The Interplay of Iron Minerals and Microflora to Accelerate Cr (VI) Reduction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Medium
2.2. Experiment
2.3. Electrochemical Experiments
2.4. Analytical Methods
3. Results and Analysis
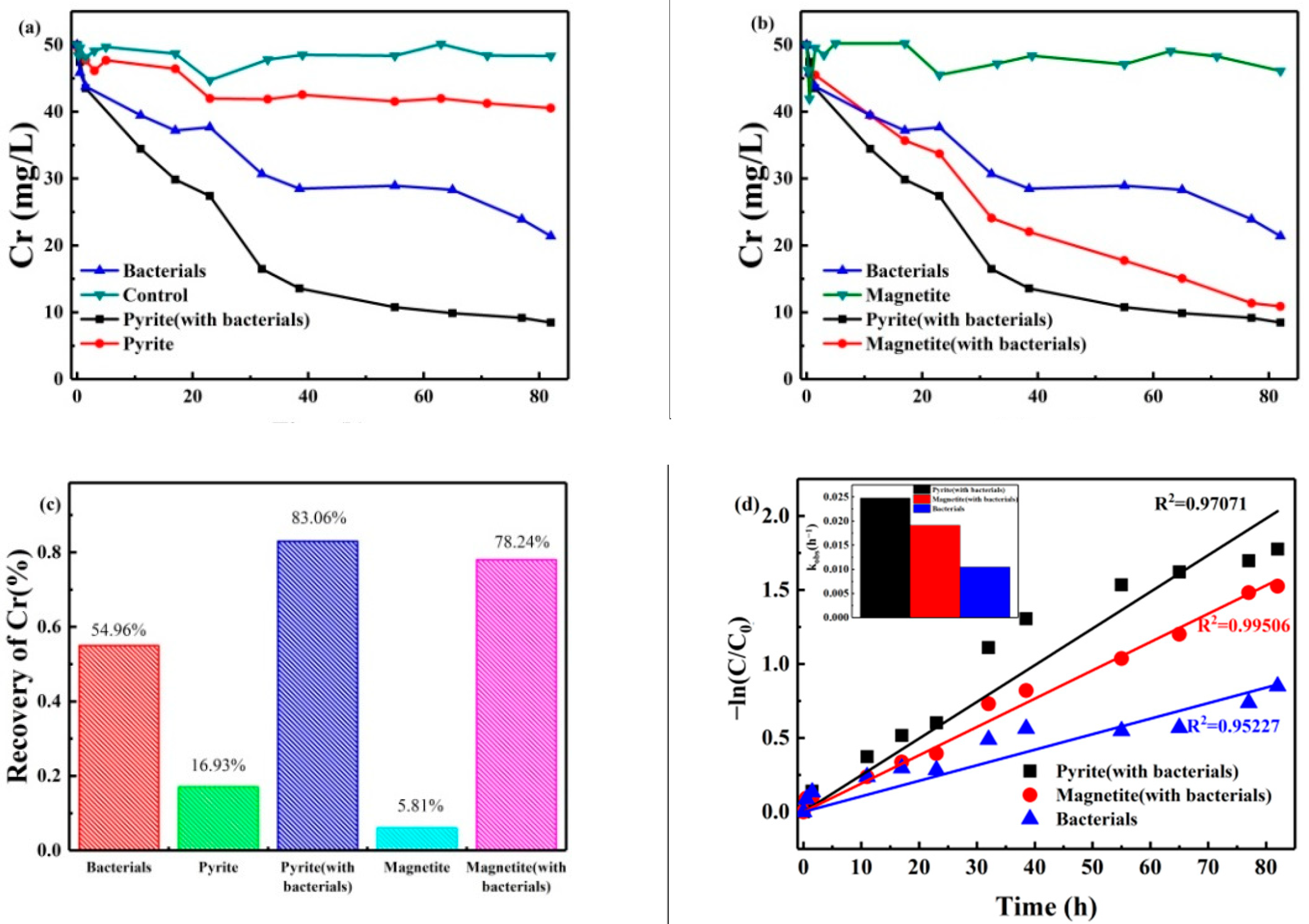
3.1. Cr (VI) Reduction Kinetics
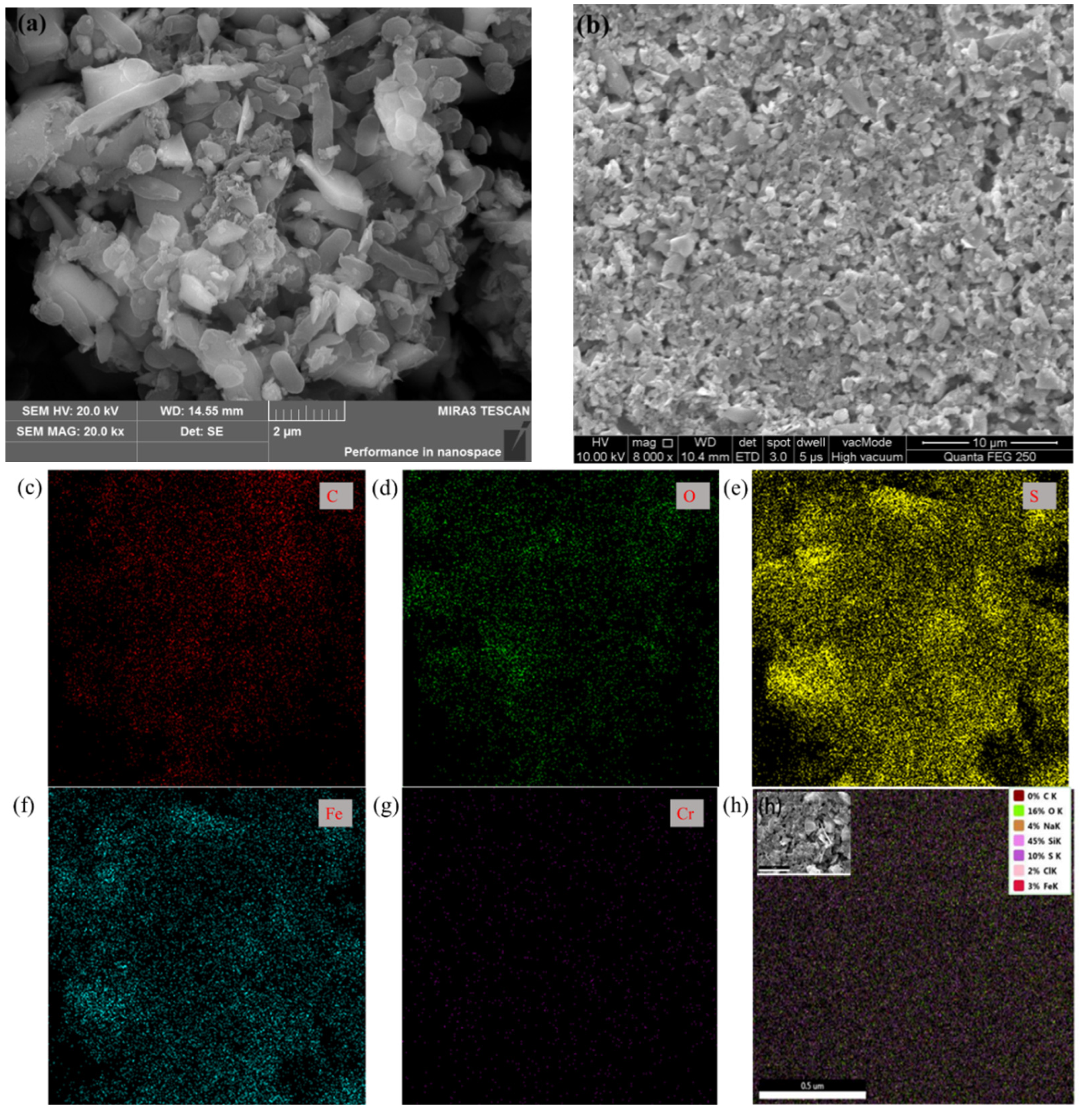
3.2. SEM and EDS Analyses of Surface Morphology
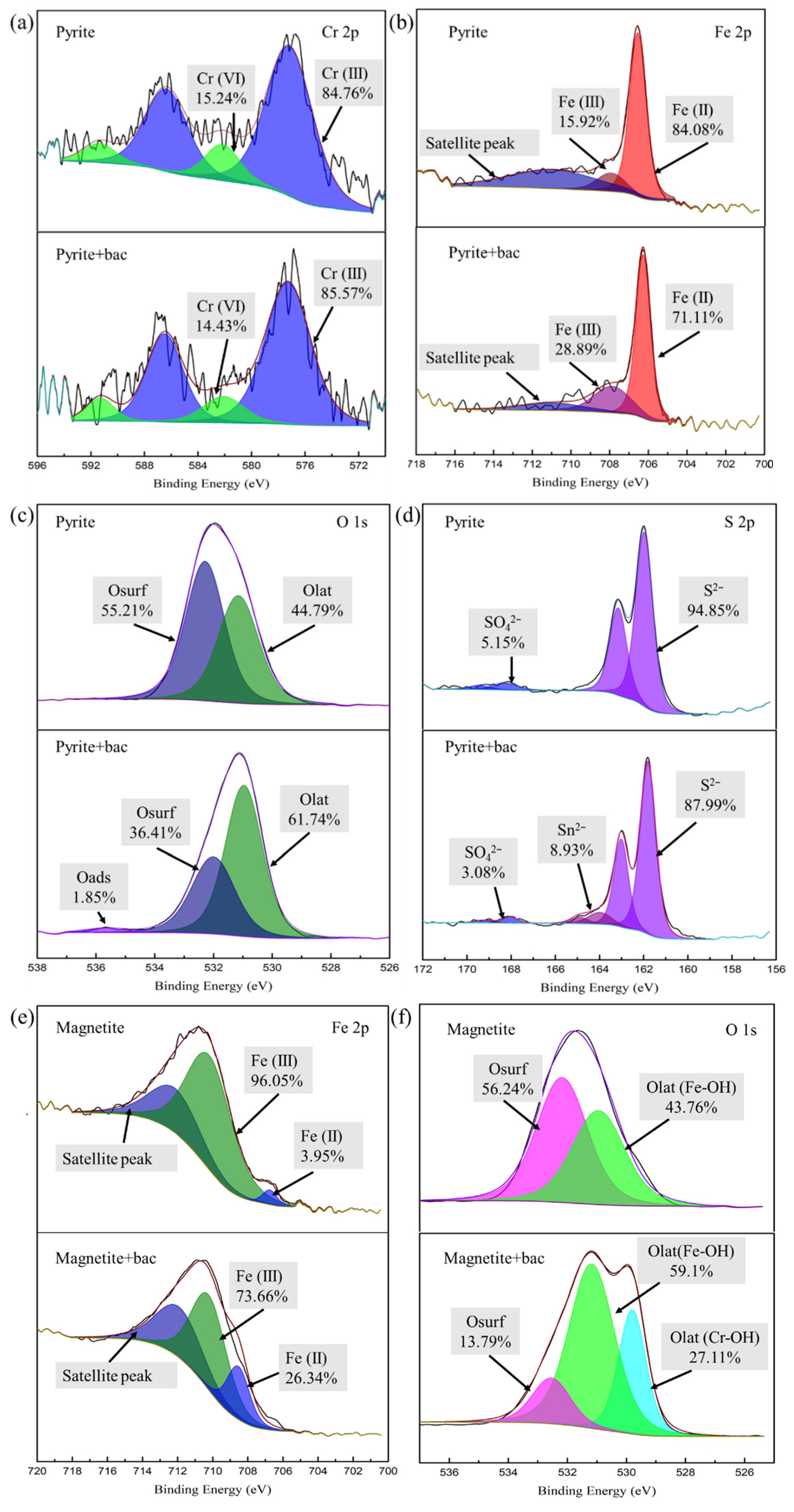
3.3. XPS Analysis of Different Iron Ore Surfaces
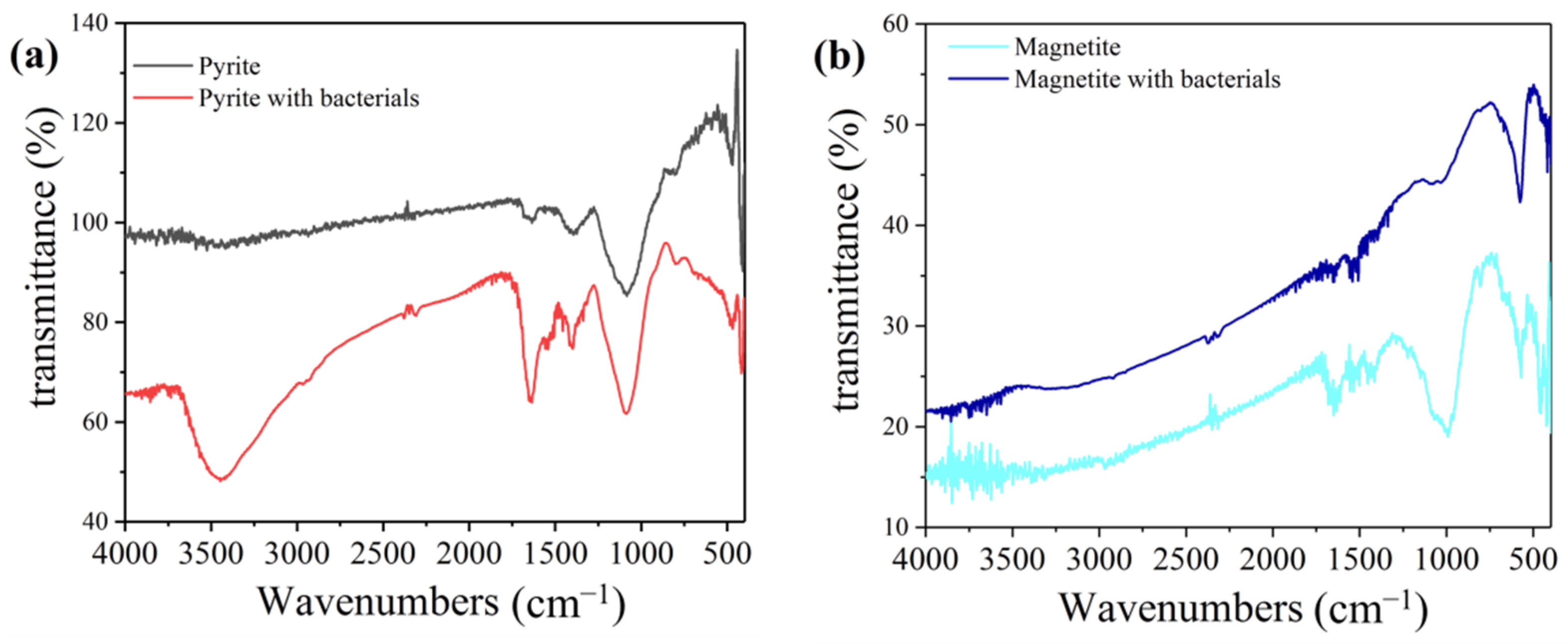
3.4. FTIR Spectroscopy
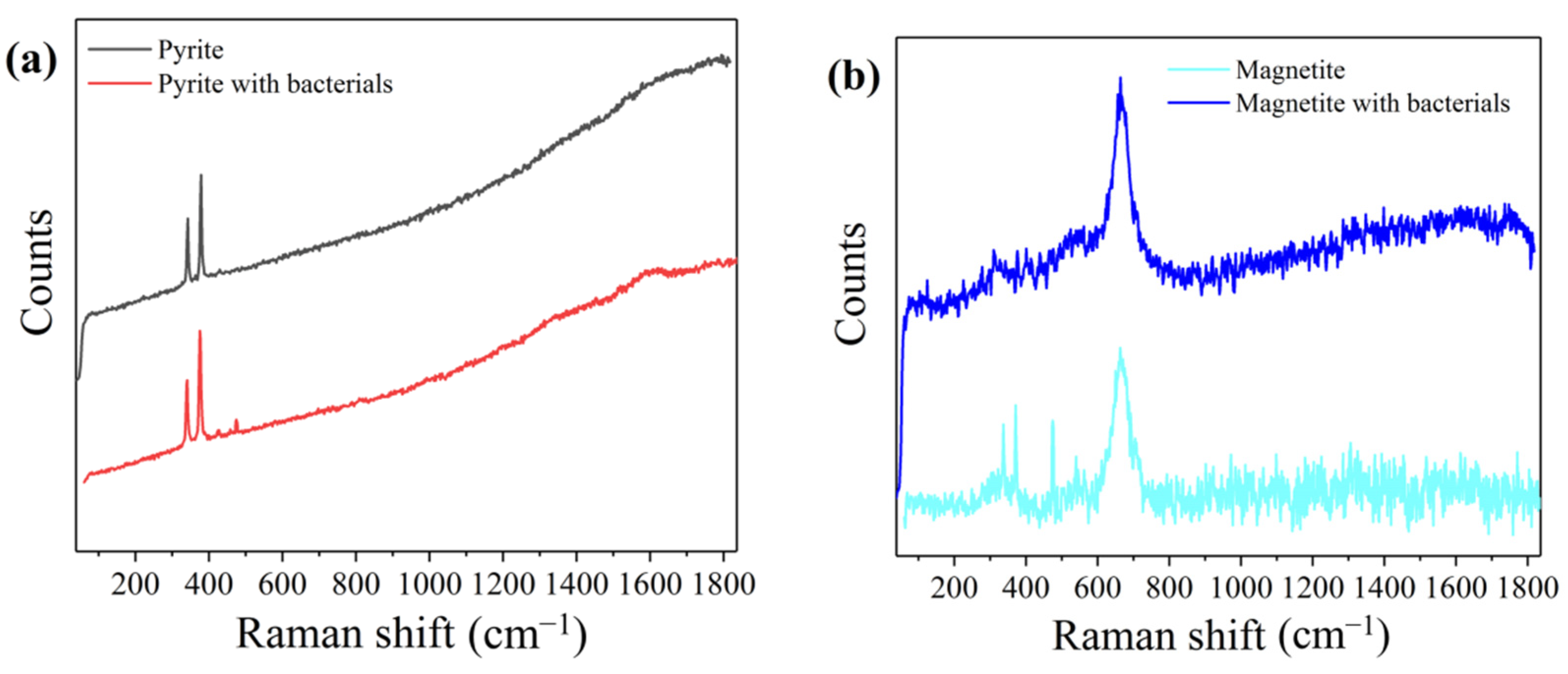
3.5. Raman Spectroscopy
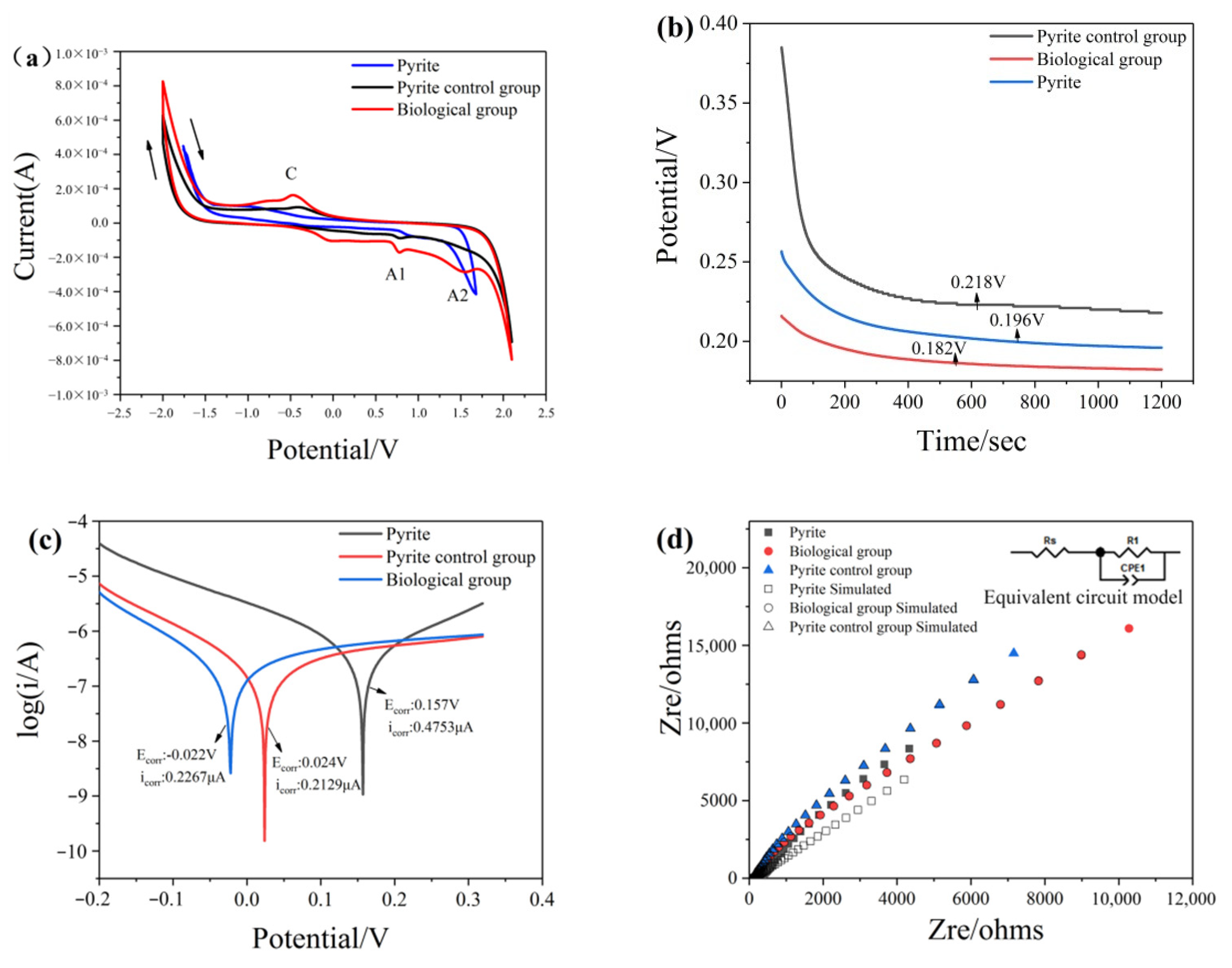
3.6. Electrochemical Analysis
3.7. Changes in Microbial Community Structure
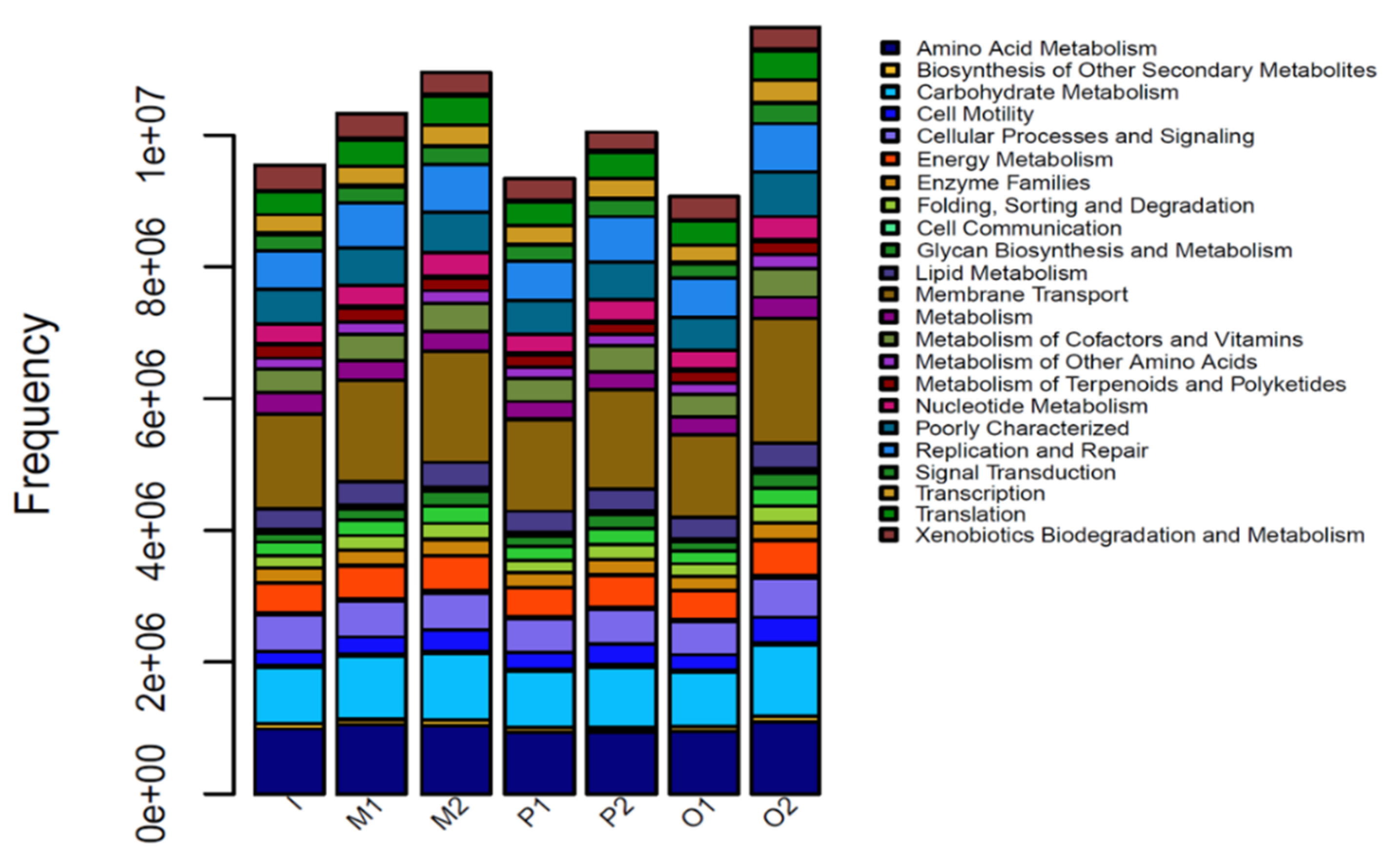
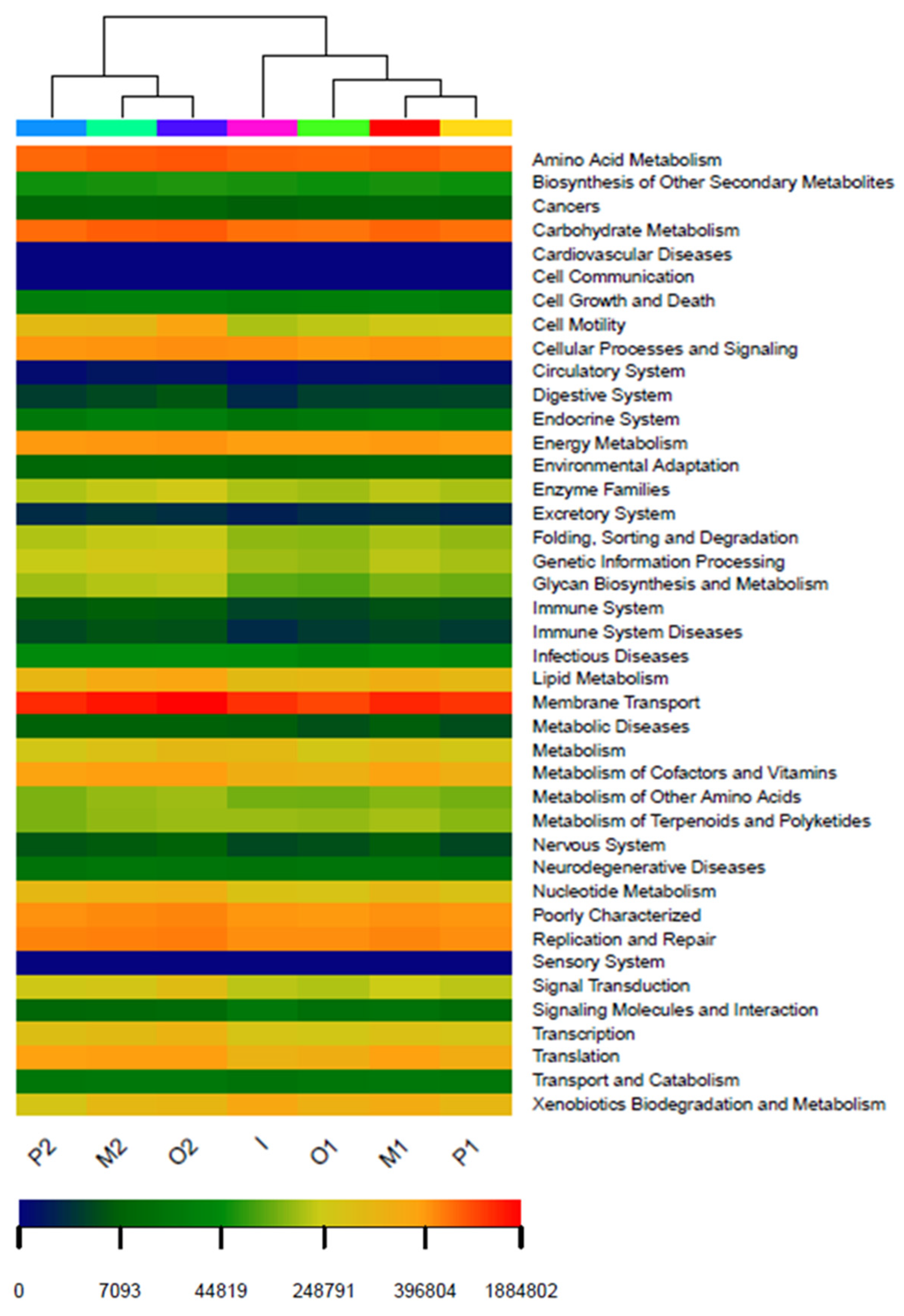
3.8. PICRUSt Function Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Locq, D.; Caron, P.; Ramusat, C.; Mevrel, R. Quaternary chromium-based alloys strengthened by Heusler phase precipitation. Mater. Sci. Eng. A 2015, 647, 322–332. [Google Scholar] [CrossRef]
- Wang, X. Research Progress of Chromium Pollution in Soil Remediation Technology. Environ. Sustain. Dev. 2014, 39, 210–212. [Google Scholar]
