Effects of Residual Xanthate on Flotation Efficiency of a Cu-Zn Sulfide Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Tests
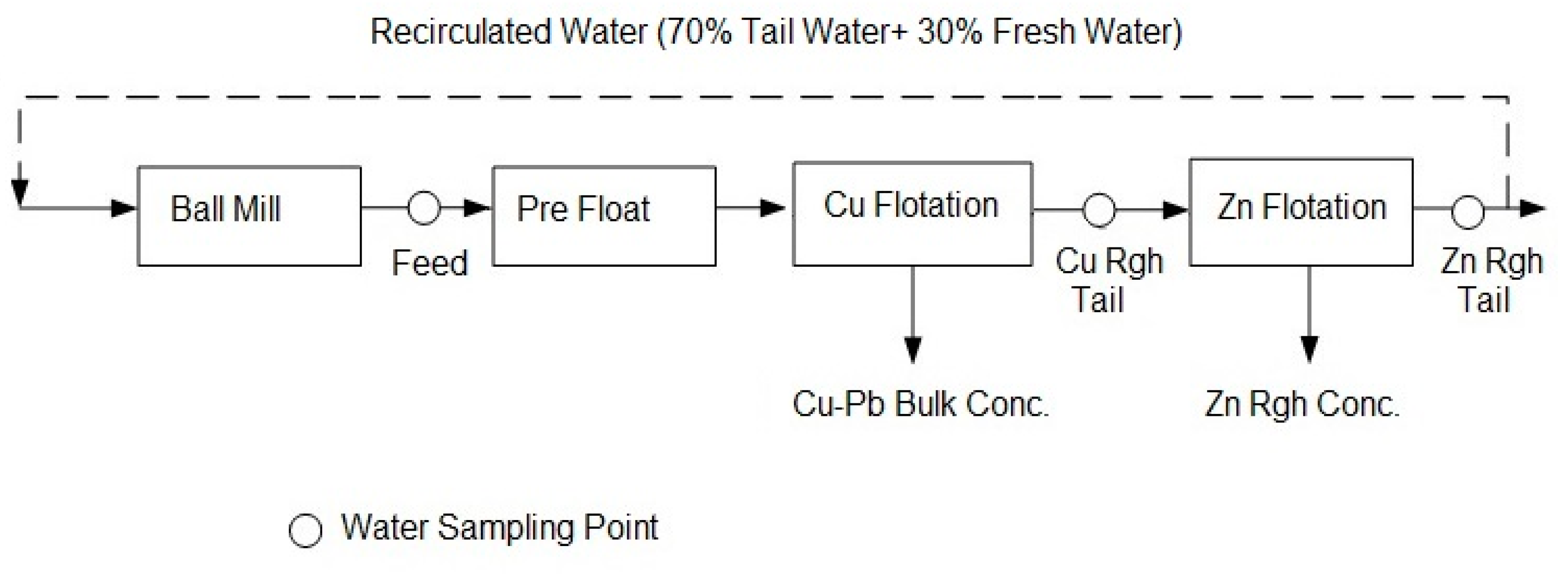
2.3. Flotation Tests
3. Results and Discussions
3.1. Adsorption Studies
3.1.1. Selection of Activated Carbon Sample
