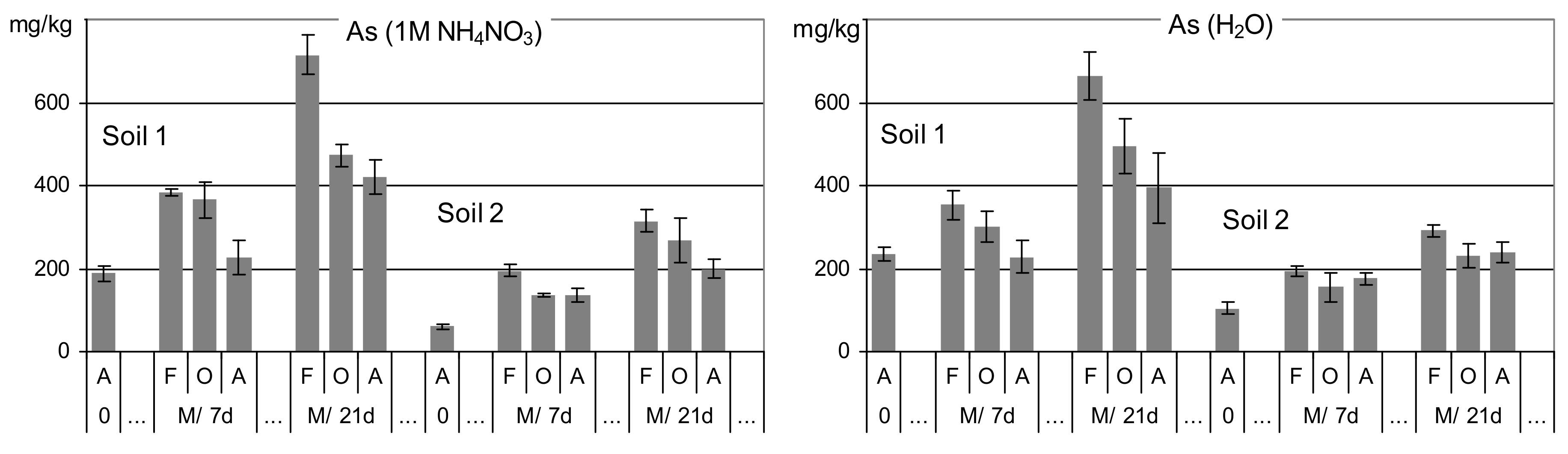
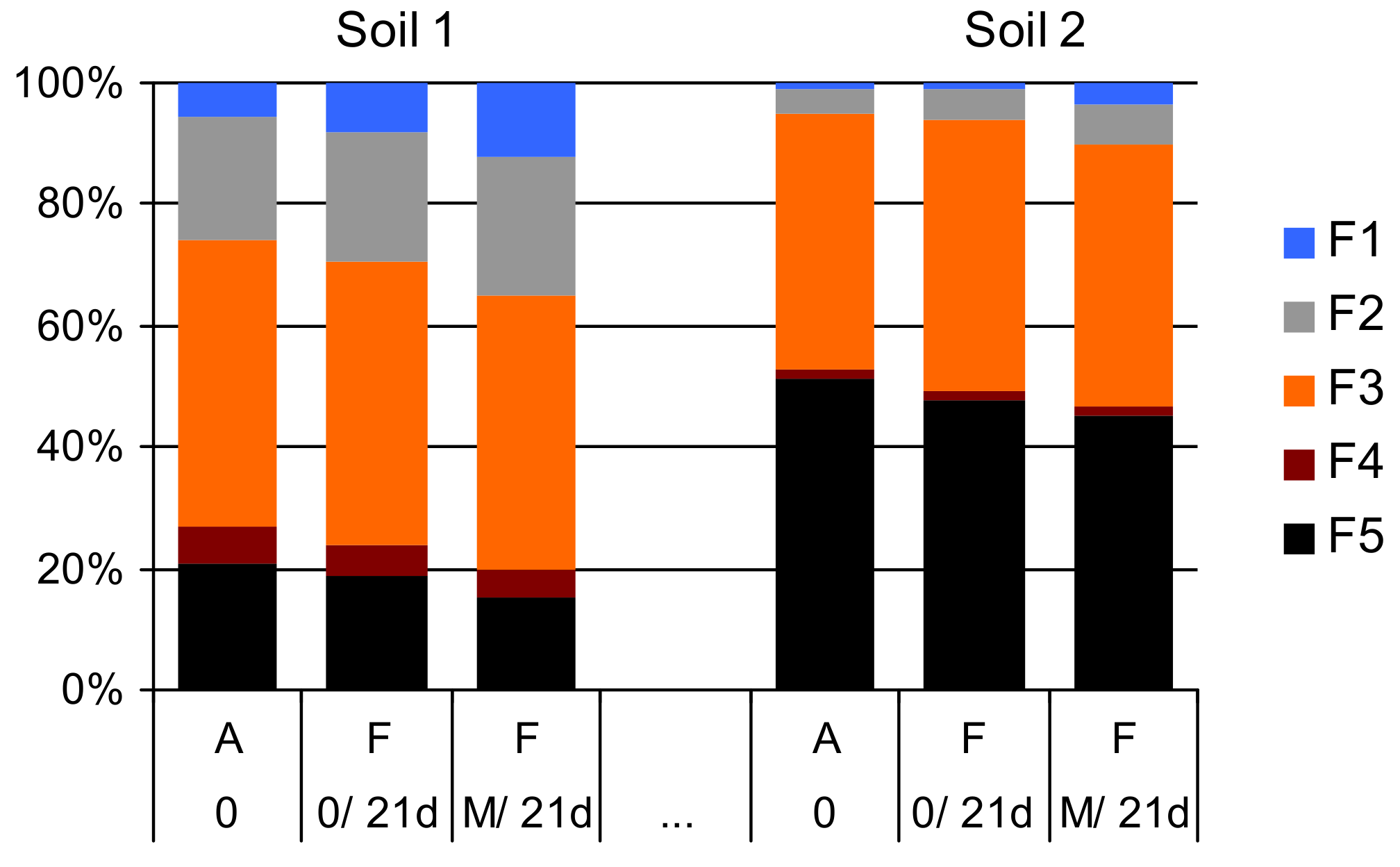
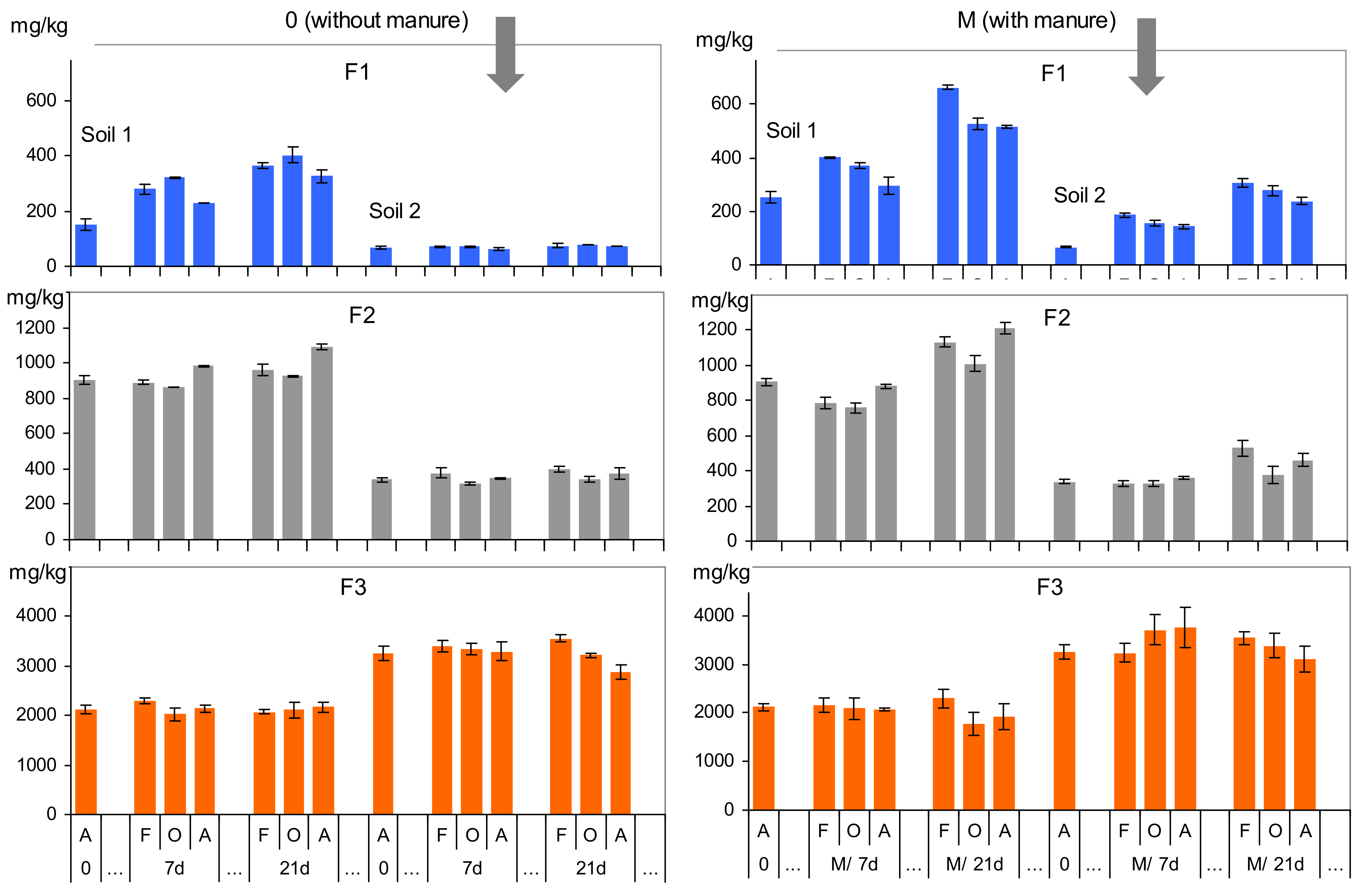
| Feature | Unit | Soil 1 | Soil 2 |
|---|---|---|---|
| Textural group (USDA) | - | Sandy loam | Loamy sand |
| <0.002 mm | % | 6 ± 1 | 3 ± 1 |
| Corg | g/kg | 26.5 ± 0.4 | 5.8 ± 0.3 |
| DOC | g/kg | 0.69 ± 0.10 | 0.24 ± 0.03 |
| pH (1 M KCl) | - | 6.30 ± 0.05 | 7.55 ± 0.10 |
| CaCO3 | % | absent | 2.0 ± 0.3 |
| As total | mg/kg | 4620 ± 180 | 7950 ± 140 |
| Water-extractable As | mg/kg | 24 ± 4 | 29 ± 5 |
| 1 M NH4NO3-extractable As | mg/kg | 19 ± 1 | 21 ± 1 |
| 0.43 M HNO3-extractable As | mg/kg | 2950 ± 120 | 3250 ± 110 |
| Fraction | Extractant | Extraction Conditions | Soil: Solution Ratio (m:v) | Washing Step |
|---|---|---|---|---|
| F1 * | 0.05 M (NH4)2SO4 | 4 h shaking, 20 °C | 1:25 | - |
| F2 | 0.05 M (NH4)H2PO4 | 16 h shaking, 20 °C | 1:25 | - |
