Experimental Modeling of Diamond Resorption during Mantle Metasomatism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Compositions
2.2. High-pressure Apparatus and Analytical Techniques
3. Results
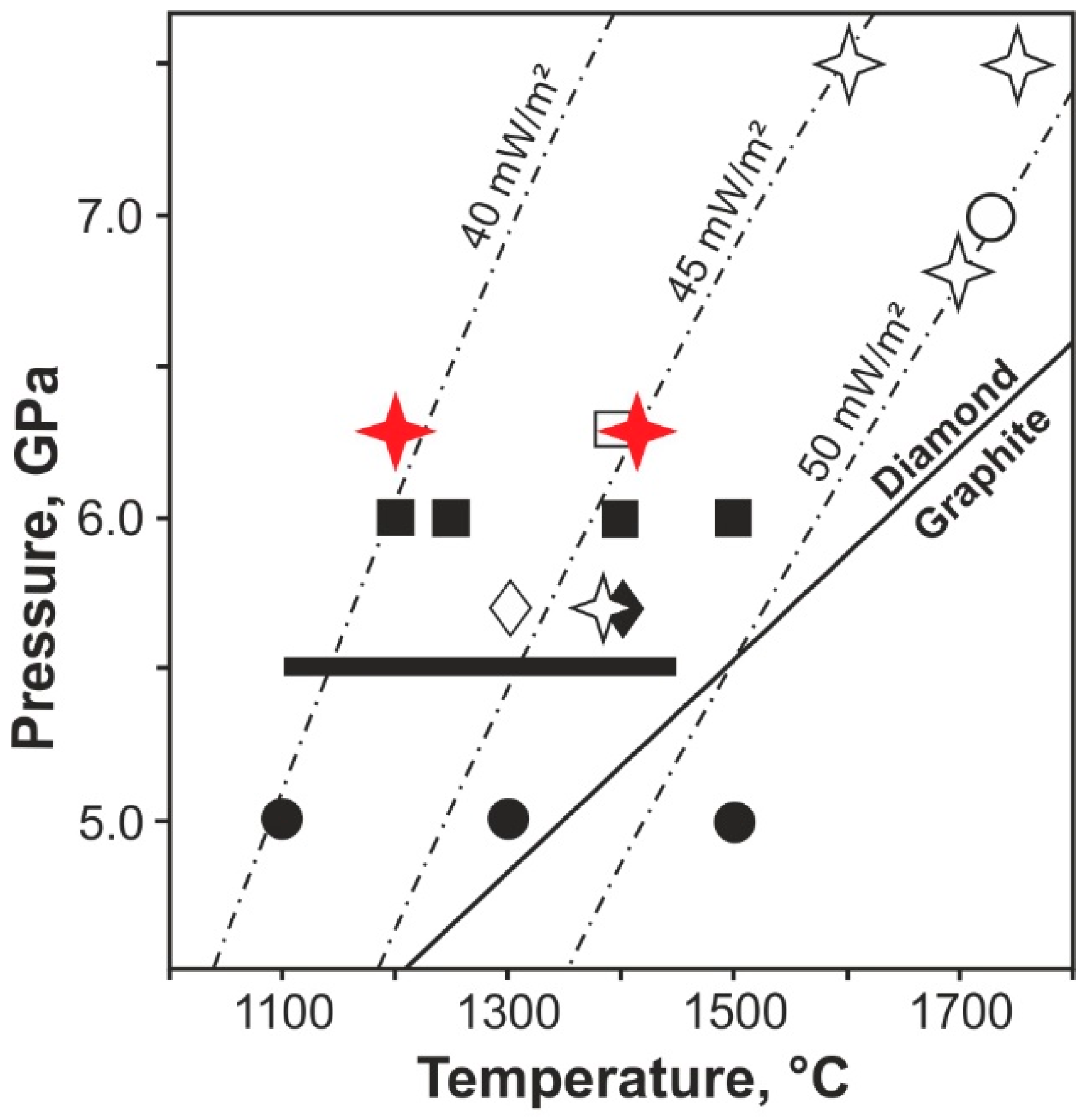
3.1. Phase Composition of Systems
3.2. Diamond Resorption Morphology
3.3. Dependence of the Resorption Rate on fO2
3.4. Temperature Dependence of the Resorption Rate
4. Discussion
4.1. Diamond Resorption Morphology
4.2. Diamond Resorption Rate
4.3. Natural Resorption of Diamond
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fedortchouk, Y.; Zang, Z. Diamond resorption: Link to metasomatic events in the mantle or record of record of magmatic fluid in kimberlitic magma? Can. Mineral. 2011, 49, 707–719. [Google Scholar] [CrossRef]
- Fedortchouk, Y.; Liebske, C.; McCammon, C. Diamond destruction and growth during mantle metasomatism: An experimental study of diamond resorption features. Earth Planet. Sci. Lett. 2019, 506, 493–506. [Google Scholar] [CrossRef]
- Fedortchouk, Y. A new approach to understanding diamond surface features based on a review of experimental and natural diamond studies. Earth-Sci. Rev. 2019, 193, 45–65. [Google Scholar] [CrossRef]
- Smit, K.V.; Shirey, S.B. Diamond from the deep. Gems. Gemol. 2020, 56, 148–155. [Google Scholar]
- Fedortchouk, Y.; Chinn, I.L.; Perritt, S.H.; Zhang, Z.; Stern, R.A.; Li, Z. Diamond-destructive mantle metasomatism: Evidence from the internal and external textures of diamonds and their nitrogen defects. Lithos 2022, 414–415, 106616. [Google Scholar] [CrossRef]
- Robinson, D.N. Surface Textures and Other Features of Diamonds. Ph.D. Thesis, The University of Cape Town, Cape Town, South Africa, 1979; 221p. [Google Scholar]
- Bulanova, G.P. Formation of diamond. J. Geochem. Explor. 1995, 53, 1–23. [Google Scholar] [CrossRef]
- Chinn, I.L. A study of unusual diamonds from the George Creek K1 Kimberlite Dyke, Colorado. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1995; p. 94. [Google Scholar]
- Haggerty, S.E. Diamond genesis in a multiply constrained model. Nature 1986, 320, 34–38. [Google Scholar] [CrossRef]
- Gurney, J.J.; Hildebrand, P.R.; Carlson, J.A.; Fedortchouk, Y.; Dyck, D.R. The morphological characteristics of diamond from the Ekati property, Northwest Territories, Canada. Lithos 2004, 77, 21–38. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Khokhryakov, A.F.; Kupriyanov, I.N. Crystallomorphological and crystallochemical indicators of diamond formation conditions. Crystallogr. Rep. 2021, 66, 142–155. [Google Scholar] [CrossRef]
