1. A Brief History of Crystal Growth in Gels
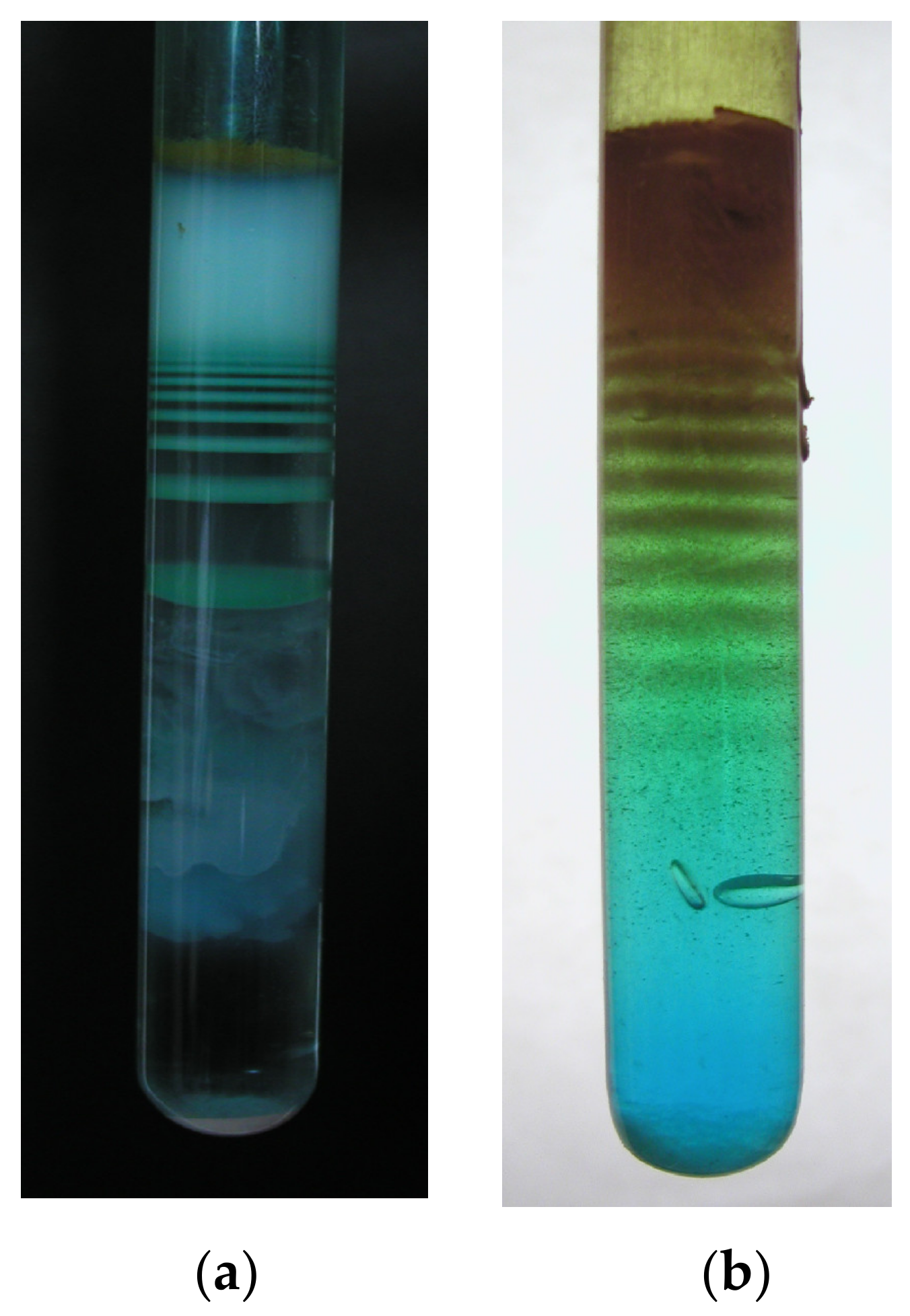
Albeit somewhat counterintuitive, the first experiments with the precipitation of solids in gel were not aimed at obtaining crystals. Instead, the investigations were focused around the phenomenon of periodic precipitation of compounds such as silver chromate and gelatin photographic emulsions, attempting to explain the formation of mostly colloid product in the form of discrete so-called ‘Liesegang rings’ [
1,
2,
3]. Such precipitation can often be observed even in modern gel experiments (
Figure 1).
The term ‘rings’, although erroneous for the given case since the precipitate forms rather as a series of discs more or less perpendicular to the direction of diffusion [
4,
5], has been so firmly established in literature that it has prevailed to this day. Ultimately, the detailed interpretation of the enigmatic process, while still not entirely understood, required the assistance of computers, enabling to model it via numerical solutions to sets of differential diffusion, precipitation and re-dissolution equations attempting to imitate the relevant chemical system [
6,
7]. Larger crystals occasionally formed between the Liesegang rings composed of fine precipitate even in the early experimental setups [
4], but were not systematically sought for.