- Yao, J.J.; Wang, M.R.; Zhang, H.X.; Peng, L.J.; Zhou, Y.X.; Tian, Y.I. Research Advances on Remediation Technology of Chromium Pollution in Nature Water. Mod. Agric. Sci. Technol. 2016, 23, 177–178. [Google Scholar]
- Mandal, S.; Sarkar, B.; Bolan, N.; Ok, Y.S.; Naidu, R. Enhancement of chromate reduction in soils by surface modified biochar. J. Environ. Manag. 2017, 186, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Thorgersen, M.P.; Andrew, L.W.; Ge, X.; Zane, G.M.; Wetmore, K.M.; Vaccaro, B.J.; Poole, F.L.; Younkin, A.D.; Deutschbauer, A.M.; Arkin, A.P. Mechanisms of Chromium and Uranium Toxicity in Pseudomonas stutzeri RCH2 Grown under Anaerobic Nitrate-Reducing Conditions. Front. Microbiol. 2017, 8, 1529. [Google Scholar] [CrossRef]
- Gan, M.; Li, J.; Sun, S.; Ding, J.; Zhu, J.; Liu, X.; Qiu, G. Synergistic effect between sulfide mineral and acidophilic bacteria significantly promoted Cr(Ⅵ) reduction. J. Environ. Manag. 2018, 219, 84–94. [Google Scholar] [CrossRef]
- Barrera-Diaz, C.E.; Lugo-Lugo, V.; Bilyeu, B. A review of chemical, electrochemical and biological methods for aqueous Cr(VI) reduction. J. Hazard. Mater. 2012, 223–224, 1–12. [Google Scholar] [CrossRef]
- Shang, J.; Zong, M.; Yu, Y.; Kong, X.; Du, Q.; Liao, Q. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef]