- Kanda, H.; Yamaoka, S.; Setaka, N.; Komatsu, H. Etching of diamond octahedrons by high pressure water. J. Cryst. Growth 1977, 38, 1–7. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Pal’yanov, Y.N. The morphology of diamond crystals, dissolved in the water containing silicate melts. Mineral. Zh. 1990, 12, 14–23. (In Russian) [Google Scholar]
- Khokhryakov, A.F.; Palyanov, Y.N. Dissolution form of diamond crystals in CaCO3 melt at 7 GPa. Russ. Geol. Geophys. 2000, 41, 682–687. [Google Scholar]
- Khokhryakov, A.F.; Palyanov, Y.N. The evolution of diamond morphology in the process of dissolution: Experimental data. Am. Mineral. 2007, 92, 909–917. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Influence of the fluid composition on diamond dissolution forms in carbonate melts. Am. Mineral. 2010, 95, 1508–1514. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Effect of crystal defects on diamond morphology during dissolution in the mantle. Am. Mineral. 2015, 100, 1528–1532. [Google Scholar] [CrossRef]
- Sokol, A.G.; Khokhryakov, A.F.; Palyanov, Y.N. Composition of primary kimberlite magma: Constraints from melting and diamond dissolution experiments. Contrib. Mineral. Petrol. 2015, 170, 26. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N.; Sobolev, N.V. Evolution of crystal morphology of natural diamond in dissolution processes: Experimental date. Dokl. Earth Sci. 2001, 381, 884–888. [Google Scholar]
- Khokhryakov, A.F.; Palyanov, Y.N.; Sobolev, N.V. Crystal morphology as an indicator of redox conditions of natural diamond dissolution at the mantle PT parameters. Dokl. Earth Sci. 2002, 385, 534–537. [Google Scholar]
- Pollanck, H.N.; Chapman, D.S. On the regional variation of head flow, geoterms, and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, C.J.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Kruk, A.N.; Sokol, A.G. The effect of oxygen fugacity on diamond resorption in ascending kimberlite melt. Lithos 2021, 394–395, 106166. [Google Scholar] [CrossRef]
- Ballhaus, C.; Berry, R.F.; Green, D.H. High pressure experimental calibration of the olivine-orthopyroxene-spinel oxygen geobarometer: Implications for the oxidation state of the upper mantle. Contrib. Mineral. Petrol. 1991, 107, 27–40. [Google Scholar] [CrossRef]
- Campbell, A.J.; Danielson, L.; Righter, K.; Seagle, C.T.; Wang, Y.; Prakapenka, V.B. Pressure–Volume–Temperature Studies of Metal–Oxide Pairs. Available online: https://www.geol.umd.edu/~ajc/Posters/CampbellCOMPRES2007poster.pdf (accessed on 23 January 2022).