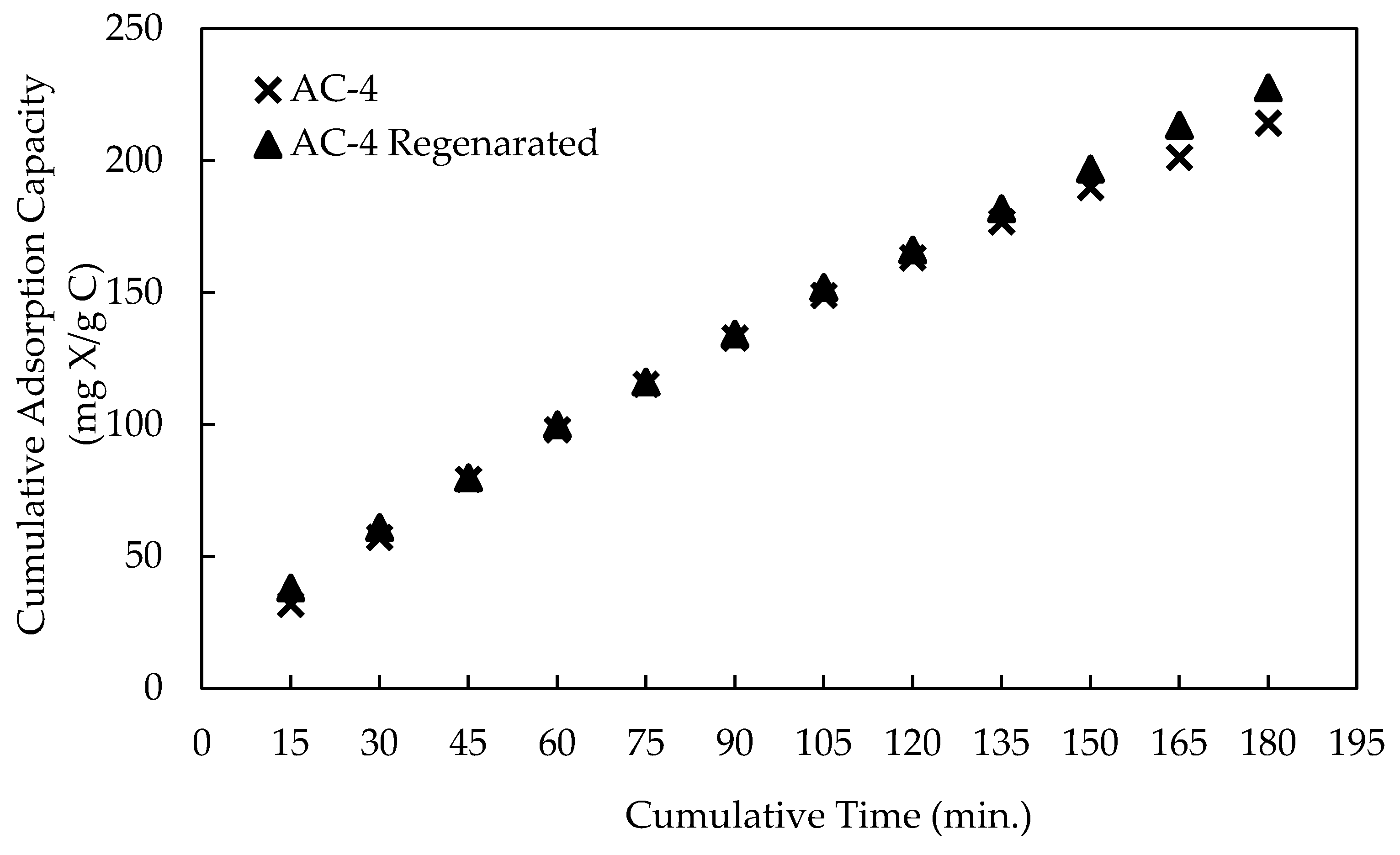
3.1.2. Regeneration of Activated Carbon
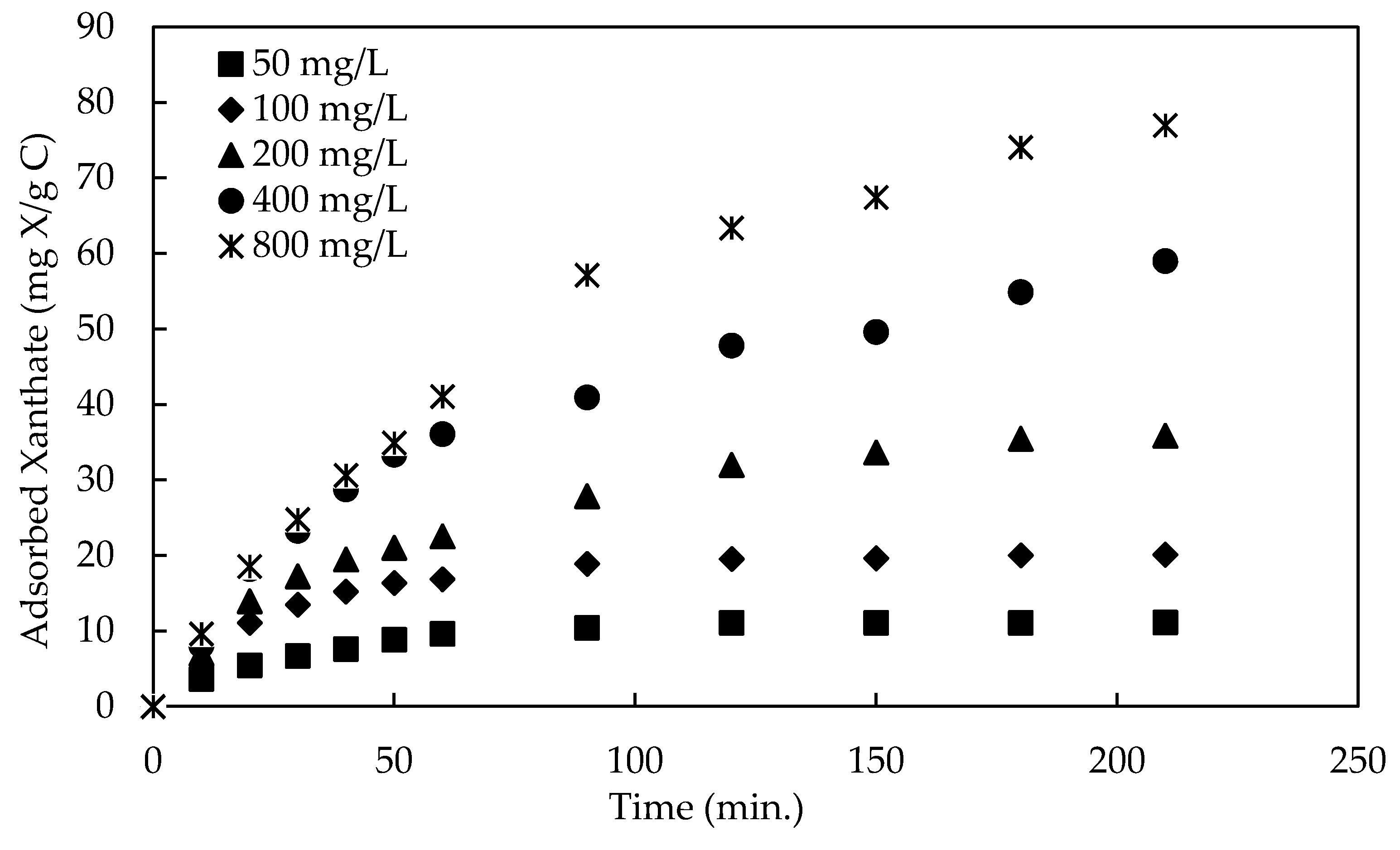
3.1.3. Effect of Initial Concentration and Contact Time
3.1.4. Adsorption Isotherm
3.2. Flotation Studies
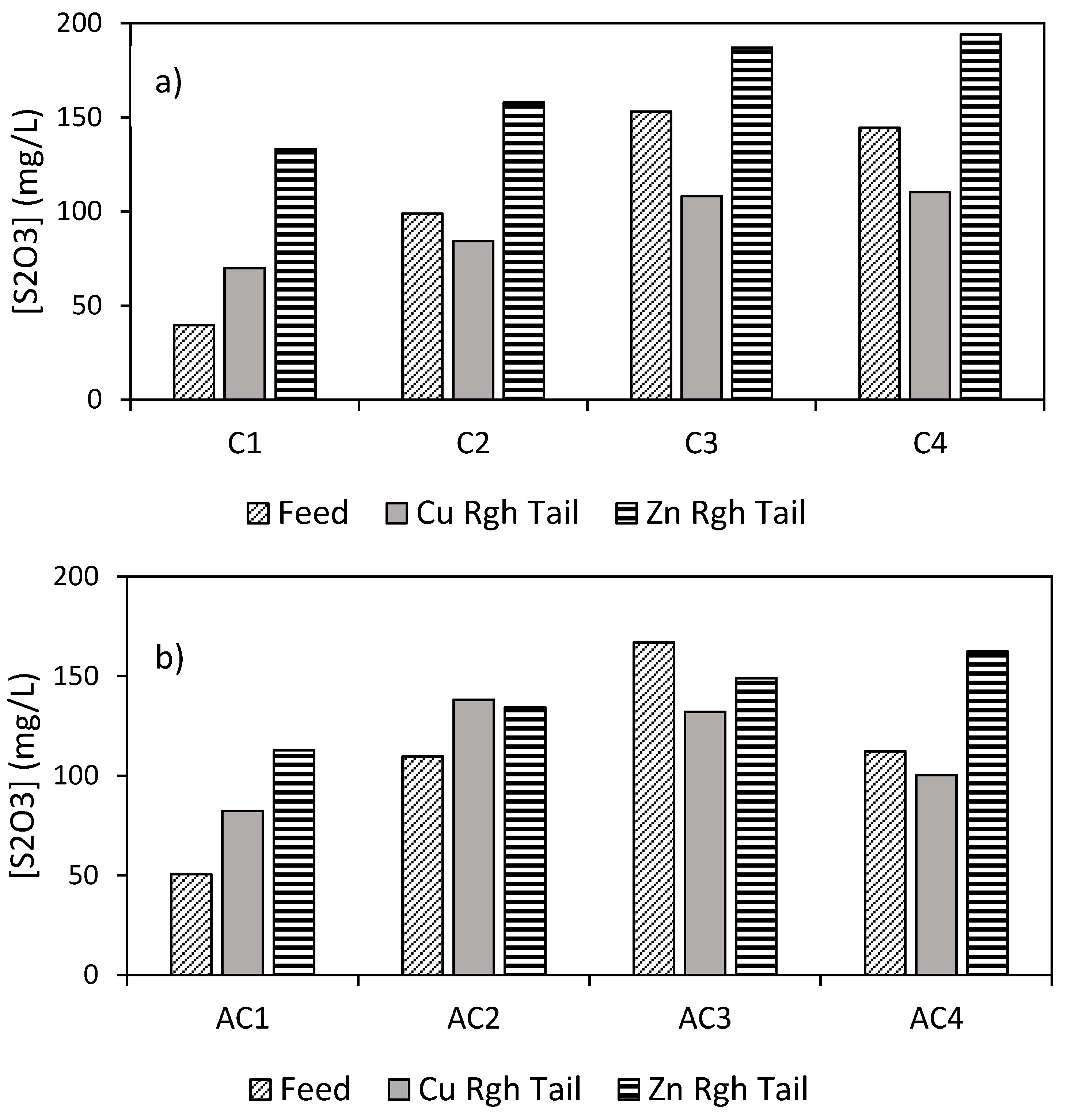
3.2.1. Water Chemistry
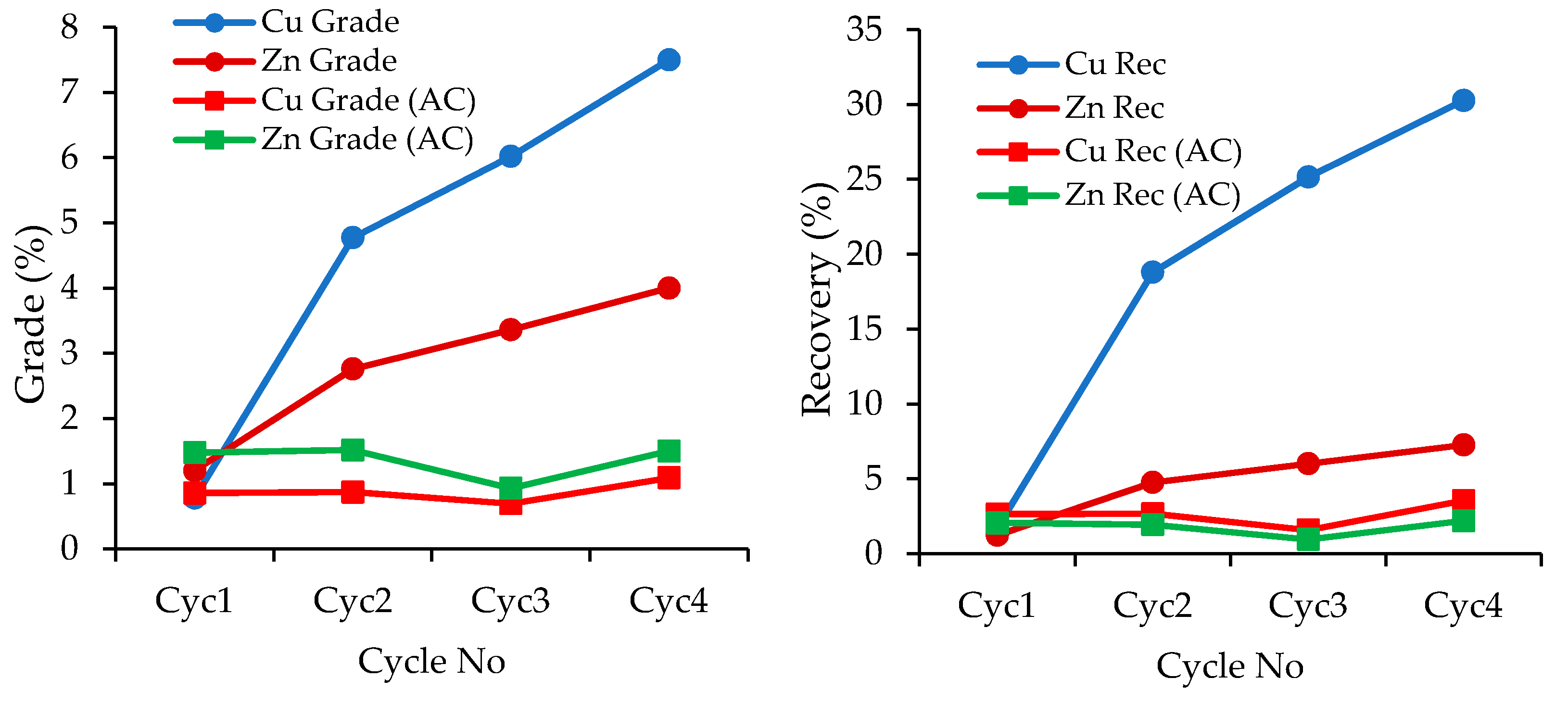
3.2.2. Pre-Flotation Section
3.2.3. Cu Rougher Flotation Section
4. Conclusions
- Naturally floatable gangue minerals (mainly talc) were removed by applying the pre-flotation stage before the copper rougher flotation stage.
- Water recirculation without treatment caused significant copper loss to the pre-flotation concentrate.
- Granular activated carbon (AC-4) was used to remove the residual xanthate in Zn rougher tail water. The adsorption data were found to follow the Langmuir adsorption isotherm model with the maximum monolayer adsorption capacity of 86.2 mg/g.
- The activated carbon could easily be regenerated by heating at 550 °C.
- Ore dissolution during grinding and flotation was found to be the most important mechanism affecting process water chemistry.
- Water treatment by AC did not significantly influence the concentration of sulfate, thiosulfate, and calcium ions.
