Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation
Abstract
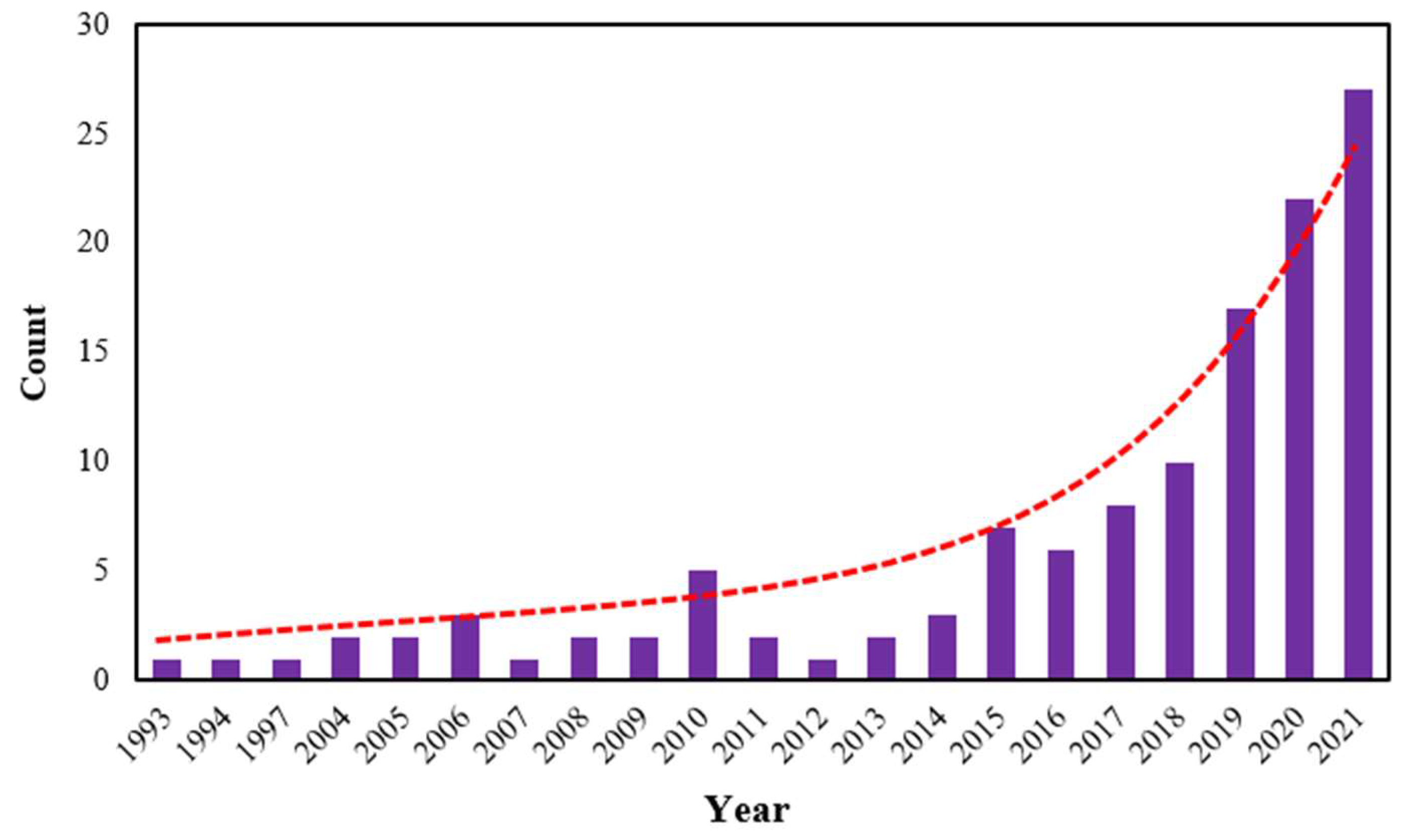
:1. Introduction
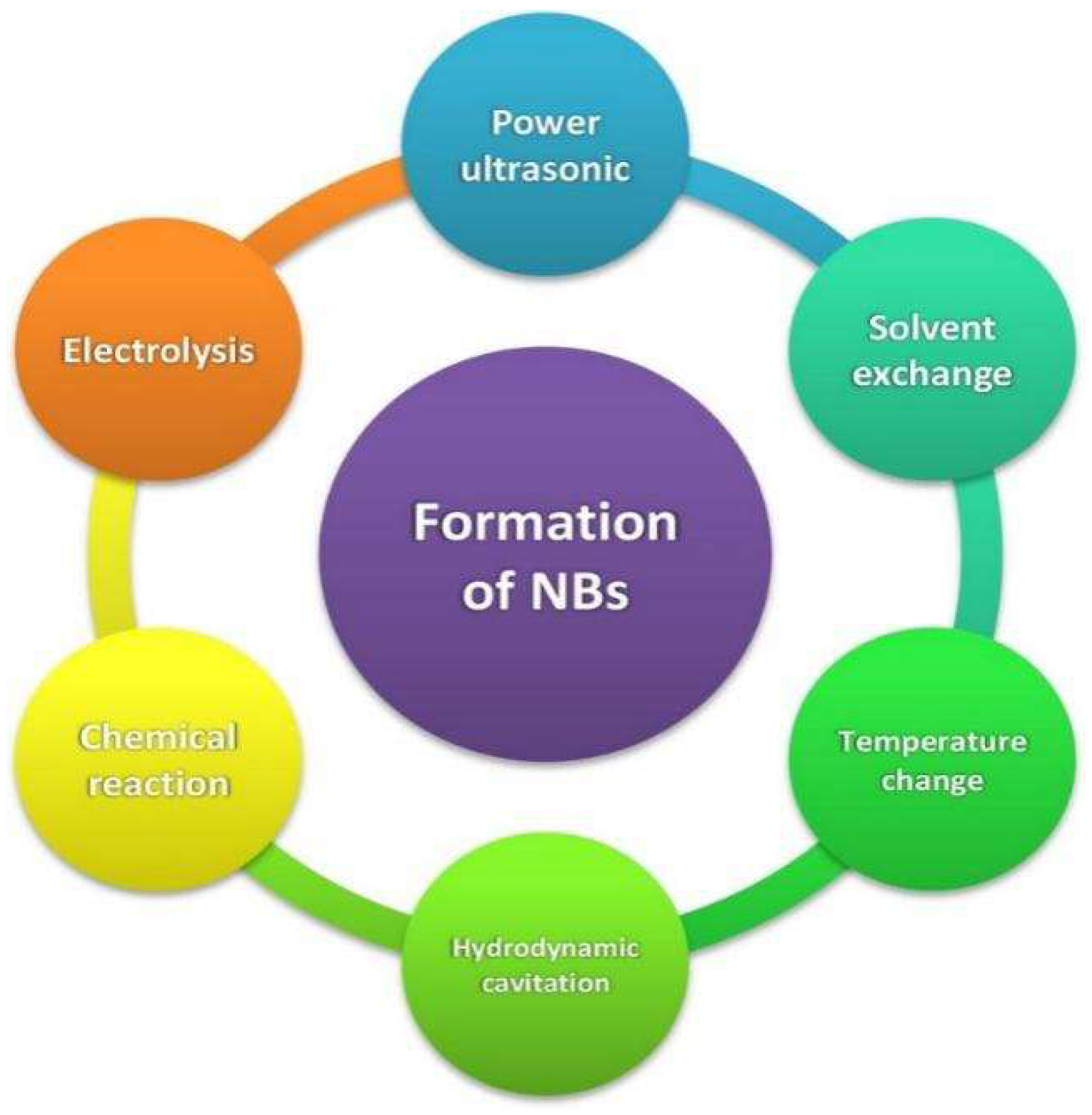
2. Generation Techniques
2.1. Power Ultrasound
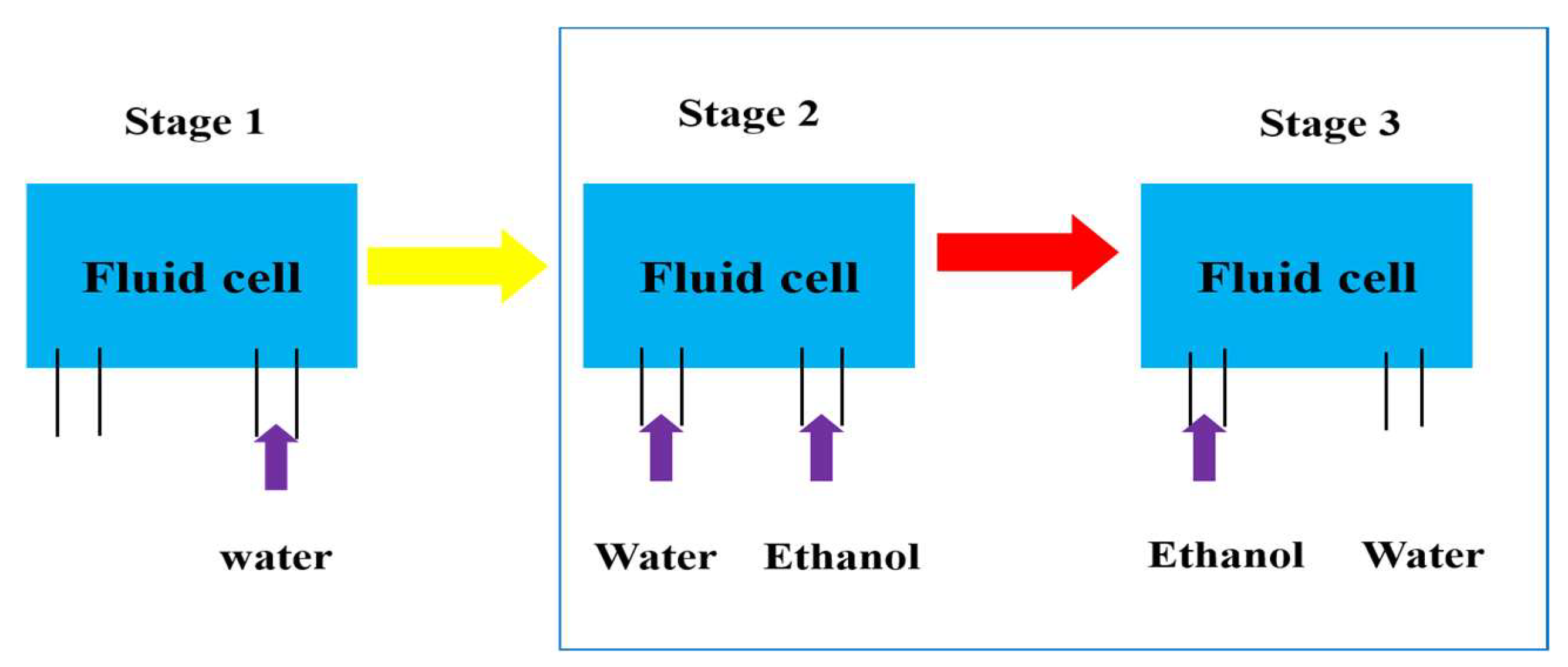
2.2. Solvent Exchange
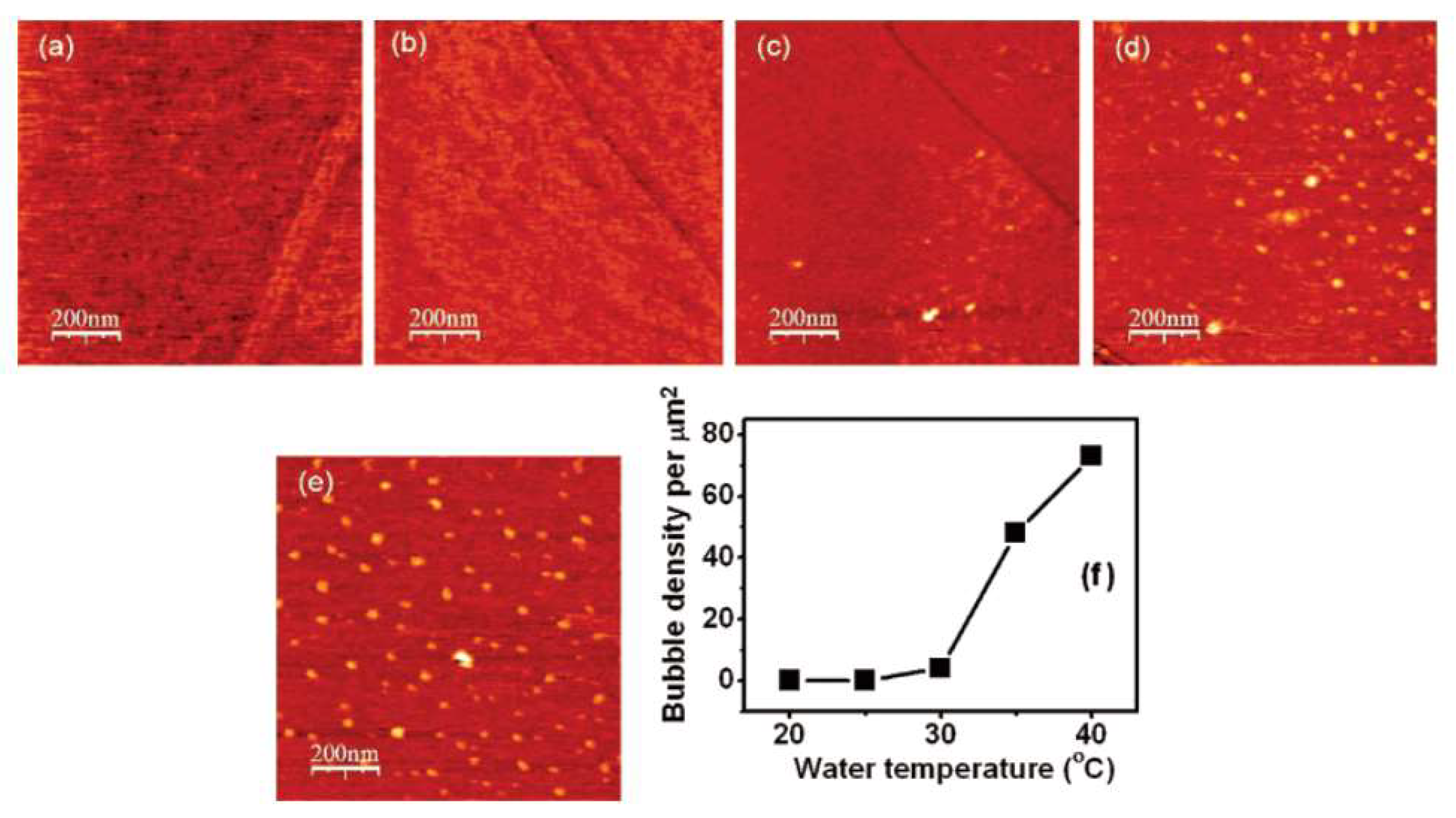
2.3. Temperature Change
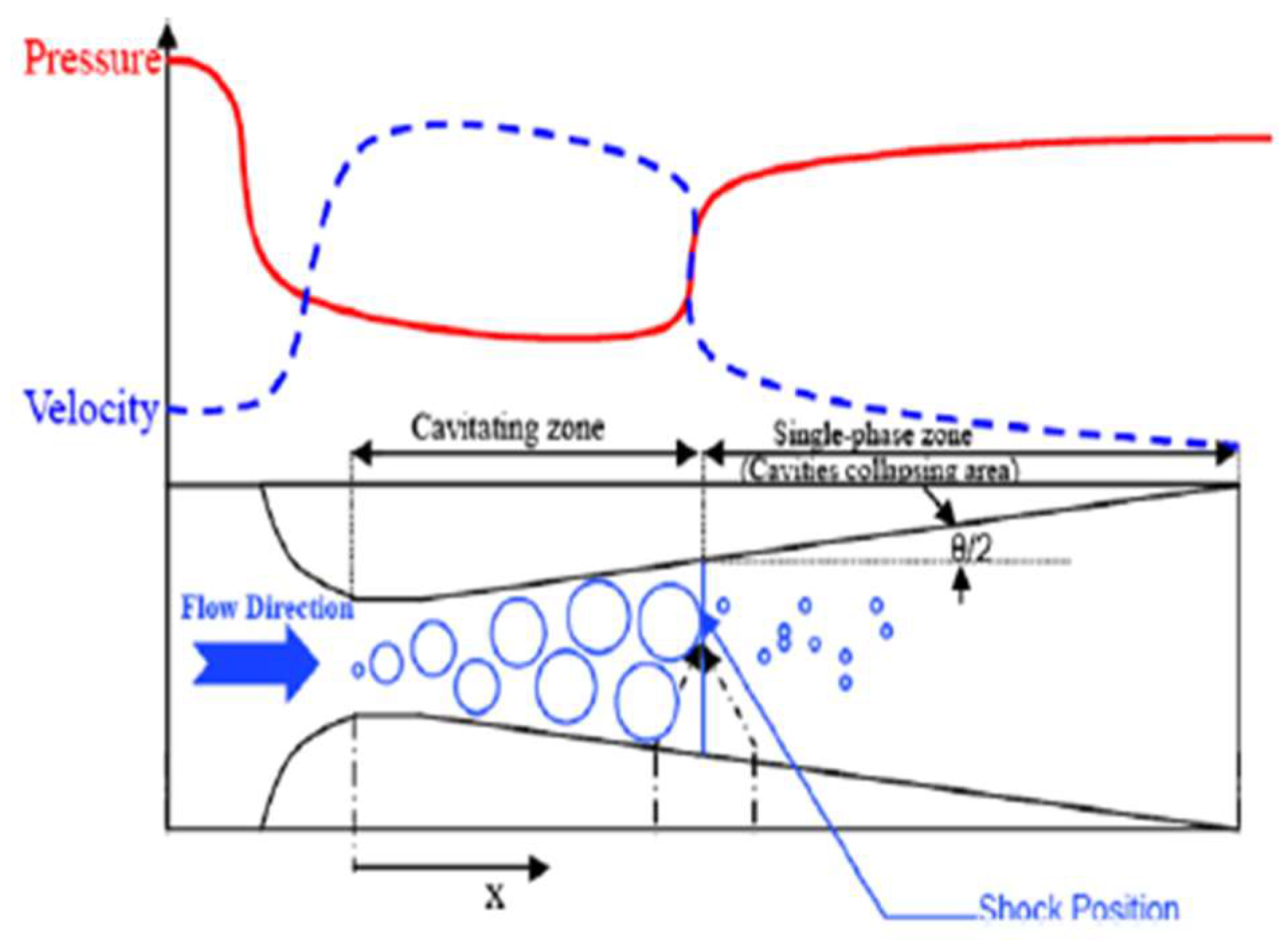
2.4. Hydrodynamic Cavitation
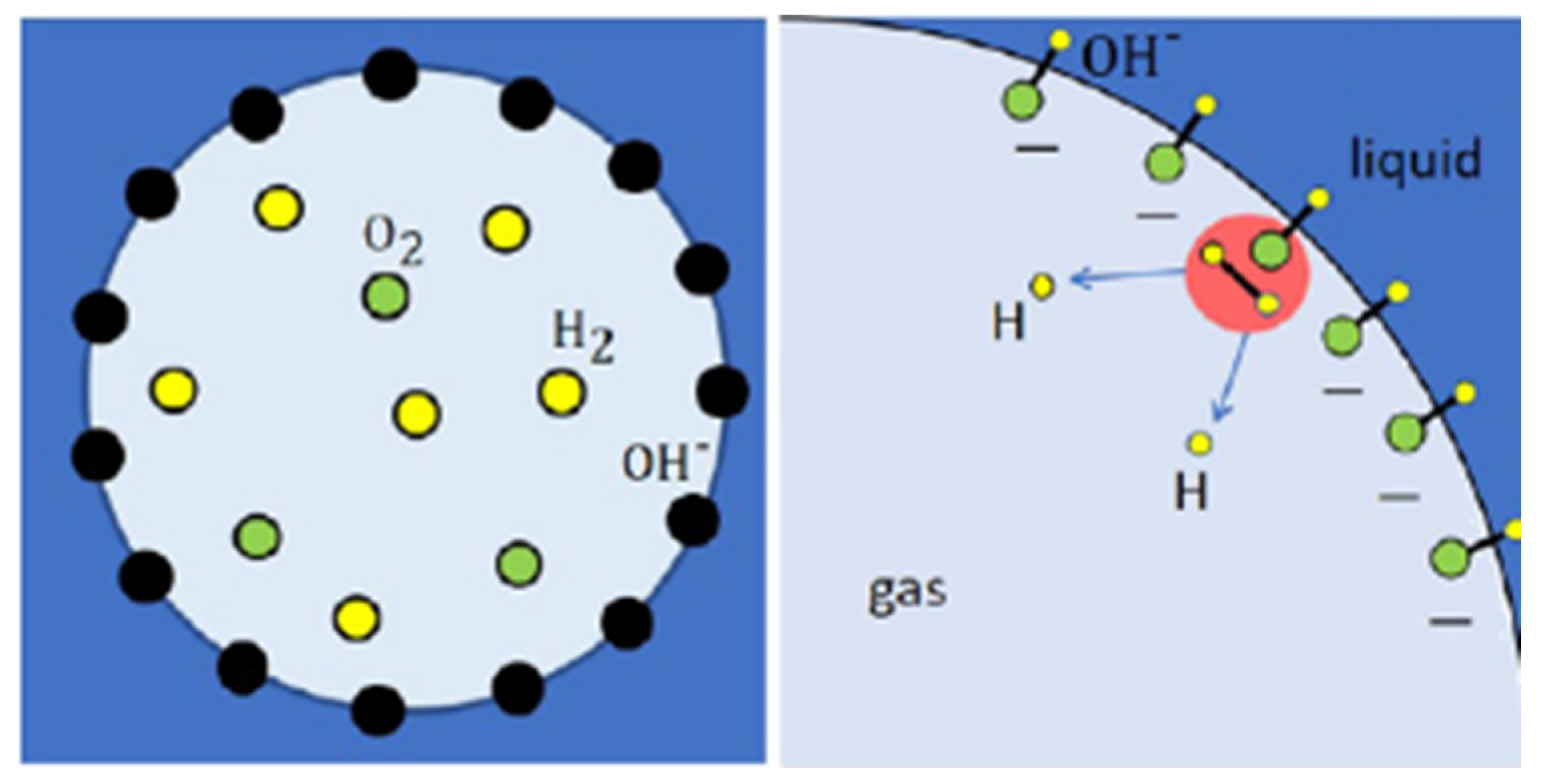
