Sulfate Sources Required for Thermochemical Sulfate Reduction in Dolostone Reservoirs in the Upper Permian Changxing Formation, Yuanba Gas Field, Sichuan Basin, China: Insights from the Origin of Celestite
Abstract
1. Introduction
2. Geological Setting
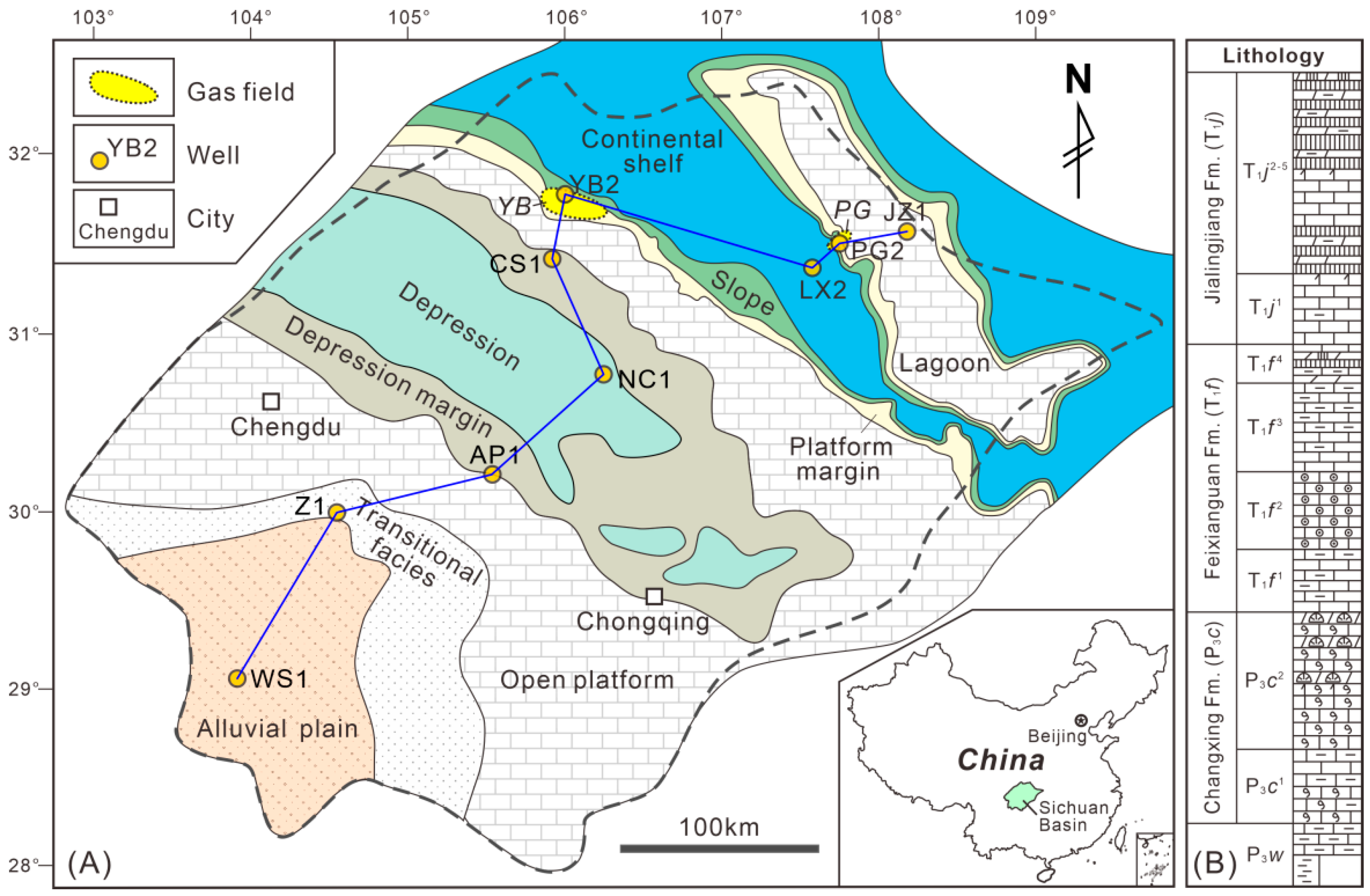
3. Materials and Methods
4. Results
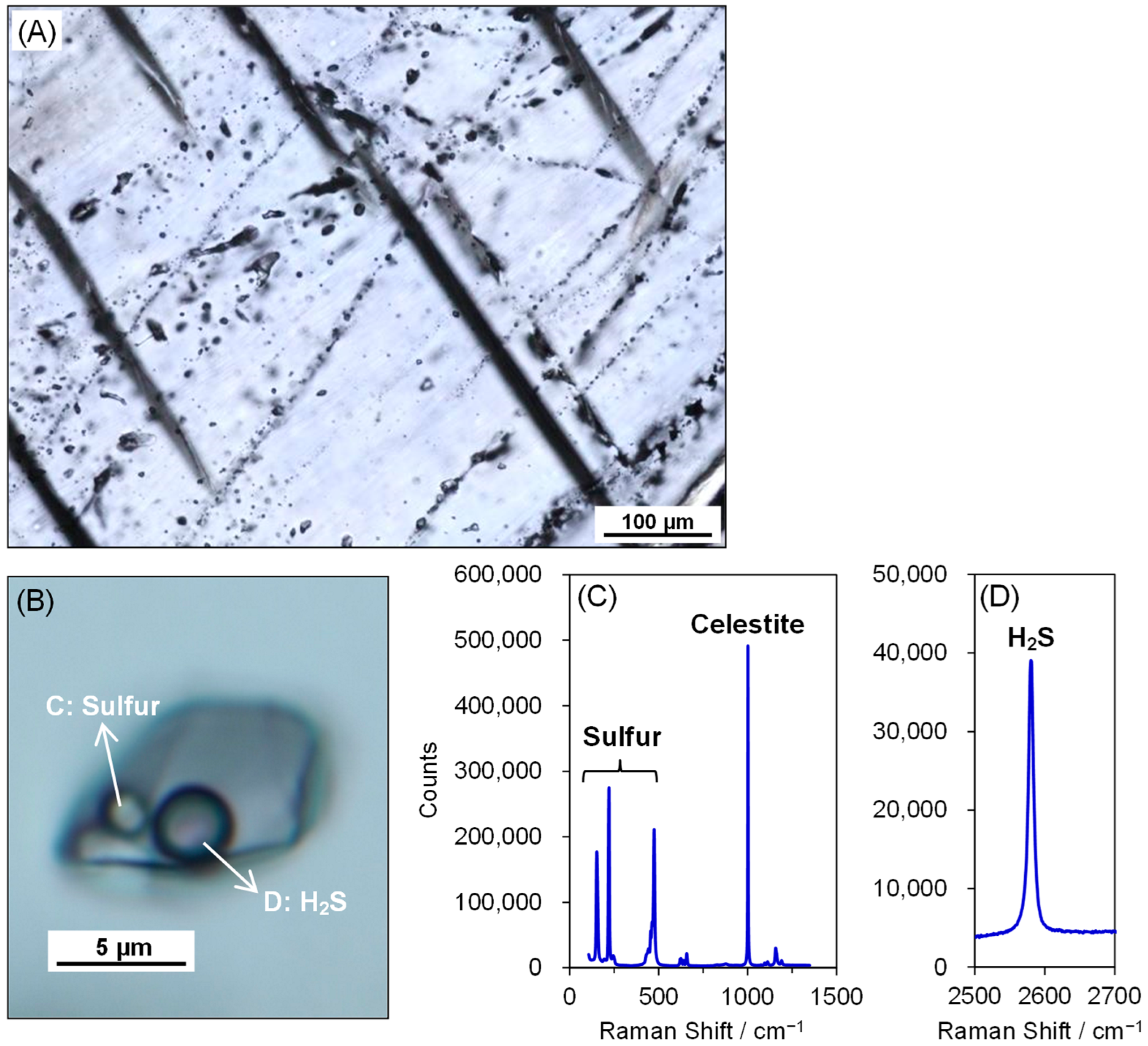
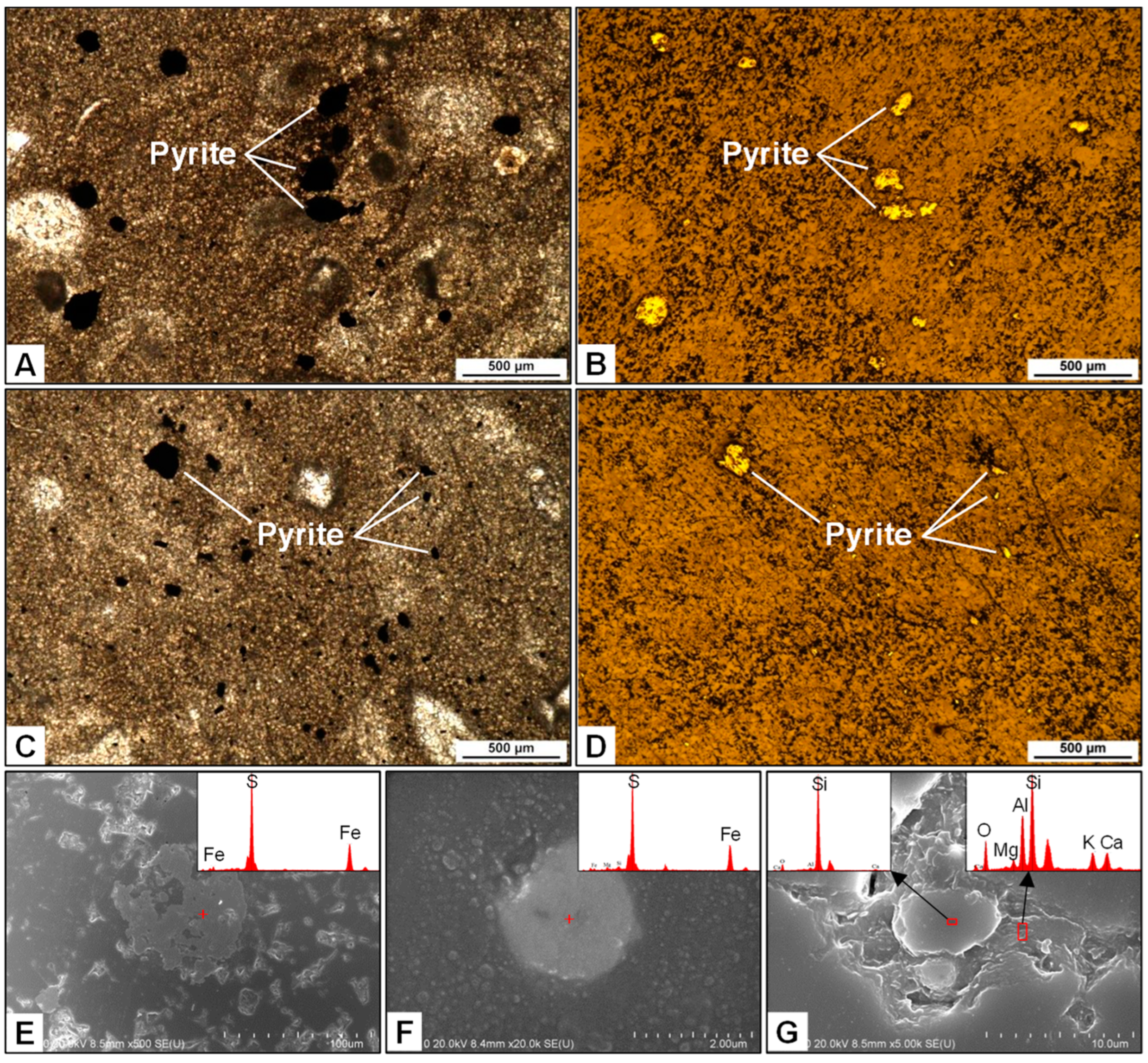
4.1. Petrography
4.2. Sulfur and Strontium Isotopic Compositions
5. Discussion
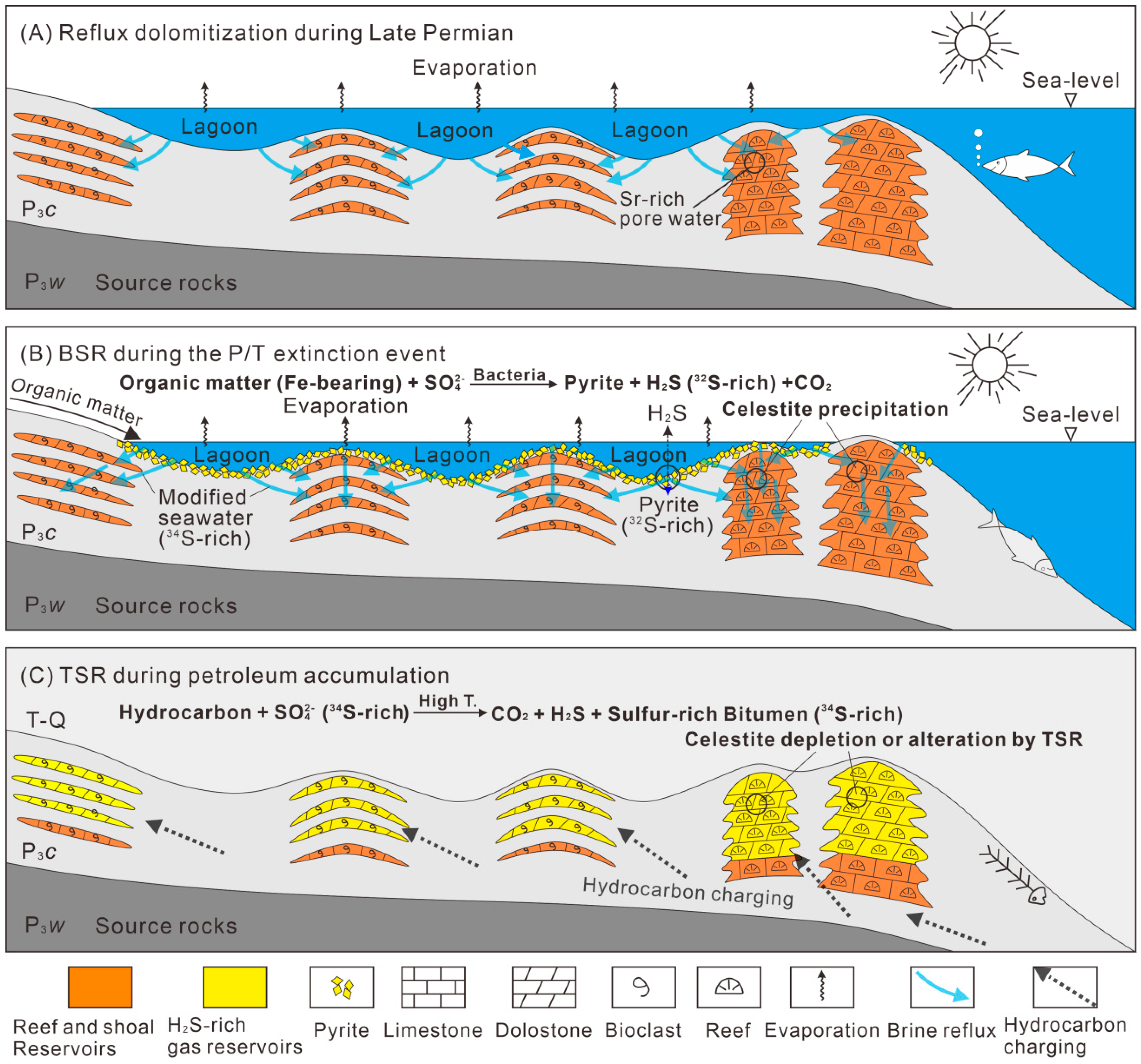
5.1. Origin of Celestite
5.1.1. Source of Sr2+ Ions
5.1.2. Source of SO42− Ions
5.2. Insights into the Sulfate Required for TSR
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Orr, W.L. Changes in Sulfur Content and Isotopic Ratios of Sulfur during Petroleum Maturation—Study of Big Horn Basin Paleozoic Oils. AAPG Bull. 1974, 58, 2295–2318. [Google Scholar] [CrossRef]
- Worden, R.H.; Smalley, P.C.; Oxtoby, N.H. Gas Souring by Thermochemical Sulfate Reduction at 140 °C. AAPG Bull. 1995, 79, 854–863. [Google Scholar] [CrossRef]
- Machel, H.G. Bacterial and Thermochemical Sulfate Reduction in Diagenetic Settings—Old and New Insights. Sediment. Geol. 2001, 140, 143–175. [Google Scholar] [CrossRef]
- Hao, F.; Guo, T.; Zhu, Y.; Cai, X.; Zou, H.; Li, P. Evidence for Multiple Stages of Oil Cracking and Thermochemical Sulfate Reduction in the Puguang Gas Field, Sichuan Basin, China. AAPG Bull. 2008, 92, 611–637. [Google Scholar] [CrossRef]
- Hao, F.; Zhang, X.; Wang, C.; Li, P.; Guo, T.; Zou, H.; Zhu, Y.; Liu, J.; Cai, Z. The Fate of CO2 Derived from Thermochemical Sulfate Reduction (TSR) and Effect of TSR on Carbonate Porosity and Permeability, Sichuan Basin, China. Earth-Sci. Rev. 2015, 141, 154–177. [Google Scholar] [CrossRef]
- Jiang, L.; Worden, R.H.; Yang, C. Thermochemical Sulphate Reduction Can Improve Carbonate Petroleum Reservoir Quality. Geochim. Cosmochim. Acta 2018, 223, 127–140. [Google Scholar] [CrossRef]
- Cai, C. Application of Organic Sulfur Isotopic Composition to Petroleum Origin and Evolution: A Review. Nat. Gas Geosci. 2018, 29, 159–167. [Google Scholar]