- Flotation tests with the recirculation of treated water showed that loss of copper and zinc to the pre-float section was prevented and the flotation efficiency was restored after treatment.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levay, G.; Schumann, R. A System Approach to Water Quality Management in the Minerals Processing Industry, Water in Mining; The Australian Institute of Mining and Metallurgy: Brisbane, Australia, 2006; pp. 277–287. [Google Scholar]
- Luukkanen, S.; Parvinen, P.; Miettinen, M.; Stén, P.; Lähteenmäki, S.; Tuikka, A. Monitoring the composition of water of flotation slurries with an on-line analyser. Miner. Eng. 2003, 16, 1075–1079. [Google Scholar] [CrossRef]
- Stén, P.; Parvinen, P.; Miettinen, M.; Luukkanen, S.; Kaskiniemi, V.; Aaltonen, J. On-line analysis of flotation process waters at Siilinjärvi (Finland) apatite concentrating plant. Min. Eng. 2003, 16, 229–236. [Google Scholar] [CrossRef]
- Broman, P.G. Water reuse at sulfide ore concentrators in Sweden: Practice, experience and current development. In Complex Sulphide Ores; Jones, M.J., Ed.; The Institution of Mining and Metallurgy: London, UK, 1980; pp. 28–39. [Google Scholar]
- Hoover, M.R. Water chemistry effects in the flotation of sulphide ores—A review and discussion for molybdenite. In Complex Sulphide Ores; Jones, M.J., Ed.; Institution of Mining and Metallurgy: London, UK, 1980; pp. 100–112. [Google Scholar]
- Levay, G.; Smart, R.S.C.; Skinner, W.M. The impact of water quality on flotation performance. J. S. Afr. Inst. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Malysiak, V.; Shackleton, N.J.; de Vaux, D. Effect of water quality on pentlandite–pyroxene floatability with emphasis on calcium ions. In Proceedings of the 22nd International Mineral Processing Congress, Cape Town, South Africa, 28 September–3 October 2003. [Google Scholar]
- Rao, S.R.; Finch, J.A. A review of water reuse in flotation. Min. Eng. 1989, 2, 65–85. [Google Scholar] [CrossRef]
- Slatter, K.A.; Plint, N.D.; Cole, M.; Dilsook, V.; De Vaux, D.; Palm, N.; Oostendorp, B. Water management in Anglo Platinum process operations: Effects of water quality on process operations. In Proceedngs of the Abstracts of International Mine Water Conference, Pretoria, South Africa, 19–23 October 2009; pp. 46–55. [Google Scholar]
- Lam, K.S. Biodegradation of Xanthate by Microbes Isolated from a Tailings Lagoon and a Potential Role for Biofilm and Plant/Microbe Associations. Ph.D. Thesis, Western Sydney University, Penrith, Australia, 1999. [Google Scholar]
- Muzinda, I.; Schreithofer, N. Water quality effects on flotation: Impacts and control of residual xanthates. Min. Eng. 2018, 125, 34–41. [Google Scholar] [CrossRef]