2.5. Electrolysis and Chemical Reaction
3. Bubble Size-Measurement Techniques
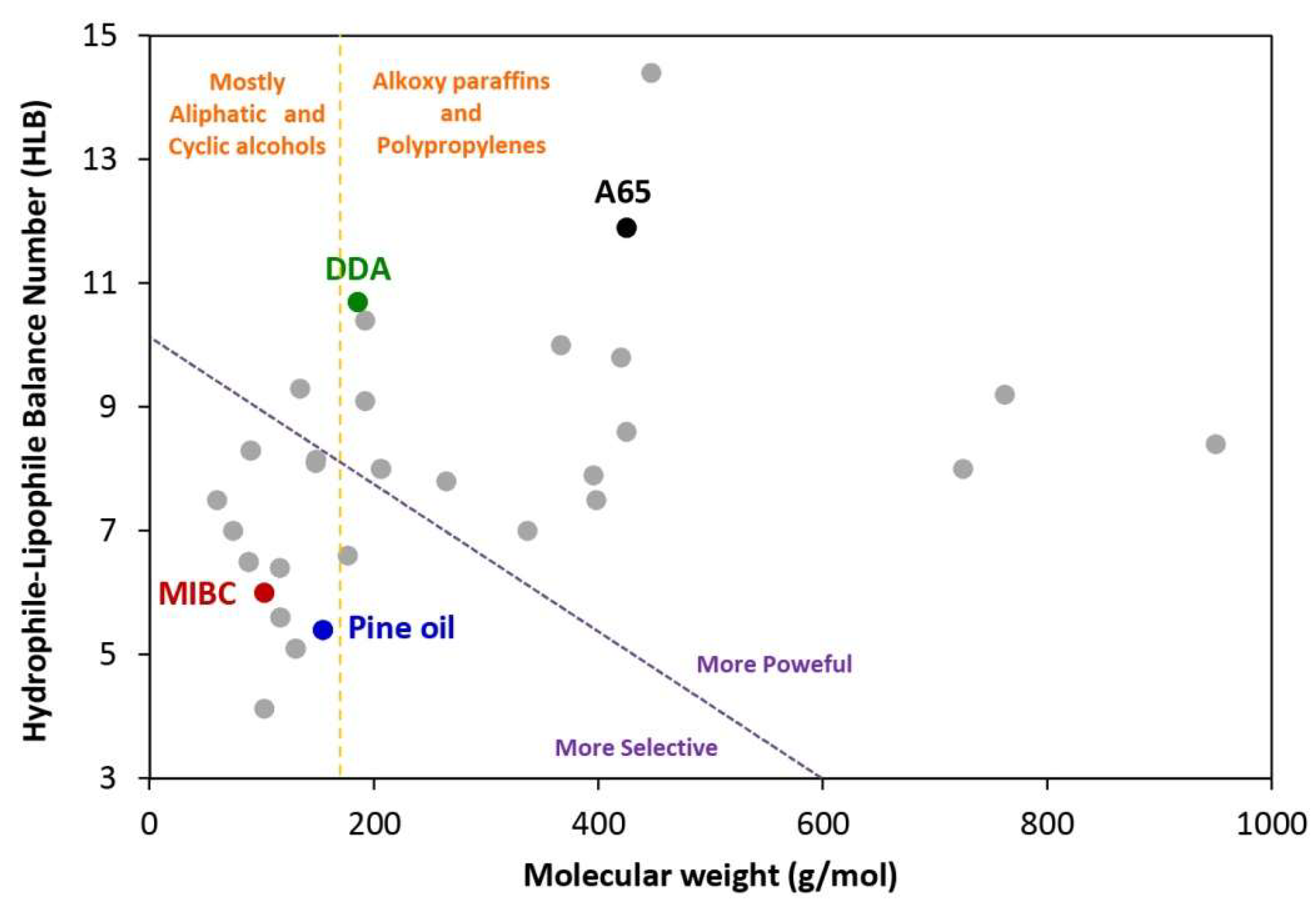
4. Commonly Used Reagents for Producing NBs
5. NB-Assisted Flotation
6. Conclusions and Future Works
- Evaluating literature data showed that while CB analyzers are used to detect ultrafine bubble sizes and distributions, the most commonly used methods are LPSA and NTA instruments, which may include a reasonable amount of bias.