It was not until later when the potential of the technique to obtain crystals of many substances, unachievable by other means, became apparent. It turned out that the products grown in gel often have a much higher degree of structural perfection in comparison with their counterparts obtained by the use of flux, hydrothermal or other high-temperature techniques [
8,
9,
10], in part due to the absence of temperature changes or thermal shock upon their extraction. Additionally, some crystals grown in gel tend to incorporate significantly higher amounts of incompatible substituents, despite the resulting structural strain. A descriptive example is the growth of calcium tartrate tetrahydrate crystals with Ca being in part replaced by the much smaller transition metal cation Ni
2+. When contacted by a hard object, the crystals undergo violent disintegration accompanied by acoustic effects [
11].
Many mineral-related compounds and oxysalts, especially hydrates, can be obtained in good quality only in gel. This was one of the causes for the general revival of the technique in the second half of the 20th century after nearly thirty years of virtual oblivion [
5]. Nowadays, it continues to serve not only as a handy tool for room-temperature synthesis, but also in the scope of biomedicinal investigations, such as the study of parameters influencing the formation of kidney stones, with the mechanical properties of the gel similar to those of the respective body tissues [
12,
13,
14].
Several basic types of reactions can be carried out:
precipitation—two soluble compounds form an insoluble product, such as 1 m Na
2SO
4 versus 1 m CaCl
2 yielding gypsum, CaSO
4∙2H
2O, to give one example [
15]. The preparation of synthetic fluorite single crystals for optical purposes in silica gel by reacting CaCl
2 with NaF [
16] prior to the introduction of significantly faster CaF
2 growth techniques from melting under vacuum remains a very rare case of limited technical application of the gel growth technique;
decomplexing—a substance soluble in a particular aqueous environment as a complex with another soluble compound is forced to precipitate upon destabilization of said complex, such as CuCl precipitating from a strongly acidic CuCl-HCl environment upon dilution or increase in pH [
9] or silver iodide from an acidic AgI-HI complex [
17] under similar circumstances;
oxidation/reduction—crystals of noble metals form via reduction of their salts (Au single crystals forming in AuCl
3-doped gel due to reduction by oxalic acid [
18], Ag crystallized in AgNO
3-doped silica gel by FeSO
4, etc.);
dilution of solvent—to provide the reader with an interesting example, even water-soluble substances such TGS (triglycene sulfate), NaCl or NaF dissolved in an H
2O-based gel can be obtained upon decreasing the H
2O concentration by means of concentrated ethanol diffusing into the gel column [
5];
temperature change—a gel with the dissolved compound is slowly cooled down to lower temperatures, the consequential supersaturation causing the formation of crystals. KDP (potassium dihydrogen phosphate) could be obtained in such a way [
19];
electrochemically assisted crystal growth—the general principle is the electrolysis of a gelled solution, allowing to obtain single crystals and dendrites of metals (Ag, Pt), thanks to the gel mitigating reactant flux to the cathode [
20,
21].
2. What Is a Gel and the General Principle
Despite the certain lack of a precise definition of a gel [
22], it is a two-component medium, consisting of a micro- to nanoporous solid phase, the pores of which are filled by water or another solution which may contain one or more dissolved reagents. With the liquid phase thusly separated into countless miniature pore-like cavities, convection enabling fast transport of dissolved compounds is no longer possible [
23]. The much slower process of diffusion limits the rate at which the reagents meet, which enables the product to form as crystals instead of a mere colloid.
The preferential formation of fewer larger crystals rather than many smaller ones is due to the second important property of the gel—its strong inhibiting effect on crystal nucleation [
5,
19]. In order for a crystal to start growing, a sufficiently large nucleus must first form. In its beginnings, the given process is thermodynamically disadvantageous due to the strong influence of the surface component of the Gibbs function, until the nucleus has reached a critical diameter depending on the local supersaturation, when the energetically favorable
volume component of the Gibbs function starts to dominate, leading to further growth [
5,
24]. Many pores in the gel have sizes well below that of the critical nucleus diameter for the particular phase at the given supersaturation level, with the pore walls posing mechanical resistance to forming nuclei. This limits successful nucleation only to the few large-enough pores, in which growth continues. The extent of nucleation suppression can be varied by using different gel strength—the stiffer the gel, the fewer nuclei form. However, too strong a gel may eventually start to hinder proper crystal growth, leading to the formation of rounded individuals or radial spherulites [
5], as shown in
Figure 2.
The average pore size varies with the type of gel. In general, organic gels (such as agar or gelatin) have pores several orders of magnitude larger (50–500 μm [
25]) than the most frequently used inorganic gel—silica gel (2–15 nm [
26])—which is, according to some authors, the preferred medium for the majority of experiments [
5]. In part, this is also due to its high inertness towards most reagents except strong bases, as well as broad thermal and chemical stability (in comparison with organic gels) across the entire acidic pH range (and up to pH~10). The average pore size can be reduced even further by using a combination of Na-silicate and aluminate to obtain a mixed Si/Al gel matrix [
27]. It must be noted that many other types of gel can been used. As further examples, a two-component polyacrylamide gel was used to produce PbHPO
4 crystals [
28] (for the principle of its preparation, the reader is referred to the Bio-Rad webpage [
29]), while a silica-free germanate gel was favored for the synthesis of Ge-analogues of zeolites [
30]. A list of other gel types used for crystal growth was also available [
31] (p. 946).
