The Passa Três Granite Intrusion-Related/Hosted Neoproterozoic Gold Deposit (Paraná State, Brazil): Mineralogical, Geochemical, Fluid Inclusion and Sulphur Isotope Constraints
Abstract
:1. Introduction
2. Geological Setting
2.1. Regional Geology
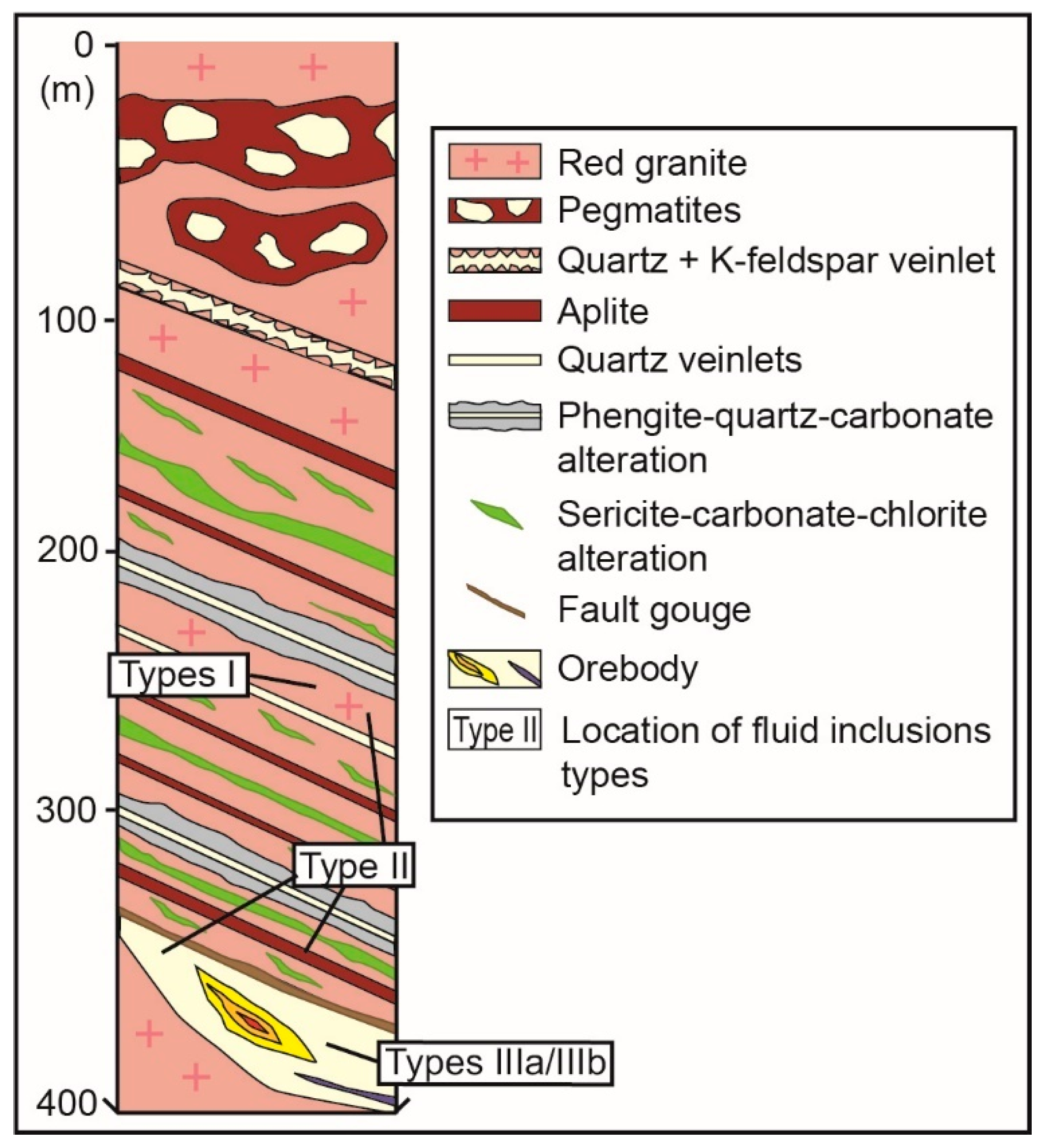
2.2. Local Geology
3. Materials and Methods
4. Results
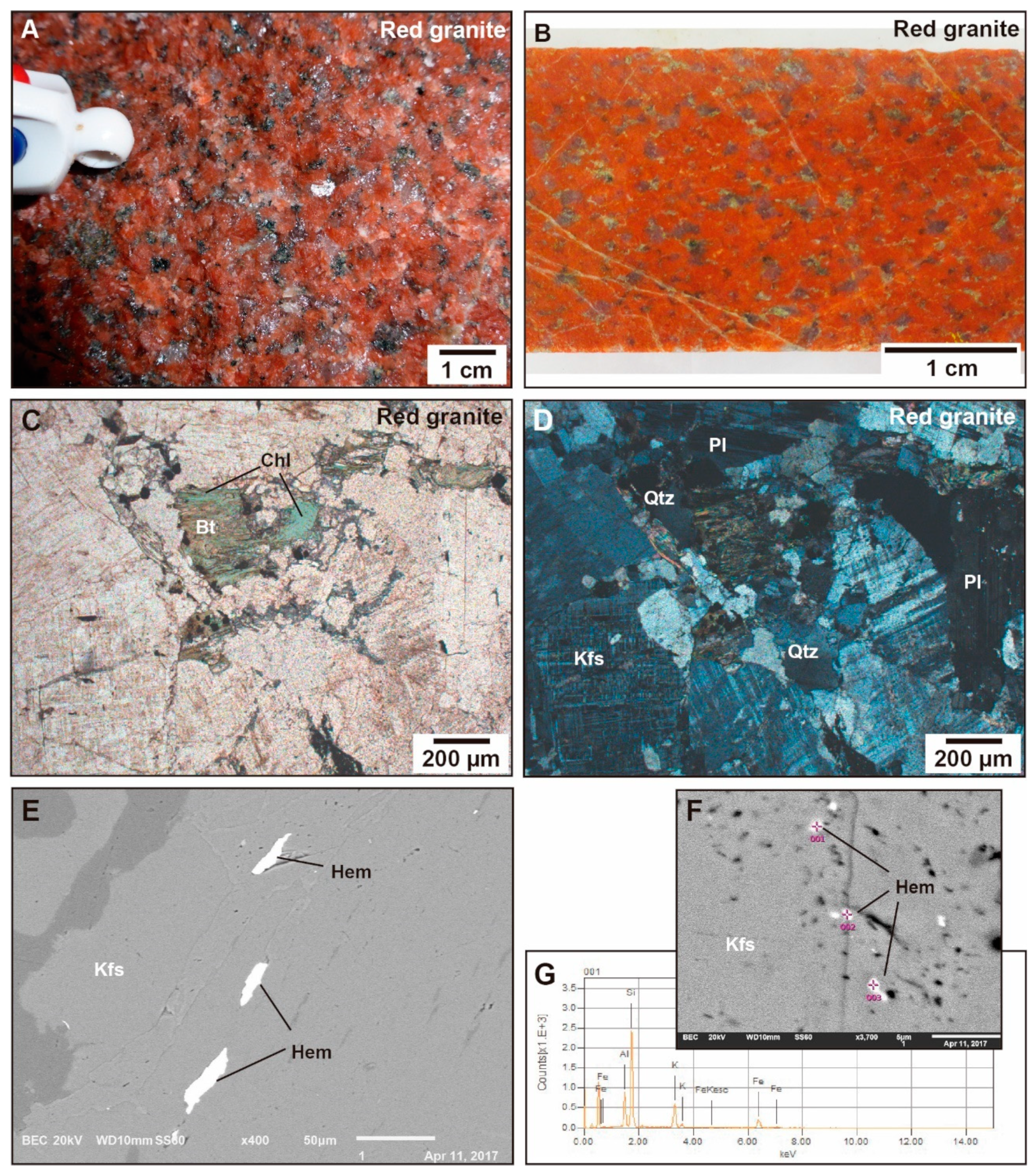
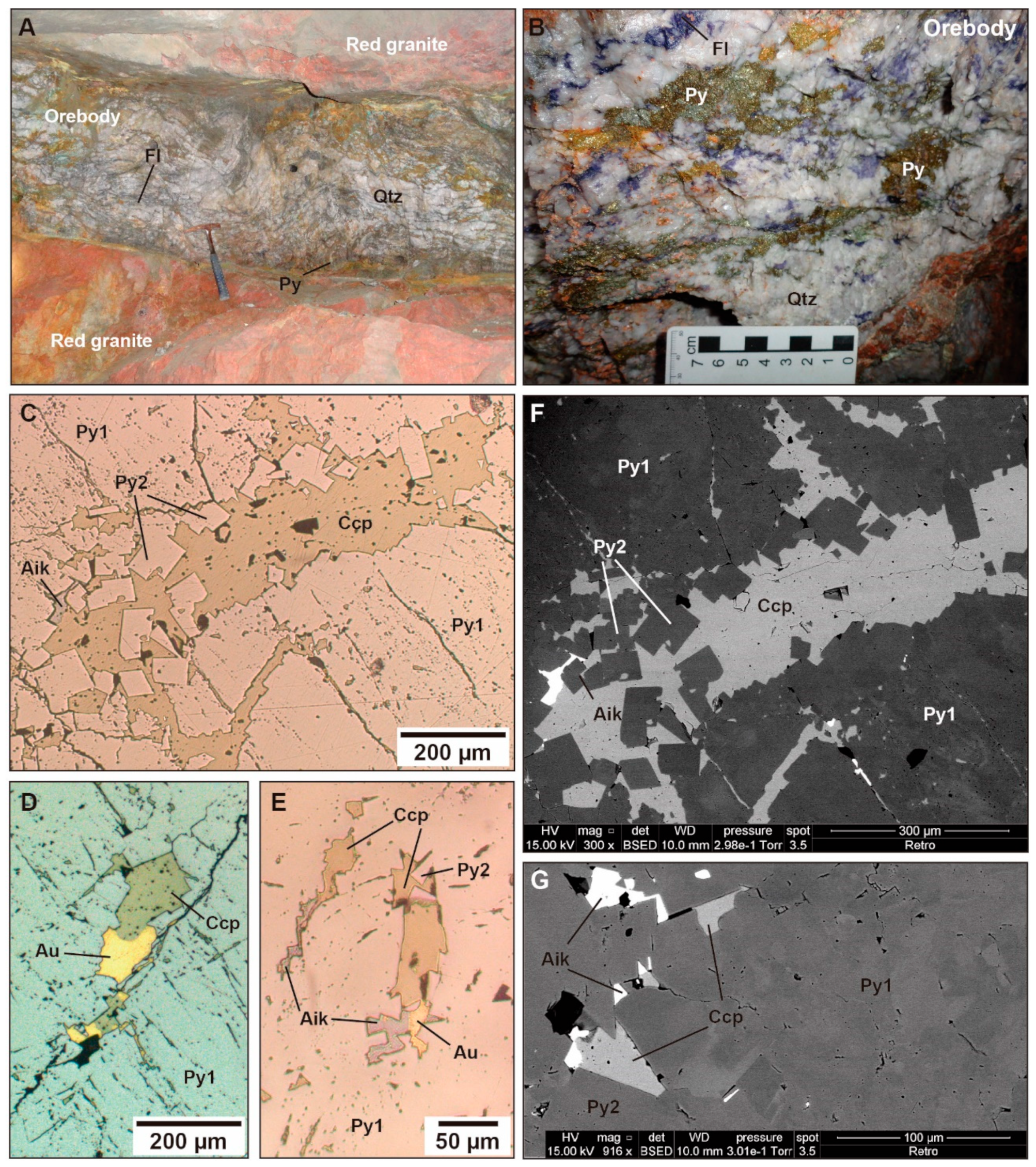
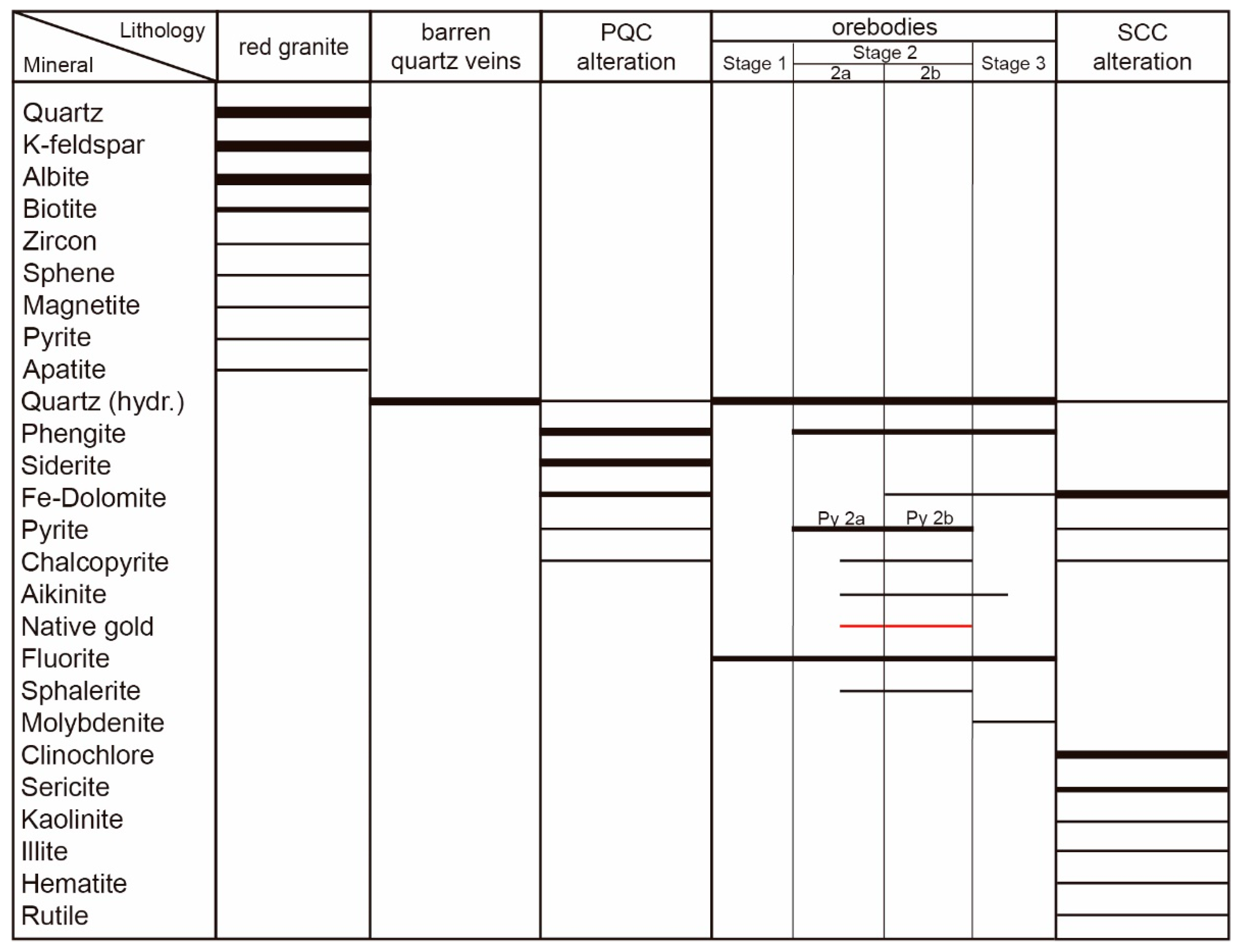
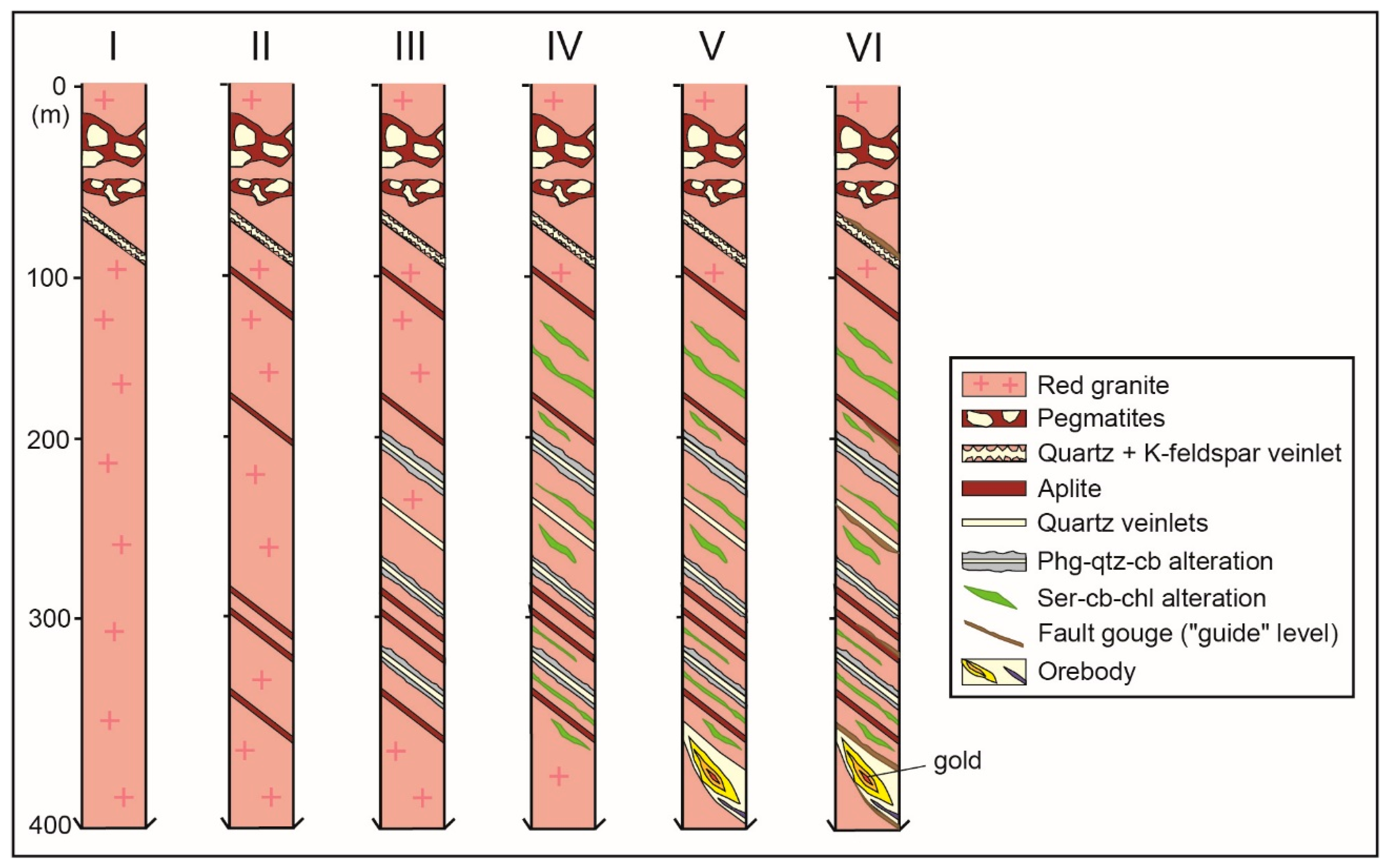
4.1. Petrology, Texture, and Mineralogy
4.1.1. Red Granite
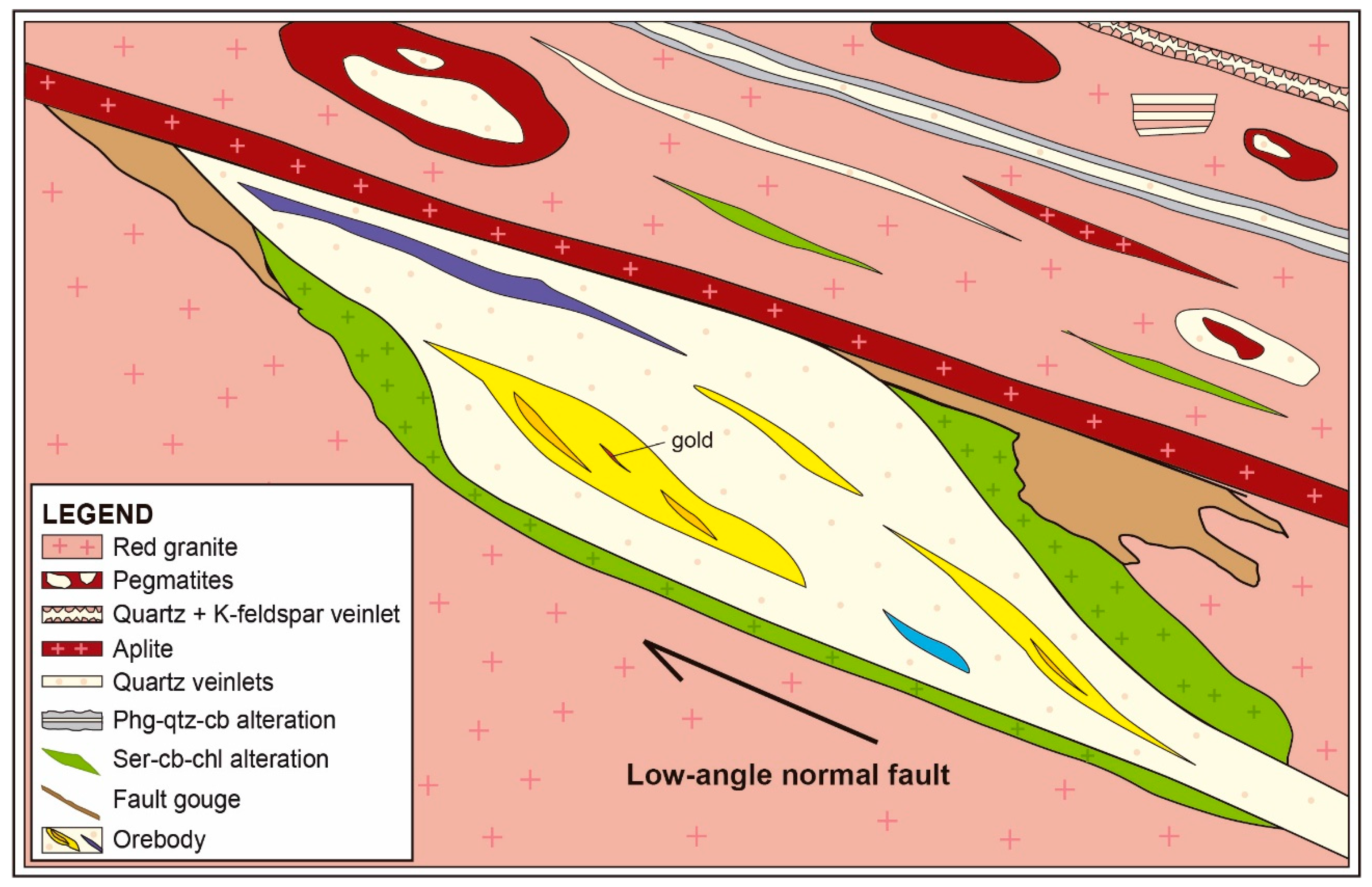
4.1.2. Gold Mineralisation
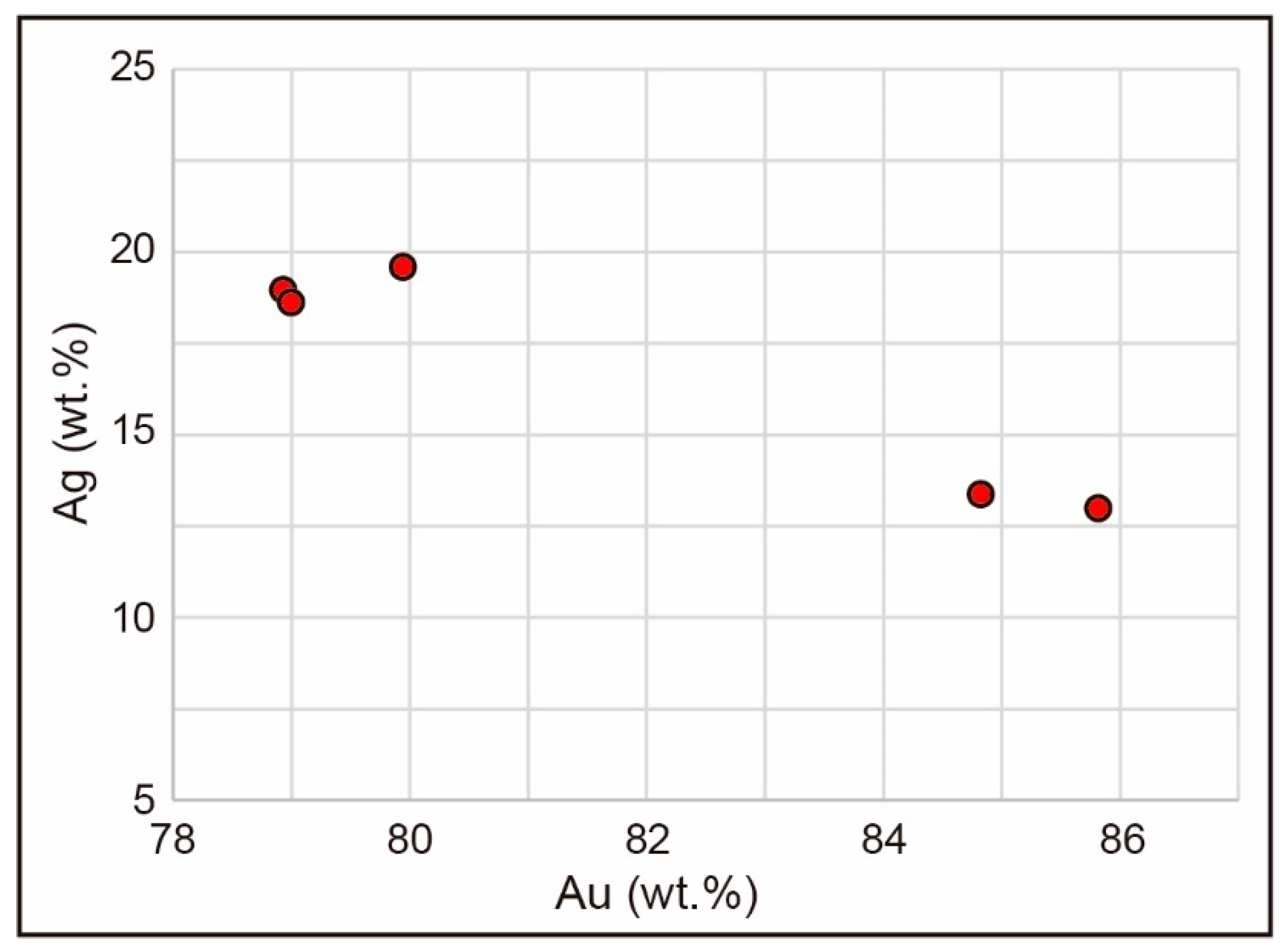
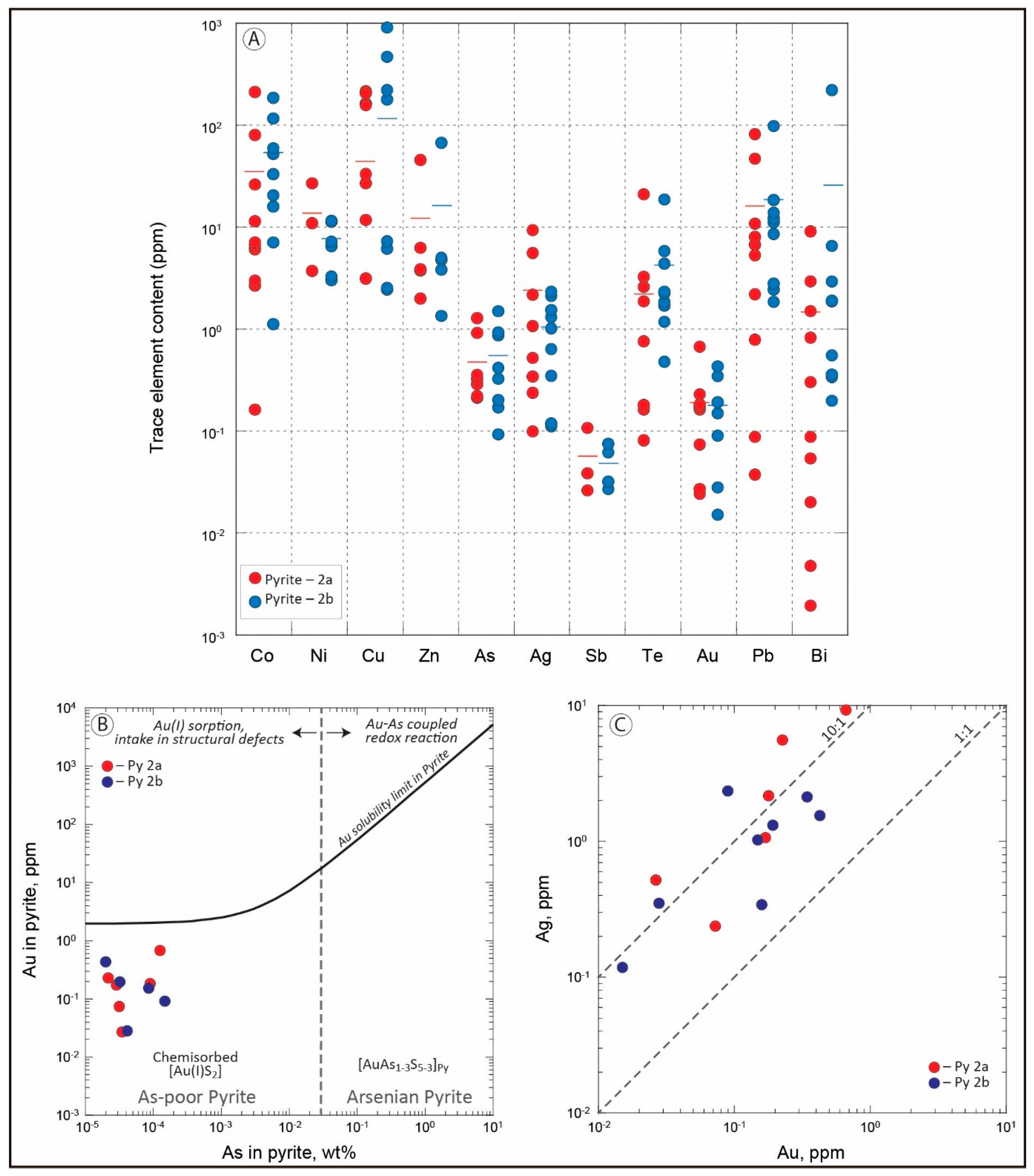
4.1.3. Chemistry of Ore Minerals (EPMA and LA-ICP-MS Analyses)
4.2. Alteration Styles
4.2.1. Phengite–Quartz–Carbonate (PQC) Alteration
4.2.2. Sericite–Carbonate–Chlorite (SCC) Alteration
4.2.3. Fault Gouge
4.3. Mineral Chemistry
4.3.1. Chlorite
4.3.2. Biotite
4.3.3. Carbonate
4.3.4. Feldspars
4.3.5. White Mica (Phengite)
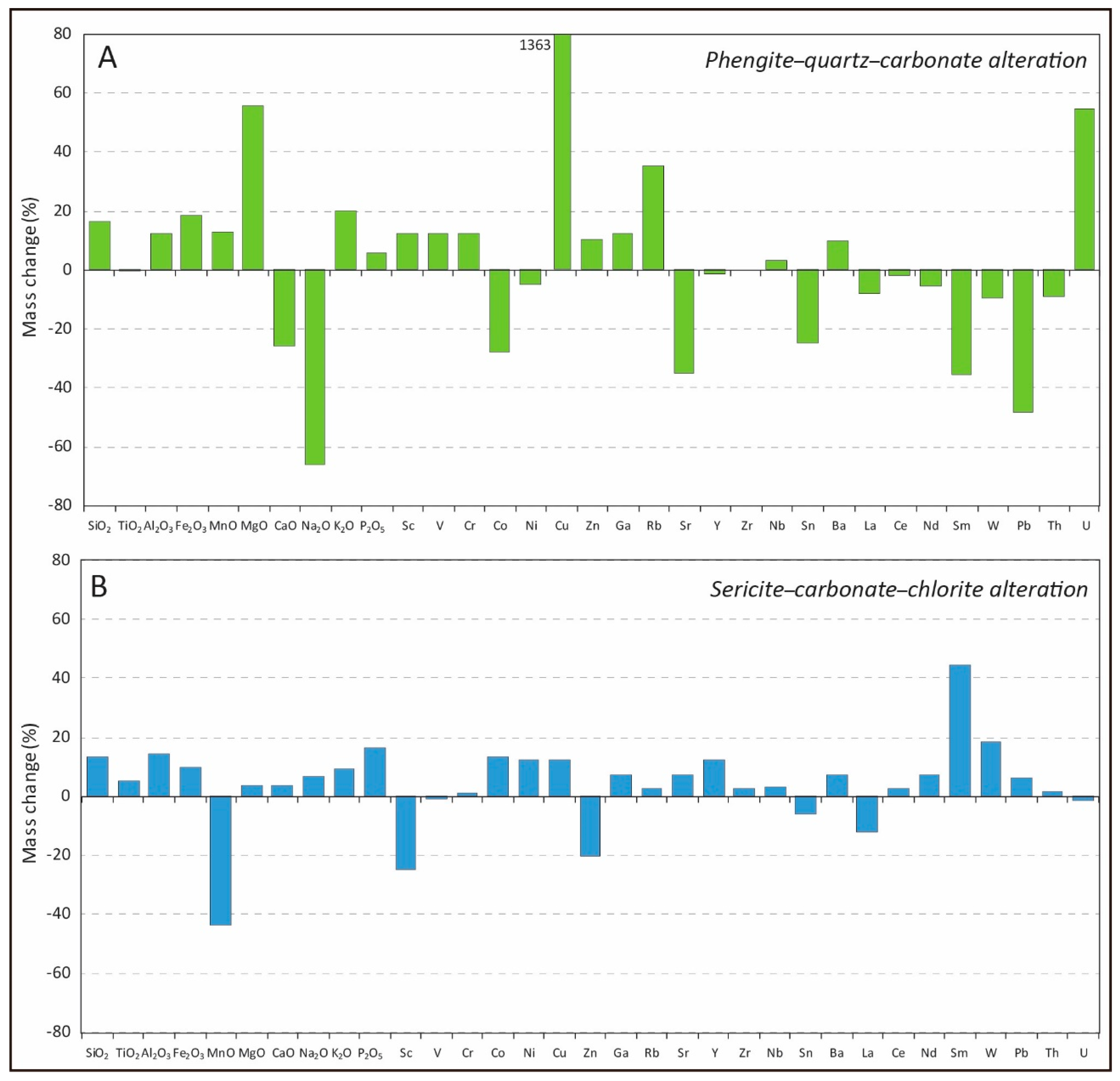
4.4. Mass Balance of Hydrothermal Alterations
4.4.1. Phengite–Quartz–Carbonate Alteration
4.4.2. Sericite–Carbonate–Chlorite Alteration