- Zhu, G.; Zhang, S.; Liang, Y.; Dai, J.; Li, J. Isotopic Evidence of TSR Origin for Natural Gas Bearing High H2S Contents within the Feixianguan Formation of the Northeastern Sichuan Basin, Southwestern China. Sci. China Ser. D Earth Sci. 2005, 48, 1960–1971. [Google Scholar] [CrossRef]
- Mankiewicz, P.J.; Pottorf, R.J.; Kozar, M.G.; Vrolijk, P. Gas Geochemistry of the Mobile Bay Jurassic Norphlet Formation: Thermal Controls and Implications for Reservoir Connectivity. AAPG Bull. 2009, 93, 1319–1346. [Google Scholar] [CrossRef]
- Jenden, P.D.; Titley, P.A.; Worden, R.H. Enrichment of Nitrogen and 13C of Methane in Natural Gases from the Khuff Formation, Saudi Arabia, Caused by Thermochemical Sulfate Reduction. Org. Geochem. 2015, 82, 54–68. [Google Scholar] [CrossRef]
- Heydari, E.; Moore, C.H. Burial Diagenesis and Thermochemical Sulfate Reduction, Smackover Formation, Southeastern Mississippi Salt Basin. Geology 1989, 17, 1080–1084. [Google Scholar] [CrossRef]
- Worden, R.H.; Smalley, P.C.; Oxtoby, N.H. The Effects of Thermochemical Sulfate Reduction upon Formation Water Salinity and Oxygen Isotopes in Carbonate Gas Reservoirs. Geochim. Cosmochim. Acta 1996, 60, 3925–3931. [Google Scholar] [CrossRef]
- Cai, C.; Xie, Z.; Worden, R.H.; Hu, G.; Wang, L.; He, H. Methane-Dominated Thermochemical Sulphate Reduction in the Triassic Feixianguan Formation East Sichuan Basin, China: Towards Prediction of Fatal H2S Concentrations. Mar. Pet. Geol. 2004, 21, 1265–1279. [Google Scholar] [CrossRef]
- Guo, T. The characteristics of sedimentation and reservoirs of reef-beach gas fields in carbonate platform margins, Northeastern Sichuan Basin. Earth Sci. Front. 2011, 18, 201–211. [Google Scholar]
- Li, P.; Hao, F.; Guo, X.; Zou, H.; Zhu, Y.; Yu, X.; Wang, G. Origin and Distribution of Hydrogen Sulfide in the Yuanba Gas Field, Sichuan Basin, Southwest China. Mar. Pet. Geol. 2016, 75, 220–239. [Google Scholar] [CrossRef]
- Li, K.; Cai, C.; Tan, X.; Jiang, H.; Fan, J. Massive Dolomitization Driven by MgSO4-Rich Seawater and Its Effects on Thermochemical Sulfate Reduction, Upper Permian Changxing Formation, Northeastern Sichuan, China. Energy Explor. Exploit. 2022, 1–29. [Google Scholar] [CrossRef]
- Wynn, J.G.; Sumrall, J.B.; Onac, B.P. Sulfur Isotopic Composition and the Source of Dissolved Sulfur Species in Thermo-Mineral Springs of the Cerna Valley, Romania. Chem. Geol. 2010, 271, 31–43. [Google Scholar] [CrossRef]