In order for the crystallization to take place within the gel, two basic experimental setups are used. The single-diffusion configuration (
Figure 3a), being the simplest of all, necessitates minimal laboratory equipment (merely a test tube, beaker or cylinder) and is sufficient for most syntheses. First, a gel is made, which contains one of the reaction partners. It is then covered by a supernatant solution containing the other reactant(s). As the reagent from the supernatant slowly diffuses into the gel (and vice versa), crystals form inside the gel and some form on the container walls above its surface. When the gel pore size is generally too low for the nucleation of the given phase, the product grows exclusively on the surface of the gel and above. We tested variants of the single-diffusion configuration by also gelling the supernatant solution itself, leading to two superimposed gels, further limiting the reaction rate. Ultimately, a triple-gel configuration was tested, with a thin layer of ‘neutral gel’ (deprived of reagents) separating both gelled solutions. However, the extra effort brought only sporadic improvement in respect to the classic setup, since an excessive nucleation rate was effectively suppressed otherwise, such as by using lower initial reagent concentrations or a stronger gel.
The double-diffusion (counter-diffusion) technique, representing the other general type of experimental setup, necessitates, in its crudest form, the use of a U-tube or a similarly shaped vessel (
Figure 3b). First, a gel is formed in the bottom portion, effectively separating both arms from one another. After it has set and aged, the reagent solutions are filled, each into one particular arm. Both components slowly diffuse one towards the other through the gel to ultimately meet and form the product. This technique is advantageous in multiple ways. Most importantly, in difference to the single-diffusion arrangement, the reagents are all added in the form of a solution. These solutions can be replaced at any time by fresh ones to promote further growth of the crystals already present in the gel. Secondly, the formation of the gel is, in some cases, hindered by using high concentrations of certain reagents when aiming for a single-diffusion arrangement. With the gel already set in the U-tube prior to reagent addition, the required high nutrient concentrations can be attained, nevertheless.
3. Gel Preparation
Organic polysaccharide or protein gels, such as agar and gelatin, respectively, involve dissolving the organic component in hot water. After cooling down to low temperature, the polysaccharide or peptide polymerizes, yielding the gel matrix. While gelatin tends to break down already above 50 °C upon warming, agar even resists boiling for some time, with the optimum temperature for its dissolution being between 95–100 °C. Polyacrylamide or other such organic gels are obtained by the addition of a cross-linking agent, causing polymerization [
28]. The strength of the resulting gel is largely determined by the concentration of the gelling agents used (
Table 1).
Organic gels, however, have too large a pore size [
32] to sufficiently inhibit nucleation of most inorganic compounds, leading to the development of numerous smaller crystals. Furthermore, given the hydrolysis caused by the dissolution of many transition metal salts leading to either acidic or basic pH, agar-based solutions will not gel if their pH becomes too low (pH < 4). Gelatin is stable in acidic pH and perfectly transparent, allowing better observation of the growth process, but has a strong tendency to become befallen by fungus.
Of the basic gel types used here, it would therefore seem that silica- or silicoalumina-gel always represent the best choice for the inorganic chemist. However, the gel itself often influences the morphology of the product. To give two examples favoring the use of agar over silica gel, gypsum, CaSO
4∙2H
2O, forms as large yet twinned crystals in silica gel, while such twinning is completely absent when using agar, also leading to better crystal transparency (
Figure 4).
The former case is also a good example of untwinned crystals sporadically nucleating and growing from solution in the supernatant section, ultimately having much better quality than the bulk of the product in gel (
Figure 5).
Chalcomenite, CuSeO
3∙2H
2O, forms as macroscopic yet relatively small and numerous crystals on top of CuCl
2-doped silica gel in comparison with the situation when agar is used, when fewer, yet well-developed and significantly larger single crystals are obtained, nested immediately below the gel surface or deeper in the gel itself (
Figure 6).
Still, for most purposes of the experimental mineralogist, inorganic gels (most commonly, silica gel) are indeed the better choice. To precipitate silica gel, an aqueous sodium silicate solution is prepared (potassium or lithium silicate can be used instead). A convenient alkali silicate concentration also used elsewhere [
5] is roughly 0.3 mol/L. To this solution, the appropriate amount of an acid (
Table 1) is quickly added while stirring vigorously (at best, using a magnetic stirrer) to effectively ‘neutralize’ the silicate, with the formation of the respective Na-salt (in case of Na
2SiO
3). This, in turn, causes the silica to precipitate out of the solution within mere seconds when the pH value is very close to neutral, forming the gel matrix. The short gelling time requires fast transfer of the acidified solution into the reaction vessel. Increasing turbidity indicates the onset of gelling. If it does not occur, further adjustment of pH is required, either by the addition of more acid, in case the pH is too basic in respect to the optimum region of gelling [
33], or a certain amount of concentrated silicate solution to increase the pH value in the opposite scenario.