- Foley, S.F. A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J. Petrol. 2011, 52, 1363–1391. [Google Scholar] [CrossRef]
- Kadik, A.A.; Lukanin, O.A. Degassing of the Upper Mantle upon Melting; Nauka PH: Moscow, Russian, 1986; 120p. (In Russian) [Google Scholar]
- Frost, D.J.; Wood, B.J. Experimental measurements of the fugacity of CO2 and graphite/diamond stability from 35 to 77 kbar at 925 to 1650 °C. Geochim. Cosmochim. Acta 1997, 61, 1565–1574. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Palyanova, G.A. Conditions for the origin of oxidized carbonate–silicate melts: Implications for mantle metasomatism and diamond formation. Lithos 2012, 128–131, 113–125. [Google Scholar] [CrossRef]
- Grassi, D.; Schmidt, M.W. Melting of carbonated pelites at 8–13 GPa: Generating K-rich carbonatites for mantle metasomatism. Contrib. Mineral. Petrol. 2011, 162, 169–191. [Google Scholar] [CrossRef] [Green Version]
- Sokol, A.G.; Kupriyanov, I.N.; Palyanov, Y.N.; Kruk, A.N.; Sobolev, N.V. Melting experiments on the Udachnaya kimberlite at 6.3–7.5 GPa: Implications for the role of H2O in magma generation and formation of hydrous olivine. Geochim. Cosmochim. Acta 2013, 101, 133–155. [Google Scholar]
- Sokol, A.G.; Pal’yanov, Y.N.; Pal’yanova, G.A.; Tomilenko, A.A. Diamond crystallization in fluid and carbonate-fluid systems under mantle P–T conditions: 1. Fluid composition. Geochem. Int. 2004, 42, 830–838. [Google Scholar]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Day, H. A revised diamond-graphite transition curve. Am. Mineral. 2012, 97, 52–62. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kruk, A.N.; Chebotarev, D.A.; Palyanov, Y.N. Carbonatite melt–peridotite interaction at 5.5–7.0 GPa: Implications for metasomatism in lithospheric mantle. Lithos 2016, 248–251, 66–79. [Google Scholar] [CrossRef]
- Hernlund, J.; Leinenweber, K.; Locke, D.; Tyburczy, J. A numerical model for steady state temperature distributions in solid-medium high-pressure cell assemblies. Am. Mineral. 2006, 91, 295–305. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V. Melting of K-rich carbonated peridotite at 6–10 GPa and the stability of K-phases in the upper mantle. Chem. Geol. 2011, 281, 333–342. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Ryabchikov, I.D. Volatile components, magmas, and critical fluids in upwelling mantle. J. Petrol. 2000, 41, 1195–1206. [Google Scholar] [CrossRef] [Green Version]
- Rock, N.M.S. Lamprophyres; Blackie: New York, NY, USA, 1991; 284p. [Google Scholar]
- Frank, F.C.; Puttic, K.E.; Wilks, E.M. Etch pits and trigons of diamond: I. Phil. Mag. 1958, 3, 1262–1279. [Google Scholar] [CrossRef]
- Orlov, Y.L. The Mineralogy of Diamond; John Wiley: New York, NY, USA, 1977; 235p. [Google Scholar]
