Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea
Abstract
:1. Introduction
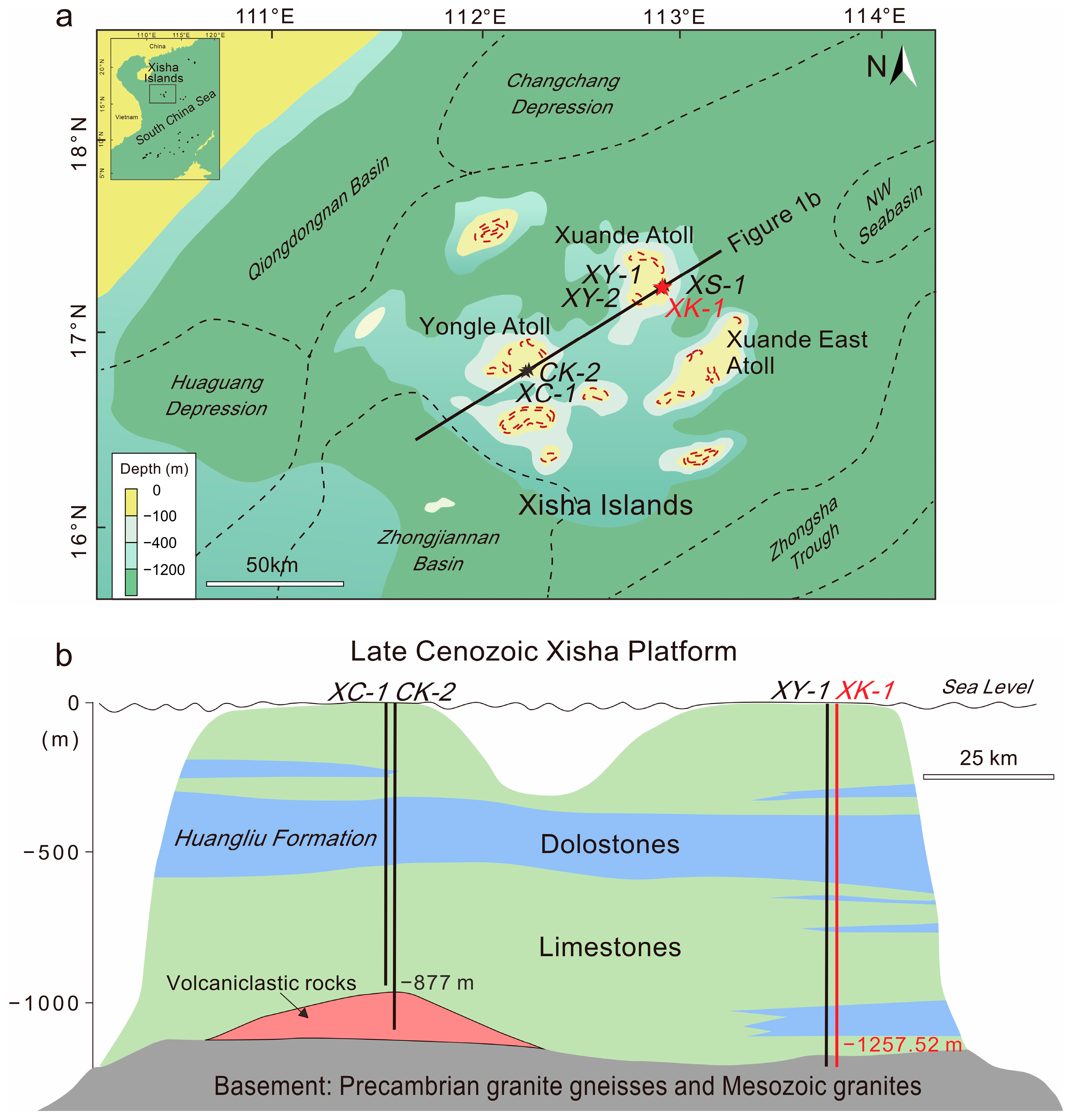
2. Geological Setting
3. Materials and Methods
3.1. X-ray Diffraction Analysis
3.2. Elemental Analysis
3.3. Expression of REY Proxies
3.4. Diagenesis Identification
4. Results
4.1. Mineralogical Composition
4.2. Diagenetic-Alteration-Sensitive Element Geochemical Proxies
4.3. REY Characteristics
5. Discussion
5.1. Diagenesis Types
5.2. Effects of Diagenetic Alteration on the REY Signatures
5.3. Potential of the Xisha Carbonates for Preserving Original Seawater REY Signatures
5.4. Implications of Using the Ce Anomaly in Marine Carbonates as a Paleo-Redox Tracer
6. Conclusions
- (1)
- The reefal carbonates in the Xisha Islands of the South China Sea well record the original seawater REY signatures during primary deposition. Meteoric diagenesis, marine burial diagenesis, and dolomitization do not drive the REY patterns and the values of typical REY proxies (Ce/Ce*, Y/Ho, and (Pr/Yb)N) away from primary seawater signatures.
- (2)
- The Ce anomaly in diagenetically altered shallow marine carbonates can still be used as a good proxy for the redox conditions of the surrounding waters during primary carbonate deposition.