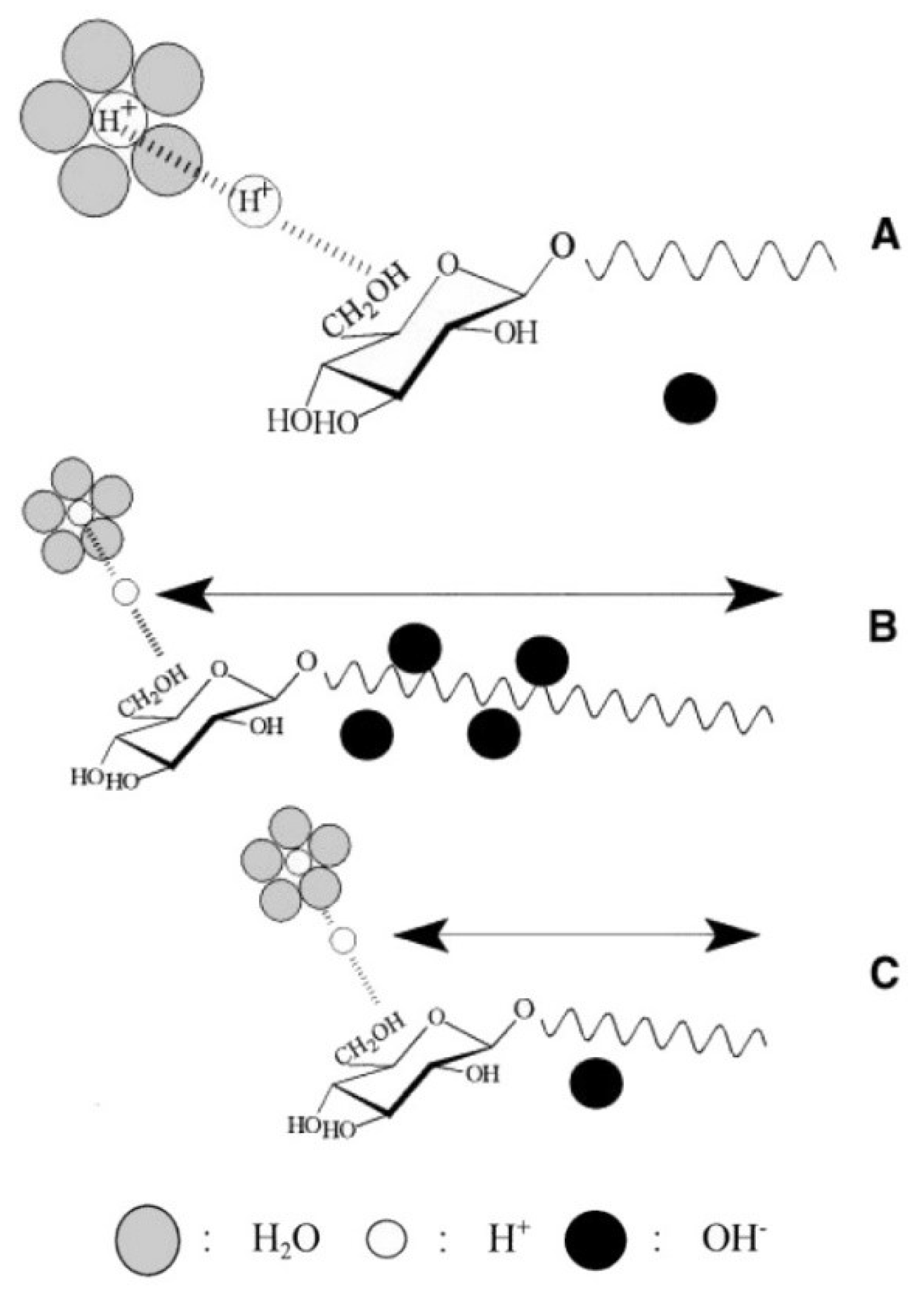
- Alkaline environments lead to the formation of smaller and stable bubbles because highly negatively charged bubbles tend to repel each other, which prevents inter-bubble aggregation and coalescence.
- Hydrodynamic cavitation was found to be the most popular technique for producing NBs, which can be likely extended to industrial applications in the future.
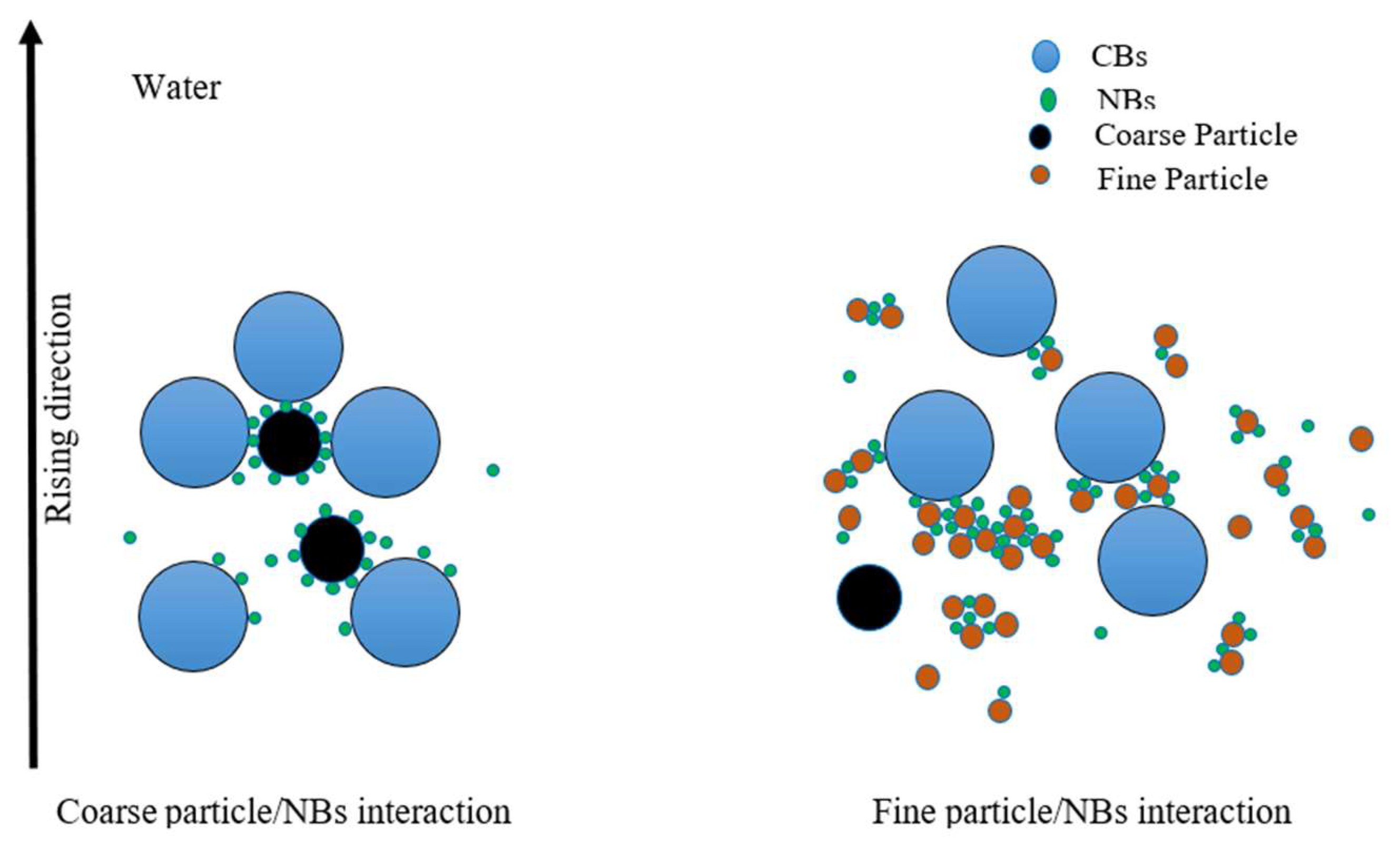
- An integrated separation of minerals in flotation using ultrafine bubbles reduces collector and frother consumption and improves not only recovery but also the flotation rate constant of fine, ultrafine, and coarse particles.