- Cai, C.; Li, K.; Zhu, Y.; Xiang, L.; Jiang, L.; Tenger; Cai, X.; Cai, L. TSR Origin of Sulfur in Permian and Triassic Reservoir Bitumen, East Sichuan Basin, China. Org. Geochem. 2010, 41, 871–878. [Google Scholar] [CrossRef]
- Li, K.; Cai, C.; Hou, D.; He, X.; Jiang, L.; Jia, L.; Cai, L. Origin of High H2S Concentrations in the Upper Permian Changxing Reservoirs of the Northeast Sichuan Basin, China. Mar. Pet. Geol. 2014, 57, 233–243. [Google Scholar] [CrossRef]
- Krouse, H.R. Sulfur Isotope Studies and Their Role in Petroleum Exploration. J. Geochem. Explor. 1977, 7, 189–211. [Google Scholar] [CrossRef]
- Machel, H.G.; Krouse, H.R.; Sassen, R. Products and Distinguishing Criteria of Bacterial and Thermochemical Sulfate Reduction. Appl. Geochem. 1995, 10, 373–389. [Google Scholar] [CrossRef]
- Cai, C.; Worden, R.H.; Bottrell, S.H.; Wang, L.; Yang, C. Thermochemical Sulphate Reduction and the Generation of Hydrogen Sulphide and Thiols (Mercaptans) in Triassic Carbonate Reservoirs from the Sichuan Basin, China. Chem. Geol. 2003, 202, 39–57. [Google Scholar] [CrossRef]
- King, H.E.; Walters, C.C.; Horn, W.C.; Zimmer, M.; Heines, M.M.; Lamberti, W.A.; Kliewer, C.; Pottorf, R.J.; Macleod, G. Sulfur Isotope Analysis of Bitumen and Pyrite Associated with Thermal Sulfate Reduction in Reservoir Carbonates at the Big Piney–La Barge Production Complex. Geochim. Cosmochim. Acta 2014, 134, 210–220. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, G.; Cai, C.; Li, M.; Chen, R.; Gao, P.; Xu, C.; Wan, W.; Zhang, Y.; Jiang, M. Alteration of Solid Bitumen by Hydrothermal Heating and Thermochemical Sulfate Reduction in the Ediacaran and Cambrian Dolomite Reservoirs in the Central Sichuan Basin, SW China. Precambrian Res. 2019, 321, 277–302. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, R.; Huang, J. Characteristics of Hydrogen Sulfide Distribution in Feixianguan Formation Gas Reservoirs in East Sichuan. Nat. Gas Ind. 2002, 22, 24–27. [Google Scholar]
- Li, P.; Zou, H.; Hao, F.; Yu, X. Sulfate Sources of Thermal Sulfate Reduction (TSR) in the Permian Changxing and Triassic Feixianguan Formations, Northeastern Sichuan Basin, China. Geofluids 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, Y.; Liu, B.; Zhang, X.; Liu, J.; Shi, K.; Wu, S. Dolomitization of the Upper Permian Changxing Formation in Yuanba Gas Field, NE Sichuan Basin. Acta Petrol. Sin. 2014, 30, 2766–2776. [Google Scholar]