- Fedortchouk, Y.; Canil, D.; Semenets, E. Mechanisms of diamond oxidation and their bearing on the fluid composition in kimberlite magmas. Am. Mineral. 2007, 92, 1200–1212. [Google Scholar] [CrossRef]
- Zhang, Z.; Fedortchouk, Y.; Hanley, J.J. Evolution of diamond resorption in a silicic aqueous fluid at 1–3 GPa: Application to kimberlite emplacement and mantle metasomatism. Lithos 2015, 227, 179–193. [Google Scholar] [CrossRef]
- Frank, F.C.; Lang, A.R. Observation by X-ray diffraction on dislocation in a diamond. Phil. Mag. 1959, 4, 383–386. [Google Scholar] [CrossRef]
- van Enckevort, W.J.P.; Seal, M. Stress birefringence microscopy of dislocations in type-Ia natural diamond. Phil. Mag. 1988, 57, 939–954. [Google Scholar] [CrossRef]
- Khokhryakov, A.F.; Palyanov, Y.N. Revealing of dislocations in diamond crystals by the selective etching method. J. Cryst. Growth 2006, 293, 469–474. [Google Scholar] [CrossRef]
- Rudenko, A.P.; Kulakova, I.I.; Shturman, V.L. Oxidation of natural diamond. In Novye Dannye o Mineralogii SSSR; Nauka: Moscow, Russian, 1979; Volume 28, pp. 105–125. (In Russian) [Google Scholar]
- Skvortsova, V.L.; Shiryaev, A.A.; Fedortchouk, Y. Influence of ions on diamond resorption. Diam. Relat. Mater. 2020, 104, 107764. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.-L. Influence of mantle CO2 in the generation of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Mysen, B.O. Silicate-COH melt and fluid structure, their physicochemical properties, and partitioning of nominally refractory oxides between melts and fluids. Lithos 2012, 148, 228–246. [Google Scholar] [CrossRef]
- Mysen, B.O. Structure-property relationships of COHN-saturated silicate melt coexisting with COHN fluid: A review of in-situ, high-temperature, high-pressure experiments. Chem. Geol. 2013, 346, 113–124. [Google Scholar] [CrossRef]
- Mysen, B.O. Water-melt interaction in hydrous magmatic systems at high temperature and pressure. Prog. Earth Planet. Sci. 2014, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Brooker, R.A.; Kohn, S.C.; Holloway, J.R.; McMillan, P.F.; Carroll, M.R. Solubility, speciation and dissolution mechanisms for CO2 in melts on the NaAlO2−2 join. Geochim. Cosmochim. Acta 1999, 63, 3549–3565. [Google Scholar] [CrossRef]
- Eggler, D.H. The effect of CO2 upon partial melting of peridotite in the system Na2O–CaO–Al2O3–MgO–SiO2–CO2 to 35 kb, with an analysis of melting in a peridotite-H2O–CO2 system. Am. J. Sci. 1978, 278, 305–343. [Google Scholar] [CrossRef]
- Luth, R.W.; Palyanov, Y.N.; Bureau, H. Experimental petrology applied to natural diamond growth. Rev. Mineral. Geochem. 2021, 87. in press. [Google Scholar]