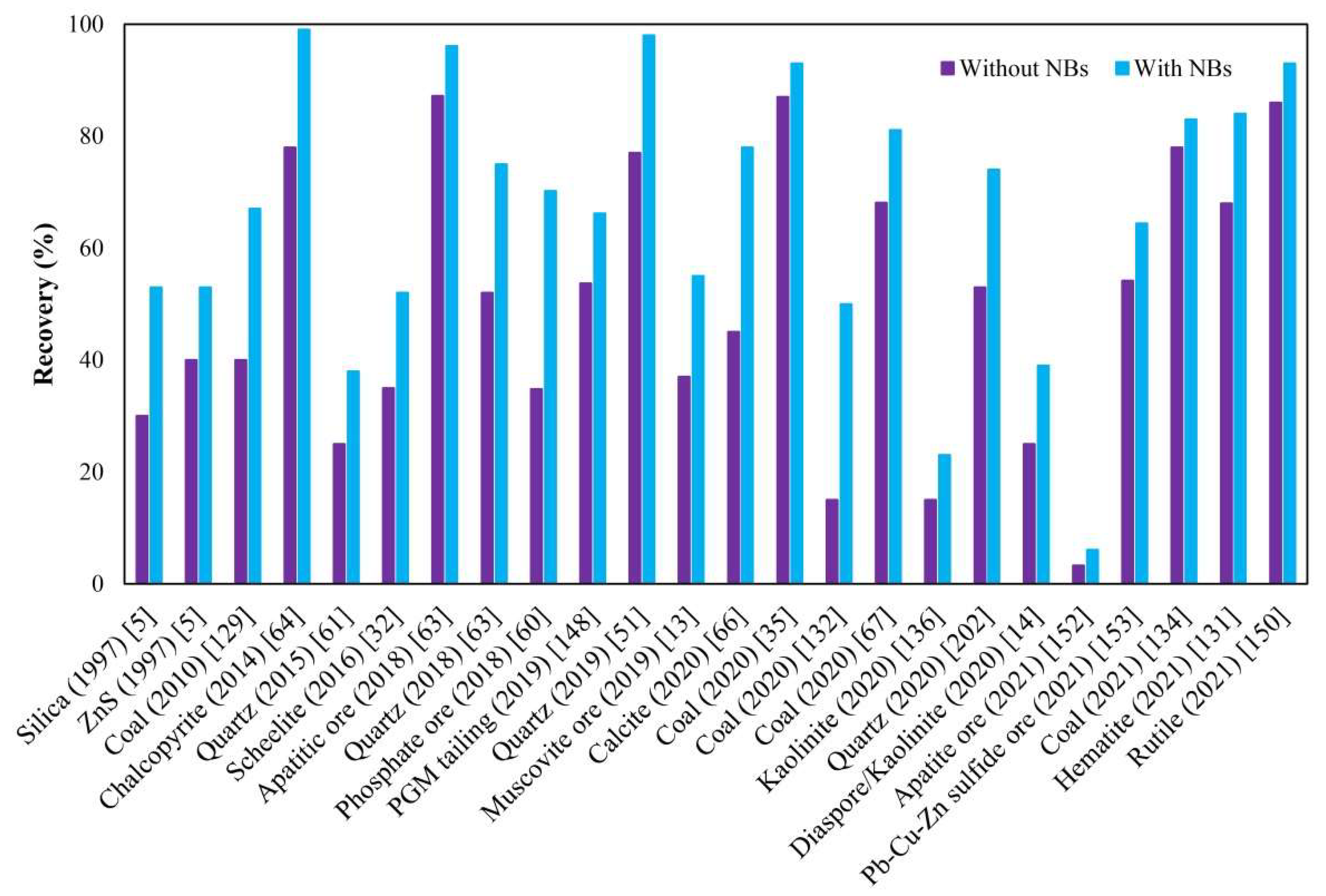
- Flotation in the presence of NBs can elevate the recoverability of mono-minerals by approximately 15% and 20% on average using mechanical and column flotation cells, respectively.
- Most of the focus in the last two decades has been on approving the existence, stability, and impact of single-mineral flotation, and little attention and few reports have been on bulk and actual ores.
- There are no solid comparative results concerning generating and observing ultrafine bubbles using commonly used apparatus. Thus, such information can help in better quantitative judgment among common techniques.
- Although some basic principles about how to generate and apply NBs to flotation research and operations are known, challenges remain in quantifying and mathematically describing their role in flotation.
- Future research should be focused on understanding the stabilization mechanisms of bubbles generated by different methods, optimizing their size ranges for maximized flotation recovery, minimizing wear and damage in industrial operations, and intensifying the role of in situ NB nucleation on particles in flotation.
- From an economic point of view, there is no information in the literature about total costs versus metallurgical beneficiations.
- Although a reasonable degree of recovery improvement has been widely reported in the literature, researchers have rarely reported the impact of NBs on grade, separation efficiency, and selectivity of separation. Thus, further studies are recommended in this sense.
- The synergy of chemical, physical, and hydrodynamic features for NB generation in an energy-efficient, technically effective, and user-friendly manner, with controlled sizes of generated bubbles, are also important goals in the future.
Author Contributions
Funding
Conflicts of Interest
References
- Nirmalkar, N.; Pacek, A.; Barigou, M. On the existence and stability of bulk nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef]
- Weijs, J.H.; Seddon, J.R.; Lohse, D. Diffusive shielding stabilizes bulk nanobubble clusters. ChemPhysChem 2012, 13, 2197–2204. [Google Scholar] [CrossRef] [Green Version]
- Fox, F.E.; Herzfeld, K.F. Gas bubbles with organic skin as cavitation nuclei. J. Acoust. Soc. Am. 1954, 26, 984–989. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Finch, J.A. On the role of cavitation in particle collection during flotation—A critical review. Miner. Eng. 1994, 7, 1073–1084. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Z.; Finch, J.; Hu, H.; Rao, S. Role of hydrodynamic cavitation in fine particle flotation. Int. J. Miner. Process. 1997, 51, 139–149. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Finch, J.A.; Masliyah, J.H.; Chow, R.S. On the role of cavitation in particle collection in flotation—A critical review. II. Miner. Eng. 2009, 22, 419–433. [Google Scholar] [CrossRef]
- Lou, S.T.; Ouyang, Z.Q.; Zhang, Y.; Li, X.J.; Hu, J.; Li, M.Q.; Yang, F.J. Nanobubbles on solid surface imaged by atomic force microscopy. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2000, 18, 2573–2575. [Google Scholar] [CrossRef]
- Zatzkis, H. Sound field of a moving cylinder and a moving sphere. J. Acoust. Soc. Am. 1954, 26, 169–173. [Google Scholar] [CrossRef]
- Eklund, F.; Swenson, J. Stable Air Nanobubbles in Water: The Importance of Organic Contaminants. Langmuir 2018, 34, 11003–11009. [Google Scholar] [CrossRef]
- Li, D.; Jing, D.; Pan, Y.; Bhushan, B.; Zhao, X. Study of the relationship between boundary slip and nanobubbles on a smooth hydrophobic surface. Langmuir 2016, 32, 11287–11294. [Google Scholar] [CrossRef]
- Fan, F.; Daniel, T.; Honaker, R.; Zhenfu, L. Nanobubble generation and its applications in froth flotation (part II): Fundamental study and theoretical analysis. Min. Sci. Technol. (China) 2010, 20, 159–177. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Shahbazi, B.; Chelgani, S.C. Study relationships between flotation variables and recovery of coarse particles in the absence and presence of nanobubble. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 284–288. [Google Scholar] [CrossRef]
- Zhou, W.; Niu, J.; Xiao, W.; Ou, L. Adsorption of bulk nanobubbles on the chemically surface-modified muscovite minerals. Ultrason. Sonochemistry 2019, 51, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, K.; Wang, L.; Zhou, B.; Niu, J.; Ou, L. The role of bulk micro-nanobubbles in reagent desorption and potential implication in flotation separation of highly hydrophobized minerals. Ultrason. Sonochemistry 2020, 64, 104996. [Google Scholar] [CrossRef]
- Lu, X.M.; Yuan, B.; Zhang, X.R.; Yang, K.; Ma, Y.Q. Molecular modeling of transmembrane delivery of paclitaxel by shock waves with nanobubbles. Appl. Phys. Lett. 2017, 110, 023701. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interface Sci. 2017, 246, 40–51. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.Y.; Chun, J.H.; Kim, J.D. Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 28–34. [Google Scholar] [CrossRef]
- Najafi, A.S.; Drelich, J.; Yeung, A.; Xu, Z.; Masliyah, J. A novel method of measuring electrophoretic mobility of gas bubbles. J. Colloid Interface Sci. 2007, 308, 344–350. [Google Scholar] [CrossRef]
- Bhondayi, C. Flotation froth phase bubble size measurement. Miner. Process. Extr. Metall. Rev. 2022, 43, 251–273. [Google Scholar] [CrossRef]
- Bournival, G.; Ata, S.; Jameson, G.J. Bubble and froth stabilizing agents in froth flotation. Miner. Process. Extr. Metall. Rev. 2017, 38, 366–387. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.; Rubio, J. Bulk nanobubbles in the mineral and environmental areas: Updating research and applications. Adv. Colloid Interface Sci. 2019, 271, 101992. [Google Scholar] [CrossRef]
- Yang, S.; Dammer, S.M.; Bremond, N.; Zandvliet, H.J.; Kooij, E.S.; Lohse, D. Characterization of nanobubbles on hydrophobic surfaces in water. Langmuir 2007, 23, 7072–7077. [Google Scholar] [CrossRef] [PubMed]
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 31–37. [Google Scholar] [CrossRef]
- Fan, F.; Daniel, T.; Honaker, R.; Zhenfu, L. Nanobubble generation and its applications in froth flotation (part III): Specially designed laboratory scale column flotation of phosphate. Min. Sci. Technol. (China) 2010, 20, 317–338. [Google Scholar] [CrossRef]
- Tuziuti, T.; Yasui, K.; Kanematsu, W. Influence of addition of degassed water on bulk nanobubbles. Ultrason. Sonochemistry 2018, 43, 272–274. [Google Scholar] [CrossRef]
- Nazari, S.; Hassanzadeh, A. The effect of reagent type on generating bulk sub-micron (nano) bubbles and flotation kinetics of coarse-sized quartz particles. Powder Technol. 2020, 374, 160–171. [Google Scholar] [CrossRef]
- Svetovoy, V.B. Spontaneous chemical reactions between hydrogen and oxygen in nanobubbles. Curr. Opin. Colloid Interface Sci. 2021, 52, 101423. [Google Scholar] [CrossRef]
- Yasuda, K.; Matsushima, H.; Asakura, Y. Generation and reduction of bulk nanobubbles by ultrasonic irradiation. Chem. Eng. Sci. 2019, 195, 455–461. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nagata, S.; Tanaka, Y.; Saihara, Y.; Ogumi, Z. Characteristics of hydrogen nanobubbles in solutions obtained with water electrolysis. J. Electroanal. Chem. 2007, 600, 303–310. [Google Scholar] [CrossRef]
- Aldrich, C.; Feng, D. The effect of mothers on bubble size distributions in flotation pulp phases and surface froths. Miner. Eng. 2000, 13, 1049–1057. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. Hydrodynamic cavitation reactors: A state of the art review. Rev. Chem. Eng. 2001, 17, 1–85. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Oliveira, H.; Azevedo, A.; Rubio, J. Nanobubbles generation in a high-rate hydrodynamic cavitation tube. Miner. Eng. 2018, 116, 32–34. [Google Scholar] [CrossRef]
- Nazari, S.; Chelgani, S.C.; Shafaei, S.; Shahbazi, B.; Matin, S.; Gharabaghi, M. Flotation of coarse particles by hydrodynamic cavitation generated in the presence of conventional reagents. Sep. Purif. Technol. 2019, 220, 61–68. [Google Scholar] [CrossRef]
- Han, H.; Liu, A.; Wang, H. Effect of hydrodynamic cavitation assistance on different stages of coal flotation. Minerals 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Rowe, P.N.; Matsuno, R. Single bubbles injected into a gas fluidised bed and observed by X-rays. Chem. Eng. Sci. 1971, 26, 923–935. [Google Scholar] [CrossRef]
- Hoang, D.H.; Hassanzadeh, A.; Peuker, U.A.; Rudolph, M. Impact of flotation hydrodynamics on the optimization of fine-grained carbonaceous sedimentary apatite ore beneficiation. Powder Technol. 2019, 345, 223–233. [Google Scholar] [CrossRef]
- Sung, J.S.; Burgess, J.M. A laser-based method for bubble parameter measurement in two-dimensional fluidised beds. Powder Technol. 1987, 49, 165–175. [Google Scholar] [CrossRef]
- Naosuke, O.; Koizumi, Y.; Kamide, H.; Ohno, S.; Ito, K. Effect of physical properties on gas entrainment rate from free surface by vortex. Int. Conf. Nucl. Eng. 2013, 55836, V006T16A029. [Google Scholar]
- Rodrigues, R.T.; Rubio, J. New basis for measuring the size distribution of bubbles. Miner. Eng. 2003, 16, 757–765. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Egiebor, N.O.; Plitt, L.R. Frother effects on bubble size estimation in a flotation column. Miner. Eng. 1993, 6, 55–67. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Wang, Q.S.; Wu, Z.X.; Tao, D.P. An experimental study on size distribution and zeta potential of bulk cavitation nanobubbles. Int. J. Miner. Metall. Mater. 2020, 27, 152–161. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhong, Y.; Zhou, Z.; Huang, Y.; Sun, C.Q. Nanobubble skin supersolidity. Langmuir 2016, 32, 11321–11327. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xie, G.; Peng, Y.; Xia, W.; Sha, J. Stability theories of nanobubbles at solid-liquid interface: A review. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 176–186. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Bu, X.; Wang, M.; An, B.; Shao, H.; Ni, C.; Peng, Y.; Xie, G. A novel flotation technique combining carrier flotation and cavitation bubbles to enhance separation efficiency of ultra-fine particles. Ultrason. Sonochemistry 2020, 64, 105005. [Google Scholar] [CrossRef]
- Wu, C.; Nesset, K.; Masliyah, J.; Xu, Z. Generation and characterization of submicron size bubbles. Adv. Colloid Interface Sci. 2012, 179, 123–132. [Google Scholar] [CrossRef]
- Tussupbayev, N.K.; Rulyov, N.N.; Kravtchenco, O.V. Microbubble augmented flotation of ultrafine chalcopyrite from quartz mixtures. Miner. Process. Extr. Metall. 2016, 125, 5–9. [Google Scholar] [CrossRef]
- Tao, D.; Sobhy, A. Nanobubble effects on hydrodynamic interactions between particles and bubbles. Powder Technol. 2019, 346, 385–395. [Google Scholar] [CrossRef]
- Knüpfer, P.; Ditscherlein, L.; Peuker, U.A. Nanobubble enhanced agglomeration of hydrophobic powders. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 117–123. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Hassanzadeh, A.; Azizi, A.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B. Study of effective parameters on generating submicron (nano)-bubbles using the hydrodynamic cavitation. Physicochem. Probl. Miner. Process. 2020, 56, 884–904. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Fan, M. Effects of nanobubble and hydrodynamic parameters on coarse quartz flotation. Int. J. Min. Sci. Technol. 2019, 29, 289–295. [Google Scholar] [CrossRef]