4.5. Sulphur Isotopes
4.6. Fluid Inclusions
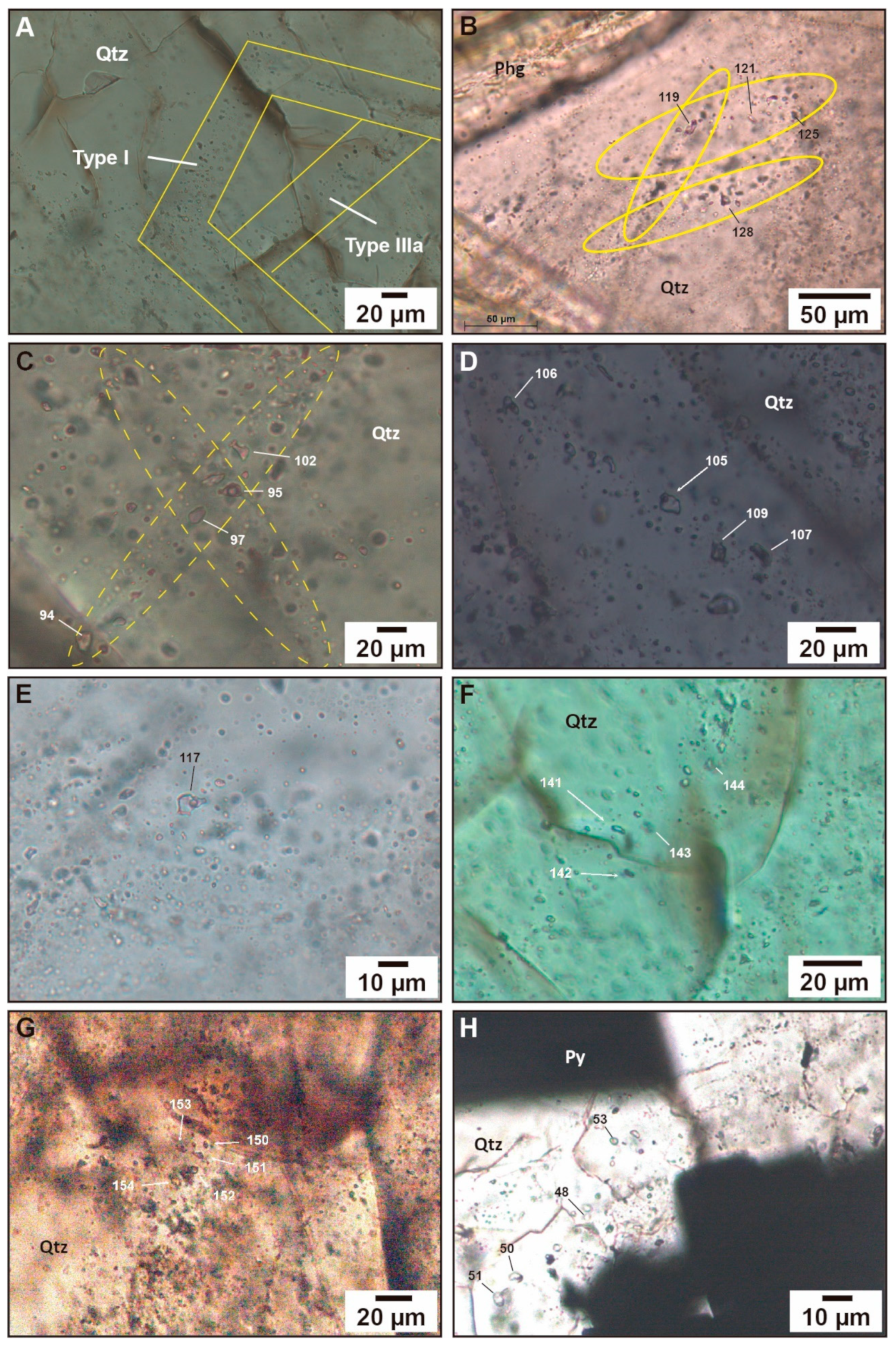
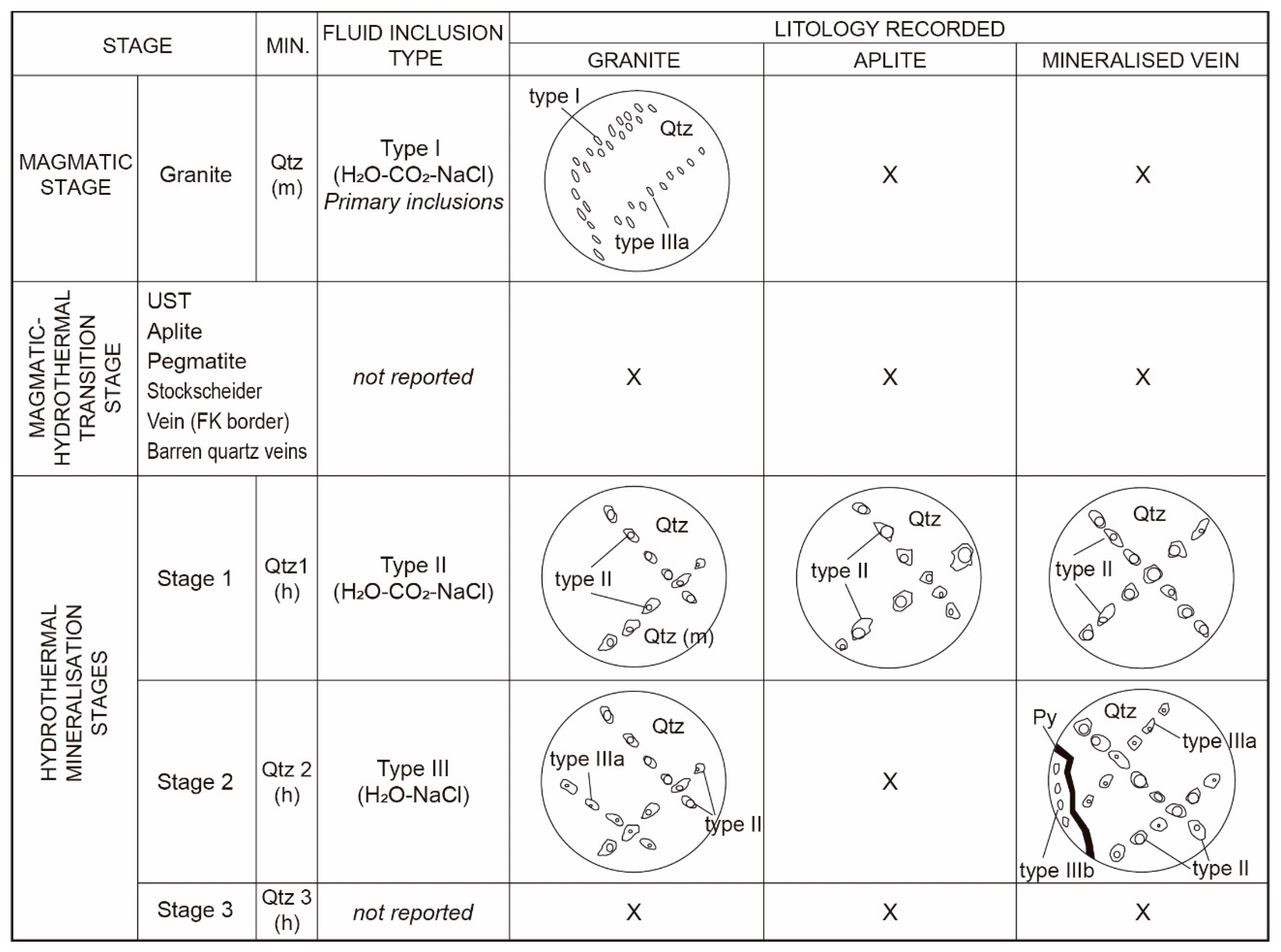
4.6.1. Fluid Inclusions Petrography
- (i)
- Type I (H2O-CO2-NaCl) fluid inclusions are dominantly subhedral, isolated, and elongated in the direction of growth, demonstrating that they are primary in origin (Figure 15A). They are two-phase, liquid–vapor (liquid H2O + vapor CO2), ranging in size from 2 to 6 µm. They were observed only in the granite sample;
- (ii)
- Type II (H2O-CO2-NaCl) fluid inclusions contain one vapor phase (vapor CO2) and one liquid phase (liquid H2O) or, possibly, two liquid phases (liquid H2O + liquid CO2). They vary in shape, from rounded, oval, triangular, to irregular, ranging in size from 3 to 18 µm and having variable vapor/liquid ratios (Figure 15B–E). They can be liquid-rich or vapor-rich as a function of their vapor/liquid proportion. Type II fluid inclusions are secondary in origin and are observed in the granite, aplite, and mineralised veins samples;
- (iii)
- Type IIIa (H2O-NaCl) fluid inclusions are rounded, occur as FIA in secondary trails, are 4–9 µm in size, and are two-phase (vapor H2O + liquid H2O; Figure 15A,F,G), while type IIIb (H2O-NaCl) inclusions are monophase (liquid H2O), 4–7 µm in size, and occur only as secondary trails in quartz 3 (Figure 15H) in association with gold-bearing minerals (e.g., pyrite 2b; [10]).