- (3)
- The Ce/Ce* characteristics indicate that water column conditions for the formation of the Xisha carbonates have been constantly oxic from the Neogene to the present, consistent with the conclusion inferred from paleontological fossils and redox-sensitive elemental proxies.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webb, G.E.; Kamber, B.S. Rare earth elements in Holocene reefal microbialites: A new shallow seawater proxy. Geochim. Cosmochim. Acta 2000, 64, 1557–1565. [Google Scholar] [CrossRef]
- Nothdurft, L.D.; Webb, G.E.; Kamber, B.S. Rare earth element geochemistry of Late Devonian reefal carbonates, Canning Basin, Western Australia: Confirmation of a seawater REE proxy in ancient limestones. Geochim. Cosmochim. Acta 2004, 68, 263–283. [Google Scholar] [CrossRef]
- Hood, A.V.S.; Planavsky, N.J.; Wallace, M.W.; Wang, X. The effects of diagenesis on geochemical paleoredox proxies in sedimentary carbonates. Geochim. Cosmochim. Acta 2018, 232, 265–287. [Google Scholar] [CrossRef]
- Li, F.; Webb, G.E.; Algeo, T.J.; Kershaw, S.; Lu, C.; Oehlert, A.M.; Gong, Q.; Pourmand, A.; Tan, X. Modern carbonate ooids preserve ambient aqueous REE signatures. Chem. Geol. 2019, 509, 163–177. [Google Scholar] [CrossRef]
- Hindshaw, R.S.; Tosca, R.; Tosca, N.J.; Tipper, E.T.; Goût, T.L.; Farnan, I.; Tosca, N.J.; Tipper, E.T. Experimental constraints on Mg isotope fractionation during clay formation: Implications for the global biogeochemical cycle of Mg. Earth Planet. Sci. Lett. 2020, 531, 115980. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-M.; Hardisty, D.S.; Lyons, T.W.; Swart, P.K. Evaluating the fidelity of the cerium paleoredox tracer during variable carbonate diagenesis on the Great Bahamas Bank. Geochim. Cosmochim. Acta 2019, 248, 25–42. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, W.; Li, S.; Yang, T.; Zhang, R.; Somerville, I.; Santosh, M.; Wei, H.; Wu, J.; Yang, J.; et al. Rare earth element geochemistry of carbonates as a proxy for deep-time environmental reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 574, 110443. [Google Scholar] [CrossRef]
- Azmy, K.; Brand, U.; Sylvester, P.; Gleeson, S.A.; Logan, A.; Bitner, M.A. Biogenic and abiogenic low-Mg calcite (bLMC and aLMC): Evaluation of seawater-REE composition, water masses and carbonate diagenesis. Chem. Geol. 2011, 280, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Caetano-Filho, S.; Paula-Santos, G.M.; Dias-Brito, D. Carbonate REE + Y signatures from the restricted early marine phase of South Atlantic Ocean (late Aptian—Albian): The influence of early anoxic diagenesis on shale-normalized REE + Y patterns of ancient carbonate rocks. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 500, 69–83. [Google Scholar] [CrossRef] [Green Version]
- Frimmel, H.E. Trace element distribution in Neoproterozoic carbonates as palaeoenvironmental indicator. Chem. Geol. 2009, 258, 338–353. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.N.; Meyers, W.J. Rare earth element and Nd isotopic variations in regionally extensive dolomites from the Burlington-Keokuk Formation (Mississippian); implications for REE mobility during carbonate diagenesis. J. Sediment. Res. 1988, 58, 415–432. [Google Scholar] [CrossRef]
- Zhao, M.-Y.; Zheng, Y.-F. Marine carbonate records of terrigenous input into Paleotethyan seawater: Geochemical constraints from Carboniferous limestones. Geochim. Cosmochim. Acta 2014, 141, 508–531. [Google Scholar] [CrossRef]
- Gong, Q.; Li, F.; Lu, C.; Wang, H.; Tang, H. Tracing seawater- and terrestrial-sourced REE signatures in detritally contaminated, diagenetically altered carbonate rocks. Chem. Geol. 2021, 570, 120169. [Google Scholar] [CrossRef]
- Budd, D.A. Cenozoic dolomites of carbonate islands: Their attributes and origin. Earth-Sci. Rev. 1997, 42, 1–47. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, B. Origin of “island dolostones”: A case study from the Cayman Formation (Miocene), Cayman Brac, British West Indies. Sediment. Geol. 2012, 243–244, 191–206. [Google Scholar] [CrossRef]
- Ren, M.; Jones, B. Spatial variations in the stoichiometry and geochemistry of Miocene dolomite from Grand Cayman: Implications for the origin of island dolostone. Sediment. Geol. 2017, 348, 69–93. [Google Scholar] [CrossRef]
- Ren, M.; Jones, B. Genesis of island dolostones. Sedimentology 2018, 65, 2003–2033. [Google Scholar] [CrossRef]
- Wang, R.; Yu, K.; Jones, B.; Wang, Y.; Zhao, J.; Feng, Y.; Bian, L.; Xu, S.; Fan, T.; Jiang, W.; et al. Evolution and development of Miocene “island dolostones” on Xisha Islands, South China Sea. Mar. Geol. 2018, 406, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Jones, B.; Yu, K. Island dolostones: Genesis by time-transgressive or event dolomitization. Sediment. Geol. 2019, 390, 15–30. [Google Scholar] [CrossRef]
