Quantitative Study on Colour and Spectral Characteristics of Beihong Agate
Abstract
:1. Introduction
2. Materials and Methods
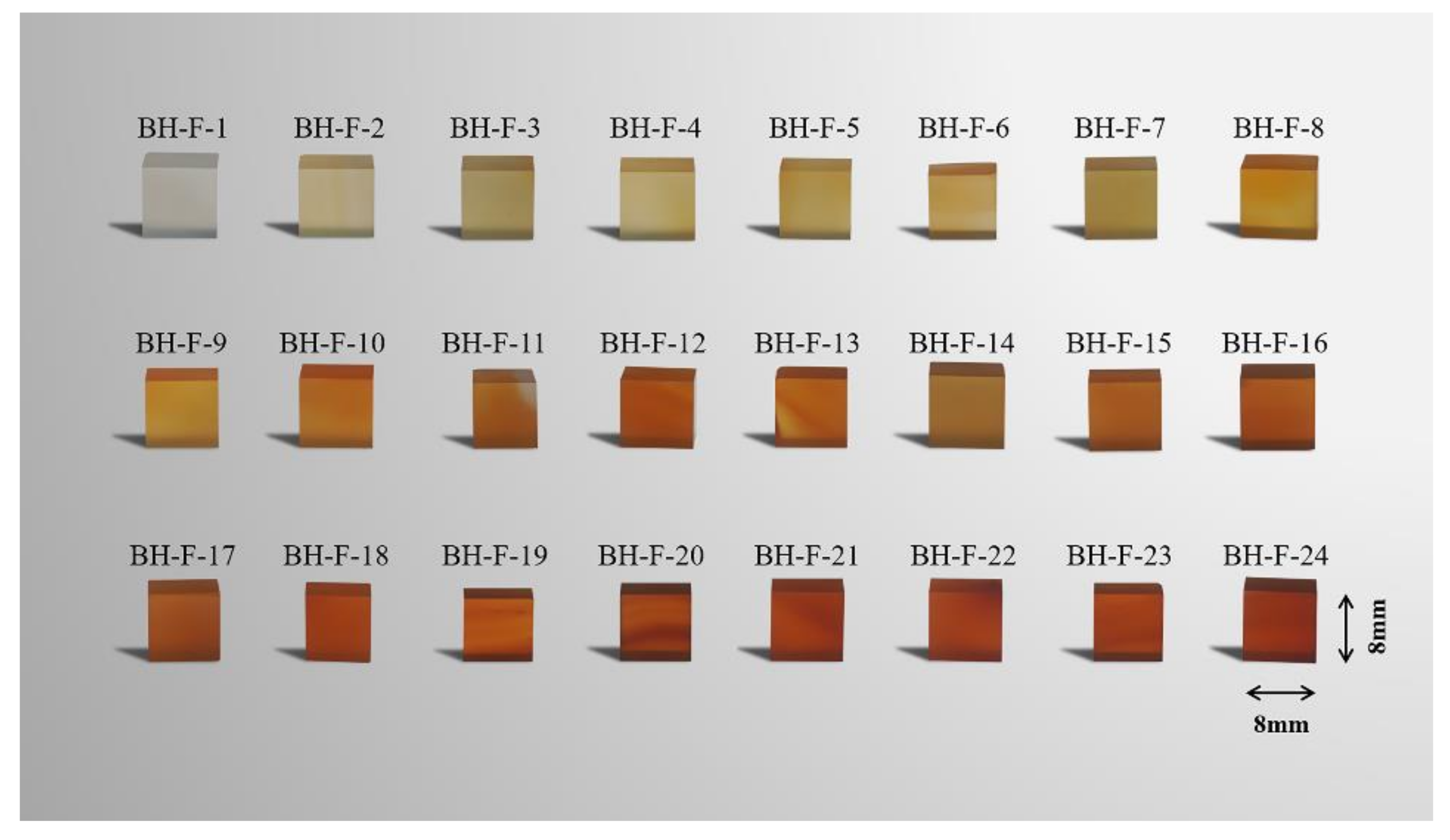
2.1. Samples
2.2. Colourimetric Analysis
2.3. Energy Dispersive X-ray Fluorescence Spectrometer
2.4. Raman Spectroscopy
2.5. UV-VIS Spectroscopy
2.6. Heating Method
3. Results and Discussion
3.1. Colour Quantification
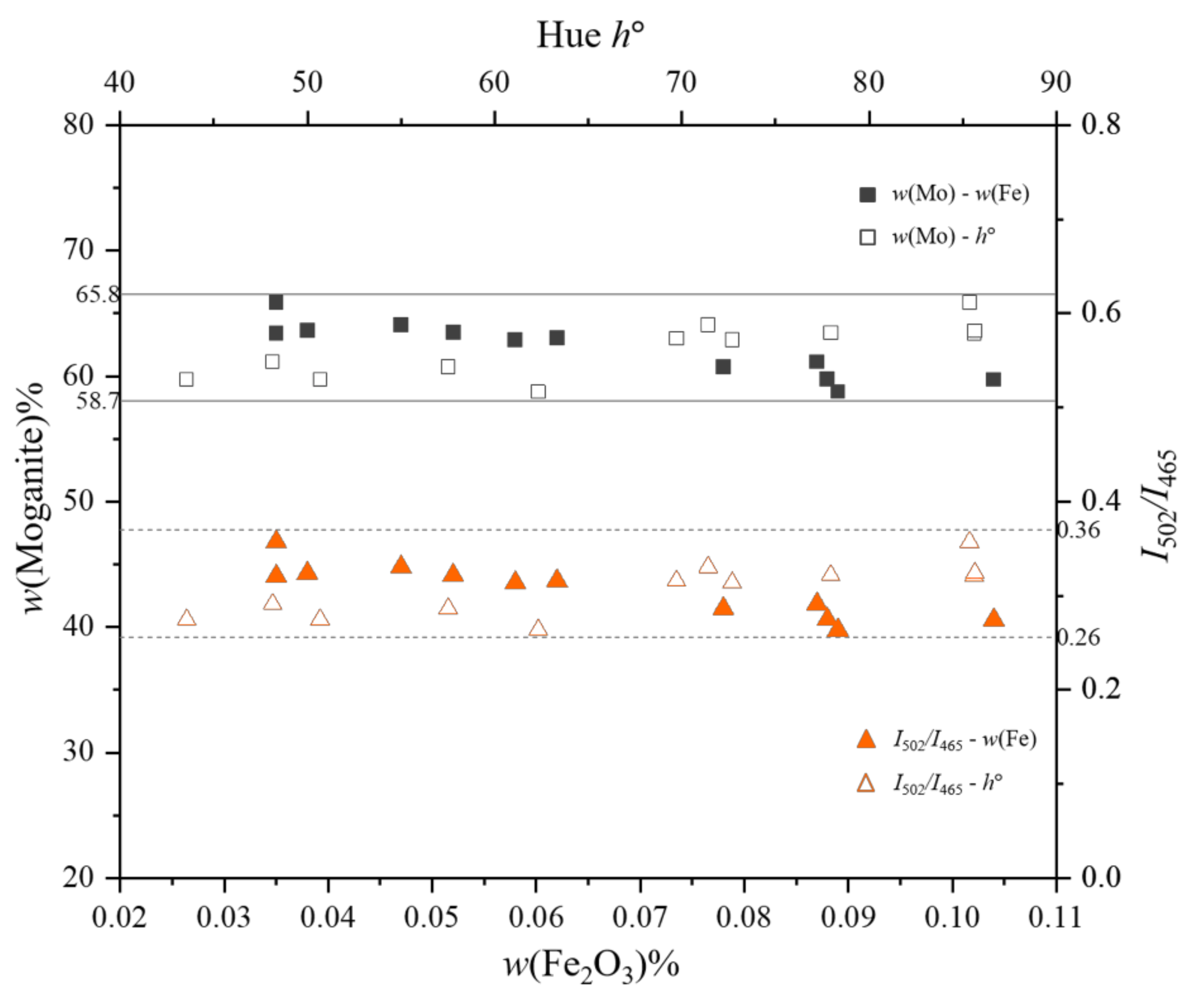
3.2. X-ray Fluorescence Measurement