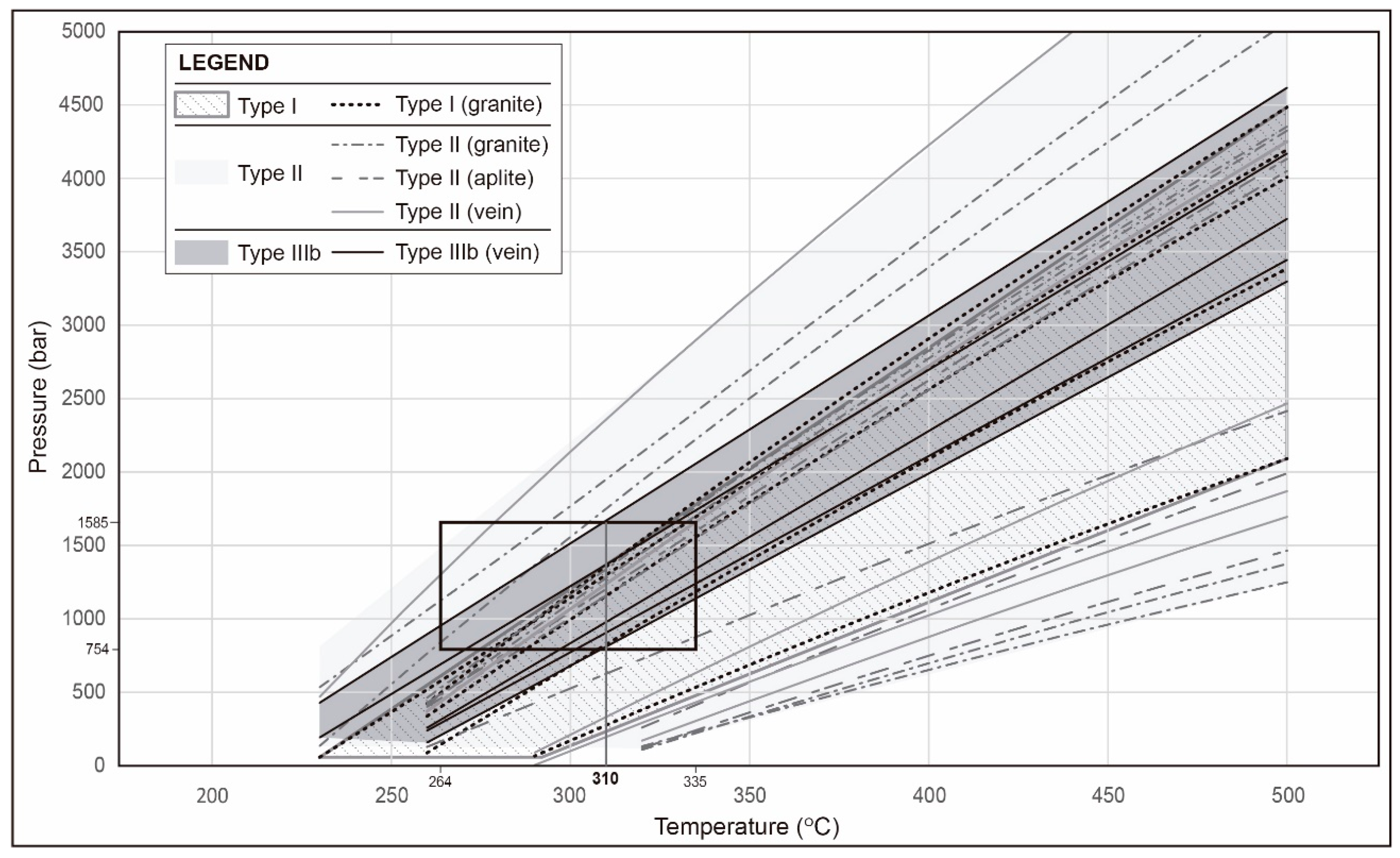
4.6.2. Microthermometry
4.6.3. Laser Raman Spectroscopy
5. Discussion
5.1. Mineralisation and Alteration Paragenetic Sequence at the Passa Três Deposit
Andesine → sericite → quartz
5.2. Ore Fluid Composition
5.3. Sources of Ore-Forming Fluids
5.4. Ore-Forming Processes
6. Conclusions
- In the Passa Três granite, there are two alteration events that affected the red granite: (i) A phengite–quartz–carbonate alteration, related to barren quartz veins (during magmatic–hydrothermal transition); and (ii) a sericite–carbonate–chlorite alteration, weakly developed but associated with the main ore-forming stage (hydrothermal stage).
- The δ34S values of pyrite from the Passa Três deposit are indicative of the magmatic origin of the sulphur in the system.
- Petrographic observations and microthermometric data show that fluid inclusions in the Passa Três deposit have a H2O-CO2-NaCl composition and were trapped at moderate to high temperatures (400 to 100 °C) and have low to moderate salinities (0.04 to 12.84 wt % NaCl eq.).