- Weber, M.E.; Paddock, D. Interceptional and gravitational collision efficiencies for single collectors at intermediate Reynolds numbers. J. Colloid Interface Sci. 1983, 94, 328–335. [Google Scholar] [CrossRef]
- Sobhy, A.; Tao, D. Nanobubble column flotation of fine coal particles and associated fundamentals. Int. J. Miner. Process. 2013, 124, 109–116. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D.; Zhao, Y.; Honaker, R. Effect of nanobubbles on the flotation of different sizes of coal particle. Min. Metall. Explor. 2013, 30, 157–161. [Google Scholar] [CrossRef]
- Xiong, Y. Bubble Size Effects in Coal Flotation and Phosphate Reverse Flotation Using a Pico-Nano Bubble Generator. Ph.D. Thesis, West Virginia University, Morgantown, VA, USA, 2014. [Google Scholar]
- Ma, F.; Tao, D.; Tao, Y. Effects of nanobubbles in column flotation of Chinese sub-bituminous coal. Int. J. Coal Prep. Util. 2019, 1–17. [Google Scholar] [CrossRef]
- Liu, Y.; Tao, X.; Jiang, H.; Chen, R. Intensification of fine apatite flotation with microbubble generation and inclined plates in the flotation column. Chem. Eng. Process. Process Intensif. 2020, 157, 108133. [Google Scholar] [CrossRef]
- Kruszelnicki, M.; Hassanzadeh, A.; Legawiec, K.J.; Polowczyk, I.; Kowalczuk, P.B. Effect of ultrasound pre-treatment on carbonaceous copper-bearing shale flotation. Ultrason. Sonochemistry 2022, 84, 105962. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Fan, M.; Honaker, R.; Parekh, B.K. Picobubble enhanced flotation of coarse phosphate particles. In Proceedings of the 23th International Mineral Processing Congress, Istanbul, Turkey, 3–8 September 2006. [Google Scholar]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M. Nanobubbles effect on the mechanical flotation of phosphate ore fine particles. Physicochem. Probl. Miner. Process. 2018, 54, 278–292. [Google Scholar]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Flotation of quartz particles assisted by nanobubbles. Int. J. Miner. Process. 2015, 137, 64–70. [Google Scholar] [CrossRef]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B. Effect of frother type and operational parameters on nano bubble flotation of quartz coarse particles. J. Min. Environ. 2018, 9, 539–546. [Google Scholar]
- Rosa, A.; Rubio, J. On the role of nanobubbles in particle-bubble adhesion for the flotation of quartz and apatitic minerals. Miner. Eng. 2018, 127, 178–184. [Google Scholar] [CrossRef]
- Ahmadi, R.; Khodadadi, D.A.; Abdollahy, M.; Fan, M. Nano-microbubble flotation of fine and ultrafine chalcopyrite particles. Int. J. Min. Sci. Technol. 2014, 24, 559–566. [Google Scholar] [CrossRef]
- Khan, P.; Zhu, W.; Huang, F.; Gao, W.; Khan, N.A. Micro- nanobubble technology and water-related application. Water Supply 2020, 20, 2021–2035. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Z.; Jiao, F.; Qin, W. Understanding bubble growth process under decompression and its effects on the flotation phenomena. Miner. Eng. 2020, 145, 106066. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Zhang, H. Efficient separation of high-ash fine coal by the collaboration of nanobubbles and polyaluminum chloride. Fuel 2020, 260, 116325. [Google Scholar] [CrossRef]
- Lohse, D.; Zhang, X. Surface nanobubbles and nanodroplets. Rev. Mod. Phys. 2015, 87, 981. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, H.; Han, M.; Kim, T. Generation of sub-micron (nano) bubbles and characterization of their fundamental properties. Environ. Eng. Res. 2019, 24, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Song, M.G.; Kim, J.D. Zeta potential of nanobubbles generated by ultrasonication in aqueous alkyl polyglycoside solutions. J. Colloid Interface Sci. 2000, 223, 285–291. [Google Scholar] [CrossRef]
- Oeffinger, B.E.; Wheatley, M.A. Development and characterization of a nano-scale contrast agent. Ultrasonics 2004, 42, 343–347. [Google Scholar] [CrossRef]
- Ohgaki, K.; Khanh, N.Q.; Joden, Y.; Tsuji, A.; Nakagawa, T. Physicochemical approach to nanobubble solutions. Chem. Eng. Sci. 2010, 65, 1296–1300. [Google Scholar] [CrossRef]
- Etchepare, R.; Oliveira, H.; Nicknig, M.; Azevedo, A.; Rubio, J. Nanobubbles: Generation using a multiphase pump, properties and features in flotation. Miner. Eng. 2017, 112, 19–26. [Google Scholar] [CrossRef]
- Michailidi, E.D.; Bomis, G.; Varoutoglou, A.; Kyzas, G.Z.; Mitrikas, G.; Mitropoulos, A.C.; Efthimiadou, E.K.; Favvas, E.P. Bulk nanobubbles: Production and investigation of their formation/stability mechanism. J. Colloid Interface Sci. 2020, 564, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Sun, L.; Yang, H.; Gui, X.; Schönherr, H.; Kappl, M.; Cao, Y.; Xing, Y. Recent advances for understanding the role of nanobubbles in particles flotation. Adv. Colloid Interface Sci. 2021, 291, 102403. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Baroot, A.; Shui, L.; Zhang, M. Nanobubbles and nanoparticles. Curr. Opin. Colloid Interface Sci. 2021, 55, 101470. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H. Surface nanobubbles and their roles in flotation of fine particles—A review. J. Ind. Eng. Chem. 2022, 106, 37–51. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H. A review of bulk nanobubbles and their roles in flotation of fine particles. Powder Technol. 2022, 395, 618–633. [Google Scholar] [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mysteries of bulk nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason. Sonochemistry 2018, 48, 259–266. [Google Scholar] [CrossRef]
- Leroy, V.; Norisuye, T. Investigating the existence of bulk nanobubbles with ultrasound. ChemPhysChem 2016, 17, 2787–2790. [Google Scholar] [CrossRef]
- Bu, X.; Alheshibri, M. The effect of ultrasound on bulk and surface nanobubbles: A review of the current status. Ultrason. Sonochemistry 2021, 76, 105629. [Google Scholar] [CrossRef]
- Chen, Y.; Truong, V.N.T.; Bu, X.; Xie, G. A review of effects and applications of ultrasound in mineral flotation. Ultrason. Sonochemistry 2020, 60, 104739. [Google Scholar] [CrossRef]
- Kursun, H.; Ulusoy, U. Zinc recovery from a lead-zinc-copper ore by ultrasonically assisted column flotation. Part. Sci. Technol. 2015, 33, 349–356. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Tesar, V.; Bandulasena, H.H. Towards energy efficient nanobubble generation with fluidic oscillation. Curr. Opin. Colloid Interface Sci. 2011, 16, 350–356. [Google Scholar] [CrossRef]
- Miastkowska, M.A.; Banach, M.; Pulit-Prociak, J.; Sikora, E.S.; Głogowska, A.; Zielina, M. Statistical analysis of optimal ultrasound emulsification parameters in thistle-oil nanoemulsions. J. Surfactants Deterg. 2017, 20, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, W.; Zhao, Y.; Yang, J.; Ren, Y.; Yang, W.; Huang, X.; Zhang, L. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface. Langmuir 2019, 35, 9239–9245. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, X.; Truong, V.N.T.; Peng, Y.; Xie, G. Study on the effects of pre-conditioning time on the floatability of molybdenite from the perspective of cavitation threshold. Miner. Eng. 2019, 141, 105845. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Sajjady, S.A.; Gholami, H.; Amini, S.; Özkan, S.G. An improvement on selective separation by applying ultrasound to rougher and re-cleaner stages of copper flotation. Minerals 2020, 10, 619. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Gholami, H.; Özkan, S.G.; Niedoba, T.; Surowiak, A. Effect of power ultrasound on wettability and collector-less floatability of chalcopyrite, pyrite and quartz. Minerals 2021, 11, 48. [Google Scholar] [CrossRef]
- Jin, F.; Ye, J.; Hong, L.; Lam, H.; Wu, C. Slow relaxation mode in mixtures of water and organic molecules: Supramolecular structures or nanobubbles? J. Phys. Chem. B 2007, 111, 2255–2261. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Liu, Y.; Guo, Z.; Liu, Z.; Lohse, D.; Zhang, X. Solvent exchange leading to nanobubble nucleation: A molecular dynamics study. Langmuir 2017, 33, 8090–8096. [Google Scholar] [CrossRef]
- Lou, S.; Gao, J.; Xiao, X.; Li, X.; Li, G.; Zhang, Y.; Li, M.; Sun, J.; Li, X.; Hu, J. Studies of nanobubbles produced at liquid/solid interfaces. Mater. Charact. 2002, 48, 211–214. [Google Scholar] [CrossRef]
- Zhou, L.M.; Wang, S.; Qiu, J.; Wang, L.; Wang, X.Y.; Li, B.; Zhang, L.J.; Hu, J. Interfacial nanobubbles produced by long-time preserved cold water. Chin. Phys. B 2017, 26, 106803. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Wu, C.; Lv, H.; Zhao, B.; Liu, K.; Ou, L. Nanobubbles heterogeneous nucleation induced by temperature rise and its influence on minerals flotation. Appl. Surf. Sci. 2020, 508, 145282. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, X.D.; Lou, S.T.; Zhang, Z.X.; Sun, J.L.; Hu, J. Degassing and temperature effects on the formation of nanobubbles at the mica/water interface. Langmuir 2004, 20, 3813–3815. [Google Scholar] [CrossRef]
- Tao, D. Role of bubble size in flotation of coarse and fine particles—A review. Sep. Sci. Technol. 2005, 39, 741–760. [Google Scholar] [CrossRef]
- Fan, F.; Daniel, T.; Honaker, R.; Zhenfu, L. Nanobubble generation and its application in froth flotation (part I): Nanobubble generation and its effects on properties of microbubble and millimeter scale bubble solutions. Min. Sci. Technol. (China) 2010, 20, 1–19. [Google Scholar] [CrossRef]
- Thornycroft, J.I.; Barnaby, S.W. Torpedo-boat destroyers. In Minutes of the Proceedings of the Institution of Civil Engineers; Thomas Telford-ICE Virtual Library: London, UK, 1895; Volume 122, pp. 51–69. [Google Scholar]
- Pease, D.C.; Blinks, L.R. Cavitation from solid surfaces in the absence of gas nuclei. J. Phys. Colloid Chem. 1947, 51, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Sayed, A.A.S. Cavitation Nanobubble Enhanced Flotation Process for More Efficient Coal Recovery. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2013. [Google Scholar]
- Xiong, Y.; Peng, F. Optimization of cavitation venturi tube design for pico and nano bubbles generation. Int. J. Min. Sci. Technol. 2015, 25, 523–529. [Google Scholar] [CrossRef]
- Favvas, E.P.; Kyzas, G.Z.; Efthimiadou, E.K.; Mitropoulos, A.C. Bulk nanobubbles, generation methods and potential applications. Curr. Opin. Colloid Interface Sci. 2021, 54, 101455. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, N. Effective parameters on generation of nanobubbles by cavitation method for froth flotation applications. Physicochem. Probl. Miner. Process. 2017, 53, 920–942. [Google Scholar]
- Wasmund, E.B. Flotation technology for coarse and fine particle recovery. In Proceedings of the Congreso Internacional De Flotacion De Minerales, Lima, Peru, 22 September 2014. [Google Scholar]
- Hart, G.; Morgan, S.; Bramall, N.; Nicol, S. Enhanced Coal Flotation Using Picobubbles, Australia, 2002, CSIRO Report-C9048. Available online: https://www.acarp.com.au/reportcategory.aspx?catId=3&subCatId=25 (accessed on 10 January 2022).