- Shen, Y.; Nagaraj, D.R.; Farinato, R.; Somasundaran, P. Study of xanthate decomposition in aqueous solutions. Min. Eng. 2016, 93, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Sun, W.; Ouyang, K.; Zhang, L.; Hu, Y. Decomposition of sodium butyl xanthate (SBX) in aqueous solution by means of OCF: Ozonator combined with flotator. Min. Eng. 2015, 70, 222–227. [Google Scholar] [CrossRef]
- Fu, P.; Wang, L.; Ma, Y.; Hou, Z. A comparative study on the degradation of ethyl xanthate collector by O3, UV254nm, UV185+254nm, O3/UV254nm and O3/UV185+254nm processes. J. Environ. Chem. Eng. 2020, 8, 103628. [Google Scholar] [CrossRef]
- García-Leiva, B.; Teixeira, L.A.C.; Torem, M.L. Degradation of xanthate in waters by hydrogen peroxide, fenton and simulated solar photo-fenton processes. J. Mater. Res. Technol. 2019, 8, 5698–5706. [Google Scholar] [CrossRef]
- Chen, S.; Gong, W.; Mei, G.; Zhou, Q.; Bai, C.; Xu, N. Primary biodegradation of sulfide mineral flotation collectors. Min. Eng. 2011, 24, 953–955. [Google Scholar] [CrossRef]
- Amrollahi, A.; Massinaei, M.; Moghaddam, A.Z. Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles. Min. Eng. 2019, 134, 142–155. [Google Scholar] [CrossRef]
- Rezaei, R.; Massinaei, M.; Moghaddam, A.Z. Removal of the residual xanthate from flotation plant tailings using modified bentonite. Min. Eng. 2018, 119, 1–10. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Rena, S.; Luo, W. Removal of ethyl, isobutyl, and isoamyl xanthates using cationic gemini surfactant-modified montmorillonites. Colloids Surf. A 2019, 580, 123723. [Google Scholar] [CrossRef]
- Dong, Y.B.; Lin, H.; Liu, Q.L.; Huo, H.X. Treatment of flotation wastewater using biological activated carbon. J. Cent. South Univ. 2014, 21, 3580–3587. [Google Scholar] [CrossRef]
- Morris, G.E.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc–water interface adsorption isotherm, microflotation and electrokinetic studies. Int. J. Miner. Process. 2002, 67, 211–227. [Google Scholar] [CrossRef]
- Wang, J.; Somasundaran, P.; Nagaraj, D.R. Adsorption mechanism of guar gum at solid–liquid interfaces. Min. Eng. 2005, 18, 77–81. [Google Scholar] [CrossRef]
- Ozturk, Y. Development of Wastewater Treatment Methods for Flotation Plants. Ph.D. Thesis, Graduate School of Science and Engineering, Hacettepe University, Ankara, Turkey, 2008. [Google Scholar]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.H.; Chen, J.; Lin, J.H. Preparation of pore-size tunable activated carbon derived from waste coffee grounds for high adsorption capacities of organic dyes. J. Environ. Chem. Eng. 2020, 8, 103929. [Google Scholar] [CrossRef]