- Zhao, Q. The Sedimentary Research about Reef Carbonate in Xisha Islands Waters; Chinese Academy of Sciences: Beijing, China, 2010. (In Chinese) [Google Scholar]
- Wu, S.; Yang, Z.; Wang, D.; Lü, F.; Lüdmann, T.; Fulthorpe, C.; Wang, B. Architecture, development and geological control of the Xisha carbonate platforms, northwestern South China Sea. Mar. Geol. 2014, 350, 71–83. [Google Scholar] [CrossRef]
- Zhai, S.; Mi, L.; Shen, X.; Liu, X.; Xiu, C.; Sun, Z.; Cao, J. Mineral compositions and their environmental implications in reef of Shidao Island, Xisha. Earth Sci. China Univ. Geosci. 2015, 40, 597–605. (In Chinese) [Google Scholar]
- Cao, J.; Zhang, D.; Zhai, S.; Luo, W.; Xiu, C.; Liu, X.; Zhang, A.; Bi, D. The characteristics and genetic model of the dolomitization in Xisha reef Islands. Haiyang Xuebao 2016, 38, 125–139. (In Chinese) [Google Scholar]
- Fan, T.; Yu, K.; Zhao, J.; Jiang, W.; Xu, S.; Zhang, Y.; Wang, R.; Wang, Y.; Feng, Y.; Bian, L.; et al. Strontium isotope stratigraphy and paleomagnetic age constraints on the evolution history of coral reef islands, northern South China Sea. GSA Bull. 2020, 132, 803–816. [Google Scholar] [CrossRef]
- He, Q.; Zhang, M. Reef Geology of the Xisha Islands, China; Science Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Wang, C.; He, X.; Qiu, S. A preliminary study on carbonate stratigraphy and micropalaeontology of well Xiyong-1, Xisha Islands. Pet. Geol. Exp. 1978, 23–38+73. [Google Scholar]
- Xiu, C.; Zhang, D.; Zhai, S.; Liu, X.; Bi, D. Zricon U-Pb age of granitic rocks from the basement beneath the Shi Island, Xisha Islands and its geological significance. Mar. Geol. Quat. Geol. 2016, 36, 115–126. (In Chinese) [Google Scholar]
- Zhu, W.L.; Xie, X.N.; Wang, Z.F.; Zhang, D.J.; Zhang, C.L.; Cao, L.C.; Shao, L. New insights on the origin of the basement of the Xisha Uplift, South China Sea. Sci. China Earth Sci. 2017, 60, 2214–2222. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Yang, Z.; Wu, T.; Gao, J.; Wang, D. Spatial and temporal evolution of Cenozoic carbonate platforms on the continental margins of the South China Sea: Response to opening of the ocean basin. Interpretation 2016, 4, SP1–SP19. [Google Scholar] [CrossRef]
- Mathew, M.; Makhankova, A.; Menier, D.; Sautter, B.; Betzler, C.; Pierson, B. The emergence of Miocene reefs in South China Sea and its resilient adaptability under varying eustatic, climatic and oceanographic conditions. Sci. Rep. 2020, 10, 7141. [Google Scholar] [CrossRef]
- Lü, C.; Wu, S.; Yao, Y.; Fulthorpe, C.S. Development and controlling factors of Miocene carbonate platform in the Nam Con Son Basin, southwestern South China Sea. Mar. Pet. Geol. 2013, 45, 55–68. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, X.; Yang, J.; Huang, B.; Sun, Z.; Wang, Y.; Jiang, H.; Yu, C.; Yang, X. Analyses on the tectonic thermal evolution and influence factors in the deep-water Qiongdongnan Basin. Acta Oceanol. Sin. 2014, 33, 107–117. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Y.; Liao, W.; Luo, W.; Ma, Z.; Xu, S.; Ma, R. Coral assemblages and their ecological environment of well Xike-1, Xisha Islands. Earth Sci. China Univ. Geosci. 2015, 40, 688–696. (In Chinese) [Google Scholar]
- Wu, F.; Xie, X.; Li, X.; Betzler, C.; Shang, Z.; Cui, Y. Carbonate factory turnovers influenced by the monsoon (Xisha Islands, South China Sea). J. Geol. Soc. 2019, 176, 885–897. [Google Scholar] [CrossRef]
- Shang, Z.; Sun, Z.; Xie, X.; Liu, X.; Lu, Y.; Liao, J.; Wang, Y.; He, Y.; Huang, L.; Jiao, X. Internal architecture and depositional model of reef-bank system since Pliocene in well Xike-1, the South China Sea. Earth Sci. China Univ. Geosci. 2015, 40, 697–710. (In Chinese) [Google Scholar]
- Zhu, Y.; Liu, X.; Ma, R.; Luo, W.; Wang, X.; Xu, S. Early Miocene to Quaternary calcareous nannofossils from the biogenetic reef complexes and their significance of well XK1, Xisha Islands, South China Sea. Acta Palaeontol. Sin. 2016, 55, 385–392. (In Chinese) [Google Scholar]
- Royse, C.F.; Wadell, J.S.; Petersen, L.E. X-ray determination of calcite-dolomite; an evaluation. J. Sediment. Res. 1971, 41, 483–488. [Google Scholar] [CrossRef]
- Cao, C.; Liu, X.M.; Bataille, C.P.; Liu, C. What do Ce anomalies in marine carbonates really mean? A perspective from leaching experiments. Chem. Geol. 2020, 532, 119413. [Google Scholar] [CrossRef]