- Zhou, W.; Ou, L.; Shi, Q.; Feng, Q.; Chen, H. Different flotation performance of ultrafine scheelite under two hydrodynamic cavitation modes. Minerals 2018, 8, 264. [Google Scholar] [CrossRef] [Green Version]
- Tao, D.; Yu, S.; Zhou, X.; Honaker, R.Q.; Parekh, B.K. Picobubble column flotation of fine coal. Int. J. Coal Prep. Util. 2008, 28, 1–14. [Google Scholar] [CrossRef]
- Ma, J.; Hsiao, C.T.; Chahine, G.L. Numerical study of acoustically driven bubble cloud dynamics near a rigid wall. Ultrason. Sonochemistry 2018, 40, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Influence of Venturi Tube Geometry and Particle Properties on the Hydrodynamic Cavitation for Fine Particle Flotation. Master’s Thesis, University of Alberta, Edmonton, AB, Canada, 2017. [Google Scholar]
- Yang, S.; Tsai, P.; Kooij, E.S.; Prosperetti, A.; Zandvliet, H.J.; Lohse, D. Electrolytically generated nanobubbles on highly orientated pyrolytic graphite surfaces. Langmuir 2009, 25, 1466–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, Y.; Zhang, X.; Li, Z.; Shen, G.; Ye, M.; Fan, C.; Fang, H.; Hu, J. Electrochemically controlled formation and growth of hydrogen nanobubbles. Langmuir 2006, 22, 8109–8113. [Google Scholar] [CrossRef] [PubMed]
- Alheshibri, M.; Jehannin, M.; Coleman, V.A.; Craig, V.S. Does gas supersaturation by a chemical reaction produce bulk nanobubbles? J. Colloid Interface Sci. 2019, 554, 388–395. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, L.; Faraji, H.; Feldberg, S.W.; White, H.S. Electrochemical measurements of single H2 nanobubble nucleation and stability at Pt nanoelectrodes. J. Phys. Chem. Lett. 2014, 5, 3539–3544. [Google Scholar] [CrossRef]
- Chen, Q.; Wiedenroth, H.S.; German, S.R.; White, H.S. Electrochemical nucleation of stable N2 nanobubbles at Pt nanoelectrodes. J. Am. Chem. Soc. 2015, 137, 12064–12069. [Google Scholar] [CrossRef]
- Ren, H.; German, S.R.; Edwards, M.A.; Chen, Q.; White, H.S. Electrochemical generation of individual O2 nanobubbles via H2O2 oxidation. J. Phys. Chem. Lett. 2017, 8, 2450–2454. [Google Scholar] [CrossRef]
- Li, M.; Tonggu, L.; Zhan, X.; Mega, T.L.; Wang, L. Cryo-EM visualization of nanobubbles in aqueous solutions. Langmuir 2016, 32, 11111–11115. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average size and zeta potential of nanobubbles in different reagent solutions. J. Nanoparticle Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- Lund, E.J.; LaBelle, J.; Torbert, R.B.; Liou, K.; Peria, W.; Kletzing, C.A.; Kelley, M.C.; Baker, S.D.; Primdahl, F.; Stenbaek-Nielsen, C.; et al. Observation of electromagnetic oxygen cyclotron waves in a flickering aurora. Geophys. Res. Lett. 1995, 22, 2465–2468. [Google Scholar] [CrossRef]
- Leifer, I.; Patro, R.K.; Bowyer, P. A study on the temperature variation of rise velocity for large clean bubbles. J. Atmos. Ocean. Technol. 2000, 17, 1392–1402. [Google Scholar] [CrossRef]
- Vinnett, L.; Sovechles, J.; Gomez, C.; Waters, K. An image analysis approach to determine average bubble sizes using one-dimensional Fourier analysis. Miner. Eng. 2018, 126, 160–166. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, O.; Seo, J.K.; Yoon, J.R. On a nonlinear partial differential equation arising in magnetic resonance electrical impedance tomography. SIAM J. Math. Anal. 2002, 34, 511–526. [Google Scholar] [CrossRef]
- Cho, J.; Perlin, M.; Ceccio, S.L. Measurement of near-wall stratified bubbly flows using electrical impedance. Meas. Sci. Technol. 2005, 16, 1021. [Google Scholar] [CrossRef]
- Manasseh, R.; LaFontaine, R.; Davy, J.; Shepherd, I.; Zhu, Y.G. Passive acoustic bubble sizing in sparged systems. Exp. Fluids 2001, 30, 672–682. [Google Scholar] [CrossRef]
- Spencer, S.J.; Bruniges, R.; Roberts, G.; Sharp, V.; Catanzano, A.; Bruckard, W.J.; Davey, K.J.; Zhang, W. An acoustic technique for measurement of bubble solids mass loading: (b) Monitoring of Jameson cell flotation performance by passive acoustic emissions. Miner. Eng. 2012, 36, 21–30. [Google Scholar] [CrossRef]
- Kracht, W.; Moraga, C. Acoustic measurement of the bubble Sauter mean diameter d32. Miner. Eng. 2016, 98, 122–126. [Google Scholar] [CrossRef]
- Khoshdast, H.; Hassanzadeh, A.; Kowalczuk, P.B.; Farrokhpay, S. Characterization techniques of flotation frothers—A Review. Miner. Process. Extr. Metall. Rev. 2022, 1–25. [Google Scholar] [CrossRef]
- Etchepare, R.; Azevedo, A.; Calgaroto, S.; Rubio, J. Removal of ferric hydroxide by flotation with micro and nanobubbles. Sep. Purif. Technol. 2017, 184, 347–353. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.M. Generation and stability of bulk nanobubbles. Langmuir 2017, 33, 3818–3823. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, K.; Sobieszuk, P.; Mroz, A.; Ciach, T. Stability of nanobubbles generated in water using porous membrane system. Chem. Eng. Process. Process Intensif. 2019, 136, 62–71. [Google Scholar] [CrossRef]
- Qiu, J.; Zou, Z.; Wang, S.; Wang, X.; Wang, L.; Dong, Y.; Zhao, H.; Zhang, L.; Hu, J. Formation and stability of bulk nanobubbles generated by ethanol- water exchange. Emphysema 2017, 18, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Daniel, T.; Honaker, R.; Zhenfu, L. Nanobubble generation and its applications in froth flotation (part IV): Mechanical cells and specially designed column flotation of coal. Min. Sci. Technol. (China) 2010, 20, 641–671. [Google Scholar] [CrossRef]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M.; Nguyen, A.V.; Chehreh Chelgani, S. Proving the existence of nanobubbles produced by hydrodynamic cavitation and their significant effects in powder flotation. Adv. Powder Technol. 2021, 32, 1810–1818. [Google Scholar] [CrossRef]
- Tao, D.; Wu, Z.; Sobhy, A. Investigation of nanobubble enhanced reverse anionic flotation of hematite and associated mechanisms. Powder Technol. 2021, 379, 12–25. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Karamoozian, M.; Saghravani, S.F. Interaction of applying stable micro-nano bubbles and ultrasonic irradiation in coal flotation. Int. J. Coal Prep. Util. 2020, 1–15. [Google Scholar] [CrossRef]
- Liu, L.; Hu, S.; Wu, C.; Liu, K.; Weng, L.; Zhou, W. Aggregates characterizations of the ultra-fine coal particles induced by nanobubbles. Fuel 2021, 297, 120765. [Google Scholar] [CrossRef]
- Lei, W.; Zhang, M.; Zhang, Z.; Zhan, N.; Fan, R. Effect of bulk nanobubbles on the entrainment of kaolinite particles in flotation. Powder Technol. 2020, 362, 84–89. [Google Scholar] [CrossRef]
- Olszok, V.; Rivas-Botero, J.; Wollmann, A.; Benker, B.; Weber, A.P. Particle-induced nanobubble generation for material-selective nanoparticle flotation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124576. [Google Scholar] [CrossRef]
- Calgaroto, S.; Azevedo, A.; Rubio, J. Separation of amine-insoluble species by flotation with nano and microbubbles. Miner. Eng. 2016, 89, 24–29. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Harbottle, D.; Masliyah, J.; Xu, Z. Studying bubble- particle interactions by zeta potential distribution analysis. J. Colloid Interface Sci. 2015, 449, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.A.; Nguyen, A.V. Accumulation of dissolved gases at hydrophobic surfaces in water and sodium chloride solutions: Implications for coal flotation. Miner. Eng. 2009, 22, 786–792. [Google Scholar] [CrossRef]
- Vaziri Hassas, B.; Jin, J.; Dang, L.X.; Wang, X.; Miller, J.D. Attachment, coalescence, and spreading of carbon dioxide nanobubbles at pyrite surfaces. Langmuir 2018, 34, 14317–14327. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Lv, B.; Cheng, G.; Lu, Y. Mechanism of micro/nano-bubble formation and cavitation effect on bubbles size distribution in flotation. Physicochem. Probl. Miner. Process. 2020, 56, 504–512. [Google Scholar] [CrossRef]
- Li, H.; Afacan, A.; Liu, Q.; Xu, Z. Study interactions between fine particles and micron size bubbles generated by hydrodynamic cavitation. Miner. Eng. 2015, 84, 106–115. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic- static microbubble flotation column with collector emulsification. J. Clean. Prod. 2017, 153, 657–672. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Filippov, L.O.; Kravchenko, O.V. Combined microflotation of glass beads. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124810. [Google Scholar] [CrossRef]
- Rulyov, N.N.; Sadovskiy, D.Y.; Rulyova, N.A.; Filippov, L.O. Column flotation of fine glass beads enhanced by their prior heteroaggregation with microbubbles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126398. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Chow, R.S.; Cleyle, P.; Xu, Z.H.; Masliyah, J.H. Effect of dynamic bubble nucleation on bitumen flotation. Can. Metall. Q. 2010, 49, 363–372. [Google Scholar] [CrossRef]
- Ross, V.; Singh, A.; Pillay, K. Improved flotation of PGM tailings with a high-shear hydrodynamic cavitation device. Miner. Eng. 2019, 137, 133–139. [Google Scholar] [CrossRef]
- Li, C.; Xu, M.; Zhang, H. The interactions between coal particles with different hydrophobicity and bulk nanobubbles in natural water. Int. J. Coal Prep. Util. 2019, 42, 463–474. [Google Scholar] [CrossRef]
