Synthesis of Iron Nanomaterials for Environmental Applications from Hydrometallurgical Liquors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Iron Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Cr(VI) Removal Experiments
3. Results
3.1. Characterization of Nanomagnetic Iron Oxides
3.1.1. X-ray Diffraction Analysis (XRD)
3.1.2. Morphology and Specific Surface
3.1.3. Magnetic Properties
3.1.4. Thermal Analysis
3.1.5. Point of Zero Charge
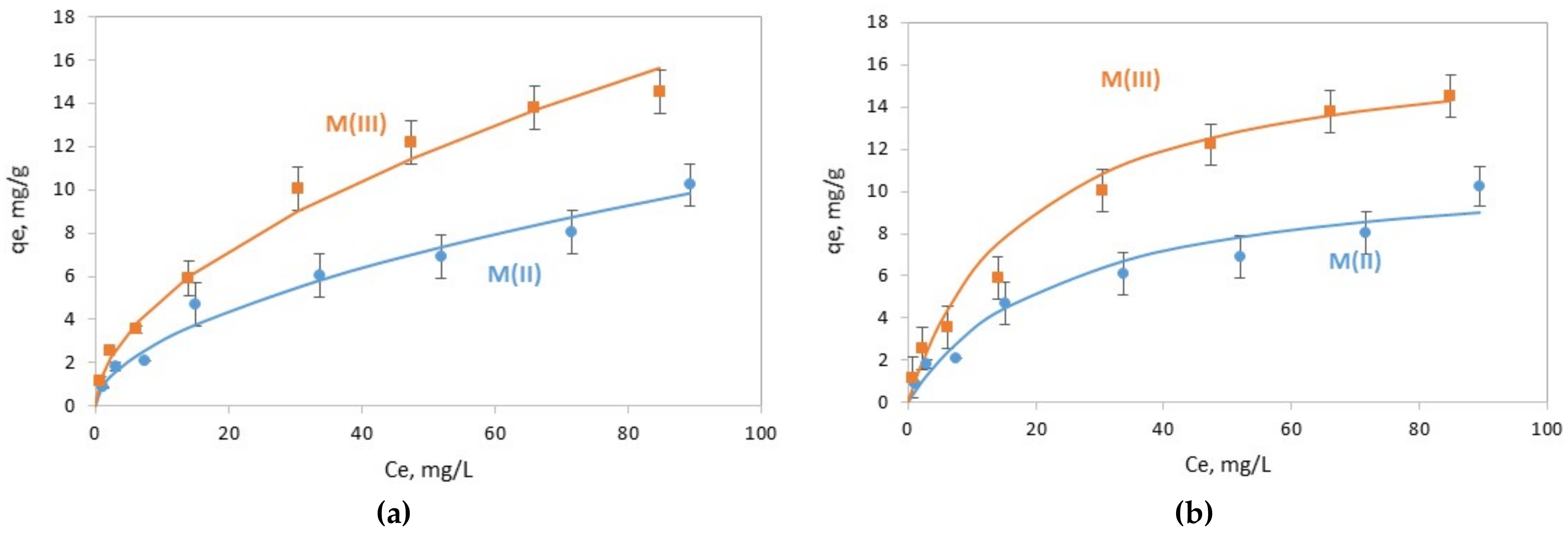
3.2. Chromate Adsorption Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winsett, J.; Moilanen, A.; Paudel, K. Quantitative determination of magnetite and maghemite in iron oxide nanoparticles using Mössbauer spectroscopy. SN Appl. Sci. 2019, 1, 1636. [Google Scholar] [CrossRef] [Green Version]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, Principles, and Properties of Magnetite Nanoparticles for In Vivo Imaging Applications—A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [Green Version]
- Suciu, M.; Ionescu, C.M.; Ciorita, A.; Tripon, S.C.; Nica, D.; Al-Salami, H.; Barbu-Tudoran, L. Applications of superparamagnetic iron oxide nanoparticles in drug and therapeutic delivery, and biotechnological advancements. Beilstein J. Nanotechnol. 2020, 11, 1092–1109. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Liu, D.; Wu, J.; Kim, C.; Fortner, J.D. Aqueous Aggregation and Surface Deposition Processes of Engineered Superparamagnetic Iron Oxide Nanoparticles for Environmental Applications. Environ. Sci. Technol. 2014, 48, 11892–11900. [Google Scholar] [CrossRef]
- Horst, M.F.; Alvarez, M.; Lassalle, V.L. Removal of heavy metals from wastewater using magnetic nanocomposites: Analysis of the experimental conditions. Sep. Sci. Technol. 2016, 51, 550–563. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Cao, N.; Sun, D.; Zhang, Z.; Wang, Y.; Wen, Y.; Yang, Y.; Lyu, T. Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis. Molecules 2021, 26, 103. [Google Scholar] [CrossRef]
- Lasheen, M.R.; El-Sherif, I.Y.; Sabry, D.Y.; El-Wakeel, S.T.; El-Shahat, M.F. Removal and recovery of Cr(VI) by magnetite nanoparticles. Desalin. Water Treat. 2014, 52, 6464–6473. [Google Scholar] [CrossRef]