- Dellinger, M.; West, A.J.; Paris, G.; Adkins, J.F.; Pogge von Strandmann, P.A.E.; Ullmann, C.V.; Eagle, R.A.; Freitas, P.; Bagard, M.-L.L.; Ries, J.B.; et al. The Li isotope composition of marine biogenic carbonates: Patterns and mechanisms. Geochim. Cosmochim. Acta 2018, 236, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Pogge von Strandmann, P.A.E.; Schmidt, D.N.; Planavsky, N.J.; Wei, G.; Todd, C.L.; Baumann, K.H. Assessing bulk carbonates as archives for seawater Li isotope ratios. Chem. Geol. 2019, 530, 119338. [Google Scholar] [CrossRef]
- Taylor, H.L.; Kell Duivestein, I.J.; Farkas, J.; Dietzel, M.; Dosseto, A. Technical note: Lithium isotopes in dolostone as a palaeo-environmental proxy-An experimental approach. Clim. Past 2019, 15, 635–646. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, X.-M.; Wang, K. Potassium isotope fractionation during chemical weathering of basalts. Earth Planet. Sci. Lett. 2020, 539, 116192. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Greig, A.; Collerson, K.D.; Kamber, B.S. Rare earth element and yttrium variability in South East Queensland waterways. Aquat. Geochemistry 2006, 12, 39–72. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Z.; Li, X.; Liu, L.; Zhang, D.; You, L.; Luo, W.; Liu, X. Reef-carbonate diagenesis in the Pleistocene–Holocene of the well Xike#1, Xisha Islands, South China Sea: Implications on sea-level changes. Carbonates Evaporites 2019, 34, 1669–1687. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Z.; Zhang, D.; Huang, K.; You, L.; Duan, X.; Li, S. Microscopic features and genesis for Miocene to Pliocene dolomite in well Xike-1, Xisha Islands. Earth Sci. China Univ. Geosci. 2015, 40, 633–644. (In Chinese) [Google Scholar]
- Bi, D.; Zhai, S.; Zhang, D.; Liu, X.; Liu, X.; Jiang, L.; Zhang, A. Constraints of fluid inclusions and C, O isotopic compositions on the origin of the dolomites in the Xisha Islands, South China Sea. Chem. Geol. 2018, 493, 504–517. [Google Scholar] [CrossRef]
- Shao, L.; Zhu, W.; Deng, C.; Zhang, Y.; Zhai, S. Sedimentology of Carbonate Reef Reservoirs in Well XK-1, South China Sea: Chronostratigraphy and Paleo-Marine Environment; China University of Geosciences Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Scholle, P.A.; Ulmer-Scholle, D.S. Carbonate Diagenesis: Eogenetic Meteoric Diagenesis. In A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis, AAPG Memoir 77; Scholle, P.A., Ulmer-Scholle, D.S., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 77, pp. 332–350. [Google Scholar]
- Melim, L.A.; Swart, P.K.; Maliva, R.G. Meteoric and marine-burial diagenesis in the subsurface of Great Bahama Bank. In Subsurface Geology of a Prograding Carbonate Platform Margin, Great Bahama Bank: Results of the Bahamas Drilling Project; Society for Sedimentary Geology: Tulsa, OK, USA, 2001; Volume 70. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Liu, X.; You, L.; Luo, W.; Yi, L.; Tan, L.; Zhu, Y.; Qin, H.; Cheng, H. Magnetostratigraphy and 230Th dating of Pleistocene biogenic reefs in XK-1 borehole from Xisha Islands, South China Sea. Chin. J. Geophys. 2017, 60, 1027–1038. [Google Scholar]
- Gavish, E.; Friedman, G.M. Progressive diagenesis in Quaternary to Late Tertiary carbonate sediments: Sequence and time scale. J. Sediment. Res. 1969, 39, 980–1006. [Google Scholar]
- Kaufman, A.J.; Knoll, A.H. Neoproterozoic variations in the C-isotopic composition of seawater: Stratigraphic and biogeochemical implications. Precambrian Res. 1995, 73, 27–49. [Google Scholar] [CrossRef]
- Dehler, C.M.; Elrick, M.; Bloch, J.D.; Crossey, L.J.; Karlstrom, K.E.; Marais, D.J. Des High-resolution δ13C stratigraphy of the Chuar Group (ca. 770–742 Ma), Grand Canyon: Implications for mid-Neoproterozoic climate change. GSA Bull. 2005, 117, 32–45. [Google Scholar] [CrossRef]
- Melim, L.A.; Swart, P.K.; Maliva, R.G. Meteoric-like fabrics forming in marine waters: Implications for the use of petrography to identify diagenetic environments. Geology 1995, 23, 755–758. [Google Scholar] [CrossRef]
- Fantle, M.S.; Maher, K.M.; Depaolo, D.J. Isotopic approaches for quantifying the rates of marine burial diagenesis. Rev. Geophys. 2010, 48, 1–38. [Google Scholar] [CrossRef]
- Dellinger, M.; Hardisty, D.S.; Planavsky, N.J.; Gill, B.C.; Kalderon-Asael, B.; Asael, D.; Croissant, T.; Swart, P.K.; West, A.J. The effects of diagenesis on lithium isotope ratios of shallow marine carbonates. Am. J. Sci. 2020, 320, 150–184. [Google Scholar] [CrossRef]
- Scholle, P.A.; Ulmer-Scholle, D.S. Carbonate Diagenesis: Meso-and Telogenetic Burial Diagenesis. In A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis, AAPG Memoir 77; Scholle, P.A., Ulmer-Scholle, D.S., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 77, pp. 352–370. [Google Scholar]