- Liang, J.; Tang, D.; Wu, H.; Wang, L. Environment corrosion behavior of cargo oil tank deck made of Cr-contained low-alloy steel. J. Southeast Univ. 2013, 43, 152–157. [Google Scholar]
- Joe-Wong, C.; Brown, G.E., Jr.; Maher, K. Kinetics and Products of Chromium(VI) Reduction by Iron(II/III)-Bearing Clay Minerals. Environ. Sci. Technol. 2017, 51, 9817–9825. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.T.; Huang, C.P. Reduction of chromium(VI) by pyrite in dilute aqueous solutions. Sep. Purif. Technol. 2008, 63, 191–199. [Google Scholar] [CrossRef]
- Thavamani, P.; Samkumar, R.A.; Satheesh, V.; Subashchandrabose, S.R.; Ramadass, K.; Naidu, R.; Venkateswarlu, K.; Megharaj, M. Microbes from mined sites: Harnessing their potential for reclamation of derelict mine sites. Environ. Pollut. 2017, 230, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Extracellular electron transfer: Wires, capacitors, iron lungs, and more. Geobiology 2008, 6, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gralnick, J.A.; Newman, D.K. Extracellular respiration. Mol. Microbiol. 2007, 65, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.-J.; Chen, T.-H. Biodegradation of phenol with chromium (VI) reduction by the Pseudomonas sp strain JF122. Desalination Water Treat. 2016, 57, 3544–3551. [Google Scholar] [CrossRef]