- Oguz, E. Adsorption characteristics and the kinetics of the Cr(VI) on the Thuja oriantalis. Colloid Surf. 2005, 252, 121–128. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Tumsek, F.; Karabacakoğlu, B. Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Rao, S.R. Resource recovery and recycling from metallurgical wastes. In Waste Management; Waste Management Series 7; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2006; pp. 473–475. ISBN -13 9780080463209. [Google Scholar]
- Öztürk, Y.; Ekmekçi, Z. Removal of sulfate ions from process water by ion exchange resins. Min. Eng. 2020, 159, 106613. [Google Scholar] [CrossRef]
- Ikumapayi, F.; Makıtalo, M.; Johansson, B.; Rao, K.H. Recycling of process water in sulphide flotation: Effect of calcium and sulphate ions on flotation of galena. Min. Eng. 2012, 39, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Kuyucak, N.; Yaschyshyn, D. Managing Thiosalts in mill effluents, studies conducted at the kidd metallurgical. In Proceedings of the Mining and the Environment IV Conference, Sudbury, ON, Canada, 19–27 October 2007. [Google Scholar]
- Liu, W.; Moran, C.J.; Vink, S. A review of the effect of water quality on flotation. Min. Eng. 2013, 53, 91–100. [Google Scholar] [CrossRef]
- Salarirad, M.M.; Behnamfard, A.; Veglio, F. Removal of xanthate from aqueous solutions by adsorption onto untreated and acid/base treated activated carbons. Desalin. Water Treat. 2021, 212, 220–233. [Google Scholar] [CrossRef]




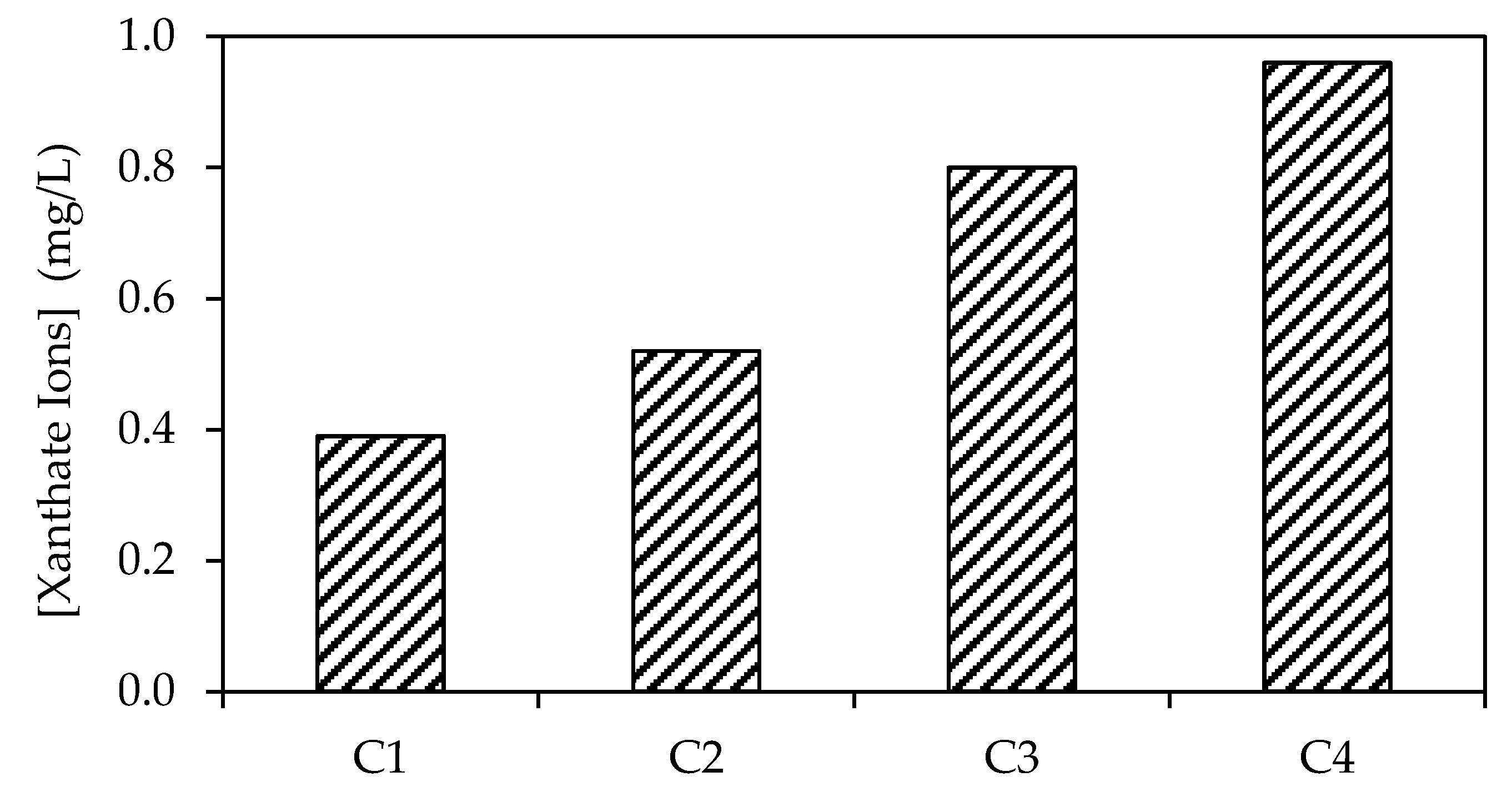

| Surface Area | Pore Volume | Pore Size | |
|---|---|---|---|
| m2/g | cc/g | Å | |
| AC-1 | 1192 | 0.49 | 5.83 |
| AC-2 | 1019 | 0.40 | 5.38 |
| AC-3 | 906 | 0.36 | 5.36 |
| AC-4 | 1699 | 0.68 | 5.43 |
| Rougher Flotation Conditions | |
|---|---|
| Grinding | 3 kg/t MBS, 0.5 kg/t Na2S and 1 kg/t ZnSO4; P80 = 45 µm., (35 min of grind.) |
| Pulp Density | %33 w/w |
| Pre-float | Frother: 20 g/t MIBC (5 min) |
| Cu Rougher Flotation | Collector: 80 g/t Na-AeroFloat, flotation time: 12 min. |
| Zn Rougher Flotation | pH 11 (lime), activator: 350 g/t CuSO4, collector: 30 g/t SIPX, flotation time: 12 min. |
| Chloride | Nitrate | Sulfate | Thiosulfate | Calcium | Magnesium |
|---|---|---|---|---|---|
| 15.9 | 8.8 | 20.9 | n.a | 30.5 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozturk, Y.; Bicak, O.; Ekmekci, Z. Effects of Residual Xanthate on Flotation Efficiency of a Cu-Zn Sulfide Ore. Minerals 2022, 12, 279. https://doi.org/10.3390/min12030279
Ozturk Y, Bicak O, Ekmekci Z. Effects of Residual Xanthate on Flotation Efficiency of a Cu-Zn Sulfide Ore. Minerals. 2022; 12(3):279. https://doi.org/10.3390/min12030279
Chicago/Turabian StyleOzturk, Yasemin, Ozlem Bicak, and Zafir Ekmekci. 2022. "Effects of Residual Xanthate on Flotation Efficiency of a Cu-Zn Sulfide Ore" Minerals 12, no. 3: 279. https://doi.org/10.3390/min12030279