For single-diffusion experiments, in which the reactant added to the gel tends towards acidic hydrolysis (as is the case with most transition metal salts), the gel preparation involves the mixing of two solutions. To the first one, containing the metal salt and the respective amount of acid for gelling, a second basic solution with the required amount of alkali silicate (at best, the most affordable Na
2SiO
3) is added dropwise while stirring. The pH value, which must be increased, at least above 3.5 [
33], has to be controlled repeatedly, at the latest, when chunks of a whitish SiO
2 precipitate start to persistently float about in the mixture. If the appropriate pH has not been reached, even after adding the whole of the basic solution, a few additional drops of an auxiliary concentrated Na
2SiO
3 solution can be used to remedy the situation. The gelling process is slower and may take even several days (the greater the deviation from neutral pH, the longer), but is mostly completed overnight. The lengthy gelling allows any SiO
2 precipitate to sink and accumulate at the bottom of the gel column, preventing it from interfering with the course of the experiment.
Both inorganic and organic gels must be left to age for a period of at least 24 h after gelling. Ageing contributes to the overall mechanical stability of the gel and reduces the amount of large pores, which benefits the formation of fewer nuclei of certain substances [
32]. Any solution placed on top of a gel should be added slowly/gently so as not to damage the gel surface.
A recurrent problem is the formation of lenticular gas bubbles in the gel, which sometimes even cross-cut the entire gel column, rendering further diffusion to its lower sections impossible. Filling the reaction vessel with distilled water and subjecting it to ultrasound for a few minutes prior to setting up the experiment has repeatedly shown itself to have an important mitigating effect on the formation of such bubbles.
4. Result Types
Most of the product typically developed in the course of five to six weeks. Later on, sluggish changes still took place, with the most important one being the so-called ‘Ostwald ripening’, when smaller crystals re-dissolved to the benefit of larger ones, which continued to grow, owing to their higher thermodynamic stability [
5,
34,
35].
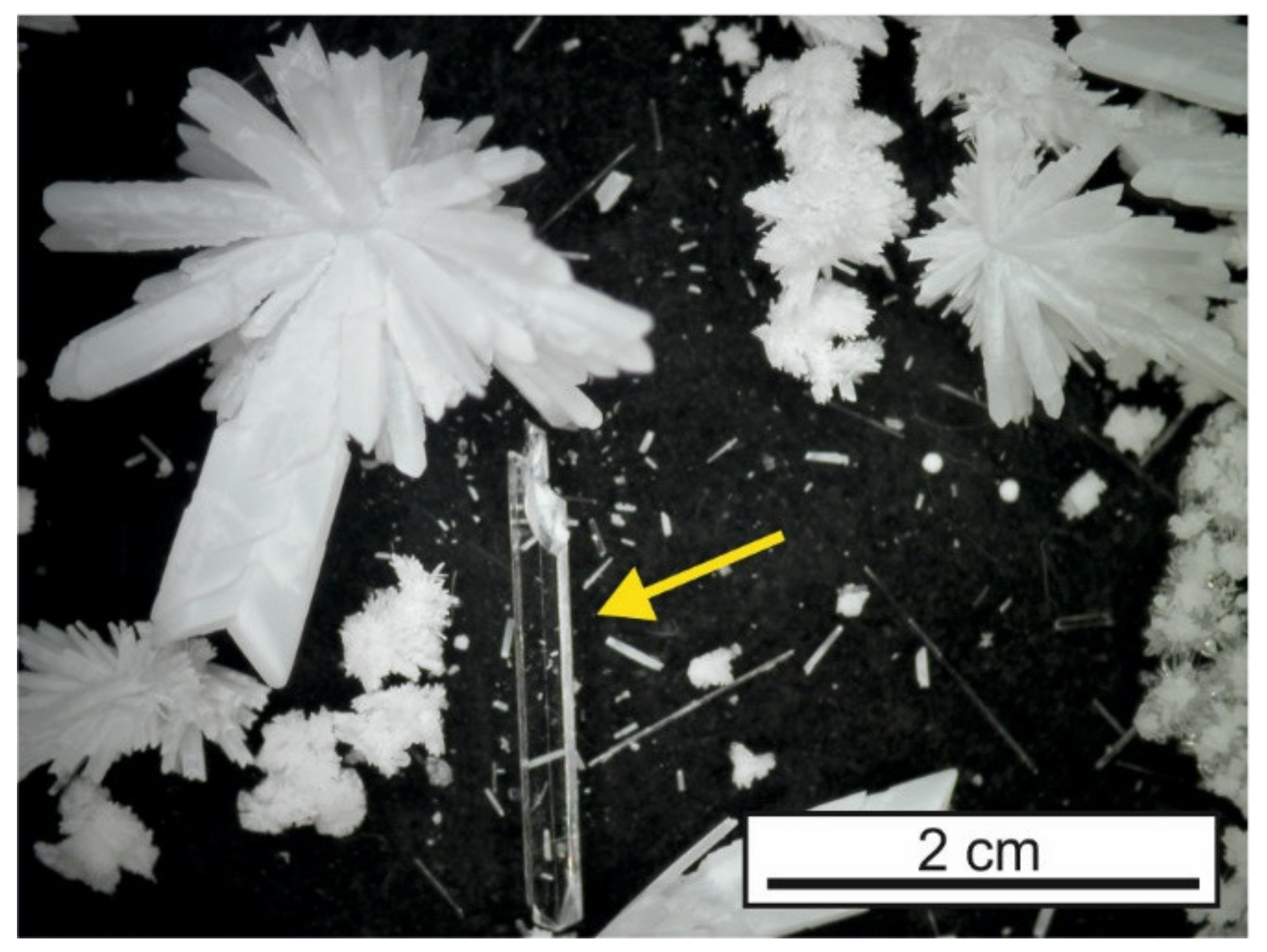
During the countless experiments made by our group, we came to the realization that only four types of results occurred (
Figure 7). In the best case, macroscopic single crystals of the title compound were obtained. Frequently, however, discrete radial spherulites formed instead. The worst outcome was the product forming as a colloid or not at all, despite the aforementioned benefits of the gel environment. Mainly, with combinations of reagents soluble, each under significantly different pH, a so-called ‘salt lid’ (
Figure 7d)—a thin but compact layer of product or hydroxide of one of the reagents—formed inside the gel, effectively blocking any further diffusion.