- Xafenias, N.; Zhang, Y.; Banks, C.J. Enhanced Performance of Hexavalent Chromium Reducing Cathodes in the Presence of Shewanella oneidensis MR-1 and Lactate. Environ. Sci. Technol. 2013, 47, 4512–4520. [Google Scholar] [CrossRef] [Green Version]
- Kato, S.; Nakamura, R.; Kai, F.; Watanabe, K.; Hashimoto, K. Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ. Microbiol. 2010, 12, 3114–3123. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Calabrese, A.F.; Stryhanyuk, B.H.; Musat, B.F.; Shrestha, B.P.M. Conductive Particles Enable Syntrophic Acetate Oxidation between Geobacter and Methanosarcina from Coastal Sediments. Mbio 2018, 9, e00218–e00226. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhao, H.; Sun, M.; Zhang, Y.; Qiu, G. The role of cupric ions in the oxidative dissolution process of marmatite: A dependence on Cu2+ concentration. Sci. Total Environ. 2019, 675, 213–223. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.; Zhang, W.; Meldrum, D.R. Optimization of whole-transcriptome amplification from low cell density deep-sea microbial samples for metatranscriptomic analysis. J. Microbiol. Methods 2011, 84, 88–93. [Google Scholar] [CrossRef]
- Gan, M.; Song, Z.; Jie, S.; Zhu, J.; Zhu, Y.; Liu, X. Biosynthesis of bifunctional iron oxyhydrosulfate by Acidithiobacillus ferroxidans and their application to coagulation and adsorption. Mater. Sci. Eng. C 2016, 59, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.M.; Bouwer, E.J. Oxidative dissolution of pyrite surfaces by hexavalent chromium: Surface site saturation and surface renewal. Geochim. Et Cosmochim. Acta 2012, 83, 379–396. [Google Scholar] [CrossRef]
- Jung, Y.; Choi, J.; Lee, W. Spectroscopic investigation of magnetite surface for the reduction of hexavalent chromium. Chemosphere 2007, 68, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, J.; Yang, Z.; Wang, H.; Liu, Y. Reduction and immobilization of hexavalent chromium in chromite ore processing residue using amorphous FeS2. Sci. Total Environ. 2018, 685, 315–323. [Google Scholar] [CrossRef]
- Derycke, V.; Kongolo, M.; Benzaazoua, M.; Mallet, M.; Barres, O.; De Donato, P.; Bussiere, B.; Mermillod-Blondin, R. Surface chemical characterization of different pyrite size fractions for flotation purposes. Int. J. Miner. Process. 2013, 118, 1–14. [Google Scholar] [CrossRef]
- Tu, Z.; Guo, C.; Zhang, T.; Lu, G.; Wan, J.; Liao, C.; Dang, Z. Investigation of intermediate sulfur species during pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Hydrometallurgy 2017, 167, 58–65. [Google Scholar] [CrossRef]
