Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Methods
3. Results and Discussion
4. Reaction Mechanism
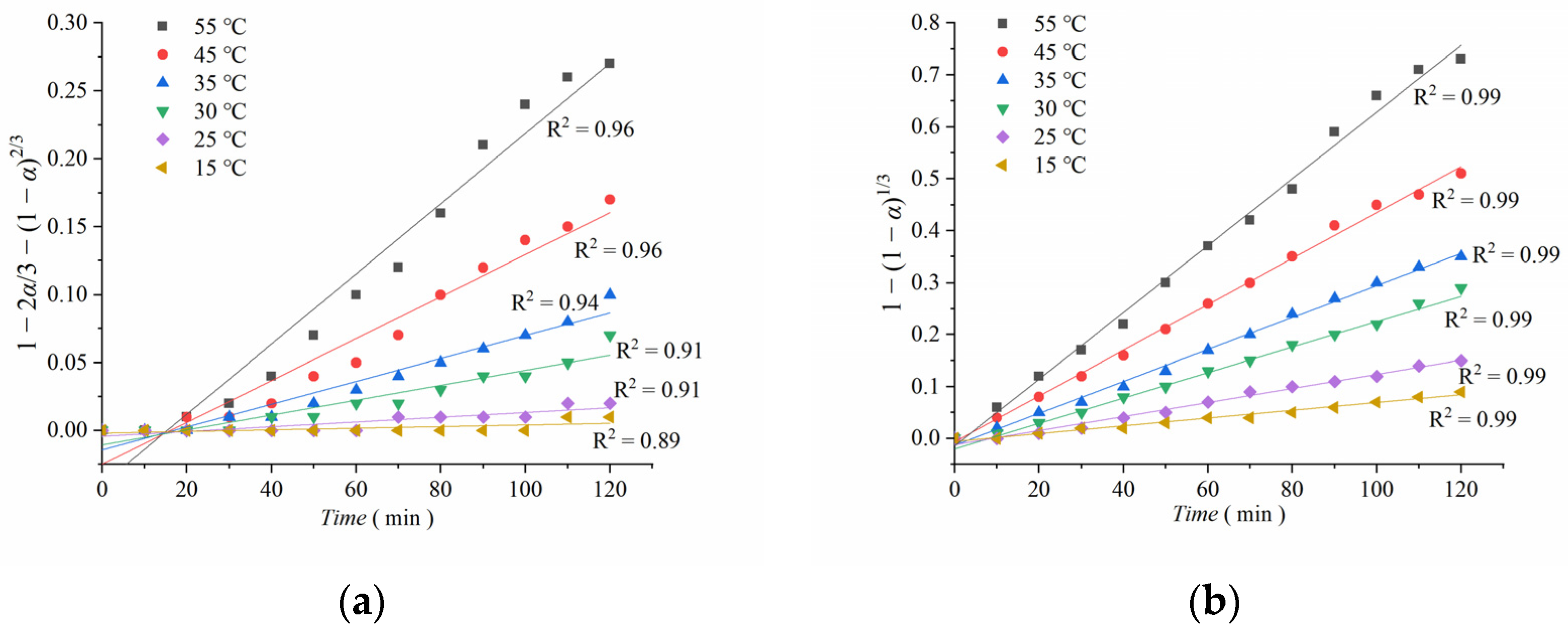
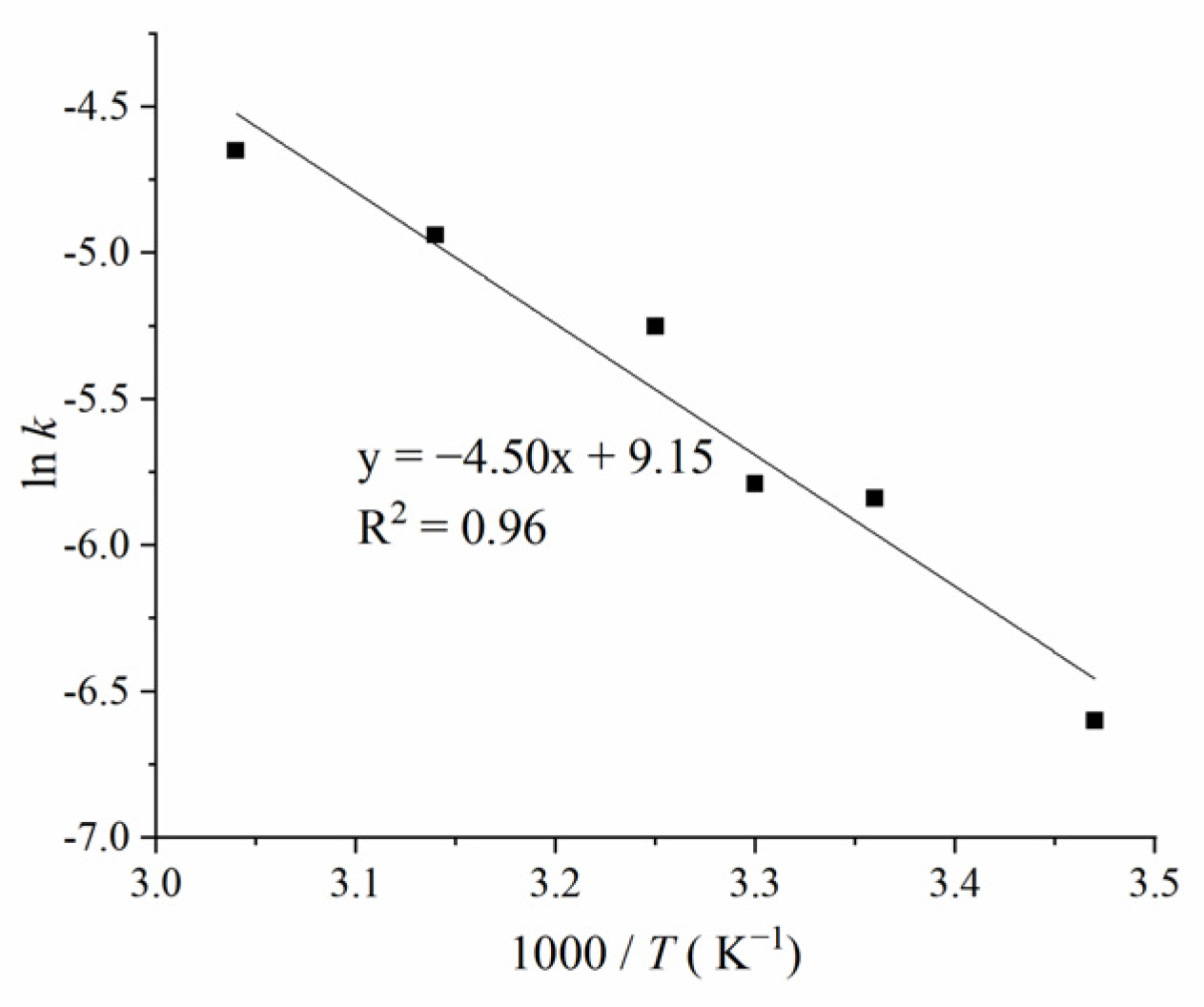
5. Apparent Activation Energy and Kinetics Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akhtar, S.; Yang, X.; Pirajno, F. Sandstone type uranium deposits in the Ordos Basin, Northwest China: A case study and an overview. J. Asian Earth Sci. 2017, 146, 367–382. [Google Scholar] [CrossRef]
- Dai, M.; Peng, Y.; Wu, C.; Jiao, Y.; Liu, L.; Miao, A.; Zhang, C.; Zhang, Z.; Chen, S. Ore characteristics of the sandstone-type Daying uranium deposit in the Ordos Basin, northwestern China. Can. J. Earth Sci. 2017, 54, 893–901. [Google Scholar] [CrossRef]
- Li, Z.; Fang, X.; Chen, A.; Ou, G.; Xiao, X.; Sun, Y.; Liu, C.; Wang, Y. Origin of gray-green sandstone in ore bed of sandstone type uranium deposit in north Ordos Basin. Sci. China Ser. D Earth Sci. 2007, 50, 165–173. [Google Scholar] [CrossRef]
- Miao, P.; Jin, R.; Li, J.; Zhao, H.; Chen, L.; Chen, Y.; Si, Q. The First Discovery of a Large Sandstone-type Uranium Deposit in Aeolian Depositional Environment. Acta Geol. Sin. 2020, 94, 583–584. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.; Li, Y.; Xiao, W.; Liu, Z.; Tan, W.; Xu, Y. The adsorption of U(VI) by albite during acid in-situ leaching mining of uranium. J. Radioanal. Nucl. Chem. 2022, 1–9. [Google Scholar] [CrossRef]
- Satybaldiyev, B.; Lehto, J.; Suksi, J.; Tuovinen, H.; Uralbekov, B.; Burkitbayev, M. Understanding sulphuric acid leaching of uranium from ore by means of 234U/238U activity ratio as an indicator. Hydrometallurgy 2015, 155, 125–131. [Google Scholar] [CrossRef]
- Ma, Q.; Feng, Z.; Liu, P.; Lin, X.; Li, Z.; Chen, M. Uranium speciation and in situ leaching of a sandstone-type deposit from China. J. Radioanal. Nucl. Chem. 2017, 311, 2129–2134. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Xu, Y.; Zhang, C. Mineral alteration and pore-plugging caused by acid in situ leaching: A case study of the Wuyier uranium deposit, Xinjiang, NW China. Arab. J. Geosci. 2018, 11, 707. [Google Scholar] [CrossRef]