- Mineralising fluid is derived, at least in part, from magmatic fluid during the late magmatic stage (aplites, pegmatites, UST, stockscheider textures) that evolved to early hydrothermal fluids (early hydrothermal veins with K-feldspar border and barren quartz veins) and, finally, formed the mineralised quartz veins. Processes such as fluid decompression, immiscibility, and cooling are also considered as important for ore deposition, in addition to the structural setting that dominantly controlled the deposit formation.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, T.; Lang, J. Fluid inclusion characteristics of intrusion-related gold mineralization, Tombstone—Tungsten magmatic belt, Yukon Territory, Canada. Miner. Depos. 2001, 36, 563–582. [Google Scholar] [CrossRef]
- Cepedal, A.; Fuertes-Fuente, M.; Martín-Izard, A.; García-Neto, J.; Boirin, M.C. An intrusion-related gold deposit (IRGD) in the NW of Spain, the Linares deposit: Igneous rocks, veins and related alterations, ore features and fluids involved. J. Geochem. Explor. 2013, 124, 101–126. [Google Scholar] [CrossRef]
- Assunção, R.F.S.; Klein, E.L. The Moreira Gomes deposit of the Caiú-Caiú goldfield: Fluid inclusions and stable isotope constraints and implications for the genesis of granite-hosted gold mineralization in the Tapajós Gold Province, Brazil. J. S. Am. Earth Sci. 2014, 49, 85–105. [Google Scholar] [CrossRef]
- Gloaguen, E.; Branquet, Y.; Chauvet, A.; Bouchot, V.; Barbanson, L.; Vigneresse, J.J. Tracing the magmatic/hydrothermal transition in regional low-strain zones: The role of magma dynamics in strain localization at pluton roof, implications for intrusion-related gold deposits. J. Struct. Geol. 2014, 58, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Marcoux, E.; Nerci, K.; Branquet, Y.; Ramboz, C.; Ruffet, G.; Peucat, J.J.; Stevenson, R.; Jébrak, M. Late-Hercynian intrusion-related gold deposits: An integrated model on the Tighza polymetallic district, central Morocco. J. Afr. Earth Sci. 2015, 107, 65–88. [Google Scholar] [CrossRef] [Green Version]
- Hart, C.; Goldfarb, R. Distinguishing Intrusion-Related from Orogenic Gold Systems. In Proceedings of the Scientific Conference on Minerals, Auckland, New Zealand, 13–16 November 2005; Available online: https://www.researchgate.net/publication/228762286_Distinguishing_intrusion-related_from_orogenic_gold_systems (accessed on 1 January 2015).