- Zhai, G. Petroleum Geology of China; Petroleum Industry Press: Beijing, China, 1989; Volume 10, pp. 1–516. [Google Scholar]
- Yao, J.; Luo, Z.; Sun, W.; Wang, R. Relationship between Emei Mantle Plume and Aulacogens of Guangwang-Kaijiang-Liangping. Xinjiang Pet. Geol. 2011, 32, 97–101. [Google Scholar]
- Wu, Y.; Fan, J. Quantitative Evaluation of the Sea-Level Drop at the End-Permian: Based on Reefs. Acta Geol. Sin. 2003, 77, 95–102. [Google Scholar]
- Yin, H.; Xie, S.; Luo, G.; Algeo, T.J.; Zhang, K. Two Episodes of Environmental Change at the Permian–Triassic Boundary of the GSSP Section Meishan. Earth-Sci. Rev. 2012, 115, 163–172. [Google Scholar] [CrossRef]
- Du, J. Natural Gas Exploration of Permian-Triassic Reef and Oolite in Sichuan Basin; Petroleum Industry Press: Beijing, China, 2010; pp. 1–160. [Google Scholar]
- Yu, X.; Li, P.; Zou, H.; Wang, G.; Zhang, Y. Rare Earth Element Geochemistry of Dolostones and Its Indicative Significance of the Permian Changxing Formation in Yuanba Gasfield, Northern Sichuan Basin. J. Palaeogeogr. 2015, 17, 309–320. [Google Scholar]
- Guo, X.; Hu, D.; Li, Y.; Duan, J.; Ji, C.; Duan, H. Discovery and Theoretical and Technical Innovation of Yuanba Gas Field in Sichuan Basin, SW China. Pet. Explor. Dev. 2018, 45, 14–26. [Google Scholar] [CrossRef]
- Guo, T. Basic Characteristics of Deep Reef-Bank Reservoirs and Major Controlling Factors of Gas Pools in the Yuanba Gas Field. Nat. Gas Ind. 2011, 31, 12–16. [Google Scholar]
- Li, P.; Hao, F.; Guo, X.; Zou, H.; Yu, X.; Wang, G. Processes Involved in the Origin and Accumulation of Hydrocarbon Gases in the Yuanba Gas Field, Sichuan Basin, Southwest China. Mar. Pet. Geol. 2015, 59, 150–165. [Google Scholar] [CrossRef]
- Wang, G.; Hao, F.; Zhang, W.; Zou, H.; Li, P. Characterization and Origin of Micropores in Tight Gas Grainstones of the Lower Triassic Feixianguan Formation in the Jiannan Gas Field, Sichuan Basin. Mar. Pet. Geol. 2022, 139, 105609. [Google Scholar] [CrossRef]