- Creighton, S.; Stachel, T.; Matveev, S.; Höfer, H.; Mc Cammon, C.; Luth, R.W. Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib. Mineral. Petrol. 2009, 157, 491–504. [Google Scholar] [CrossRef]
- Woodland, A.B.; Koch, M. Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal Craton, southern Africa. Earth Planet. Sci. Lett. 2003, 214, 295–310. [Google Scholar]
- Becker, M.; Le Roex, A.P. Geochemistry of South African on- and off-craton, group I and group II kimberlites: Petrogenesis and source region evolution. J. Petrol. 2006, 162, 169–191. [Google Scholar] [CrossRef] [Green Version]
- Kogarko, L.N. Alkaline magmatism and enriched mantle reservoirs: Mechanisms, time, and depth of formation. Geochem. Int. 2006, 44, 3–10. [Google Scholar] [CrossRef]
- Bowen, D.C.; Ferraris, R.D.; Palmer, C.E.; Ward, J.D. On the unusual characteristics of the diamonds from Letšent-la-Terai kimberlites, Lesotho. Lithos 2009, 112 (Suppl. S2), 767–774. [Google Scholar] [CrossRef]
- Hetman, C.M.; Smith, B.S.; Robey, J.; Nkotsi, T.; Mohapi, M.; Mohapi, T. Letšeng diamond mine, Lesotho: A variant of Kimberley-type pyroclastic kimberlite emplacement. Mineral. Petrol. 2018, 112 (Suppl. S2), S365–S382. [Google Scholar] [CrossRef]
- Moore, A.E. Type II diamonds: Flamboyant Megacrysts? South Afr. J. Geol. 2009, 112, 23–38. [Google Scholar] [CrossRef]
- Kozai, Y.; Arima, M. Experimental study on diamond dissolution in kimberlitic and lamproitic melts at 1300–1420 °C and 1 GPa with controlled oxygen partial pressure. Am. Mineral. 2005, 90, 1759–1766. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Weiss, Y.; Navon, O.; Nielsen, T.F.D.; Mernagh, T.P. How unique is the Udachnaya-East kimberlite? Comparison with kimberlites from the Slave Craton (Canada) and SW Greenland. Lithos 2009, 112, 334–346. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Giuliani, A.; O’Brien, H. What is a Kimberlite? Petrology and Mineralogy of Hypabyssal Kimberlites. Elements 2019, 15, 381–386. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.U.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Kruk, A.N. Conditions of diamond crystallization in kimberlite melt: Experimental data. Russ. Geol. Geophys. 2015, 36, 196–210. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Pal’yanova, G.A.; Borzdov, Y.U.M.; Sobolev, N.V. Diamond and graphite crystallization in COH fluid at PT parameters of the natural diamond formation. Dokl. Earth Sci. 2000, 375, 1395–1399. [Google Scholar]











| GS-H2O | GS-OA | Ud-H2O | Ud-OA | |
|---|---|---|---|---|
| SiO2 | 0.56 | 0.57 | 25.03 | 25.19 |
| TiO2 | 0.02 | 0.02 | 1.23 | 1.24 |
| Al2O3 | 0.66 | 0.67 | 2.67 | 2.68 |
| Cr2O3 | - | - | 0.11 | 0.11 |
| Fe2O3 | - | - | 2.82 | 2.84 |
| FeO | 3.61 | 3.63 | 4.58 | 4.61 |