- Wang, X.-N.; Sun, G.-X.; Zhu, Y.-G. Thermodynamic energy of anaerobic microbial redox reactions couples elemental biogeochemical cycles. J. Soils Sediments 2017, 106, 37–47. [Google Scholar] [CrossRef]
- Xu, X.; Yang, Y.; Jia, Y.; Lian, X.; Zhang, Y.; Feng, F.; Liu, Q.; Xi, B.; Jiang, Y. Heterogeneous catalytic degradation of 2,4-dinitrotoluene by the combined persulfate and hydrogen peroxide activated by the as-synthesized Fe-Mn binary oxides. Chem. Eng. J. 2019, 374, 776–786. [Google Scholar] [CrossRef]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Mills, A.L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Et Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Vedavathi, A.; Reddy, Y.M.; Reddy, K. Effect of Precursor Concentration on Structural and Morphological Properties of Iron Pyrite Thin Films. Procedia Mater. Sci. 2015, 10, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.L.; Zhang, P.; Chen, Y.H.; Pan, J.Y.; Wang, J.; Qi, J.Y.; Li, X.P.; Liu, J. Study on the purification property of pyrite and its spectra on the processing of metal-bearing wastewater. Environ. Earth Sci. 2010, 61, 939–945. [Google Scholar] [CrossRef]
- Fleischer, K.; Caffrey, D.; Farrell, L.; Norton, E.; Mullarkey, D.; Arca, E.; Shvets, I.V. Raman spectra of p-type transparent semiconducting Cr2O3:Mg. Thin Solid Film 2015, 594, 245–249. [Google Scholar] [CrossRef] [Green Version]