- Chen, W. In-Situ Leaching of Uranium; China Atomic Energy Press: Beijing, China, 2018; pp. 145–148. [Google Scholar]
- Lasheen, T.A.; El-Ahmady, M.E.; Hassib, H.B.; Helal, A.S. Oxidative leaching kinetics of molybdenum-uranium ore in H2SO4 using H2O2 as an oxidizing agent. Front. Chem. Sci. Eng. 2013, 7, 95–102. [Google Scholar] [CrossRef]
- Amme, M.; Bors, W.; Michel, C.; Stettmaier, K.; Rasmussen, G.; Betti, M. Effects of Fe(II) and Hydrogen Peroxide Interaction upon Dissolving UO2 under Geologic Repository Conditions. Environ. Sci. Technol. 2005, 39, 221–229. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, H.; Sun, Z.; Liu, Y.; Xu, L.; Shi, W.; Liu, J. Characteristics of uranium dissolution and migration in acidic Solution containing Fe3+. Acta Geol. Sin. 2016, 90, 3554–3562. [Google Scholar]
- Filippov, A.P.; Kanevski, E.A. Oxidation-reduction potentials and the dearee ofuraniumm leaching in sulphuric acid solutions. J. Nucl. Energy Parts A/B React. Sci. Technol. 1965, 19, 575–580. [Google Scholar] [CrossRef]
- Safari, V.; Arzpeyma, G.; Rashchi, F.; Mostoufi, N. A shrinking particle—shrinking core model for leaching of a zinc ore containing silica. Int. J. Miner. Process. 2009, 93, 79–83. [Google Scholar] [CrossRef]
- Faraji, F.; Alizadeh, A.; Rashchi, F.; Mostoufi, N. Kinetics of leaching: A review. Rev. Chem. Eng. 2022, 38, 113–148. [Google Scholar] [CrossRef]
- Pan, Z.; Bartova, B.; LaGrange, T.; Butorin, S.M.; Hyatt, N.C.; Stennett, M.C.; Kvashnina, K.O.; Bernier-Latmani, R. Nanoscale mechanism of UO2 formation through uranium reduction by magnetite. Nat. Commun. 2020, 11, 4001. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.P.; Voelker, B.M. Decomposition of Hydrogen Peroxide and Organic Compounds in the Presence of Dissolved Iron and Ferrihydrite. Environ. Sci. Technol. 2002, 36, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Leaching Uranium Mining; China Atomic Energy Press: Beijing, China, 1998; pp. 23–28. [Google Scholar]