- Thompson, J.F.H.; Sillitoe, R.H.; Baker, T.; Lang, J.R.; Mortensen, J.K. Intrusion-related gold deposits associated with tungsten-tin provinces. Miner. Depos. 1999, 34, 323–334. [Google Scholar] [CrossRef]
- Lang, J.R.; Baker, T. Intrusion-related gold systems: The present level of understanding. Miner. Depos. 2001, 36, 477–489. [Google Scholar] [CrossRef]
- Hart, C.J.R. Reduced intrusion-related gold systems. In Mineral Deposits of Canada: A Synthesis of Major Deposit Types, District Metallogeny, the Evolution of Geological Provinces, and Exploration Methods; Goodfellow, W.D., Ed.; Special Publication n. 5; Geological Association of Canada: St. John, NL, Canada, 2007; pp. 95–112. [Google Scholar]
- Dressel, B.C.; Chauvet, A.; Trzaskos, B.; Biondi, J.C.; Bruguier, O.; Monié, P.; Villanova, S.N.; Newton, J.B. The Passa Três lode gold deposit (Paraná State, Brazil): An example of structurally-controlled mineralisation formed during magmatic-hydrothermal transition and hosted within granite. Ore Geol. Rev. 2018, 102, 701–727. [Google Scholar] [CrossRef]
- Soares, P.C.; Góis, J.R. Geologia do Granito Passa Três (Paraná) e suas mineralizações auríferas. In 3rd Simpósio Sul Brasileiro de Geologia (Atas…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): Curitiba, Brazil, 1987; Volume 2, p. 497. [Google Scholar]
- Chiodi Filho, C.; Santos, J.F.; Soares, P.C.; Moretzshon, J.S. Estudo ETR para caracterização e avaliação metalogenética de granitóides no escudo paranaense. In 2nd Congresso Brasileiro de Geoquímica (Anais…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): Rio de Janeiro, Brazil, 1989; Volume 1, pp. 487–498. [Google Scholar]
- Piekarz, G.F.; Schrank, A.; Choudhuri, A.; Figueiredo, B.; Xavier, R. A porphyry-type gold deposit in the Passa Três Granite, South Brazil. In Brazil Gold 91: The Economics Geology Geochemistry and Genesis of Gold Deposit; Ladeira, E.A., Ed.; Balkema: Rotterdam, The Netherlands, 1991; pp. 541–546. [Google Scholar]
- Riedel, W. Zur Mechanick Geologischer Brucherscheinungen. Cent. Mineral. Geol. Paläontologie B 1929, 8, 354–368. [Google Scholar]
- Piekarz, G.F. O Granito Passa Três—PR e as Mineralizações Auríferas Associadas. Master’s Thesis, Universidade Estadual de Campinas (Instituto de Geociências), Campinas, Brazil, 1992; 221p. [Google Scholar]
- Picanço, J.L. Composição Isotópica e Processos Hidrotermais Associados aos Veios Auríferos do Maciço Granítico Passa Três, Campo Largo, PR. Ph.D. Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2000; 175p. [Google Scholar] [CrossRef] [Green Version]
- Dressel, B.C. Análise Estrutural, Petrológica e Metalogenética da Mineralização Aurífera Neorpoterozoica do Granito Passa Três (Campo Largo—PR)—Implicações Sobre as Relações Granito/Mineralização. Analyse Structurale, Pétrologique et Métallogénique de la Minéralisation Aurifère du Granite Passa Três (Campo Largo—PR, Sud du Brésil)—Implications sur les Relations Granite/Minéralisation. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, Université Montpellier (Géosciences Montpellier), Montpellier, France, 2018; 193p. Available online: https://hdl.handle.net/1884/59959 (accessed on 18 December 2018).
- Turini Neto, G. Estudos Geoquímicos da Mineralização Aurífera do Granito Passa Três—PR. Master’s Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2012; 47p. [Google Scholar]
- Sacoman, D. Microestruturas em Veios de Quartzo-Sulfetados Mineralizados em Ouro, Granito Passa Três, Campo Largo—Paraná. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2015; 58p. [Google Scholar]
- Pontes, J.B. Geologia e potencialidades econômicas da formação Água Clara (PR). In 32nd Congresso Brasileiro de Geologia (Anais…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): Salvador, Brazil, 1982; Volume 3, pp. 1002–1016. [Google Scholar]
- Biondi, J.C. Typological and quantitative classification of mineral deposits with gold. In Brazil Gold 91: The Economics Geology Geochemistry and Genesis of Gold Deposit; Ladeira, E.A., Ed.; Balkema: Rotterdam, The Netherlands, 1991; pp. 523–534. [Google Scholar]
- Mesquita, M.J.; Marczyski, E.S.; Vasconcelos, E.M.G.; Picanço, J.; Salamuni, E. Alteração hidrotermal precoce das mineralizações de Au tipo veio da Mina do Morro, Campo Largo, PR. In 41st Congresso Brasileiro de Geologia (Anais…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): João Pessoa, Brazil, 2002; Volume 1, p. 216. [Google Scholar]
- Heilbron, M.; Pedrosa-Soares, A.C.; Campos Neto, M.d.C.; da Silva, L.C.; Trouw, R.A.J.; Janasi, V.A. Província Mantiqueira. In Geologia do Continente Sul-Americano: Evolução da Obra de Fernando Flávio Marques de Almeida; Mantesso-Neto, V., Bartorelli, A., Carneiro, C.D.R., de Orgs Brito-Neves, B.B., Eds.; Deca: São Paulo, Brazil, 2004; pp. 203–234. [Google Scholar]
- Fiori, A.P.; Fassbinder, E.; Góis, J.R.; Fumagalli, C.E. Compartimentação tectônica do Grupo Açungui a norte de Curitiba. In 3rd Simpósio Sul Brasileiro de Geologia (Atas…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): Curitiba, Brazil, 1987; Volume 1, pp. 183–196. [Google Scholar]
- Fiori, A.P. Tectônica e estratigrafia do Grupo Açungui, PR. Bol. IG-USP Série Científica 1992, 23, 55–74. [Google Scholar] [CrossRef]
- Fiori, A.P. Evolução geológica da Bacia Açungui. Bol. Parana. Geociências 1994, 42, 7–27. [Google Scholar]
- Fassbinder, E. A Unidade Água Clara No Contexto do Grupo Açungui: Um Modelo Transpressivo de Colisão Oblíqua No Neoproterozóico Paranaense. Ph.D. Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 1996; 207p. [Google Scholar] [CrossRef] [Green Version]
- Kaulfuss, G.A. Geocronologia dos Núcleos de Embasamento Setuva, Betara e Tigre, Norte de Curitiba-PR. Master’s Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2001; 115p. [Google Scholar] [CrossRef] [Green Version]
- Cury, L.F.; Kaulfuss, G.A.; Siga, O., Jr.; Basei, M.A.S.; Harara, O.M.; Sato, K. Idades U-Pb (Zircões) de 1.75 Ga em granitóides alcalinos deformados dos núcleos Betara e Tigre: Evidências de regimes extensionais do Estateriano na Faixa Apiaí. Geol. USP Série Científica 2002, 2, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Basei, M.A.S.; Brito Neves, B.B.; Siga, O., Jr.; Babinski, M.; Pimentel, M.M.; Tassinari, C.C.G.; Hollanda, M.H.B.; Nutman, A.; Cordani, U.G. Contribution of SHRIMP U–Pb zircon geochronology to unravelling the evolution of Brazilian Neoproterozoic fold belts. Precambrian Res. 2010, 183, 112–144. [Google Scholar] [CrossRef]
- Campanha, G.A.C.; Sadowki, G.R. Tectonics of the southern portion of the Ribeira Belt (Apiaí Domain). Precambrian Res. 1999, 98, 31–51. [Google Scholar] [CrossRef]
- Siga, O., Jr.; Basei, M.A.S.; Passarelli, C.R.; Sato, K.; Cury, L.F.; McReach, I. Lower and Upper Neoproterozoic magmatic records in Itaiacoca Belt (Paraná-Brazil): Zircon ages and lithostratigraphy studies. Gondwana Res. 2009, 15, 197–208. [Google Scholar] [CrossRef]
- Fragoso Cesar, A.R.S.; Soares, P.C. As placas brasilianas do sul e sudeste da Plataforma Sul-Americana. In 4st Simpósio Nacional de Estudos Tectônicos (Atas…); Brazilian Society of Geology (Sociedade Brasileira de Geologia): Belo Horizonte, Brazil, 1993. [Google Scholar]