- Lazor, S.P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar]
- Chen, G.; Yang, H. Electrochemical study on Surface Oxidation of Natural Pyrite in Ferric Sulfate Solution. Int. J. Electrochem. Sci. 2019, 14, 7047–7061. [Google Scholar] [CrossRef]
- Bonaglia, S.; Broman, E.; Brindefalk, B.; Hedlund, E.; Hjorth, T.; Rolff, C.; Nascimento, F.J.A.; Udekwu, K.; Gunnarsson, J.S. Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 2020, 248, 126023. [Google Scholar] [CrossRef] [PubMed]


| Amplification System | |
|---|---|
| 2 × Taq master Mix | 15 μL |
| primer F (10 μM) | 1 μL |
| Primer R (10 μM) | 1 μL |
| Genomic DNA | 10–20 ng |
| H20 | Add to 30 μL |
| Atomic % | C K | O K | S K | FeK | CrK |
|---|---|---|---|---|---|
| Pyrite with bacterials | 15.56 | 8.02 | 49.75 | 26.07 | 0.2 |
| Pyrite | 7,87 | 57.48 | 6.4 | 37.8 | / |
| Eletment | Rs (Ω) | R1 (Ω) | CPE(Ss1/2) | |
|---|---|---|---|---|
| CPE-T | CPE-P | |||
| Pyrite | 127.3 | 53,642 | 9.10 × 10−6 | 0.79377 |
| Pyrite biological group | 125.1 | 45,681 | 8.85 × 10−6 | 0.81858 |
| Pyrite control group | 125.8 | 61,016 | 1.15 × 10−5 | 0.84449 |
| Magnetite | 113.8 | 73,669 | 7.0 × 10−6 | 0.70527 |
| Magnetite biological group | 109.7 | 34,485 | 4.67 × 10−6 | 0.74593 |