- Zhang, Z.; Ren, L.; Zhang, Y. Role of nanobubbles in the flotation of fine rutile particles. Miner. Eng. 2021, 172, 107140. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Li, H.; Chow, R.S.; Liu, Q.; Xu, Z.; Masliyah, J. Role of mineral flotation technology in improving bitumen extraction from mined Athabasca oil sands- II. Flotation hydrodynamics of water-based oil sand extraction. Can. J. Chem. Eng. 2020, 98, 330–352. [Google Scholar] [CrossRef]
- Xu, G.; Chen, Y.; Bu, X.; Dong, X.; Xie, G.; Sun, Y. Separation performance of mechanical flotation cell and cyclonic microbubble flotation column: In terms of the beneficiation of high-ash coal fines. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 2845–2855. [Google Scholar] [CrossRef]
- Chipakwe, V.; Jolstera, R.; Chelgani, S.C. Nanobubble-Assisted Flotation of Apatite Tailings: Insights on Beneficiation Options. ACS Omega 2021, 6, 13888–13894. [Google Scholar] [CrossRef]
- Chipakwe, V.; Sand, A.; Chelgani, S.C. Nanobubble assisted flotation separation of complex Pb–Cu–Zn sulfide ore—Assessment of process readiness. Sep. Sci. Technol. 2021, 57, 1351–1358. [Google Scholar] [CrossRef]
- Lee Black, D.; McQuay, M.Q.; Bonin, M.P. Laser-based techniques for particle-size measurement: A review of sizing methods and their industrial applications. Prog. Energy Combust. Sci. 1996, 22, 267–306. [Google Scholar] [CrossRef]
- Grubbs, J.; Tsaknopoulos, K.; Massar, C.; Young, B.; O’Connell, A.; Walde, C.; Birt, A.; Siopis, M.; Cote, D. Comparison of laser diffraction and image analysis techniques for particle size-shape characterization in additive manufacturing applications. Powder Technol. 2021, 391, 20–33. [Google Scholar] [CrossRef]
- Yan-ge, H. Rectification study of particle analysing result between laser instrument and sieving method. Mathematics 2012, 30, 716–723. [Google Scholar]
- Zhang, S.Y.; Lv, F.Y.; Xia, Z.M.; Li, N.; Wu, M. The feasibility study of laser particle size analyzer for thick pastes. Appl. Mech. Mater. 2013, 372, 428–432. [Google Scholar] [CrossRef]
- Ilic, M.; Budak, I.; Vucinic Vasic, M.; Nagode, A.; Kozmidis-Luburić, U.; Hodolic, J.; Puskar, T. Size and shape particle analysis by applying image analysis and laser diffraction—Inhalable dust in a dental laboratory. Measurement 2015, 66, 109–117. [Google Scholar] [CrossRef]
- Hou, J.; Ci, H.; Wang, P.; Wang, C.; Lv, B.; Miao, L.; You, G. Nanoparticle tracking analysis versus dynamic light scattering: Case study on the effect of Ca2+ and alginate on the aggregation of cerium oxide nanoparticles. J. Hazard. Mater. 2018, 360, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Sheetz, M.P.; Elson, E.L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by nanosight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, K.; Nowack, B.; Arroyo Rojas Dasilva, Y.; Mitrano, D.M. Improvements in nanoparticle tracking analysis to measure particle aggregation and mass distribution: A case study on engineered nanomaterial stability in incineration landfill leachates. Environ. Sci. Technol. 2017, 51, 5611–5621. [Google Scholar] [CrossRef] [Green Version]
- Babick, F. Dynamic light scattering (DLS). In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 3.2.1; pp. 137–172. [Google Scholar]
- Jin, J.; Wang, R.; Tang, J.; Yang, L.; Feng, Z.; Xu, C.; Yang, F.; Gu, N. Dynamic tracking of bulk nanobubbles from microbubbles shrinkage to collapse. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124430. [Google Scholar] [CrossRef]
- Sartor, M. Dynamic Light Scattering to Determine the Radius of Small Beads in Brownian Motion in a Solution. Course Material. University of California: San Diego, CA, USA, 2014. Available online: https://neurophysics.ucsd.edu/courses/physics_173_273/dynamic_light_scattering_03.pdf (accessed on 10 January 2022).
- Aljamali, N. Zetasizer technique in biochemistry. Biochem. Anal. Biochem. 2015, 4, 2. [Google Scholar]
- Sjogreen, C.; Tellez, D.A.; Perez, J.E.R.; Hurtado, P.C.P.; Roa-Rojas, J. Experimental study of nanobubbles in salt solutions. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2018, 42, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Jhe, W. Atomic force microscopy and spectroscopy. Rep. Prog. Phys. 2007, 71, 016101. [Google Scholar] [CrossRef]
- Rabinowitz, J.; Whittier, E.; Liu, Z.; Jayant, K.; Frank, J.; Shepard, K. Nanobubble-controlled nanofluidic transport. Sci. Adv. 2020, 6, eabd0126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, S.; Zhang, L.; Hu, J. Generation and stability of bulk nanobubbles: A review and perspective. Curr. Opin. Colloid Interface Sci. 2021, 53, 101439. [Google Scholar] [CrossRef]
- Wesley, D.J.; Toolan, D.T.; Brittle, S.A.; Howse, J.R.; Zimmerman, W.B. Development of an optical microscopy system for automated bubble cloud analysis. Appl. Opt. 2016, 55, 6102–6107. [Google Scholar] [CrossRef] [PubMed]
- Karpitschka, S.; Dietrich, E.; Seddon, J.R.; Zandvliet, H.J.; Lohse, D.; Riegler, H. Nonintrusive optical visualization of surface nanobubbles. Phys. Rev. Lett. 2012, 109, 066102. [Google Scholar] [CrossRef]
- Chan, C.U.; Ohl, C.D. Total internal reflection fluorescence microscopy for the study of nanobubble dynamics. Phys. Rev. Lett. 2012, 109, 174501. [Google Scholar] [CrossRef]
- Drzymala, J.; Kowalczuk, P.B. Classification of flotation frothers. Minerals 2018, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Ng, C.Y.; Wang, L. Bubble size in a flotation column with oscillatory air supply in the presence of frothers. Miner. Process. Extr. Metall. Rev. 2021, 1–9. [Google Scholar] [CrossRef]
- Urbina, R.H. Recent developments and advances in formulations and applications of chemical reagents used in froth flotation. Miner. Process. Extr. Metall. Rev. 2003, 24, 139–182. [Google Scholar] [CrossRef]
- Moreno, Y.S.; Bournival, G.; Ata, S. Classification of flotation frothers—A statistical approach. Chem. Eng. Sci. 2022, 248, 117252. [Google Scholar] [CrossRef]
- Calgaroto, S.; Wilberg, K.Q.; Rubio, J. On the nanobubbles interfacial properties and future applications in flotation. Miner. Eng. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Jia, W.; Ren, S.; Hu, B. Effect of water chemistry on zeta potential of air bubbles. Int. J. Electrochem. Sci. 2013, 8, 5828–5837. [Google Scholar]
- Phan, K.; Truong, T.; Wang, Y.; Bhandari, B. Effect of electrolytes and surfactants on generation and longevity of carbon dioxide nanobubbles. Food Chem. 2021, 363, 130299. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.; Shafaei, S.Z.; Gharabaghi, M.; Ahmadi, R.; Shahbazi, B.; Tehranchi, A. New approach to quartz coarse particles flotation using nanobubbles, with emphasis on the bubble size distribution. Int. J. Nanosci. 2020, 19, 1850048. [Google Scholar] [CrossRef]
- Laskowski, J.S. Testing flotation frothers. Physicochem. Probl. Miner. Process. 2004, 38, 13–22. [Google Scholar]
- Laskowski, J.S. Frothers and Flotation Froth. Miner. Process. Extr. Metall. Rev. 1993, 12, 61–89. [Google Scholar] [CrossRef]
- Khoshdast, H.; Sam, A. Flotation frothers: Review of their classifications, properties and preparation. Open Miner. Process. J. 2011, 4, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Aveyard, R.; Binks, B.; Fletcher, P.; Peck, T.; Rutherford, C. Aspects of aqueous foam stability in the presence of hydrocarbon oils and solid particles. Adv. Colloid Interface Sci. 1994, 48, 93–120. [Google Scholar] [CrossRef]
- Yoon, R.H.; Luttrell, G.H. The effect of bubble size on fine particle flotation. Miner. Process. Extr. Metall. Rev. 1989, 5, 101–122. [Google Scholar] [CrossRef]
- Farrokhpay, S.; Filippov, L.; Fornasiero, D. Flotation of fine particles: A review. Miner. Process. Extr. Metall. Rev. 2021, 42, 473–483. [Google Scholar] [CrossRef]
- Cho, Y.S.; Laskowski, J.S. Bubble coalescence and its effect on dynamic foam stability. Can. J. Chem. Eng. 2002, 80, 299–305. [Google Scholar] [CrossRef]
- Khoshdast, H.; Abbasi, H.; Sam, A.; Noghabi, K.A. Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem. Eng. J. 2012, 60, 127–134. [Google Scholar] [CrossRef]
- Zhang, X.H.; Khan, A.; Ducker, W.A. A nanoscale gas state. Phys. Rev. Lett. 2007, 98, 136101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Switkes, M.; Ruberti, J. Rapid cryofixation/freeze fracture for the study of nanobubbles at solid-liquid interfaces. Appl. Phys. Lett. 2004, 84, 4759–4761. [Google Scholar] [CrossRef]
- Steitz, R.; Gutberlet, T.; Hauss, T.; Klösgen, B.; Krastev, R.; Schemmel, S.; Simonsen, A.C.; Findenegg, G.H. Nanobubbles and their precursor layer at the interface of water against a hydrophobic substrate. Langmuir 2003, 19, 2409–2418. [Google Scholar] [CrossRef]
- Johnson, B.D.; Cooke, R.C. Generation of stabilized microbubbles in seawater. Science 1981, 213, 209–211. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of seawater on sulfide ore flotation: A review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Bournival, G.; Zhang, F.; Ata, S. Coal flotation in saline water: Effects of electrolytes on interfaces and industrial practice. Miner. Process. Extr. Metall. Rev. 2021, 42, 53–73. [Google Scholar] [CrossRef]
- Hewage, S.A.; Kewalramani, J.; Meegoda, J.N. Stability of nanobubbles in different salts solutions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 609, 125669. [Google Scholar] [CrossRef]