| MnO | - | - | 0.14 | 0.14 |
| NiO | - | - | 0.19 | 0.19 |
| MgO | 4.34 | 4.36 | 25.18 | 25.33 |
| CaO | 14.25 | 14.34 | 12.12 | 12.19 |
| Na2O | 0.16 | 0.16 | 2.33 | 2.34 |
| K2O | 28.45 | 28.64 | 1.67 | 1.68 |
| H2O | 9.09 | 1.83 | 11.36 | 4.11 |
| P2O5 | - | - | 0.37 | 0.38 |
| CO2 | 38.85 | 45.78 | 8.58 | 15.34 |
| S | - | - | 0.30 | 0.30 |
| F | - | - | 0.13 | 0.13 |
| Cl | - | - | 1.20 | 1.21 |
| Total | 99.99 | 100.00 | 100 | 100 |
| Run No. | T (°C) | Duration (h) | fO2 | Capsule | Outer Container | Ol | Grt | Cpx | Opx | Mst | Prv | Liq |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ud-H2O system | ||||||||||||
| 704_8_1 | 1400 | 5 | RRO | Pt+Re | Fe2O3 | 0.26 | 0.13 | 0.05 | - | - | - | 0.56 |
| 1176_5_1 | 1200 | 40 | CW-CCO | Pt+Re | no | 0.39 | 0.08 | 0.03 | - | - | 0.01 | 0.49 |
| 1666_3_3 | 1200 | 10 | RRO | Pt+Re | Fe2O3 | 0.4 | 0.08 | 0.03 | - | - | 0.01 | 0.47 |
| 2223_2_1 | 1200 | 10 | IRM | Au | no | 0.34 | - | 0.16 | - | - | - | 0.5 |
| Ud-OA system | ||||||||||||
| 704_8_2 | 1400 | 5 | RRO | Pt+Re | Fe2O3 | 0.09 | - | 0.37 | - | 0.26 | - | 0.28 |
| 1753_1 | 1200 | 40 | CW-CCO | Pt+Re | no | n.d | n.d | n.d | n.d | n.d | n.d | n.d |
| 1294_7_5 | 1200 | 10 | RRO | Pt+Re | Fe2O3 | 0.04 | 0.01 | 0.36 | 0.03 | 0.35 | 0.01 | 0.2 |
| 2223_2_3 | 1200 | 10 | IRM | Au | no | - | 0.01 | - | 0.43 | - | - | 0.56 |
| GS-H2O system | ||||||||||||
| 706_8_1 | 1400 | 5 | RRO | Pt+Re | Fe2O3 | - | - | - | - | - | - | 1 |
| 1176_5_2 | 1200 | 40 | CW-CCO | Pt+Re | no | - | - | - | - | - | - | 1 |
| 1666_3_4 | 1200 | 10 | RRO | Pt+Re | Fe2O3 | - | - | - | - | - | - | 1 |
| 2223_2_5 | 1200 | 10 | IRM | Au | no | - | - | - | - | - | - | 1 |
| GS-OA system | ||||||||||||
| 706_8_2 | 1400 | 5 | RRO | Pt+Re | Fe2O3 | - | - | - | - | - | - | 1 |
| 2178_2_5 | 1200 | 40 | CW-CCO | Pt+Re | no | - | - | - | - | - | - | 1 |
| 1294_7_6 | 1200 | 10 | RRO | Pt+Re | Fe2O3 | - | - | - | - | - | - | 1 |
| 2223_2_7 | 1200 | 10 | IRM | Au | no | - | - | - | - | - | - | 1 |
| Run No. | T (°C) Duration (h) | fO2 | Phase | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO | MnO | NiO | MgO | CaO | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ud-H2O system | |||||||||||||||
| 704_8_1 | 1400 5 | RRO | Ol | 41.1 | - | - | - | 7.37 | - | 0.3 | 51.1 | - | - | - | 99.87 |
| Grt | 42.3 | 0.9 | 19.68 | 2.57 | 4.61 | - | - | 18.7 | 10.7 | - | - | 99.46 | |||
| Cpx | 55.1 | 0.2 | 2.39 | 0.33 | 2.08 | - | - | 17.6 | 20.3 | 1.3 | - | 99.3 | |||
| Liq | 11.2 | 1.4 | 1.9 | - | 6.13 | - | - | 15.5 | 18.2 | 4.4 | 1.9 | 60.63 | |||
| 1176_5_1 | 1200 40 | CW-CCO | Ol | 40.3 | - | - | - | 9.1 | - | 0.2 | 49.5 | 0.2 | 0.2 | - | 99.5 |
| Grt | 40 | 2.8 | 17.3 | 1.13 | 7.34 | 0.3 | - | 12.7 | 17.3 | 0.3 | - | 99.17 | |||