- Jiang, W.; Pelaez, M.; Dionysiou, D.D.; Entezari, M.H.; Tsoutsou, D. Chromium (VI) removal by maghemite nanoparticles. Chem. Eng. Sci. 2013, 222, 527–533. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, S.; Han, M.; Su, Q.; Xia, L.; Hui, Z. Adsorption Properties of Magnetic Magnetite Nanoparticle for Coexistent Cr (VI) and Cu(II) in Mixed Solution. Water 2020, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Fato, P.; Li, D.W.; Zhao, L.J.; Qiu, K.; Long, Y.T. Simultaneous Removal of Multiple Heavy Metal Ions from River Water Using Ultrafine Mesoporous Magnetite Nanoparticles. ACS Omega 2019, 4, 7543–7549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makie, P.; Westin, G.; Persson, P.; Osterlund, L. Adsorption of Trimethyl Phosphate on Maghemite, Hematite, and Goethite Nanoparticles. J. Phys. Chem. A 2011, 115, 8948–8959. [Google Scholar] [CrossRef]
- de Melo, F.M.; Almeida, S.N.; Toma, H.E. Magnetic Nanohydrometallurgy Applied to Lanthanide Separation. Minerals 2020, 10, 530. [Google Scholar] [CrossRef]
- Almeida, S.N.; Toma, H.E. Neodymium(III) and lanthanum(III) separation by magnetic nanohydrometallurgy using DTPA functionalized magnetite nanoparticles. Hydrometallurgy 2016, 161, 22–28. [Google Scholar] [CrossRef]
- Monhemius, A.J. The iron elephant: A brief history of hydrometallurgists’ struggles with element no. 26. CIM J. 2017, 8, 197–206. [Google Scholar] [CrossRef]
- Gordon, A.R.; Pickering, R.W. Improved leaching technologies in the electrolytic zinc industry. Metall. Mater. Trans. B 1975, 6, 43–53. [Google Scholar] [CrossRef]
- Société de la Vieille Montagne, S. Appln. Belgian Patent No. 724214, 20 November 1968.
- Von Röpenack, A. Hematite—The Solution to a Disposal Problem—An Example from the Zinc Industry. In Iron Control in Hydrometallurgy; Dutrizac, J.E., Monhemius, A.J., Eds.; Ellis Horwood Ltd.: Chichester, UK, 1986; pp. 731–741. [Google Scholar]
- Riveros, P.A.; Dutrizac, J.E.; Benguerel, E.; Houlachi, G. The recovery of iron from zinc sulphate–sulphuric acid processing solutions by solvent extraction or ion exchange. Miner. Proc. Extr. Metall. Rev. 1998, 18, 105–145. [Google Scholar] [CrossRef]