While the solution to obtaining single crystals instead of spherulites or diffuse colloid is, in some cases, a mere issue of using higher initial reagent concentrations (such as in the case of gypsum and calcite), many phases tend to remain in their given peculiar form despite all attempts at remediating the situation, including the ‘salt lid’ scenario (
Figure 7d), requiring often unrealistically low starting concentrations, in turn, negatively influencing the product quality and amount.
One key factor explaining the varied form of the resulting product is the so-called ‘crystallization pressure’, a thermodynamically governed property, specific to each substance. Its value/extent determines the ability of the crystal to grow despite the mechanical resistance of its environment. The best-known example of a crystallizing phase exerting physical pressure on its surroundings is the use of water to split rock as it freezes to ice [
36]. The basic formula, describing the parameters governing the value of crystallization pressure is [
37]:
where Δ
P is the difference between ambient pressure and that at the loaded crystal face (ranging to hundreds or even thousands of MPa at very high supersaturation [
38]), R represents the molar gas constant,
T the thermodynamic temperature and
Vm the molar volume of the title compound. The variable
S defines the level of solution supersaturation with respect to the forming product via the ratio of the actual concentration of the reacting components in solution (
c) against their saturation concentration (
c0) under the prevailing conditions.
As is evident from Formula (1) above, the amount of crystallization pressure can actively be changed to higher values in several ways. Apart from conducting the experiment at an elevated temperature, the simplest way is to enhance local supersaturation around the growing crystals, imploring merely the use of sufficiently concentrated reagents to obtain single crystals (
Figure 8). Alternatively, one can attempt to use a softer gel made from a less concentrated gelling solution, which, in turn, poses lower mechanical resistance to crystal growth.
The difference in crystallization pressure can cause another substance to precipitate instead of the intended one. Similar to the case of mirabilite versus thenardite [
35], hydroxyapatite, despite proper pH of the gel environment, forms exclusively as minor spherulites and clumps arranged into ‘Liesegang rings’. Simultaneously, the dominant yet unwanted product are single-crystal laths of brushite, CaHPO
4∙2H
2O, ranging to several centimeters in size (
Figure 9).
The other key parameter influencing the character of the product is the extent of nucleation, also inherently specific to each substance. For many setups, even the gel environment proves ineffective at hindering excessive amounts of crystal nuclei from forming. The result is an extremely fine crystalline product.
The worst-case scenario occurs when a phase is, in itself, unable to exert sufficient crystallization pressure to repel the surrounding gel and nucleates uncontrollably, leading to the obtainment of the product in the form of a colloid, a ‘salt lid’ or as fine-grained polycrystalline cement impregnating the gel matrix.
From the perspective of the aforesaid, the recurrent product types of a gel synthesis always represent one of the following three situations: single crystals are the evidence of the title compound nucleating sparingly and exerting enough crystallization pressure to repel the surrounding gel; discreet macroscopic radial spherulites are evidence of acceptable nucleation rate, but low crystallization pressure. A colloid habit, as mentioned earlier, represents the most unfavorable combination of both parameters.
A dilemma presents itself—a higher starting reagent concentration (at the given temperature) leads to the desired increase in crystallization pressure necessary for better product quality, but, in turn, also raises the nucleation rate of the wanted phase. Determining the initial reagent concentrations, which represent a viable compromise between both effects, is often a challenge.
Several other parameters often play a substantial role as to the character and quality of the resulting product. Surprisingly, in the scope of single-diffusion experiments, it matters significantly which of the two reagents involved is present in gel. This especially applies to syntheses involving salts of transition metals, when coarser well-developed crystals always form with the transition metal reagent fixed in gel, possibly due to adsorption and partial immobilization of the metal cations on the large surface of the gel matrix, in analogy with the effect of clay minerals adsorbing heavy metals [
39,
40].