| Cpx | 54.1 | 0.3 | 1.8 | 0.22 | 2.52 | - | - | 16.1 | 22.9 | 1.3 | - | 99.24 | |||
| Prv | - | 58.7 | 0.33 | - | 1.6 | - | - | 39.2 | - | - | - | 99.83 | |||
| Liq | 8.9 | 1 | 4.43 | 0.35 | 3.99 | - | - | 8.2 | 19.7 | 5.8 | 3.2 | 55.57 | |||
| 1666_3_3 | 1200 10 | RRO | Ol | 40.5 | - | - | - | 9.4 | - | 0.3 | 49.3 | - | - | - | 99.5 |
| Grt | 40.4 | 2.2 | 17.73 | 1.17 | 6.63 | 0.2 | - | 10.8 | 19.8 | 0.3 | 0.2 | 99.43 | |||
| Cpx | 54.2 | 0.3 | 1.86 | 0.32 | 2.19 | - | - | 16.2 | 22.9 | 1.1 | - | 99.07 | |||
| Prv | - | 57.9 | 0.31 | - | 2.2 | - | - | 39.4 | - | - | - | 99.81 | |||
| Liq | 8.1 | - | 2.18 | - | 1.74 | - | - | 7 | 18.7 | 4.4 | 2.7 | 44.82 | |||
| 2223_2_1 | 1200 10 | IRM | Ol | 40.2 | - | - | - | 10.53 | - | 0.2 | 48.6 | 0.1 | - | - | 99.63 |
| Cpx | 54.8 | 0.3 | 1.77 | 0.26 | 4.45 | - | - | 16.7 | 19.4 | 1.5 | - | 99.18 | |||
| Liq | 6.4 | 1 | 1.58 | - | 8.86 | - | - | 9.8 | 12.9 | 2.3 | 2.1 | 44.94 | |||
| Ud-OA system | |||||||||||||||
| 704_8_2 | 1400 5 | RRO | Ol | 40.7 | - | - | - | 9.83 | - | 0.3 | 48.9 | 0.1 | - | - | 99.83 |
| Cpx | 56 | 0.5 | 2.33 | 0.25 | 3.9 | - | - | 20.7 | 14.5 | 1.6 | - | 99.78 | |||
| Mst | - | - | - | - | 5.05 | - | - | 37.2 | 3.2 | - | - | 45.45 | |||
| Liq | 2.9 | 0.4 | 1.38 | - | 5.53 | - | - | 12.8 | 21.4 | 5.2 | 2.8 | 52.41 | |||
| 1294_7_5 | 1200 10 | RRO | Ol | 40.8 | - | - | - | 8.19 | - | 0.3 | 50.2 | - | - | - | 99.49 |
| Cpx | 54.6 | 0.3 | 2.58 | 0.5 | 2.67 | - | - | 17.4 | 19.5 | 1.5 | - | 99.05 | |||
| Opx | 56.1 | - | 3.02 | 0.38 | 4.9 | - | - | 34.1 | 0.5 | - | - | 99 | |||
| Grt | 41.8 | 1.78 | 20.16 | 1.06 | 7.67 | 0 | 0 | 19.84 | 7.14 | 0 | 0 | 99.45 | |||
| Mst | - | - | - | - | 4.37 | - | - | 37.6 | 1.7 | - | - | 43.67 | |||
| Prv | - | 54.21 | 0 | 0.72 | 2 | 0 | - | 1.48 | 38.9 | 1.42 | 0.48 | 99.21 | |||
| Liq | 9 | 0.2 | 1.05 | - | 3.72 | - | - | 9.7 | 18.9 | 10.1 | 3.5 | 56.17 | |||
| 2223_2_3 | 1200 10 | IRM | Opx | 56.1 | - | 0.79 | - | 9.82 | - | - | 31 | 1.2 | 0.3 | - | 99.21 |
| Grt | 42.5 | 1.4 | 18.88 | 1.68 | 14.81 | 0.3 | - | 15.4 | 5.1 | 0.2 | - | 100.27 | |||
| Liq | 5.5 | 1.6 | 1.62 | - | 12.81 | - | - | 6.7 | 9.3 | 2.9 | 1.6 | 42.03 | |||
| GS-H2O system | |||||||||||||||
| 706_8_1 | 1400 5 | RRO | Liq | 0.7 | - | 0.85 | - | 3.32 | - | - | 3.7 | 14.9 | 0.4 | 26.5 | 50.37 |
| 1176_5_2 | 1200 40 | CW-CCO | Liq | 0.6 | - | 0.77 | - | 2.85 | - | - | 4 | 14.3 | 0.3 | 19.4 | 42.22 |
| 1666_3_4 | 1200 10 | RRO | Liq | 0.7 | - | 0.95 | - | 3.03 | - | - | 4.5 | 16.4 | - | 29.2 | 54.78 |
| 2223_2_5 | 1200 10 | IRM | Liq | 2.1 | 2.2 | 0.5 | - | 14.14 | - | - | 2.7 | 12.1 | - | 24.1 | 57.84 |
| GS-OA system | |||||||||||||||
| 706_8_2 | 1400 5 | RRO | Liq | 1.8 | - | 1.96 | - | 3.68 | - | - | 4.4 | 16.8 | 0.5 | 18.5 | 47.64 |