- Scholle, P.A.; Ulmer-Scholle, D.S. Carbonate Diagenesis: Syngenetic/Eogenetic Marine Diagenesis. In A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis: AAPG Memoir 77; Scholle, P.A., Ulmer-Scholle, D.S., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003; Volume 77, pp. 313–327. [Google Scholar]
- Swart, P.K. The geochemistry of carbonate diagenesis: The past, present and future. Sedimentology 2015, 62, 1233–1304. [Google Scholar] [CrossRef]
- Sibley, D.F. The origin of common dolomite fabrics: Clues from the Pliocene. J. Sediment. Res. 1982, 52, 1087–1110. [Google Scholar] [CrossRef]
- Kyser, T.K.; James, N.P.; Bone, Y. Shallow Burial Dolomitization and Dedolomitization of Cenozoic Cool-Water Limestones, Southern Australia: Geochemistry and Origin. J. Sediment. Res. 2002, 72, 146–157. [Google Scholar] [CrossRef]
- Murray, S.T.; Swart, P.K. Evaluating formation fluid models and calibrations using clumped isotope paleothermometry on Bahamian dolomites. Geochim. Cosmochim. Acta 2017, 206, 73–93. [Google Scholar] [CrossRef] [Green Version]
- Ahm, A.S.C.; Bjerrum, C.J.; Blättler, C.L.; Swart, P.K.; Higgins, J.A. Quantifying early marine diagenesis in shallow-water carbonate sediments. Geochim. Cosmochim. Acta 2018, 236, 140–159. [Google Scholar] [CrossRef]
- Jiang, L.; Cai, C.; Worden, R.H.; Crowley, S.F.; Jia, L.; Zhang, K.; Duncan, I.J. Multiphase dolomitization of deeply buried Cambrian petroleum reservoirs, Tarim Basin, north-west China. Sedimentology 2016, 63, 2130–2157. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, M. Dolomite and dolomitization model—A short review. Int. J. Hydrol. 2018, 2, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L. Diagenesis of the San Andres Formation in the Seminole unit in Central Basin platform, western Texas. Am. Assoc. Pet. Geol. Bull. 2022, 106, 267–287. [Google Scholar] [CrossRef]
- Pleydell, S.M.; Jones, B.; Longstaffe, F.J.; Baadsgaard, H. Dolomitization of the Oligocene–Miocene Bluff Formation on Grand Cayman, British West Indies. Can. J. Earth Sci. 1990, 27, 1098–1110. [Google Scholar] [CrossRef]
- Fouke, B.W.; Beets, C.J.; Meyers, W.J.; Hanson, G.N.; Melillo, A.J. 87Sr/86Sr Chronostratigraphy and dolomitization history of the Seroe Domi Formation, Curaçao (Netherlands Antilles). Facies 1996, 35, 293–320. [Google Scholar] [CrossRef]
- Meyers, W.J.; Lu, F.H.; Zachariah, J.K. Dolomitization by mixed evaporative brines and freshwater, upper Miocene carbonates, Nijar, Spain. J. Sediment. Res. 1997, 67, 898–912. [Google Scholar] [CrossRef]
- Brand, U.; Veizer, J. Chemical diagenesis of a multicomponent carbonate system; 1, Trace elements. J. Sediment. Res. 1980, 50, 1219–1236. [Google Scholar] [CrossRef]
- Korte, C.; Kozur, H.W.; Bruckschen, P.; Veizer, J. Strontium isotope evolution of Late Permian and Triassic seawater. Geochim. Cosmochim. Acta 2003, 67, 47–62. [Google Scholar] [CrossRef]
- Kaufman, A.J.; Knoll, A.H.; Awramik, S.M. Biostratigraphic and chemostratigraphic correlation of Neoproterozoic sedimentary successions: Upper Tindir Group, northwestern Canada, as a test case. Geology 1992, 20, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Alibo, D.S.; Nozaki, Y. Dissolved rare earth elements in the South China Sea: Geochemical characterization of the water masses. J. Geophys. Res. Ocean. 2000, 105, 28771–28783. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Rare earth elements in seawater: Particle association, shale-normalization, and Ce oxidation. Geochim. Cosmochim. Acta 1999, 63, 363–372. [Google Scholar] [CrossRef]
- Nozaki, Y.; Zhang, J.; Amakawa, H. The fractionation between Y and Ho in the marine environment. Earth Planet. Sci. Lett. 1997, 148, 329–340. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Piepgras, D.J.; Jacobsen, S.B. The pore water chemistry of rare earth elements in Buzzards Bay sediments. Geochim. Cosmochim. Acta 1989, 53, 2847–2856. [Google Scholar] [CrossRef]
- Banner, J.L.; Hanson, G.N. Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochim. Cosmochim. Acta 1990, 54, 3123–3137. [Google Scholar] [CrossRef]
- Webb, G.E.; Nothdurft, L.D.; Kamber, B.S.; Kloprogge, J.T.; Zhao, J.-X. Rare earth element geochemistry of scleractinian coral skeleton during meteoric diagenesis: A sequence through neomorphism of aragonite to calcite. Sedimentology 2009, 56, 1433–1463. [Google Scholar] [CrossRef]
- Johannesson, K.H.; Palmore, C.D.; Fackrell, J.; Prouty, N.G.; Swarzenski, P.W.; Chevis, D.A.; Telfeyan, K.; White, C.D.; Burdige, D.J. Rare earth element behavior during groundwater–seawater mixing along the Kona Coast of Hawaii. Geochim. Cosmochim. Acta 2017, 198, 229–258. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Liu, X.M. Spatial and temporal distribution of rare earth elements in the Neuse River, North Carolina. Chem. Geol. 2018, 488, 34–43. [Google Scholar] [CrossRef]