| F3 |
0.2 M
NH4-oxalate buffer; pH 3.25 |
4 h shaking in the dark, 20 °C | 1:25 |
0.2 M
NH4-oxalate, pH 3.25: m:v 1:12.5; 10 min shaking in the dark |
| F4 |
0.2 M
NH4-oxalate buffer + 0.1 M ascorbic acid; pH 3.25 |
30 min in a water bath, 96 ± 3 °C, in the light | 1:25 |
0.2 M
NH4-oxalate, pH 3.25: m:v 1:12.5; 10 min shaking in the dark |
| F5 | HCl:HNO3 (3:1) | Microwave digestion | 1:50 | - |
| Incubation Time | Redox Potential, Eh, mV | |||
|---|---|---|---|---|
| Soil 1 (0) | Soil 1 (M) | Soil 2 (0) | Soil 2 (M) | |
| 7 d | 95 ± 10 | −36 ± 8 | 165 ± 7 | −7 ± 6 |
| 21 d | −53 ± 12 | −160 ± 21 | −97 ± 13 | −134 ± 18 |
| Method of Drying | Incubation Time | Reduction of As Extractability, % | |||||
|---|---|---|---|---|---|---|---|
| Soil 1 | Soil 2 | ||||||
| 1 M NH4NO3 | H2O | 0.05 M (NH4)2SO4 (F1 in SE) | 1 M NH4NO3 | H2O | 0.05 M (NH4)2SO4 (F1 in SE) | ||
| Oven | 7 d | 5 | 14 | 7 | 30 | 19 | 16 |
| 21 d | 34 | 26 | 21 | 15 | 21 | 10 | |
| Air | 7 d | 41 | 36 | 27 | 30 | 9 | 23 |
| 21 d | 41 | 41 | 22 | 37 | 18 | 23 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karczewska, A.; Dradrach, A.; Gałka, B.; Szopka, K. Does Soil Drying in a Lab Affect Arsenic Speciation in Strongly Contaminated Soils? Minerals 2022, 12, 223. https://doi.org/10.3390/min12020223
Karczewska A, Dradrach A, Gałka B, Szopka K. Does Soil Drying in a Lab Affect Arsenic Speciation in Strongly Contaminated Soils? Minerals. 2022; 12(2):223. https://doi.org/10.3390/min12020223
Chicago/Turabian StyleKarczewska, Anna, Agnieszka Dradrach, Bernard Gałka, and Katarzyna Szopka. 2022. "Does Soil Drying in a Lab Affect Arsenic Speciation in Strongly Contaminated Soils?" Minerals 12, no. 2: 223. https://doi.org/10.3390/min12020223