- Li, C.; Planavsky, N.J.; Love, G.D.; Reinhard, C.T.; Hardisty, D.; Feng, L.; Bates, S.M.; Huang, J.; Zhang, Q.; Chu, X.; et al. Marine Redox Conditions in the Middle Proterozoic Ocean and Isotopic Constraints on Authigenic Carbonate Formation: Insights from the Chuanlinggou Formation, Yanshan Basin, North China. Geochim. Cosmochim. Acta 2015, 150, 90–105. [Google Scholar] [CrossRef]
- Jin, C.; Li, C.; Algeo, T.J.; Planavsky, N.J.; Cui, H.; Yang, X.; Zhao, Y.; Zhang, X.; Xie, S. A Highly Redox-Heterogeneous Ocean in South China during the Early Cambrian (~529–514 Ma): Implications for Biota-Environment Co-Evolution. Earth Planet. Sci. Lett. 2016, 441, 38–51. [Google Scholar] [CrossRef]
- Gao, S.; Rudnick, R.L.; Yuan, H.; Liu, X.; Liu, Y.; Xu, W.; Ling, W.; Ayers, J.; Wang, X.; Wang, Q. Recycling Lower Continental Crust in the North China Craton. Nature 2004, 432, 888–892. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Y.; Wang, X.; Zong, K.; Hu, Z.; Chen, H.; Zhou, L. Crust Recycling Induced Compositional-Temporal-Spatial Variations of Cenozoic Basalts in the Trans-North China Orogen. Lithos 2017, 274–275, 383–396. [Google Scholar] [CrossRef]
- Olaussen, S. Formation of Celestite in the Wenlock, Oslo Region Norway—Evidence for Evaporitic Depositional Environments. J. Sediment. Petrol. 1981, 51, 37–45. [Google Scholar] [CrossRef]
- Taberner, C.; Marshall, J.D.; Hendry, J.P.; Pierre, C.; Thirlwall, M.F. Celestite Formation, Bacterial Sulphate Reduction and Carbonate Cementation of Eocene Reefs and Basinal Sediments (Igualada, Northeastern Spain). Sedimentology 2002, 49, 171–190. [Google Scholar] [CrossRef]
- Martin, J.M.; Ortega-Huertas, M.; Torres-Ruiz, J. Genesis and Evolution of Strontium Deposits of the Granada Basin (Southeastern Spain): Evidence of Diagenetic Replacement of a Stromatolite Belt. Sediment. Geol. 1984, 39, 281–298. [Google Scholar] [CrossRef]
- Scholle, P.A.; Stemmerik, L.; Harpøth, O. Origin of Major Karst-Associated Celestite Mineralization in Karstryggen, Central East Greenland. J. Sediment. Petrol. 1990, 60, 397–410. [Google Scholar] [CrossRef]
- Skinner, H.C.W. Precipitation of Calcian Dolomites and Magnesian Calcites in the Southeast of South Australia. Am. J. Sci. 1963, 261, 449–472. [Google Scholar] [CrossRef]