| 1294_7_6 | 1200 10 | RRO | Liq | 0.7 | - | 0.82 | - | 3.42 | - | - | 3.8 | 14.2 | 0.3 | 22.6 | 45.84 |
| 2223_2_7 | 1200 10 | IRM | Liq | 1.9 | 1.9 | 0.47 | - | 17.68 | 0.3 | - | 2.8 | 11 | - | 22.7 | 58.75 |
| Run No. | T (°C) | Duration (h) | fO2 | Diamond | |||
|---|---|---|---|---|---|---|---|
| Initial Weight (mg) | Final Weight (mg) | WL % | V | ||||
| Ud-H2O system | |||||||
| 704_8_1 | 1400 | 5 | RRO | 0.31 | 0.16 | 48 | 0.369 |
| 0.31 | 0.11 | 65 | 0.488 | ||||
| 1176_5_1 | 1200 | 40 | CW-CCO | 0.33 | 0.29 | 12 | 0.011 |
| 0.33 | 0.28 | 15 | 0.014 | ||||
| 1666_3_3 | 1200 | 10 | RRO | 0.30 | 0.26 | 13 | 0.048 |
| 0.30 | 0.25 | 16.5 | 0.060 | ||||
| 2223_2_1 | 1200 | 10 | IRM | 0.36 | 0.21 | 42 | 0.170 |
| Ud-OA system | |||||||
| 704_8_2 | 1400 | 5 | RRO | 0.29 | 0.23 | 21 | 0.148 |
| 0.29 | 0.24 | 17 | 0.123 | ||||
| 1753_1 | 1200 | 40 | CW-CCO | 0.40 | 0.40 | 0 | 0 |
| 1294_7_5 | 1200 | 10 | RRO | 0.27 | 0.24 | 11 | 0.037 |
| 0.27 | 0.23 | 15 | 0.049 | ||||
| 2223_2_3 | 1200 | 10 | IRM | 0.39 | 0.33 | 15 | 0.066 |
| 2223_2_4 | 1200 | 10 | IRM | 0.39 | 0.34 | 13 | 0.055 |
| GS-H2O system | |||||||
| 706_8_1 | 1400 | 5 | RRO | 0.34 | 0.05 | 85 | 0.672 |
| 0.34 | 0.01 | 97 | 0.764 | ||||
| 1176_5_2 | 1200 | 40 | CW-CCO | 0.30 | 0.26 | 13 | 0.012 |
| 0.30 | 0.25 | 17 | 0.015 | ||||
| 1666_3_4 | 1200 | 10 | RRO | 0.30 | 0.24 | 20 | 0.072 |
| 0.30 | 0.22 | 27 | 0.980 | ||||
| 2223_2_5 | 1200 | 10 | IRM | 0.36 | 0.24 | 33 | 0.136 |
| GS-OA system | |||||||
| 706_8_2 | 1400 | 5 | RRO | 0.25 | 0.13 | 48 | 0.308 |
| 0.25 | 0.01 | 96 | 0.615 | ||||
| 2178_2_5 | 1200 | 40 | CW-CCO | 0.38 | 0.38 | ˂2 | ˂0.003 |
| 1294_7_6 | 1200 | 10 | RRO | 0.30 | 0.22 | 27 | 0.096 |
| 0.30 | 0.24 | 20 | 0.072 | ||||
| 2223_2_7 | 1200 | 10 | IRM | 0,36 | 0,26 | 27 | 0.113 |
| 2223_2_8 | 1200 | 10 | IRM | 0.36 | 0.27 | 25 | 0.102 |
| Pressure, GPa | Activation Energy of Diamond Resorption, kJ/mol | |||
|---|---|---|---|---|
| Ud-H2O System | Ud-OA System | GS-H2O System | GS-OA System | |
| 3.0 1 | 125 | 195 | 240 | 260 |
| 6.3 | 215 | 120 | 240 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhryakov, A.F.; Kruk, A.N.; Sokol, A.G.; Nechaev, D.V. Experimental Modeling of Diamond Resorption during Mantle Metasomatism. Minerals 2022, 12, 414. https://doi.org/10.3390/min12040414
Khokhryakov AF, Kruk AN, Sokol AG, Nechaev DV. Experimental Modeling of Diamond Resorption during Mantle Metasomatism. Minerals. 2022; 12(4):414. https://doi.org/10.3390/min12040414
Chicago/Turabian StyleKhokhryakov, Alexander F., Alexey N. Kruk, Alexander G. Sokol, and Denis V. Nechaev. 2022. "Experimental Modeling of Diamond Resorption during Mantle Metasomatism" Minerals 12, no. 4: 414. https://doi.org/10.3390/min12040414