- Campos Neto, M.C.; Figueiredo, M.C.H. The Rio Doce Orogeny, Southeastern Brazil. J. S. Am. Earth. Sci. 1995, 8, 143–162. [Google Scholar] [CrossRef]
- Rogers, J.J.W.; Unrug, R.; Sultan, M. Tectonic assembly of Gondwana. J. Geodyn. 1995, 19, 1–34. [Google Scholar] [CrossRef]
- Gimenez Filho, A.G.; Janasi, V.A.; Campanha, G.A.C.; Teixeira, W.; Trevizoli, L.E., Jr. U-Pb dating and Rb-Sr isotope geochemistry of the Eastern portion of the Três Córregos Batolith Ribeira Fold Belt, São Paulo, Brazil. Rev. Bras. Geociências 2000, 30, 45–50. [Google Scholar] [CrossRef]
- Prazeres Filho, H.J. Litogeoquímica, Geocronologia (U-Pb) e Geologia Isotópica dos Complexos Graníticos Cunhaporanga e Três Córregos, Estado do Paraná. Master’s Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2000; 180p. [Google Scholar] [CrossRef] [Green Version]
- Janasi, V.A.; Leite, R.J.; Van Schmu, W.R. U-Pb chronostratigraphy of the granitic magmatism in the Agudos Grandes Batholith (west of São Paulo)—Implications for the evolution of the Ribeira Belt. J. S. Am. Earth. Sci. 2001, 14, 363–376. [Google Scholar] [CrossRef]
- Prazeres Filho, H.J.; Basei, M.A.S.; Passarelli, C.R.; Harara, O.M.M.; Siga, O., Jr. U-Pb Zircon ages of post-orogenetic granitic magmatism in Apiaí Folded Belt (Paraná State, Southern Brazil): Petrological and geotectonic significance. In 4st South American Symposium on Isotope Geology (Short Papers); CBPM: Salvador, Brazil, 2003. [Google Scholar]
- Prazeres Filho, H.J.; Harara, O.M.; Basei, M.A.S.; Passarelli, C.R.; Siga, O., Jr. Litogeoquímica, geocronologia U-Pb e geologia isotópica (Sr-Nd-Pb) das rochas graníticas dos batólitos Cunhaporanga e Três Córregos na porção sul do Cinturão Ribeira, Estado do Paraná. Geol. USP Série Científica 2003, 3, 51–70. [Google Scholar] [CrossRef] [Green Version]
- Fiori, A.P. Aplicação do modelo de cisalhamento simples na análise da deformação dúctil de alguns granitos paranaenses. Bol. Parana. Geociências 1985, 36, 31–40. [Google Scholar]
- Cury, L.F. Geocronologia e Litogeoquímica dos Stocks Graníticos da Porção Sudeste da Faixa Apiaí, Estado do Paraná. Master’s Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2003; 125p. [Google Scholar] [CrossRef] [Green Version]
- Faleiros, F.M. Evolução de Terrenos Tectono-Metamórficos da Serrania do Ribeira e Planalto Alto Turvo (SP, PR). Ph.D. Thesis, Universidade de São Paulo (Instituto de Geociências), São Paulo, Brazil, 2008; 318p. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulfide calibration standards for the laser ablation inductivelt-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Pirrie, D.; Butcher, A.R.; Power, M.R.; Gottlieb, P.; Miller, G.L. Rapid Quantitative Mineral and Phase Analysis Using Automated Scanning Electron Microscopy (QEMSCAN); Potential Applications in Forensic Geoscience. In Geological Society, London, Special Publication 232; Geological Society: London, UK, 2004; pp. 123–136. [Google Scholar]
- Nakano, S.; Akai, J.; Shimobayashi, N. Contrasting Fe-Ca distributions and related microtextures in syenite alkali feldspar from the Patagonian Andes, Chile. Mineral Mag. 2005, 69, 521–535. [Google Scholar] [CrossRef]
- Putnis, A.; Hinrichs, R.; Putnis, C.V.; Golla-Schindler, U.; Collins, L.G. Hematite in porous red-clouded feldpars: Evidence of large-scale crustal fluid-rock interaction. Lithos 2007, 95, 10–18. [Google Scholar] [CrossRef]
- Parsons, I.; Fitz Gerald, J.D.; Lee, M.R. Routine characterization and interpretation of complex alkali feldspar intergrowths. Am. Min. 2015, 100, 1277–1303. [Google Scholar] [CrossRef]
- Crespo, J.; Holley, E.; Pfaff, K.; Guillen, M.; Huamani, R. Ore mineralogy, trace element geochemistry and geochronological constraints at the Mollehuaca and San Juan de Chorunga Au-Ag vein deposits in the Nazca-Ocoña metallogenic belt, Arequipa, Peru. Minerals. Minerals 2020, 10, 1112. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Escoda, C.; Blanchard, M.; Testemale, D.; Hazeman, J.-L.; Gouy, S.; Kokh, M.A.; Boiron, M.-C.; de Parseval, F.; Aigouy, T.; et al. An arsenic-driven pump for invisible gold in hydrothermal systems. Geochem. Perspect. Lett. 2021, 17, 39–44. [Google Scholar] [CrossRef]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmocnim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Cathelineau, M.; Izquierdo, G.; Nieva, D. Thermobarometry of hydrothermal alteration in the Los Azufres geothermal system (Michoacan, Mexico): Significance of fluid-inclusion data. Chem. Geol. 1989, 76, 229–238. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Minerais Constituintes das Rochas, 4th ed.; Fundação Calouste Gulbenkian: Lisboa, Portugal, 2010; 727p. [Google Scholar]
- MacLean, W.H. Mass change calculations in altered rock series. Miner. Depos. 1990, 25, 44–49. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions (Reviews in Mineralogy, Volume 12); Mineralogical Society of America: Chantilly, WV, USA, 1984; 646p. [Google Scholar]
- Goldstein, R.H.; Reynolds, T.J. Systematics of fluid inclusions in diagenetic minerals. In Society for Sedimentary Geology, Society of Economic Paleontologists and Mineralogists, Short Course 31; SEPM (Society for Sedimentary Geology): Tulsa, OK, USA, 1994; p. 199. [Google Scholar]
- Diamond, L.W. Review of the systematics of CO2–H2O fluid inclusions. Lithos 2001, 55, 69–99. [Google Scholar] [CrossRef]
- Hollister, L.S.; Burrus, R.C. Phase equilibria in fluid inclusions from Khatada Lake metamorphic complex. Geochim. Cosmochim. Acta 1976, 40, 163–175. [Google Scholar] [CrossRef]
- Bakker, R.J. Package FLUIDS 1. Computer programs for analysis of fluid inclusion data and for modelling bulk fluid properties. Chem. Geol. 2003, 194, 3–23. [Google Scholar] [CrossRef]
- Knight, C.L.; Bodnar, R.J. Synthetic fluid inclusions: IX. Critical PVTX properties of NaCl-H2O solutions. Geochim. Cosmochim. Acta 1989, 53, 3–8. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O–NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications; de Vivo, B., Frezzotti, M.L., Eds.; Short Course IMA: Pontignano-Siena, Italy, 1994; pp. 117–130. [Google Scholar]
- Bowers, T.S.; Helgeson, H.C. Calculation of the thermodynamic and geochemical consequences of nonideal mixing in the system H2O-CO2-NaCl on phase relations in geological systems: Equation of state for H2O-CO2-NaCl fluids at high pressures and temperatures. Geochim. Cosmochim. Acta 1983, 47, 1247–1275. [Google Scholar] [CrossRef]
- Bakker, R.J. Adaptation of the Bowers and Helgeson 1983 equation of state to the H2O-CO2-CH4-N2-NaCl system. Chem. Geol. 1999, 154, 225–236. [Google Scholar] [CrossRef]
- Pirajno, F. Hydrothermal Processes and Mineral Systems; Springer & Geological Survey of Western Australia: Dordrecht, Australia, 2009; 1250p. [Google Scholar]
- Maloof, T.L.; Baker, T.; Thompson, J.F.H. The Dublin Gulch intrusion-hosted gold deposit, Tombstone plutonic suite, Yukon Territory, Canada. Miner. Depos. 2001, 36, 583–593. [Google Scholar] [CrossRef]