- Li, K.; Cai, C.; Jiang, L.; Cai, L.; Jia, L.; Zhang, B.; Xiang, L.; Yuan, Y. Sr Evolution in the Upper Permian and Lower Triassic Carbonates, Northeast Sichuan Basin, China: Constraints from Chemistry, Isotope and Fluid Inclusions. Appl. Geochem. 2012, 27, 2409–2424. [Google Scholar] [CrossRef]
- Zheng, R.; Dang, L.; Wen, H.; Chen, Z.; Chen, F.; Zhang, H. Diagenesis Characteristics and System for Dolostone in Feixianguan Formation of Northeast Sichuan. Earth Sci. 2011, 36, 659–669. [Google Scholar]
- Meng, W.; Wu, H.; Li, G.; Zhang, X.; Lv, Z. Dolomitization Mechanisms and Influence on Reservoir Development in the Upper Permian Changxing Formation in Yuanba Area, Northern Sichuan Basin. Acta Petrol. Sin. 2014, 30, 699–708. [Google Scholar]
- Jones, G.D.; Xiao, Y. Dolomitization, Anhydrite Cementation, and Porosity Evolution in a Reflux System: Insights from Reactive Transport Models. AAPG Bull. 2005, 89, 577–601. [Google Scholar] [CrossRef]
- Al-Helal, A.B.; Whitaker, F.F.; Xiao, Y. Reactive Transport Modeling of Brine Reflux: Dolomitization, Anhydrite Precipitation, and Porosity Evolution. J. Sediment. Res. 2012, 82, 196–215. [Google Scholar] [CrossRef]
- Huang, S.; Qing, H.; Pei, C.; Hu, Z.; Wu, S.; Sun, Z. Strontium Concentration, Isotope Composition and Dolomitization Fluids in the Feixianguan Formation of Triassic, Eastern Sichuan of China. Acta Petrol. Sin. 2006, 22, 2123–2132. [Google Scholar]
- Hu, Z.; Huang, S.; Li, Z.; Zhang, Y.; Xu, E.; Qi, S. Geochemical Characteristics of the Permian Changxing Formation Reef Dolomites, Northeastern Sichuan Basin, China. Pet. Sci. 2013, 10, 38–49. [Google Scholar] [CrossRef][Green Version]
- Jiang, L.; Cai, C.F.; Worden, R.H.; Li, K.K.; Xiang, L. Reflux Dolomitization of the Upper Permian Changxing Formation and the Lower Triassic Feixianguan Formation, NE Sichuan Basin, China. Geofluids 2013, 13, 232–245. [Google Scholar] [CrossRef]
- Song, H.; Tong, J.; Algeo, T.J.; Song, H.; Qiu, H.; Zhu, Y.; Tian, L.; Bates, S.; Lyons, T.W.; Luo, G.; et al. Early Triassic Seawater Sulfate Drawdown. Geochim. Cosmochim. Acta 2014, 128, 95–113. [Google Scholar] [CrossRef]
- Martin, E.E.; Macdougall, J.D. Sr and Nd Isotopes at the Permian/Triassic Boundary; a Record of Climate Change. Chem. Geol. 1995, 125, 73–99. [Google Scholar] [CrossRef]