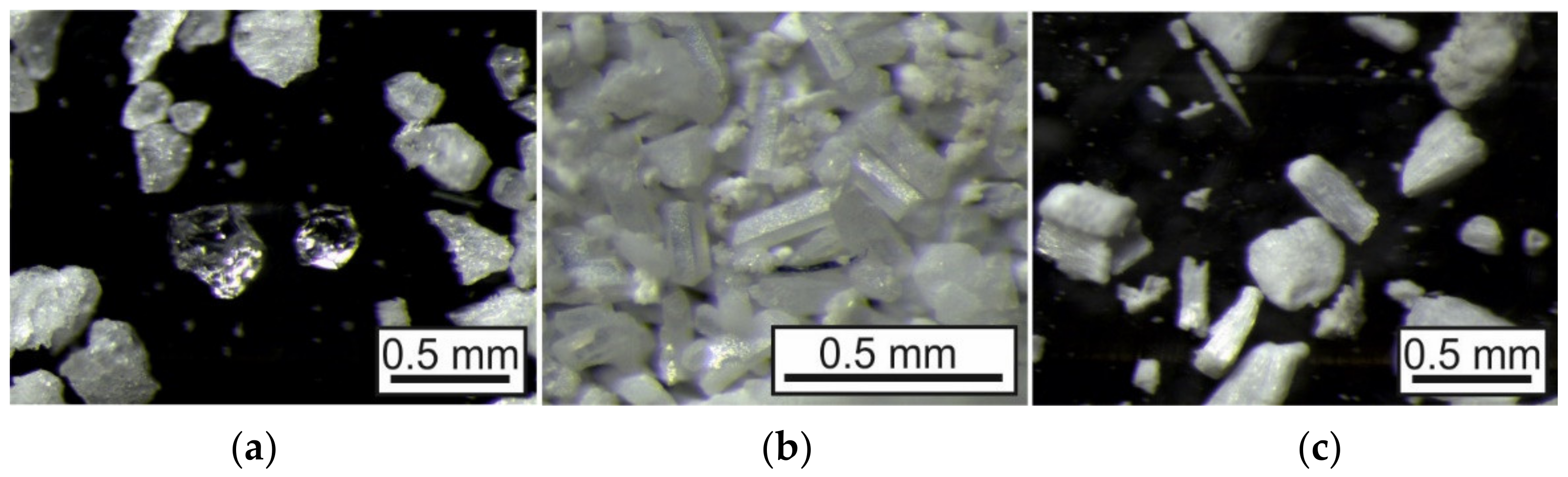
Figure 10 illustrates the comparison of metallic copper grown by a reaction between 0.3 m CuSO
4 and 1 m L-ascorbic acid with CuSO
4 in gel and L-ascorbic acid as the supernatant solution and vice versa. In the latter case, only very fine copper crystals formed instead of the expected macroscopic results.
Each of the several chemicals, which in themselves should have a comparable effect on the reaction, influences the nucleation and growth of the title compound in another manner. One good example is the preparation of gold crystals. Out of several effective reducing agents (FeSO
4, MnSO
4, L-ascorbic acid, citric acid, oxalic acid, hydrazine) with the same concentration (0.8 mol/L) acting upon 0.3 m AuCl
3 dissolved in silica gel, only the use of oxalic acid led to well-developed gold crystals instead of varicolored Au colloid obtained in all other cases. Similarly, good results, as to metallic Cu, can be obtained only with the aforementioned L-ascorbic acid and hydrazine in use. The comparison between both types of Cu crystals (
Figure 11) also shows their entirely different habit, dependent on the reducing agent.
At times, certain additives can be employed to intentionally change the habit or significantly enhance the quality of the product. In comparison to the twinned cloudy gypsum crystals obtained in silica gel by reacting 1 m Na
2SO
4 in gel with 1 m CaCl
2 in solution (
Figure 4a and
Figure 5), the same configuration with an admixture of AlCl
3 (0.6 mol/L) to the supernatant solution yields perfectly transparent, untwinned and elongated crystals of the title compound (
Figure 12). Finding out which additives are effective to enhance the growth of a given compound are even, nowadays, more alchemy than anything else. In the example above, there is no apparent explanation as to why AlCl
3 should influence gypsum habit or quality in any way, using current scientific knowledge. In fact, it is common that every type of grown crystal requires an individual approach and specific set of ‘tweaks’ to optimize the process [
5].
Lastly, due not only to thermodynamic effects, but also to mistakes which will be discussed further in the text, a different product than the one expected can form. Sometimes, the situation can be remedied by using one of the reagents in excess, as is described for the preparation of various CaCO
3 polymorphs [
41]. Many phases, however, simply cannot be obtained by the gel technique, in contrast to the optimistic considerations of the mid-19th century [
42].
5. Most Common Pitfalls of the Gel Growth Technique and Product Extraction
Several mistakes are repetitively made by anyone not skilled in the art of gel synthesis. The most trivial are errors such as overcharging organic gels with reagent in the case of single-diffusion arrangements, heating the gel solution for too long or overheating it, which denaturates the organic gelling compound, preventing the gel to set in all mentioned cases. The use of the wrong amount of acid to cause gelling of an alkali silicate solution (mostly Na2SiO3) leads to the same result.