- Robert, F. Syenite-associated disseminated gold deposits in the Abitibi greenstone belt, Canada. Miner. Depos. 2001, 36, 503–516. [Google Scholar] [CrossRef]
- Stephens, J.L.; Mair, J.L.; Oliver, N.H.S.; Hart, C.J.R.; Baker, T. Structural and mechanical controls on intrusion-related deposits of the Tombstone Gold Belt, Yukon, Canada, with comparisons to other vein-hosted ore-deposit types. J. Struct. Geol. 2004, 26, 1025–1041. [Google Scholar] [CrossRef]
- Wen, B.J.; Fan, H.R.; Hu, F.F.; Liu, X.; Yang, K.F.; Sun, Z.F. Fluid evolution and ore genesis of the giant Sanshandao gold deposit, Jiaodong gold province, China: Constrains from geology, fluid inclusions and H–O–S–He–Ar isotopic compositions. J. Geochem. Explor. 2016, 171, 96–112. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Marsh, E.E.; Hart, C.J.R.; Mair, J.L.; Miller, M.L.; Johnson, C. Geology and Origin of Epigenetic Lode Gold Deposits, Tintina Gold Province, Alaska and Yukon. In Recent U.S. Geological Survey Studies in the Tintina Gold Province, Alaska, United States, and Yukon, Canada—Results of a 5-Year Project; Scientific Investigations Report; Gough, L.P., Day, W.C., Eds.; U.S. Geological Survey: Reston, VA, USA, 2007. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Lowenstern, J.B. The role of magmas in the formation of hydrothermal ore deposits. Nature 1994, 370, 519–527. [Google Scholar] [CrossRef]
- Biondi, J.C. Processos Metalogenéticos e os Depósitos Minerais Brasileiros, 2nd ed.; Oficina de Textos: São Paulo, Brazil, 2015; 552p. [Google Scholar]
- Audétat, A.; Pettke, T.; Heinrich, C.A.; Bodnar, R.J. The Composition of Magmatic-Hydrothermal Fluids in Barren and Mineralized Intrusions. Econ. Geol. 2008, 103, 877–908. [Google Scholar] [CrossRef]
- Fournier, R.O. Hydrothermal processes related to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ. Geol. 1999, 94, 1193–1211. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Gebre-Mariam, M.; Hagemann, S.G.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Goldfarb, R.J.; Baker, T.; Dubé, B.; Groves, D.I.; Hart, C.J.R.; Gosselin, P. Distribution, character, and genesis of gold deposits in metamorphic terrain. In One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: McLean, WV, USA, 2005. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Hurai, V.; Huraiová, M.; Slobodni’k, M.; Thomas, R. Geofluids Developments in Microthermometry, Spectroscopy, Thermodynamics, and Stable Isotopes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; 504p. [Google Scholar]
- Fan, H.R.; Hu, F.F.; Yang, J.H.; Shen, K.; Zhai, M.G. Fluid inclusion and large-scale gold metallogeny during Meozoic tectonic transition in the eastern Shandong province. Acta Petrol. Sin. 2005, 21, 1317. [Google Scholar]
- Deng, J.; Liu, X.; Wang, Q.; Pan, R. Origin of the Jiaodong-type Xinli gold deposit, Jiaodong Peninsula, China: Constraints from fluid inclusion and C–D–O–S–Sr isotope compositions. Ore Geol. Rev. 2015, 65, 674–686. [Google Scholar] [CrossRef]
- Faleiros, A.M.; Campanha, G.A.C.; Faleiros, F.M.; Bello, R.M.S. Fluid regimes, fault-valve behavior and formation of gold-quartz veins—The Morro do Ouro Mine, Ribeira Belt, Brazil. Ore Geol. Rev. 2014, 56, 442–456. [Google Scholar] [CrossRef]
- Boullier, A.M.; Robert, F. Palaeoseismic events recorded in Archaean gold-quartz vein networks, Val d’Or, Abitibi, Quebec, Canada. J. Struct. Geol. 1992, 14, 161–179. [Google Scholar] [CrossRef]
- Sibson, R.H. Fluid involvement in normal faulting. J. Geodyn. 2000, 29, 469–499. [Google Scholar] [CrossRef]
- Famin, V.; Hébert, R.; Philippot, P.; Jolivet, L. Ion probe and fluid inclusion evidence for co-seismic fluid infiltration in a crustal detachment. Contrib. Mineral. Petrol. 2005, 150, 354–367. [Google Scholar] [CrossRef]
- Bigot, L.; Jébrak, M. Gold mineralization at the syenite-hosted Beattie gold deposit, Duparquet, Neoarchean Abitibi Belt, Canada. Econ. Geol. 2015, 110, 315–335. [Google Scholar] [CrossRef]
- Bailey, S.W. Chlorites: Structures and crystal chemistry. Rev. Mineral. 1988, 19, 347–403. [Google Scholar]
- Beaufort, D. Etude Pétrographique des Altérations Hydrothermales Superposées Dans le Porphyre Cuprifère de Sibert (Rhône): Influence des Microsystèmes Géochimiques dans la Différenciation des Micas Blancs et des Phases Trioctaédriques. Ph.D. Thesis, Faculté des Sciences, Poitiers, France, 1981; 147p. [Google Scholar]







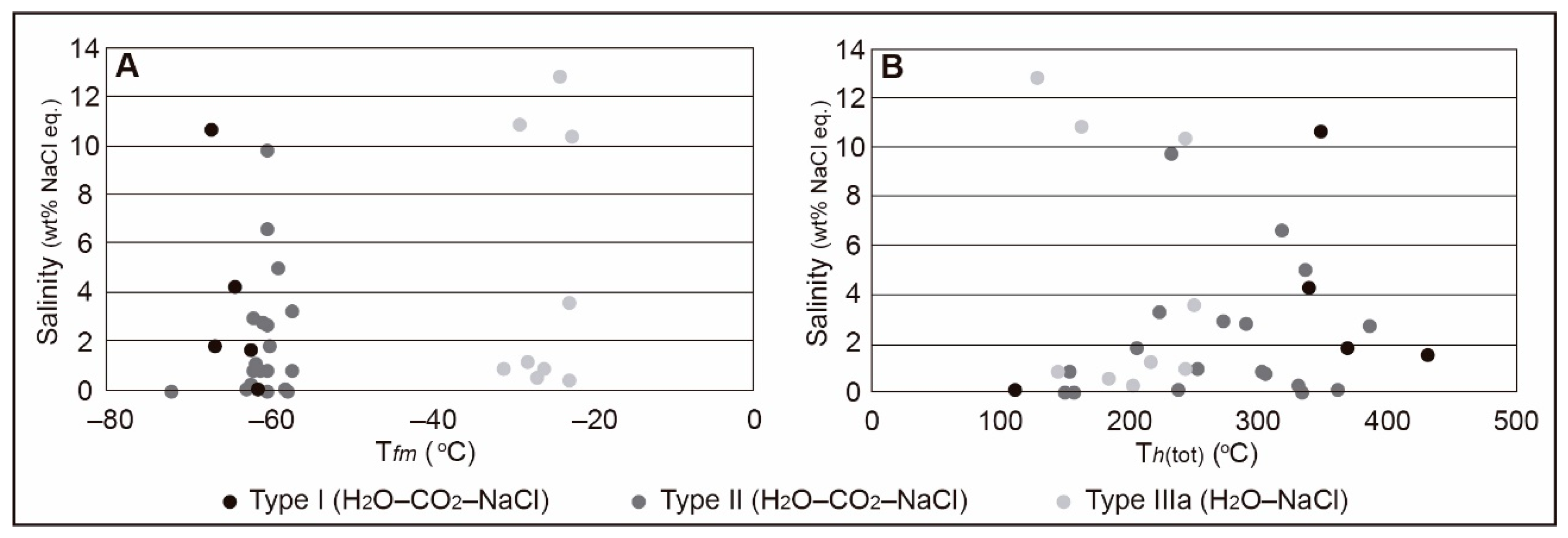
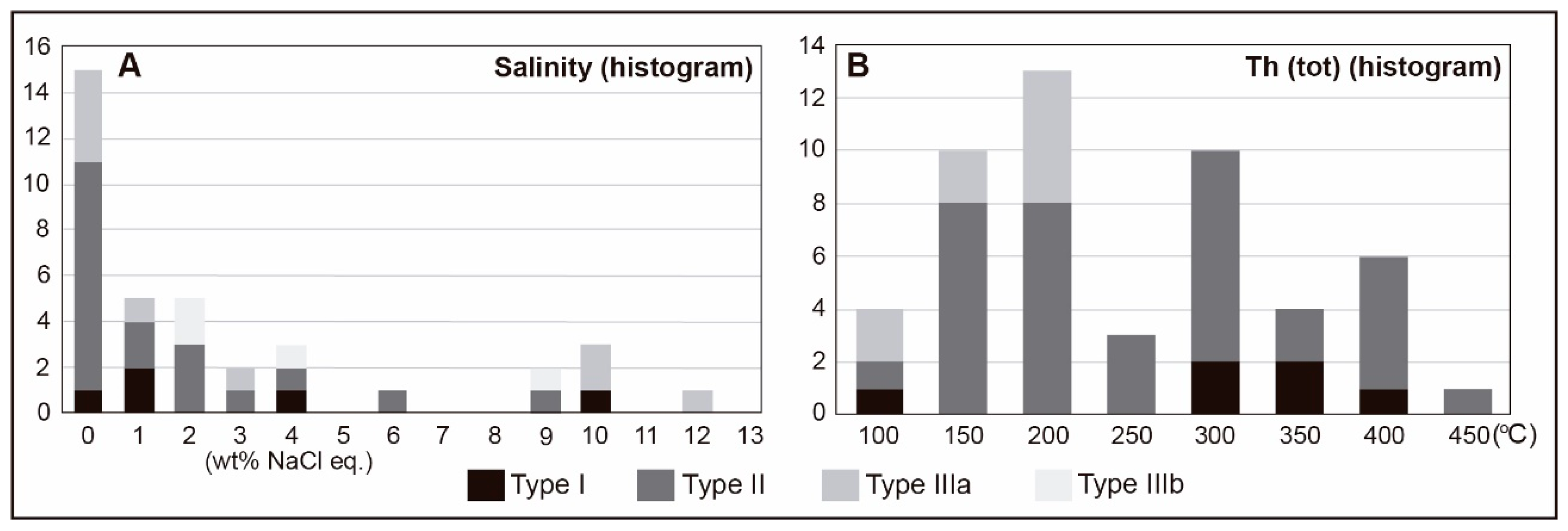
| Sample | Mineral | Lithology | Type | Number | Tfm (°C) | Tm clath (°C) (Average) | Tm-ice (°C) (Average) | Th-tot (°C) (Average) | Salinity (wt.% NaCl eq.) (Average) | Density (g/cm3) (Average) |
|---|---|---|---|---|---|---|---|---|---|---|
| GEF | Quartz | Red granite | I | 6 | −67.2 to −61.4 | 4.0 to 16.4 (9.3) | −8.1 to −1.2 (−3.6) | 432 to 112 (329.7) | 0.04 to 10.63 (3.65) | 0.74 to 0.94 (0.87) |
| GEF | Quartz | Red granite | II | 10 | −67.6 to −56.0 | 4.5 to 14.7 (9.3) | −10.2 to −0.2 (−5) | 150 to 453 (246.19) | 0.40 to 9.77 (2.72) | 0.62 to 1.02 (0.88) |
| BD-76d | Quartz | Aplite | II | 14 | −64.3 to −57.3 | 6.3 to 12.2 (9.4) | −11.6 to −1.6 (−6.3) | 177 to 430 (301.1) | 2.23 to 4.97 (2.64) | 0.65 to 1.07 (0.93) |
| BD-26b | Quartz | Mineralised vein (Qtz 1) | II | 10 | −62.5 to −57.3 | 8.4 to 15.0 (11.4) | −5.6 to −0.2 (−3.1) | 147 to 430 (272.3) | 0.81 to 2.74 (1.52) | 0.71 to 0.92 (0.85) |
| BD-86a | Quartz | Mineralised vein (Qtz 1) | IIIa | 9 | −31.0 to −22.6 | - | −9.0 to −0.2 (−3.1) | 183 to 250 (197.6) | 0.35 to 12.84 (4.61) | 0.81 to 1.01 (0.90) |
| BD-85b | Quartz | Mineralised vein (Qtz 3) | IIIb | 5 | −27.5 to −24.1 | - | −6.4 to −0.7 (−2.6) | - | 2.40 to 9.70 (4.88) | - |
| GEF | Quartz | Red granite | I | 6 | −67.2 to −61.4 | 4.0 to 16.4 (9.3) | −8.1 to −1.2 (−3.6) | 432 to 112 (329.7) | 0.04 to 10.63 (3.65) | 0.74 to 0.94 (0.87) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dressel, B.C.; Chauvet, A.; Kouzmanov, K.; Trzaskos, B.; Bruguier, O.; Monié, P.; Villanova, S.N.; Newton, J.B. The Passa Três Granite Intrusion-Related/Hosted Neoproterozoic Gold Deposit (Paraná State, Brazil): Mineralogical, Geochemical, Fluid Inclusion and Sulphur Isotope Constraints. Minerals 2022, 12, 407. https://doi.org/10.3390/min12040407
Dressel BC, Chauvet A, Kouzmanov K, Trzaskos B, Bruguier O, Monié P, Villanova SN, Newton JB. The Passa Três Granite Intrusion-Related/Hosted Neoproterozoic Gold Deposit (Paraná State, Brazil): Mineralogical, Geochemical, Fluid Inclusion and Sulphur Isotope Constraints. Minerals. 2022; 12(4):407. https://doi.org/10.3390/min12040407
Chicago/Turabian StyleDressel, Bárbara Carolina, Alain Chauvet, Kalin Kouzmanov, Barbara Trzaskos, Olivier Bruguier, Patrick Monié, Sandro Notto Villanova, and José Bazille Newton. 2022. "The Passa Três Granite Intrusion-Related/Hosted Neoproterozoic Gold Deposit (Paraná State, Brazil): Mineralogical, Geochemical, Fluid Inclusion and Sulphur Isotope Constraints" Minerals 12, no. 4: 407. https://doi.org/10.3390/min12040407