| Magnetite contral group | 128.4 | 93,788 | 1.20 × 10−5 | 0.6927 |
| Sample | Shannon | ACE | Chao1 | Coverage | Simpson |
|---|---|---|---|---|---|
| I | 1.396 | 643.051 | 381.428 | 0.998 | 0.457 |
| M1 | 1.832 | 551.874 | 322.036 | 0.999 | 0.249 |
| M2 | 1.880 | 1069.374 | 484.564 | 0.998 | 0.226 |
| P1 | 1.853 | 350.333 | 265.029 | 0.999 | 0.220 |
| P2 | 2.084 | 349.812 | 231.189 | 0.999 | 0.185 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Lei, P.; Liu, M.; He, P.; Chen, Y.; Gan, M.; Zhu, J. The Interplay of Iron Minerals and Microflora to Accelerate Cr (VI) Reduction. Minerals 2022, 12, 460. https://doi.org/10.3390/min12040460
Zhu J, Lei P, Liu M, He P, Chen Y, Gan M, Zhu J. The Interplay of Iron Minerals and Microflora to Accelerate Cr (VI) Reduction. Minerals. 2022; 12(4):460. https://doi.org/10.3390/min12040460
Chicago/Turabian StyleZhu, Jinglei, Pan Lei, Mengfei Liu, Peng He, Yaozong Chen, Min Gan, and Jianyu Zhu. 2022. "The Interplay of Iron Minerals and Microflora to Accelerate Cr (VI) Reduction" Minerals 12, no. 4: 460. https://doi.org/10.3390/min12040460
APA StyleZhu, J., Lei, P., Liu, M., He, P., Chen, Y., Gan, M., & Zhu, J. (2022). The Interplay of Iron Minerals and Microflora to Accelerate Cr (VI) Reduction. Minerals, 12(4), 460. https://doi.org/10.3390/min12040460