- Haley, B.A.; Klinkhammer, G.P.; McManus, J. Rare earth elements in pore waters of marine sediments. Geochim. Cosmochim. Acta 2004, 68, 1265–1279. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawabe, I. REE abundances in ancient seawater inferred from marine limestone and experimental REE partition coefficients between calcite and aqueous solution. Geochem. J. 2006, 40, 425–435. [Google Scholar] [CrossRef] [Green Version]
- Allwood, A.C.; Kamber, B.S.; Walter, M.R.; Burch, I.W.; Kanik, I. Trace elements record depositional history of an Early Archean stromatolitic carbonate platform. Chem. Geol. 2010, 270, 148–163. [Google Scholar] [CrossRef]
- Moore, C.H.; Wade, W.J. Concepts of Sequence Stratigraphy as Applied to Carbonate Depositional Systems. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 55, pp. 19–36. [Google Scholar]
- Bau, M.; Koschinsky, A.; Dulski, P.; Hein, J.R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Kamber, B.S.; Webb, G.E.; Gallagher, M. The rare earth element signal in Archaean microbial carbonate: Information on ocean redox and biogenicity. J. Geol. Soc. Lond. 2014, 171, 745–763. [Google Scholar] [CrossRef]
- German, C.R.; Elderfield, H. Application of the Ce anomaly as a paleoredox indicator: The ground rules. Paleoceanography 1990, 5, 823–833. [Google Scholar] [CrossRef]
- Xiu, C.; Luo, W.; Yang, H.; Zhai, S.; Liu, X.; Cao, J.; Liu, X.; Chen, H.; Zhang, A. Geochemical characteristics of reef carbonate rocks in well Xike-1 of Shidao Island, Xisha area. Earth Sci. China Univ. Geosci. 2015, 40, 645–652. (In Chinese) [Google Scholar]
- Wallace, M.W.; Hood, A.; Shuster, A.; Greig, A.; Planavsky, N.J.; Reed, C.P. Oxygenation history of the Neoproterozoic to early Phanerozoic and the rise of land plants. Earth Planet. Sci. Lett. 2017, 466, 12–19. [Google Scholar] [CrossRef]
- Liu, X.-M.; Kah, L.C.; Knoll, A.H.; Cui, H.; Wang, C.; Bekker, A.; Hazen, R.M. A persistently low level of atmospheric oxygen in Earth’s middle age. Nat. Commun. 2021, 12, 351. [Google Scholar] [CrossRef] [PubMed]






| Depth | LMC | DOL | Mineral Unit | Al | Mn | Fe | Sr | Y | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Th | ∑REY | Ce/Ce* | (Pr/Yb)N | Y/Ho | Mn/Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | % | % | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | μg/g | |||||
| 0.3 | HMC: 43 LMC: 31 ARA: 26 | — | 106 | 20 | 71 | 3231 | 11.23 | 2.36 | 1.47 | 0.49 | 2.36 | 0.59 | 0.14 | 0.83 | 0.12 | 0.85 | 0.17 | 0.53 | 0.06 | 0.49 | 0.05 | 0.02 | 21.77 | 0.46 | 0.31 | 65 | 0.01 | |
| 37.0 | 100 | 0 | Cal 1 | 359 | 49 | 225 | 1777 | 5.07 | 1.36 | 1.42 | 0.29 | 1.34 | 0.31 | 0.08 | 0.40 | 0.06 | 0.38 | 0.08 | 0.23 | 0.03 | 0.23 | 0.02 | 0.06 | 11.28 | 0.69 | 0.40 | 67 | 0.03 |
| 100.3 | 100 | 0 | Cal 1 | 69 | 16 | 64 | 1113 | 3.08 | 0.48 | 0.39 | 0.10 | 0.48 | 0.12 | 0.03 | 0.18 | 0.03 | 0.20 | 0.04 | 0.14 | 0.02 | 0.14 | 0.02 | 0.01 | 5.44 | 0.61 | 0.22 | 74 | 0.01 |
| 173.3 | 100 | 0 | Cal 1 | 15 | 34 | 16 | 1913 | 2.51 | 0.43 | 0.28 | 0.08 | 0.39 | 0.10 | 0.03 | 0.15 | 0.02 | 0.16 | 0.03 | 0.11 | 0.01 | 0.11 | 0.01 | 0.01 | 4.44 | 0.54 | 0.22 | 73 | 0.02 |
| 259.3 | 90.4 | 9.6 | — | 76 | 32 | 124 | 464 | 2.82 | 0.65 | 0.63 | 0.14 | 0.69 | 0.17 | 0.04 | 0.23 | 0.03 | 0.23 | 0.05 | 0.14 | 0.02 | 0.15 | 0.01 | 0.02 | 6.00 | 0.67 | 0.31 | 61 | 0.07 |
| 289.3 | 4.4 | 95.6 | Dol 1 | 49 | 11 | 38 | 267 | 1.46 | 0.41 | 0.32 | 0.08 | 0.37 | 0.09 | 0.02 | 0.12 | 0.02 | 0.12 | 0.02 | 0.07 | 0.01 | 0.07 | 0.01 | 0.02 | 3.18 | 0.63 | 0.35 | 62 | 0.04 |
| 296.3 | 0 | 100 | Dol 1 | 55 | 18 | 78 | 229 | 1.63 | 0.58 | 0.47 | 0.11 | 0.55 | 0.13 | 0.03 | 0.17 | 0.03 | 0.18 | 0.03 | 0.10 | 0.01 | 0.09 | 0.01 | 0.03 | 4.14 | 0.64 | 0.38 | 48 | 0.08 |
| 346.3 | 90.2 | 9.8 | — | 212 | 27 | 205 | 748 | 2.70 | 0.91 | 1.01 | 0.20 | 0.91 | 0.21 | 0.05 | 0.25 | 0.03 | 0.22 | 0.04 | 0.13 | 0.01 | 0.12 | 0.01 | 0.07 | 6.80 | 0.74 | 0.50 | 65 | 0.04 |
| 392.1 | 2.2 | 97.8 | Dol 2 | 267 | 20 | 293 | 162 | 2.02 | 0.83 | 0.79 | 0.17 | 0.79 | 0.19 | 0.05 | 0.24 | 0.04 | 0.23 | 0.04 | 0.13 | 0.01 | 0.11 | 0.01 | 0.05 | 5.63 | 0.69 | 0.48 | 47 | 0.12 |
| 404.7 | 0 | 100 | Dol 2 | 54 | 3 | 37 | 171 | 0.71 | 0.31 | 0.31 | 0.06 | 0.28 | 0.07 | 0.02 | 0.08 | 0.01 | 0.08 | 0.02 | 0.04 | 0.005 | 0.04 | 0.004 | 0.01 | 2.02 | 0.77 | 0.50 | 46 | 0.02 |
| 424.7 | 0 | 100 | Dol 3 | 30 | 26 | 99 | 205 | 1.34 | 0.43 | 0.24 | 0.07 | 0.33 | 0.08 | 0.02 | 0.10 | 0.01 | 0.10 | 0.02 | 0.06 | 0.01 | 0.06 | 0.01 | 0.01 | 2.88 | 0.53 | 0.36 | 70 | 0.13 |