- Uchida, T.; Liu, S.; Enari, M.; Oshita, S.; Yamazaki, K.; Gohara, K. Effect of NaCl on the lifetime of micro-and nanobubbles. Nanomaterials 2016, 6, 31. [Google Scholar] [CrossRef]
- Basarova, P.; Zawala, J.; Zednikova, M. Interactions between a small bubble and a greater solid particle during the flotation process. Miner. Process. Extr. Metall. Rev. 2019, 40, 410–426. [Google Scholar] [CrossRef]
- Fan, M.; Zhao, Y.; Tao, D. Fundamental Studies of Nanobubble Generation and Applications in Flotation. In Separation Technologies for Minerals, Coal, and Earth Resources; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2012; pp. 457–469. [Google Scholar]
- Farrokhpay, S.; Filippova, I.; Filippov, L.; Picarra, A.; Rulyov, N.; Fornasiero, D. Flotation of fine particles in the presence of combined microbubbles and conventional bubbles. Miner. Eng. 2020, 155, 106439. [Google Scholar] [CrossRef]
- Fan, M.; Tao, D. A study on picobubble enhanced coarse phosphate froth flotation. Sep. Sci. Technol. 2008, 43, 1–10. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Liu, J.; Wang, D. Cyclonic flotation column of siliceous phosphate ore. Int. J. Miner. Process. 2012, 110–111, 6–11. [Google Scholar] [CrossRef]
- Johansson, G.; Pugh, R. The influence of particle size and hydrophobicity on the stability of mineralized froths. Int. J. Miner. Process. 1992, 34, 1–21. [Google Scholar] [CrossRef]
- Kennedy, D.L. Redesign of Industrial Column Flotation Circuits Based on a Simple Residence Time Distribution Model. Master’s Thesis, Virginia Tech., Blacksburg, VA, USA, 2008. [Google Scholar]

| Analysis Technique | Size (µm) | Analysis Speed | Data Output | Advantages and Disadvantages |
|---|---|---|---|---|
| Acoustical methods | 34–1200 | Fast and highly automated | Size, size distribution | Available in non-transparent media. High cost and limited data output. |
| Light scattering | <100 | Fast and automated | Size, size distribution | Small range, limited output and generally used in backlighting illumination |
| Photographic | >50 | Slow and manual | Size, size distribution, rise velocity, shape analysis, formation process | Available for obtaining more information, modifiable, relatively low cost, viscous liquids. |
| Size Analyzer | Equipment | Material | Refs. |
|---|---|---|---|
| LPSA | Venturi tube | Coal | [107] |
| Venturi tube and static mixer | Coal and phosphate | [11,24,131] | |
| Venturi tube and the static mixer | Coal | [53] | |
| Static mixer- venturi tube | Coal | [55] | |
| Venturi tube | Chalcopyrite | [64] | |
| Venturi tube | Coarse quartz particles | [34,51] | |
| Venturi tube and static mixer | Coal | [101] | |
| Venturi tube | Phosphate ore | [60,132] | |
| Venturi tube | Sub-bituminous coal | [56] | |
| Venturi tube | Hematite | [133] | |
| Venturi tube | Coal | [35] | |
| Hydrodynamic cavitation | Coal | [134] | |
| DLS | Venturi tube | Fine silica and zinc sulphide | [5] |
| Porous membrane system | UN * | [129] | |
| Hydrodynamic cavitation | Coal | [135] | |
| NTA | Needle valves | Ferric hydroxide | [73,127] |
| Depressurization of DI water | Quartz and apatitic minerals | [63] | |
| Venturi tube | UN * | [33] | |
| Venturi tube | Muscovite | [13] | |
| Ultrasonic | Mica | [86] | |
| Decompression method (Vacuum drying oven) | Kaolinite | [136] | |
| Depressurization with a gas vent | Platinum nanoparticles | [137] | |
| Zetasizer | Steel needle valve | Quartz | [61] |
| Flow constrictor (needle valve) | Quartz | [138] | |
| Venturi tube | Scheelite | [32] | |
| Venturi tube | Scheelite | [106] | |
| Venturi tube | Diaspore and kaolinite | [14] | |
| Venturi tube | UN * | [74] | |
| ZetaPALS | baffled high intensity agitation (BHIA) | UN * | [46] |
| High speed agitator | Alumina and silica | [139] | |
| Venturi tube | Coal | [45] | |
| AFM | Solvent-exchange | Graphite | [140] |
| Blowing N2 and CO2 gas into deionized (DI) water | Pyrite | [141] | |
| Temperature rise | Muscovite | [94] | |
| Venturi tube | Au, Pb | [142] | |
| Beam Reflectance Measurement (FBRM) | Venturi tube | Subbituminous coal | [143] |
| Camera | Ultrasonic | Zinc ore | [83] |
| high-speed camera system | Venture cavitation sparger | Coal | [144] |
| A 405 nm laser beam | YBM Fubby (cavitation bubbles and vortex flow) | UN * | [25] |
| High speed camera | Venturi tube | Apatite | [57] |
| Photocamera- Microscope | Air-in-water microdispersion generator | Glass beads | [145,146] |
| UN * | Temperature rise | Bitumen | [147] |
| High-speed venturi nozzles | Platinum Group Metal (PGM) tailings | [148] | |
| Temperature rise | Coal | [149] | |
| Ultrasonic cavitation | Coal | [67] | |
| Ultrasonic cavitation | Rutile | [150] | |
| Venturi tube | Bitumen | [151] | |
| Venturi tube | High-ash coal | [152] | |
| Venturi tube | Apatite ore | [153] | |
| Venturi tube | Pb–Cu–Zn sulfide ore | [154] |
| Surfactant | Formula | Structure | MW (g/mol) | HLB | Used by |
|---|---|---|---|---|---|
| Methyl isobutyl carbinol (MIBC) | (CH3)2CHCH2CHOHCH3 | 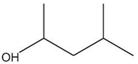 | 102.17 | 6.00 | [11,24,26,50,53,60,97] |
| Pine Oil (PO) | C10H18O | 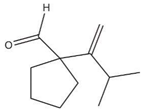 | 154.25 | 5.40 | [26] |
| Dipropylene glycol (DPG) | C6H14O3 | 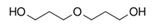 | 134.17 | 9.30 | [26,50] |
| Dodecylamine (DDA) | CH3(CH2)11NH2 | 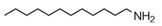 | 185.35 | 10.70 | [13,26] |
| Flotigam EDA 3B * | [R–(O–CH2)3–NH3] + CH3COO– ** | Commercial cationic alkyl methyl ether monoamine | 195.00 | NA *** | [63,179] |
| Dodecylamine hydrochloride (DAH) | C12H28ClN | 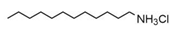 | 221.81 | NA | [18] |
| PEB70 * | CH3(CH2)3O(C2H4O)nH | 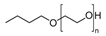 | ~250 | NA | [103] |
| Dodecyltrimethyl ammonium chloride (DTAC) | C15H34ClN | 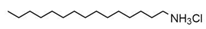 | 263.89 | NA | [180] |
| Sodium dodecyl sulphate (SDS) | CH3(CH2)11SO4Na |  | 288.37 | 40 | [18,179,180,181] |
| FLO-YS-20 * | Collector–frother based on fatty acids | Straight structure with long hydrocarbon chain | >300 | NA | [60,103] |
| F507 | H(C3H6O)6.5OH | 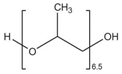 | 425.00 | 8.63 | [11,24,53,97] |
| Polysorbate 80 (Tween 80) | C64H124O26 | 1310.00 | 15.00 | [181] | |
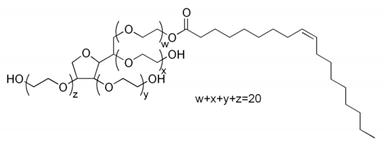 | |||||
| Flotation Cell | Materials | Scale | NBs Size (nm) | Equipment | Relative Change (%) | Refs. |
|---|---|---|---|---|---|---|
| Mechanical flotation | Silica | Laboratory | UN | Venturi tube | 23 | [5] |
| ZnS | Laboratory | UN | Venturi tube | 13 | [5] | |
| Quartz | Laboratory | 171 | Venturi tube | 21 | [34] | |
| Quartz | Laboratory | 150–200 | Depressurization of DI water | 23 | [63] | |
| Quartz | Laboratory | 200–720 | Steel needle valve | 13 | [61] | |
| Chalcopyrite | Laboratory | 358 | Venturi tube | 21 | [64] | |
| P2O5 | Laboratory | 150–200 | Depressurization of DI water | 30 | [63] | |
| Coal | Laboratory | ~300 | Hydrodynamic cavitation | 35 | [134] | |
| Kaolinite | Laboratory | <120 | Decompression | 8 | [136] | |
| Diaspore/kaolinite | Laboratory | 100–300 | Venturi tube | 14 | [14] | |
| Coal | Laboratory | 100–200 | Ultrasonic cavitation | 13 | [67] | |
| Scheelite | Laboratory | UN | Venturi tube | 17 | [32] | |
| Muscovite | Laboratory | 100 | Venturi tube | 18 | [13] | |
| Hematite | Laboratory | 150–280 | Venturi tube | 16 | [133] | |
| P2O5 | Laboratory | <1 μm | Venturi tube | 30 | [24] | |
| Column flotation | Coal | Pilot | <1 μm | Venturi tube | 27 | [131] |
| P2O5 | Laboratory | 150–240 | Venturi tube | 14 | [55] | |
| Coal | Laboratory | <1 μm | Venturi tube | 50 | [53] | |
| Coal | Pilot | 700 | Hydrodynamic cavitation | 46 | [54] | |
| Coal | Laboratory | 160–250 | Venturi tube | 39 | [56] |
| Flotation Cell | Materials | Relative Change (%) | Refs. |
|---|---|---|---|
| Mechanical flotation | Silica | 40 | [5] |
| Zns | 65 | [5] | |
| Coal | 98.4 | [131] | |
| PGM | 61 | [148] | |
| Quartz | 36 | [51] | |
| Muscovite | 26 | [13] | |
| Coal | 33.6 | [35] | |
| Quartz | 75 | [200] | |
| Apatite ore | 10.4 | [153] | |
| Coal | 14.4 | [135] | |
| Rutile | 42.7 | [150] | |
| Column flotation | Coal | 40 | [97] |
| Phosphate | 109 | [24] | |
| Coal | 160 | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, S.; Hassanzadeh, A.; He, Y.; Khoshdast, H.; Kowalczuk, P.B. Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation. Minerals 2022, 12, 462. https://doi.org/10.3390/min12040462
Nazari S, Hassanzadeh A, He Y, Khoshdast H, Kowalczuk PB. Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation. Minerals. 2022; 12(4):462. https://doi.org/10.3390/min12040462
Chicago/Turabian StyleNazari, Sabereh, Ahmad Hassanzadeh, Yaqun He, Hamid Khoshdast, and Przemyslaw B. Kowalczuk. 2022. "Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation" Minerals 12, no. 4: 462. https://doi.org/10.3390/min12040462
APA StyleNazari, S., Hassanzadeh, A., He, Y., Khoshdast, H., & Kowalczuk, P. B. (2022). Recent Developments in Generation, Detection and Application of Nanobubbles in Flotation. Minerals, 12(4), 462. https://doi.org/10.3390/min12040462