- Pehrman, R.; Trummer, M.; Lousada, C.M.; Jonsson, M. On the redox reactivity of doped UO2 pellets—Influence of dopants on the H2O2 decomposition mechanism. J. Nucl. Mater. 2012, 430, 6–11. [Google Scholar] [CrossRef]
- Ray, H.S.; Ray, S. Kinetics of Metallurgical Process; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Tan, K.; Li, C.; Liu, J.; Qu, H.; Xia, L.; Hu, Y.; Li, Y. A novel method using a complex surfactant for in-situ leaching of low permeable sandstone uranium deposits. Hydrometallurgy 2014, 150, 99–106. [Google Scholar] [CrossRef]
- Samadifard, N.; Devine, C.; Edwards, E.; Mahadevan, K.; Papangelakis, V. Ferric Sulfate Leaching of Pyrrhotite Tailings between 30 to 55 °C. Minerals 2015, 5, 801–814. [Google Scholar] [CrossRef]
- Park, B.H.; Seo, C.S. A semi-empirical model for the air oxidation kinetics of UO2. Korean J. Chem. Eng. 2008, 25, 59–63. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Tan, K.; Li, Y.; Liu, Z.; Li, C.; Tan, W.; Tian, Y.; Huang, W. Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium. Minerals 2022, 12, 570. https://doi.org/10.3390/min12050570
Wang P, Tan K, Li Y, Liu Z, Li C, Tan W, Tian Y, Huang W. Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium. Minerals. 2022; 12(5):570. https://doi.org/10.3390/min12050570
Chicago/Turabian StyleWang, Peng, Kaixuan Tan, Yongmei Li, Zhenzhong Liu, Chunguang Li, Wanyu Tan, Yunting Tian, and Wuyang Huang. 2022. "Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium" Minerals 12, no. 5: 570. https://doi.org/10.3390/min12050570
APA StyleWang, P., Tan, K., Li, Y., Liu, Z., Li, C., Tan, W., Tian, Y., & Huang, W. (2022). Effect of Pyrite on the Leaching Kinetics of Pitchblende in the Process of Acid In Situ Leaching of Uranium. Minerals, 12(5), 570. https://doi.org/10.3390/min12050570