| 436.6 | 0 | 100 | Dol 3 | 30 | 13 | 20 | 165 | 0.96 | 0.42 | 0.32 | 0.08 | 0.37 | 0.08 | 0.02 | 0.11 | 0.02 | 0.10 | 0.02 | 0.06 | 0.01 | 0.05 | 0.01 | 0.01 | 2.60 | 0.61 | 0.50 | 52 | 0.08 |
| 450.6 | 0 | 100 | Dol 3 | 12 | 1 | 3 | 170 | 0.62 | 0.22 | 0.14 | 0.04 | 0.19 | 0.04 | 0.01 | 0.06 | 0.01 | 0.05 | 0.01 | 0.03 | 0.004 | 0.03 | 0.003 | 0.005 | 1.47 | 0.51 | 0.41 | 59 | 0.004 |
| 462.7 | 2 | 98 | Dol 4 | 36 | 4 | 26 | 188 | 0.92 | 0.29 | 0.23 | 0.05 | 0.25 | 0.06 | 0.01 | 0.08 | 0.01 | 0.07 | 0.01 | 0.04 | 0.01 | 0.04 | 0.004 | 0.01 | 2.08 | 0.67 | 0.40 | 64 | 0.02 |
| 476.7 | 0 | 100 | Dol 4 | 49 | 8 | 41 | 181 | 2.66 | 0.82 | 0.61 | 0.15 | 0.71 | 0.17 | 0.04 | 0.22 | 0.03 | 0.21 | 0.04 | 0.12 | 0.01 | 0.10 | 0.01 | 0.02 | 5.89 | 0.61 | 0.45 | 66 | 0.04 |
| 489.9 | 0 | 100 | Dol 4 | 30 | 5 | 36 | 170 | 2.07 | 0.69 | 0.44 | 0.12 | 0.58 | 0.13 | 0.03 | 0.18 | 0.03 | 0.17 | 0.03 | 0.10 | 0.01 | 0.08 | 0.01 | 0.02 | 4.67 | 0.55 | 0.46 | 61 | 0.03 |
| 504.8 | 0 | 100 | Dol 4 | 34 | 6 | 8 | 212 | 1.43 | 0.66 | 0.47 | 0.13 | 0.64 | 0.15 | 0.03 | 0.17 | 0.02 | 0.15 | 0.03 | 0.07 | 0.01 | 0.06 | 0.01 | 0.01 | 4.03 | 0.53 | 0.72 | 56 | 0.03 |
| 528.8 | 0 | 100 | Dol 4 | 16 | 3 | 9 | 162 | 1.23 | 0.47 | 0.30 | 0.08 | 0.41 | 0.10 | 0.02 | 0.13 | 0.02 | 0.11 | 0.02 | 0.06 | 0.01 | 0.05 | 0.01 | 0.01 | 3.02 | 0.56 | 0.55 | 57 | 0.02 |
| 549.8 | 0 | 100 | Dol 4 | 46 | 7 | 59 | 167 | 3.30 | 0.89 | 0.66 | 0.13 | 0.66 | 0.15 | 0.04 | 0.24 | 0.03 | 0.24 | 0.05 | 0.14 | 0.02 | 0.12 | 0.01 | 0.03 | 6.68 | 0.75 | 0.35 | 70 | 0.04 |
| 565.0 | 1.9 | 98.1 | Dol 4 | 155 | 10 | 143 | 169 | 1.32 | 0.63 | 1.00 | 0.13 | 0.62 | 0.14 | 0.03 | 0.16 | 0.02 | 0.14 | 0.02 | 0.07 | 0.01 | 0.06 | 0.01 | 0.04 | 4.36 | 1.14 | 0.69 | 53 | 0.06 |
| 579.8 | 100 | 0 | Cal 2 | 35 | 26 | 20 | 689 | 1.42 | 0.32 | 0.28 | 0.06 | 0.30 | 0.07 | 0.02 | 0.10 | 0.01 | 0.09 | 0.02 | 0.05 | 0.01 | 0.05 | 0.005 | 0.01 | 2.79 | 0.72 | 0.37 | 82 | 0.04 |
| 618.8 | 2.1 | 97.9 | Dol 5 | 37 | 5 | 23 | 172 | 0.86 | 0.26 | 0.24 | 0.05 | 0.24 | 0.06 | 0.01 | 0.07 | 0.01 | 0.07 | 0.01 | 0.04 | 0.004 | 0.03 | 0.003 | 0.01 | 1.96 | 0.79 | 0.52 | 68 | 0.03 |
| 630.4 | 2 | 98 | Dol 5 | 28 | 7 | 18 | 207 | 2.61 | 1.08 | 0.86 | 0.18 | 0.91 | 0.21 | 0.05 | 0.26 | 0.04 | 0.21 | 0.04 | 0.10 | 0.01 | 0.08 | 0.01 | 0.01 | 6.65 | 0.72 | 0.71 | 67 | 0.03 |
| 633.9 | 2.1 | 97.9 | Dol 5 | 48 | 8 | 22 | 197 | 1.73 | 0.66 | 0.56 | 0.13 | 0.61 | 0.15 | 0.04 | 0.17 | 0.02 | 0.15 | 0.03 | 0.07 | 0.01 | 0.06 | 0.01 | 0.02 | 4.38 | 0.67 | 0.69 | 65 | 0.04 |
| 643.8 | 93.9 | 6.1 | Cal 3 | 24 | 5 | 20 | 334 | 2.61 | 0.80 | 0.53 | 0.14 | 0.68 | 0.16 | 0.04 | 0.21 | 0.03 | 0.18 | 0.03 | 0.09 | 0.01 | 0.08 | 0.01 | 0.003 | 5.61 | 0.61 | 0.53 | 77 | 0.01 |
| 683.8 | 100 | 0 | Cal 3 | 61 | 21 | 104 | 707 | 3.26 | 0.88 | 0.57 | 0.13 | 0.66 | 0.15 | 0.04 | 0.22 | 0.03 | 0.19 | 0.04 | 0.11 | 0.01 | 0.10 | 0.01 | 0.01 | 6.39 | 0.64 | 0.43 | 86 | 0.03 |
| 716.4 | 100 | 0 | Cal 3 | 168 | 50 | 187 | 661 | 2.89 | 0.88 | 0.83 | 0.17 | 0.81 | 0.19 | 0.05 | 0.25 | 0.03 | 0.22 | 0.04 | 0.12 | 0.01 | 0.11 | 0.01 | 0.04 | 6.62 | 0.72 | 0.49 | 70 | 0.08 |
| 759.9 | 1 | 98 | Dol 6 | 49 | 6 | 34 | 176 | 1.78 | 0.68 | 0.48 | 0.12 | 0.58 | 0.13 | 0.03 | 0.18 | 0.02 | 0.15 | 0.03 | 0.07 | 0.01 | 0.06 | 0.01 | 0.02 | 4.34 | 0.61 | 0.66 | 66 | 0.03 |
| 767.7 | 2 | 97 | Dol 6 | 22 | 6 | 13 | 179 | 1.39 | 0.57 | 0.35 | 0.09 | 0.46 | 0.11 | 0.03 | 0.14 | 0.02 | 0.12 | 0.02 | 0.06 | 0.01 | 0.05 | 0.005 | 0.01 | 3.42 | 0.56 | 0.61 | 65 | 0.03 |
| 775.1 | 2 | 96 | Dol 6 | 35 | 10 | 28 | 184 | 2.89 | 1.10 | 0.68 | 0.19 | 0.93 | 0.21 | 0.05 | 0.28 | 0.04 | 0.23 | 0.04 | 0.11 | 0.01 | 0.09 | 0.01 | 0.02 | 6.85 | 0.57 | 0.69 | 70 | 0.06 |
| 824.8 | 99 | 0 | Cal 4 | 31 | 11 | 38 | 656 | 3.98 | 1.06 | 0.78 | 0.18 | 0.88 | 0.20 | 0.05 | 0.27 | 0.04 | 0.25 | 0.05 | 0.14 | 0.02 | 0.11 | 0.01 | 0.01 | 8.02 | 0.63 | 0.52 | 81 | 0.02 |
| 856.8 | 98 | 2 | Cal 4 | 5 | 8 | 14 | 352 | 4.34 | 0.93 | 0.55 | 0.16 | 0.81 | 0.19 | 0.05 | 0.28 | 0.04 | 0.27 | 0.05 | 0.16 | 0.02 | 0.13 | 0.01 | 0.01 | 8.00 | 0.52 | 0.39 | 81 | 0.02 |
| 896.7 | 1 | 98 | — | 26 | 6 | 14 | 159 | 1.92 | 0.55 | 0.39 | 0.10 | 0.49 | 0.12 | 0.03 | 0.16 | 0.02 | 0.15 | 0.03 | 0.08 | 0.01 | 0.07 | 0.01 | 0.02 | 4.14 | 0.58 | 0.43 | 68 | 0.03 |
| 934.8 | 95 | 4 | — | 10 | 11 | 29 | 461 | 3.93 | 0.86 | 0.92 | 0.15 | 0.75 | 0.18 | 0.05 | 0.27 | 0.04 | 0.26 | 0.05 | 0.15 | 0.02 | 0.13 | 0.01 | 0.01 | 7.78 | 0.95 | 0.35 | 77 | 0.02 |
| 973.8 | 4 | 96 | Dol 7 | 43 | 9 | 36 | 171 | 2.28 | 0.67 | 0.48 | 0.12 | 0.60 | 0.15 | 0.04 | 0.20 | 0.03 | 0.18 | 0.03 | 0.10 | 0.01 | 0.09 | 0.01 | 0.02 | 5.00 | 0.59 | 0.44 | 67 | 0.05 |