More complex mistakes include the use of an inappropriate acid for the gelling of silica gel in respect to the intended experiment. The consequently obtained alkali salt present in the gel may get involved in the reaction scheme with significant or even destructive influence on the resulting product. To give two practical examples, the use of HCl to obtain a silica gel leads, in turn, to the formation of CuCl single crystals instead of metallic copper in a reaction involving the reduction of CuSO
4 by L-ascorbic acid (
Figure 13a). With HNO
3 or CH
3COOH as the acidification agent, crystals of the desired metal do form. The use of HCl should be avoided in all syntheses involving soluble reagents of lead (most commonly Pb(NO
3)
2), such as to obtain cerussite (PbCO
3) by a double-diffusion process using Na
2CO
3 as the reaction partner. Instead of forming the desired carbonate, all Pb
2+ cations in the system are exhausted by joining with the Cl
− anions in gel to form sparingly soluble colorless PbCl
2 single crystals when HCl is used for gelling.
Despite that the title compound indeed crystallizes in acceptable form in the course of the few weeks the synthesis had been running, the danger of spoiling everything is not over yet. The crystals still need to be removed from the gel. The extraction from gelatin or agar involves heating the vessels containing the gel with the product to temperatures close to the boiling point of water (at best, using a water bath). Despite that the temperature of a mere 90–100 °C may seem harmless, many phases corresponding to supergene or secondary minerals already dehydrate or decompose, such as (yet again) cerussite, which converts to brightly colored lead oxide. CuCl single crystals obtained by a reaction of a copper salt with a reducing agent in the presence of a chloride phase partly decompose and coat themselves with metallic Cu during the heating phase (
Figure 13). Gypsum, in turn, does not handle the temperature increase from the mechanical point of view, developing numerous cracks along its principal cleavage direction (010), becoming more or less opaque.
While the dissolution of most organic gels involves mere heating, silica gel must be decomposed using a strong base. However, many of the desired compounds are also susceptible to dissolution in basic conditions. Such is the case for the difficult synthesis of krokoite single crystals (PbCrO4), which are sensitive to both strongly acidic and basic pH. Under the mentioned conditions, the product dissolves together with the gel. These demanding phases require the use of ‘hybrid’ gel techniques, allowing (beside others) their easy extraction from a liquid environment. The given strategy is discussed further below in the text.
The use of ultrasound to break up the gel or clean the obtained crystals is often not advisable, mainly with easily cleavable substances. Cerussite is the example of a compound, which becomes reduced to fine powder after a mere few seconds of ultrasound treatment.
The most secure extraction method would therefore seem to be pure ‘mechanical’ extraction at room temperature. There are a few prerequisites, though. The crystals need to be large enough (>0.2 mm) to be effectively separated from the gel. In cases of silica- and softer agar gels, it is advisable to use a thin jet of liquid (mostly distilled water) from a dash bottle to break up the gel, with the crystals directly inside the reaction vessel instead of using mechanical aids, followed by pouring the debris out into a large Petri bowl. Consequently, a soft tool (at best, one’s own fingers in a glove) must be used for further gel disintegration not to damage the product. The gel must be broken up thoroughly, since the density of its fragments, even when still containing crystals of the title compound, is very low. They can easily suffer the fate of being washed away or decanted with the incorporated crystals.
6. Hybrid Gel Growth Techniques
Phases with sufficient crystallization pressure and low nucleation rates are the most easy to obtain in the classic setup of a gel synthesis. However, some, such as carbonates, tend to incorporate the gel matrix directly into the crystals [
43] instead of pulling the gel apart and forming a series of cracks, so-called ‘cusps’ [
5], as shown in
Figure 14. The gel content in the crystals may be undesirable for some applications, such as electron microprobe standards. The plethora of substances, which exert low crystallization pressure and thus merely form as spherulites in gel, also necessitates an alternative preparation route to obtain single crystals. The so-called ‘hybrid’ gel techniques satisfy these goals.
In the general scheme, the hybrid setup takes the advantage of the gel environment, restricting reactant mobility to slow diffusion rates dictated by the properties of the gel, which, in this configuration, serves as a mere diffusion barrier. The product itself forms in an alcove within or under the gel filled by solution, which poses near to no resistance to the growing crystals. As such, phases with poor crystallization pressure can be obtained in well-developed form and, as mentioned for the case of carbonates, no gel becomes incorporated into them.
The challenge is how to create the required solution-filled void encompassed by gel. A similar strategy applies to the use of single- and double-diffusion hybrid configurations [
43]. The given portion of the test tube or U-tube in which the void is to form, is filled by a saturated solution of a compound inert towards the reagents involved. For most cases, ammonium nitrate and ammonium sulfate are good choices, provided that none of the reagents tends to form ammonium complexes or an insoluble sulfate, respectively. The saturated solution enables, due to its high density, the possibility of being overlaid by the less dense gelling solution. If possible, it is advisable to place seed crystals of the desired compound into the intended crystallization void prior to adding the gelling solution, with the aim of obtaining a thick overgrowth of the seeds instead of many small spontaneously nucleated individuals. Typical hybrid setups used in the scope of a single- and double- diffusion similar experimental configuration are depicted in
Figure 15.