- Stefanakis, M.; Monhemius, J. The extraction of iron(III) from aluminium nitrate solutions with Versatic acid. In Proceedings of the International Solvent Extraction Conference, Moscow, Russia, 18–24 July 1988; Volume 83, pp. 401–402. [Google Scholar]
- Principe, F.; Demopoulos, G.P. Comparative study of iron (III) separation from zinc sulphate-sulphuric acid solution using the organophosphorous extractant, OPAP and D2EHPA Part I: Extraction. Hydrometallurgy 2004, 74, 93. [Google Scholar] [CrossRef]
- Cai, X.; Han, J.; Pang, M.; Cui, Y.; Li, Y.; Sun, G. Structural effect of diamide extractants on the extraction behaviour of Fe(III) from hydrochloric acid. Hydrometallurgy 2016, 164, 48–53. [Google Scholar] [CrossRef]
- Sokolov, A.; Valeev, D.; Kasikov, A. Solvent extraction of iron(III) from Al chloride solution of bauxite HCl leaching by mixture of aliphatic alcohol and ketone. Metals 2021, 11, 321. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Dreisinger, D.B.; Espinosa, D.C.R.; Tenório, J.A.S. Pre-Reducing Process Kinetics to Recover Metals from Nickel Leach Waste Using Chelating Resins. Int. J. Chem. Eng. 2018, 2018, 9161323. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Vicente, A.A.; Espinosa, D.C.R.; Tenório, J.A.S. Effect of iron oxidation state for copper recovery from nickel laterite leach solution using chelating resin. Sep. Sci. Technol. 2020, 55, 788–798. [Google Scholar] [CrossRef]
- Mystrioti, C.; Papassiopi, N.; Xenidis, A. Selective Recovery of Iron by Solvent Extraction from Ni-Laterite Leach Solutions, as Precursor for the Synthesis of High Added-Value Nanomaterials. Circ. Econ. Sust. 2021. [Google Scholar] [CrossRef]
- Sun, J.; O’Keefe, T.J. An evaluation of steel scrap as a reducing agent in the galvanic stripping of iron from D2EHPA. Miner. Eng. 2002, 15, 177–185. [Google Scholar] [CrossRef]
- Principe, F.; Demopoulos, G.P. Comparative study of iron (III) separation from zinc sulphate-sulphuric acid solution using the organophosphorous extractant, OPAP and D2EHPA Part II Stripping. Hydrometallurgy 2005, 79, 97. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matijević, E. Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J. Colloid Interface Sci. 1980, 74, 227–243. [Google Scholar] [CrossRef]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2018, 8, 48201. [Google Scholar] [CrossRef] [Green Version]