- Song, H.; Wignall, P.B.; Tong, J.; Song, H.; Chen, J.; Chu, D.; Tian, L.; Luo, M.; Zong, K.; Chen, Y.; et al. Integrated Sr Isotope Variations and Global Environmental Changes through the Late Permian to Early Late Triassic. Earth Planet. Sci. Lett. 2015, 424, 140–147. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, L.; Wen, Y.; Zhang, J.; Liu, H. Origin of H2S in Triassic Feixianguan Formation Gas Pools, Northeastern Sichuan Basin, China. Geochimica 2002, 31, 517–524. [Google Scholar]
- Zhu, G.; Fei, A.; Zhao, J.; Liu, C. Sulfur Isotopic Fractionation and Mechanism for Thermochemical Sulfate Reduction Genetic H2S. Acta Petrol. Sin. 2014, 30, 3772–3786. [Google Scholar]
- Zhu, Y.; Wang, J.; Hao, F.; Zou, H.; Cai, X. Geochemical Characteristics and Origin of Natural Gases from Xuanhan Area, Eastern Sichuan. Chin. J. Geol. 2008, 43, 518–532. [Google Scholar]
- Zhang, X. The Reservoir Formation and Preservation of Lower Triassic Feixianguan Formation, Northeastern Sichuan Basin. Ph. D Thesis, China University of Petroleum, Beijing, China, 2009. [Google Scholar]
- Bernasconi, S.M.; Meier, I.; Wohlwend, S.; Brack, P.; Hochuli, P.A.; Bläsi, H.; Wortmann, U.G.; Ramseyer, K. An Evaporite-Based High-Resolution Sulfur Isotope Record of Late Permian and Triassic Seawater Sulfate. Geochim. Cosmochim. Acta 2017, 204, 331–349. [Google Scholar] [CrossRef]
- Xu, X.; Liu, B.; Zhao, Y.; Lu, Y. Sequence Stratigraphy and Basin—Mountain Transformation in the Western Margin of Upper Yangtze Landmass during the Permian to Triassic; Geological Publishing House: Beijing, China, 1997; pp. 1–124. [Google Scholar]
- Kaplan, I.R.; Rittenberg, S.C. Microbiological Fractionation of Sulphur Isotopes. J. Gen. Microbiol. 1964, 34, 195–212. [Google Scholar] [CrossRef]
- Shen, L.; Wang, L.; Liu, C.; Zhao, Y. Sr, S, and O isotope compositions of evaporites in the Lanping–Simao Basin, China. Minerals 2021, 11, 96. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2021; p. 504. [Google Scholar]







| Well | Depth (m) | Sample | Occurrence | δ34S (VCDT, ‰) | 87Sr/86Sr |
|---|---|---|---|---|---|
| YB2 | 6583.10 | Celestite | Vug-filling | 35.53 | 0.707168 |
| YB205 | 6461.73 | Celestite | Vein-filling | 38.83 | 0.707411 |
| YB29 | 6640.15 | Celestite | Vein-filling | 39.78 | 0.707415 |