7. Hybrid Gel Growth with Sparingly Soluble Reagents
So far, the discussion regarding crystal growth in gels was restricted to all reagents being in the form of a solution. Despite the effect of insufficient crystallization pressure of the intended product mitigated by the hybrid approach discussed above, the prerequisite of maintaining a sufficiently low nucleation rate of the title compound remains. If that is not the case, a further modification must be adopted to inhibit this second factor. Limiting nucleation becomes all the more crucial with phases such as krokoite (PbCrO
4), which display another peculiar property—the so-called ‘cooperative’ crystal growth [
5]. The phenomenon is in sharp contrast to the ‘classic’ scheme of larger crystals growing at the expense of smaller ones due to their enhanced thermodynamic stability and crystallization pressure [
44]. Instead of a few large individuals, krokoite forms countless micro thin needles of comparable dimensions along a reaction front between the gel and the solution in a classic single-diffusion setup. The most effective way to reduce the nucleation rate was to significantly decrease the availability of one of the reagents. In the above-mentioned case, the alternate source of Pb (instead of Pb(NO
3)
2) was sparingly soluble Pb(OH)
2. Its powder was placed on the bottom of a large test tube (100 mL inner volume) and covered by 10 mL of a saturated dense NH
4NO
3 solution to form the reaction alcove. The heavy solution was, in turn, overlaid by 40 mL of a 0.3 m Na
2SiO
3 solution acidified by the respective amount of HNO
3 for gelling (
Table 1). After the gel has set and aged, 40 mL of a supernatant solution containing 0.05 m K
2CrO
4 were added. In the course of a few months, the first well-developed long-prismatic crystals of PbCrO
4 started to appear in the solution-filled alcove above the Pb(OH)
2 powder (
Figure 16). After roughly a year, a sufficient quantity of PbCrO
4 crystals could be recovered from the said pocket. In addition, a minor amount of PbO(CrO
4)
2 (phoenicochroite) crystal aggregates was also present.
The scheme adopted above can be used for the synthesis of many other types of minerals including hydrous REE-phosphates, such as churchite-(Y), YPO
4∙2H
2O, or rhabdophane-(Ce), CePO
4∙H
2O. The setup involves the use of nearly insoluble Y(OH)
3 for the former and synthetically obtained hydroxylapatite, Ca
5(PO
4)
3(OH) for the latter in powdered form, acting as the respective sparingly soluble reagents. The reaction alcove was again formed directly above the powders using NH
4NO
3. After the placement of an HNO
3-acidified 0.3 m Na
2SiO
3 gelling solution and ageing of the obtained gel in both examples, a 0.3 m NaPO
3 solution was administered in the former and a 0.3 m CeCl
3 solution in the latter case. In approximately five months, beautiful multifaceted churchite-(Y) single crystals formed in the former case (
Figure 17a). As for the rhabdophane setup, the whole of the synthetic hydroxylapatite reagent was converted to the respective title compound, albeit with the crystals having a somewhat fibrous form (
Figure 17c). The successful use of the given configuration to obtain anglesite single crystals (
Figure 17b) with Pb(OH)
2 as the near-to-insoluble reagent and 0.5 m Na
2SO
4 as supernatant is yet another justification for the usefulness of the hybrid gel growth techniques.
9. Is the Gel Growth Technique Always the Best Choice?
While all types of methodology using gel described above may seem to be the optimum solution for the synthesis of countless phases, one should always consider all possible (and potentially faster) alternatives. Barite, BaSO
4, has been documented to be obtainable by the gel technique [
45]. It should, however, already have become apparent, that the growth rates of crystals in gel are quite sluggish. For this particular phase, a much swifter and more reliable route to produce transparent single crystals up to the size of several millimeters is the use of the flux technique, with precipitated and washed BaSO
4 as nutrient and Na
2SO
4 in the role of flux by slow cooling of the mixture from a peak temperature of 850 °C [
46]. Instead of weeks, the preparation of the very same phase takes but a few days, provided that a platinum crucible and a programmable muffle furnace are available.
In a different manner, it has been shown above that one of the main functions of the gel is to ‘slow down’ the rate at which the reagents meet, and that even this is often not enough, requiring one of the compounds to be present in nearly insoluble form. The arising question is whether some phases cannot crystallize even without the presence of any gel and its effects, as in the particular case of extremely low solubility of one of the reagents when, despite direct contact with the aqueous solution of its counterpart, good crystals are obtained, nonetheless.
The specific need to obtain teineite, CuTeO
3∙2H
2O, for future high-pressure studies provided the answer to this question, also putting forth another general flaw of the average crystal grower—impatience. It was only thanks to the COVID-19 pandemic and subsequent lockdowns that several test tubes, in which no product was forming even nearly after a year, were not discarded. In these, a 0.3 m CuCl
2 solution acted upon solid TeO
2, ultimately yielding beautiful prismatic teineite crystals up to 0.5 mm in length, but no sooner than two years since the start of the experiment (
Figure 19). Although we perfected the procedure to make it somewhat faster by using a filtered and washed pastel green Cu-Te-O precipitate (obtained by mixing 1:1 stoichiometric amounts of K
2TeO
3 and CuSO
4) in contact with a CuSO
4 solution [
47] of pH 2.5–3.0, the process still takes nearly one entire year to run its course. We are currently synthesizing zemannite endmember samples along a similar reaction route.