- Schwertmann, U.; Cornell, R. Iron Oxides in the Laboratory; WILEY-VCH Verlag: Weinheim, Germany, 2000. [Google Scholar]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C. Static and dynamic magnetic properties of spherical magnetite nanoparticles. J. Appl. Phys. 2003, 94, 3520. [Google Scholar] [CrossRef] [Green Version]
- Pankratov, D.A.; Anuchina, M.M. Nature-inspired synthesis of magnetic non-stoichiometric Fe3O4 nanoparticles by oxidative in situ method in a humic medium. Mater. Chem. Phys. 2019, 231, 216–224. [Google Scholar] [CrossRef]
- Pankratov, D.A.; Anuchina, M.M.; Spiridonov, F.M.; Krivtsov, G.G. Fe3–δO4 Nanoparticles Synthesized in the Presence of Natural Polyelectrolytes. Crystallogr. Rep. 2020, 65, 393–397. [Google Scholar] [CrossRef]
- Melinkov, P.; Nascimento, V.; Arkhangelsky, I.; Zanoni Consolo, L.; de Oliveira, L. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J. Therm. Anal. Calorim. 2014, 115, 145–151. [Google Scholar] [CrossRef]
- Gallagher, P.; Johnson, W.; Schrey, F. Thermal Decomposition of Iron(II) Sulfates. J. Am. Ceram. Soc. 1970, 53, 666–670. [Google Scholar] [CrossRef]
- Ventruti, G.; Della Ventura, G.; Gomez, M.; Capitani, G.; Sbroscia, M.; Sodo, A. High-temperature study of basic ferric sulfate, FeOHSO4. Phys. Chem. Miner. 2020, 47, 43. [Google Scholar] [CrossRef]
- Alexandrovskaya, Y.; Pavley, Y.; Grigoriev, Y.; Grebenev, V.; Shatalov, T.; Obrezkov, V. Thermal behavior of magnetite nanoparticles with various coatings in the range 30–1000 °C. Thermochim. Acta 2022, 708, 179120. [Google Scholar] [CrossRef]
- Darezereshki, E. Synthesis of maghemite (γ-Fe2O3) nanoparticles by wet chemical method at room temperature. Mater. Lett. 2010, 64, 1471–1472. [Google Scholar] [CrossRef]
- Hu, J.; Lo, I.M.C.; Chen, G. Removal of Cr(VI) by magnetite nanoparticle. Water Sci. Technol. 2004, 50, 139–146. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res. 2005, 39, 4528–4536. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Yanful, E.K. Arsenic and chromium removal by mixed magnetite maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 2010, 91, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Cai, Q.; Xu, W.; Yang, M.; Cai, Y.; Dionysiou, D.D. Cr(VI) Adsorption and Reduction by Humic Acid Coated on Magnetite. Environ. Sci. Technol. 2014, 48, 8078–8085. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Angove, M.J.; Morton, D.W. Recent innovative research on chromium (VI) adsorption mechanism. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100267. [Google Scholar] [CrossRef]


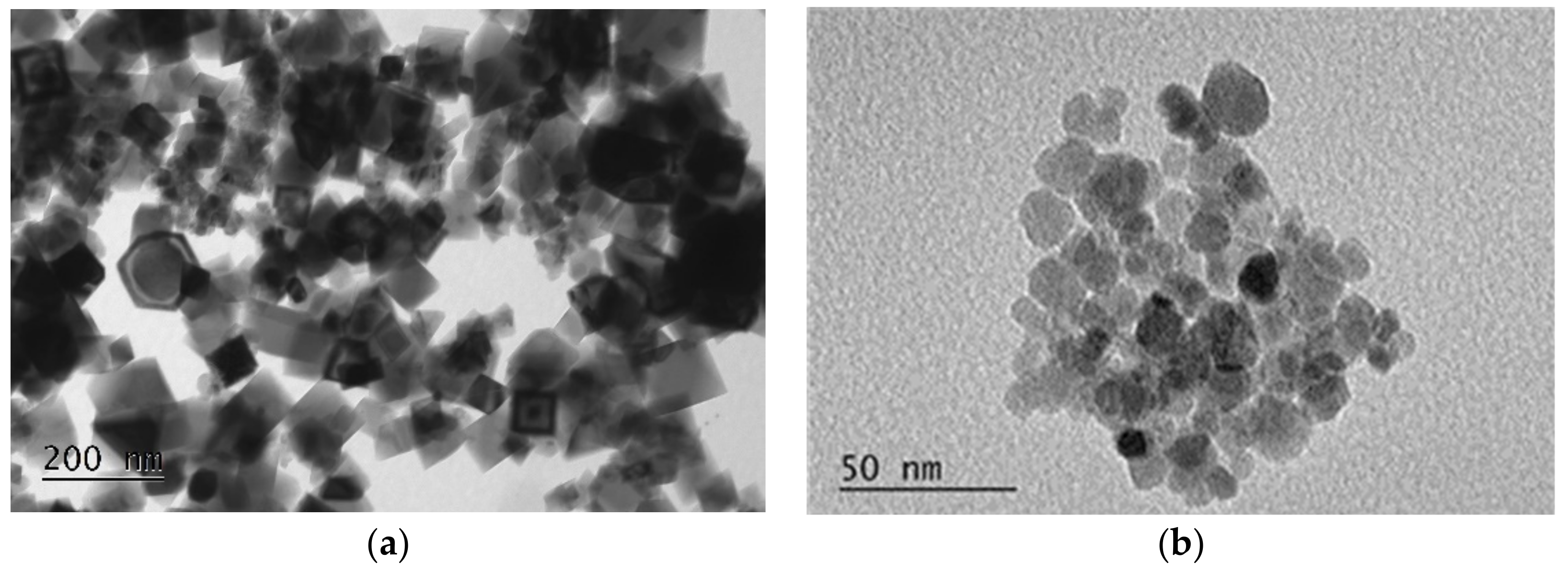

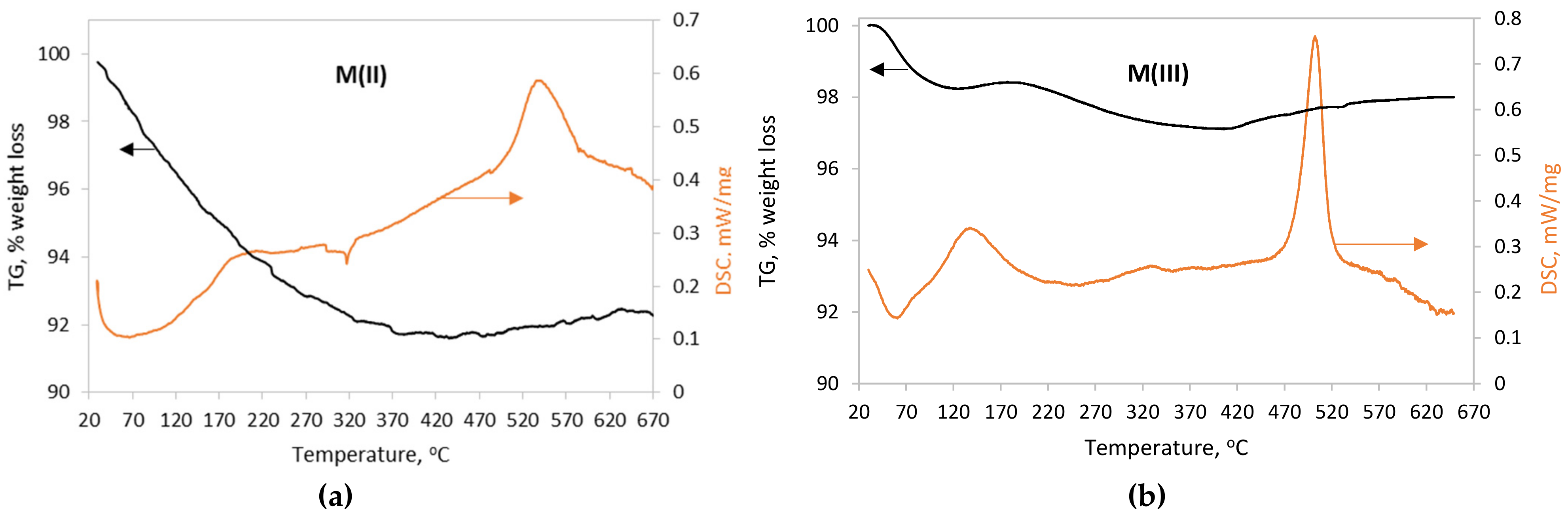
| Sample | Composition (Mössbauer) | Crystallite Size (XRD) nm | Particle Size (TEM) nm | SSA * m2/g | Msat emu·g−1 | pHpzc |
|---|---|---|---|---|---|---|
| M(II) | Fe3O4 (100%) | 35 | 20–100 | 17.0 | 95 | 7.3 |
| M(III) | Fe3O4 (25%), γ-Fe2O3 (75%) | 11 | 10–15 | 88.7 | 80 | 6.1 |
| Sample | Freundlich Isotherm: | ||
| n | KF (mg(1−1/n) L(1/n) g−1) | R2 | |
| M(II) | 1.85 | 0.871 | 0.976 |
| M(III) | 1.87 | 1.451 | 0.992 |
| Sample | Langmuir Isotherm: | ||
| qmax (mg/g) | KL (L/mg) | R2 | |
| M(II) | 11.4 | 0.043 | 0.928 |
| M(III) | 17.4 | 0.054 | 0.968 |
| Mionp Type | Particle Size (nm) | Adsorption Capacity at pH 4–5 (mg/g) | Reference |
|---|---|---|---|
| Magnetite | 10 | 14 | [43] |
| Maghemite | 30 | 4 | [44] |
| Mixture magnetite, maghemite | 20–40 | 6 | [45] |
| Humic acid-Magnetite | 15 | 3.4 | [46] |
| Magnetite | 2–7 | 121 | [11] |
| Magnetite (MII) | 35 | 11.4 | Present study |
| Mixture magnetite, maghemite (M(III) | 11 | 17.4 | Present Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mystrioti, C.; Papassiopi, N.; Xenidis, A. Synthesis of Iron Nanomaterials for Environmental Applications from Hydrometallurgical Liquors. Minerals 2022, 12, 556. https://doi.org/10.3390/min12050556
Mystrioti C, Papassiopi N, Xenidis A. Synthesis of Iron Nanomaterials for Environmental Applications from Hydrometallurgical Liquors. Minerals. 2022; 12(5):556. https://doi.org/10.3390/min12050556
Chicago/Turabian StyleMystrioti, Christiana, Nymphodora Papassiopi, and Anthimos Xenidis. 2022. "Synthesis of Iron Nanomaterials for Environmental Applications from Hydrometallurgical Liquors" Minerals 12, no. 5: 556. https://doi.org/10.3390/min12050556