| 997.1 | 5 | 95 | Dol 7 | 21 | 10 | 12 | 184 | 2.46 | 0.71 | 0.44 | 0.13 | 0.63 | 0.16 | 0.04 | 0.21 | 0.03 | 0.19 | 0.04 | 0.10 | 0.01 | 0.10 | 0.01 | 0.02 | 5.25 | 0.53 | 0.42 | 69 | 0.05 |
| 1006.8 | 6 | 94 | Dol 7 | 72 | 11 | 52 | 204 | 2.19 | 0.73 | 0.53 | 0.13 | 0.64 | 0.15 | 0.04 | 0.20 | 0.03 | 0.18 | 0.03 | 0.10 | 0.01 | 0.09 | 0.01 | 0.03 | 5.05 | 0.61 | 0.49 | 68 | 0.06 |
| 1035.8 | 1 | 99 | Dol 7 | 71 | 12 | 84 | 171 | 2.31 | 0.86 | 0.86 | 0.16 | 0.78 | 0.19 | 0.04 | 0.23 | 0.03 | 0.20 | 0.04 | 0.11 | 0.01 | 0.09 | 0.01 | 0.05 | 5.92 | 0.79 | 0.55 | 63 | 0.07 |
| 1048.7 | 1 | 99 | Dol 7 | 89 | 16 | 97 | 203 | 4.46 | 1.16 | 1.78 | 0.28 | 1.37 | 0.37 | 0.09 | 0.44 | 0.07 | 0.42 | 0.08 | 0.22 | 0.03 | 0.20 | 0.02 | 0.07 | 10.99 | 0.95 | 0.44 | 57 | 0.08 |
| 1064.6 | 1 | 99 | Dol 7 | 55 | 31 | 117 | 180 | 2.25 | 0.53 | 0.66 | 0.10 | 0.49 | 0.13 | 0.03 | 0.17 | 0.03 | 0.17 | 0.03 | 0.10 | 0.01 | 0.09 | 0.01 | 0.03 | 4.79 | 0.98 | 0.36 | 70 | 0.17 |
| 1078.4 | 1 | 99 | Dol 7 | 67 | 22 | 105 | 198 | 5.48 | 1.65 | 1.08 | 0.30 | 1.45 | 0.34 | 0.09 | 0.45 | 0.06 | 0.41 | 0.08 | 0.23 | 0.03 | 0.20 | 0.02 | 0.06 | 11.86 | 0.53 | 0.47 | 68 | 0.11 |
| 1094.8 | 1 | 99 | Dol 7 | 81 | 13 | 88 | 183 | 3.31 | 0.96 | 0.67 | 0.18 | 0.85 | 0.21 | 0.05 | 0.27 | 0.04 | 0.25 | 0.05 | 0.14 | 0.02 | 0.13 | 0.01 | 0.04 | 7.13 | 0.56 | 0.45 | 69 | 0.07 |
| 1102.8 | 1 | 99 | Dol 7 | 62 | 10 | 70 | 187 | 2.61 | 0.87 | 0.78 | 0.18 | 0.82 | 0.20 | 0.05 | 0.24 | 0.03 | 0.23 | 0.04 | 0.13 | 0.01 | 0.10 | 0.01 | 0.03 | 6.31 | 0.64 | 0.53 | 63 | 0.05 |
| 1112.0 | 1 | 99 | Dol 7 | 49 | 20 | 48 | 175 | 1.53 | 0.48 | 0.35 | 0.09 | 0.42 | 0.10 | 0.02 | 0.13 | 0.02 | 0.12 | 0.02 | 0.07 | 0.01 | 0.06 | 0.01 | 0.02 | 3.41 | 0.59 | 0.48 | 67 | 0.12 |
| 1122.0 | 1 | 99 | Dol 7 | 33 | 9 | 25 | 196 | 2.03 | 0.54 | 0.40 | 0.10 | 0.48 | 0.12 | 0.03 | 0.15 | 0.02 | 0.14 | 0.03 | 0.08 | 0.01 | 0.07 | 0.01 | 0.02 | 4.21 | 0.61 | 0.43 | 73 | 0.04 |
| 1137.0 | 1 | 99 | Dol 7 | 23 | 12 | 22 | 189 | 2.03 | 0.68 | 0.46 | 0.12 | 0.56 | 0.14 | 0.03 | 0.18 | 0.02 | 0.15 | 0.03 | 0.08 | 0.01 | 0.06 | 0.01 | 0.02 | 4.56 | 0.59 | 0.57 | 68 | 0.07 |
| 1147.0 | 1 | 99 | Dol 7 | 40 | 13 | 39 | 230 | 1.40 | 0.57 | 0.46 | 0.11 | 0.49 | 0.11 | 0.03 | 0.13 | 0.02 | 0.12 | 0.02 | 0.06 | 0.01 | 0.05 | 0.005 | 0.02 | 3.59 | 0.63 | 0.61 | 67 | 0.06 |
| 1152.0 | 1 | 99 | Dol 7 | 25 | 23 | 36 | 195 | 1.13 | 0.48 | 0.40 | 0.08 | 0.39 | 0.09 | 0.02 | 0.11 | 0.02 | 0.10 | 0.02 | 0.05 | 0.01 | 0.05 | 0.004 | 0.02 | 2.95 | 0.70 | 0.54 | 63 | 0.12 |
| 1167.0 | 1 | 99 | Dol 7 | 32 | 21 | 71 | 231 | 2.22 | 0.79 | 0.57 | 0.13 | 0.64 | 0.14 | 0.03 | 0.18 | 0.03 | 0.16 | 0.03 | 0.09 | 0.01 | 0.07 | 0.01 | 0.03 | 5.11 | 0.62 | 0.61 | 72 | 0.09 |
| 1179.5 | 4 | 96 | Dol 7 | 232 | 33 | 163 | 223 | 7.13 | 2.32 | 1.68 | 0.43 | 1.99 | 0.44 | 0.11 | 0.57 | 0.08 | 0.51 | 0.10 | 0.28 | 0.03 | 0.23 | 0.02 | 0.07 | 15.91 | 0.56 | 0.59 | 73 | 0.15 |
| 1196.0 | 96 | 4 | Cal 5 | 68 | 24 | 62 | 587 | 2.38 | 0.93 | 0.70 | 0.16 | 0.76 | 0.16 | 0.04 | 0.20 | 0.03 | 0.17 | 0.03 | 0.09 | 0.01 | 0.08 | 0.01 | 0.01 | 5.75 | 0.63 | 0.65 | 73 | 0.04 |
| 1249.7 | 95 | 5 | — | 110 | 75 | 150 | 281 | 2.66 | 0.95 | 1.19 | 0.20 | 0.91 | 0.22 | 0.06 | 0.27 | 0.04 | 0.25 | 0.05 | 0.13 | 0.02 | 0.12 | 0.01 | 0.08 | 7.08 | 0.85 | 0.51 | 59 | 0.27 |
| Average | 66 | 16 | 66 | 404 | 2.59 | 0.78 | 0.63 | 0.14 | 0.70 | 0.17 | 0.04 | 0.22 | 0.03 | 0.20 | 0.04 | 0.11 | 0.01 | 0.10 | 0.01 | 0.03 | 5.77 | 0.66 | 0.48 | 66 | 0.06 | |||
| Average Calcite | 89 | 28 | 90 | 768 | 3.12 | 0.82 | 0.72 | 0.15 | 0.74 | 0.18 | 0.04 | 0.23 | 0.03 | 0.22 | 0.04 | 0.13 | 0.01 | 0.12 | 0.01 | 0.03 | 6.57 | 0.68 | 0.42 | 73 | 0.05 | |||
| Average Dolomite | 57 | 12 | 57 | 190 | 2.15 | 0.72 | 0.58 | 0.13 | 0.64 | 0.15 | 0.04 | 0.19 | 0.03 | 0.18 | 0.03 | 0.10 | 0.01 | 0.08 | 0.01 | 0.03 | 5.04 | 0.65 | 0.51 | 64 | 0.06 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.-F.; Zhai, S.; Wang, X.-K.; Liu, X.; Liu, X.-M. Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea. Minerals 2022, 12, 578. https://doi.org/10.3390/min12050578
Liu X-F, Zhai S, Wang X-K, Liu X, Liu X-M. Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea. Minerals. 2022; 12(5):578. https://doi.org/10.3390/min12050578
Chicago/Turabian StyleLiu, Xiao-Feng, Shikui Zhai, Xi-Kai Wang, Xinyu Liu, and Xiao-Ming Liu. 2022. "Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea" Minerals 12, no. 5: 578. https://doi.org/10.3390/min12050578
APA StyleLiu, X. -F., Zhai, S., Wang, X. -K., Liu, X., & Liu, X. -M. (2022). Rare Earth Element Geochemistry of Late Cenozoic Island Carbonates in the South China Sea. Minerals, 12(5), 578. https://doi.org/10.3390/min12050578