| YB29 | 6640.15 | Strontianite | Vein-filling | / | 0.707471 |
| YB29 | 6640.15 | Dolostone | Matrix | / | 0.707399 |
| YB205 | 6461.73 | Dolostone | Matrix | / | 0.707396 |
| YB2 | 6583.10 | Dolostone | Matrix | / | 0.707232 |
| Well | Depth (m) | Sample | δ34S (V-CDT, ‰) |
|---|---|---|---|
| YB123 | 6872.7 | CAS in limestone | 22.97 |
| YB123 | 6951.9 | CAS in limestone | 19.10 |
| YB224 | 6621.8 | CAS in limestone | 26.51 |
| YB224 | 6640.9 | CAS in dolostone | 27.53 |
| YB2 | 6556.6 | CAS in dolostone | 18.96 |
| YB2 | 6559.2 | CAS in dolostone | 25.72 |
| YB2 | 6580.6 | CAS in dolostone | 23.29 |
| YB2 | 6584.9 | CAS in dolostone | 22.56 |
| YB2 | 6549.1 | Pyrite in dolomicrite | 1.09 |
| YB2 | 6549.4 | Pyrite in dolomicrite | 4.53 |
| YB2 | 6549.7 | Pyrite in dolomicrite | 4.93 |
| YB2 | 6549.9 | Pyrite in dolomicrite | 1.93 |
| YB2 | 6550.0 | Pyrite in dolomicrite | 10.79 |
| YB2 | 6550.4 | Pyrite in dolomicrite | 2.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhu, D.; Zou, H.; Hao, F. Sulfate Sources Required for Thermochemical Sulfate Reduction in Dolostone Reservoirs in the Upper Permian Changxing Formation, Yuanba Gas Field, Sichuan Basin, China: Insights from the Origin of Celestite. Minerals 2022, 12, 605. https://doi.org/10.3390/min12050605
Yu X, Zhu D, Zou H, Hao F. Sulfate Sources Required for Thermochemical Sulfate Reduction in Dolostone Reservoirs in the Upper Permian Changxing Formation, Yuanba Gas Field, Sichuan Basin, China: Insights from the Origin of Celestite. Minerals. 2022; 12(5):605. https://doi.org/10.3390/min12050605
Chicago/Turabian StyleYu, Xinya, Dancheng Zhu, Huayao Zou, and Fang Hao. 2022. "Sulfate Sources Required for Thermochemical Sulfate Reduction in Dolostone Reservoirs in the Upper Permian Changxing Formation, Yuanba Gas Field, Sichuan Basin, China: Insights from the Origin of Celestite" Minerals 12, no. 5: 605. https://doi.org/10.3390/min12050605
APA StyleYu, X., Zhu, D., Zou, H., & Hao, F. (2022). Sulfate Sources Required for Thermochemical Sulfate Reduction in Dolostone Reservoirs in the Upper Permian Changxing Formation, Yuanba Gas Field, Sichuan Basin, China: Insights from the Origin of Celestite. Minerals, 12(5), 605. https://doi.org/10.3390/min12050605