3.3. Raman Spectroscopic Analysis
3.3.1. Silica Matrix
3.3.2. Ferruginous Inclusions
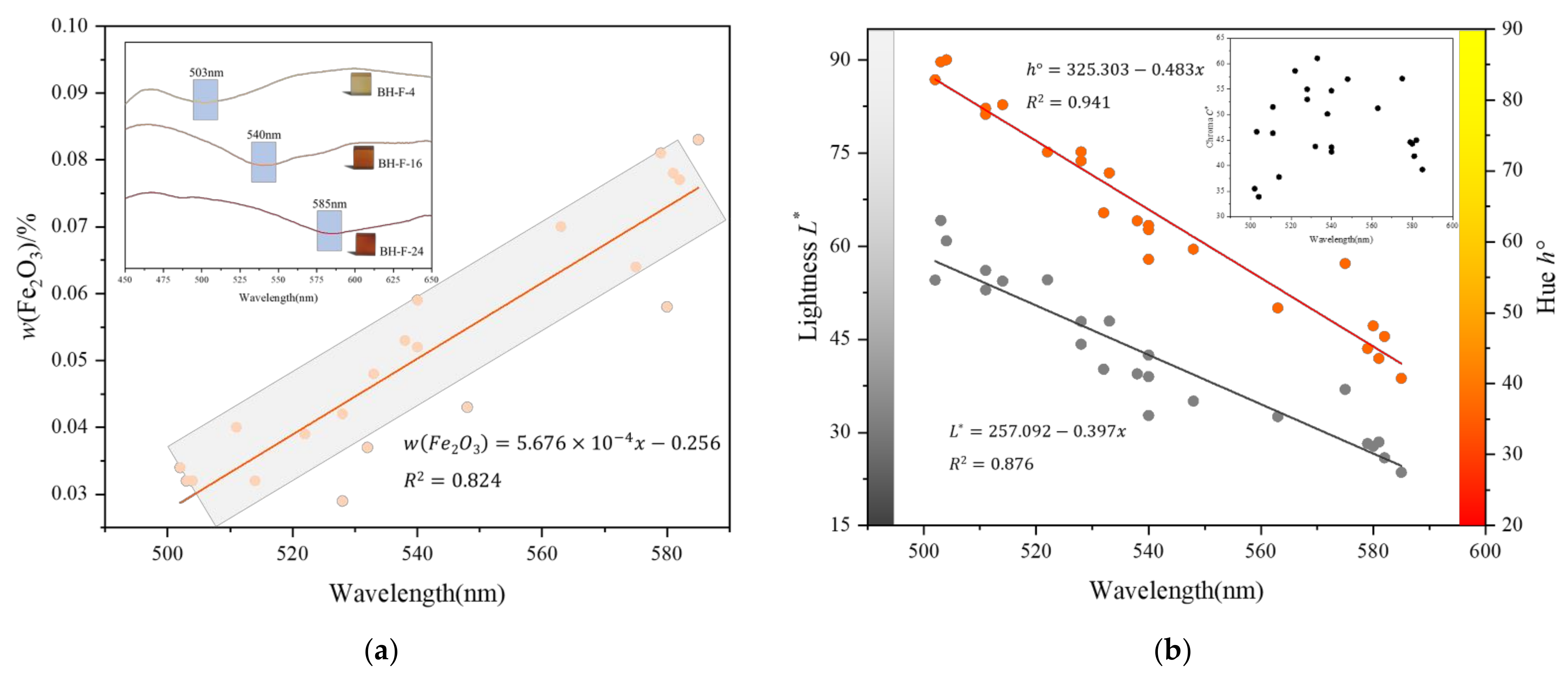
3.4. UV-VIS Spectral Analysis
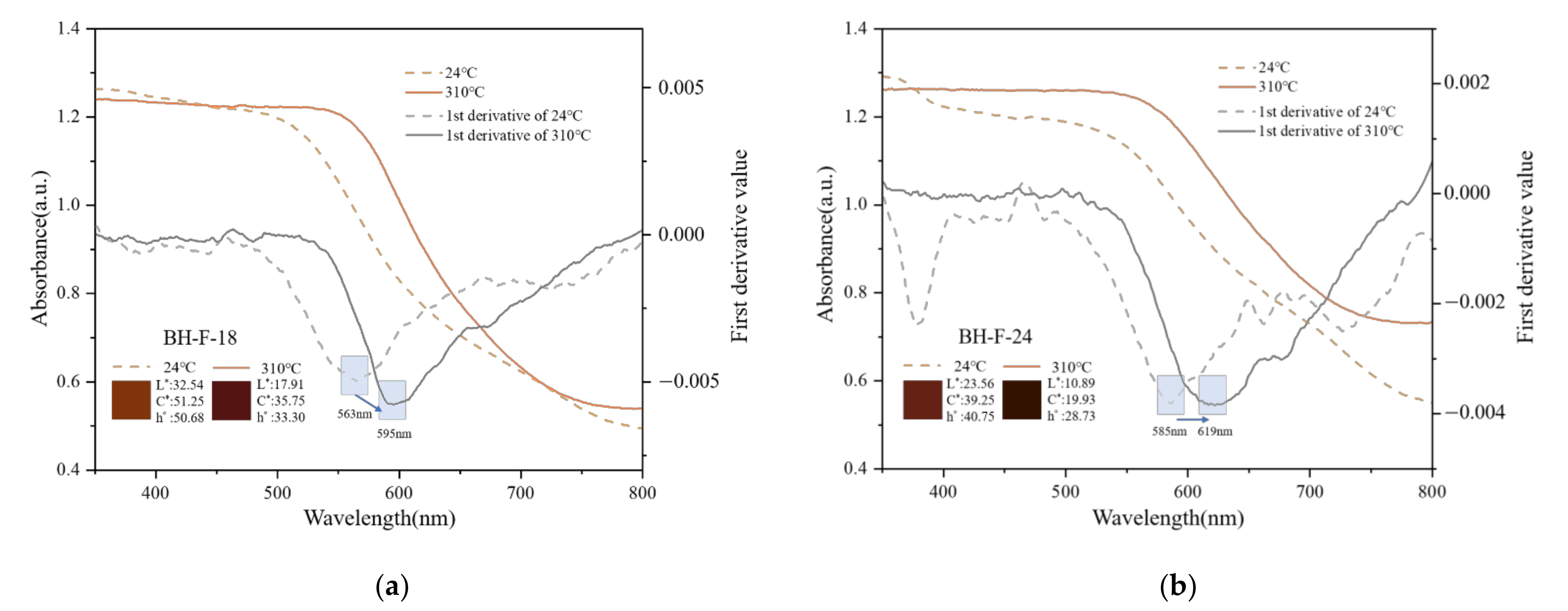
3.4.1. First Derivative Characteristics of UV-VIS Spectra
3.4.2. Heat Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Florke, O.W.; Kohlerherbertz, B.; Langer, K.; Tonges, I. Water in Microcrystalline Quartz of Volcanic Origin—Agates. Contrib. Miner. Petrol. 1982, 80, 324–333. [Google Scholar] [CrossRef]
- Moxon, T. A re-examination of water in agate and its bearing on the agate genesis enigma. Mineral. Mag. 2018, 81, 1223–1244. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, Geochemistry and Genesis of Agate—A Review. Minerals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Saminpanya, S.; Saiyasombat, C.; Chanlek, N.; Thammajak, N.; Sirisurawong, E.; Viriyasunsakun, R.; Kingkanlaya, P.; Rakponramuang, P. Trace elements content and cause of color in ancient treated carnelian and its natural counterpart from SE Asia. Archaeol. Anthropol. Sci. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, L.; He, X. Gemological Characteristics and Origin of the Zhanguohong Agate from Beipiao, Liaoning Province, China: A Combined Microscopic, X-ray Diffraction, and Raman Spectroscopic Study. Minerals 2020, 10, 401. [Google Scholar] [CrossRef]
- Zhou, D.; Shi, G.; Liu, S.; Wu, B. Mineralogy and Magnetic Behavior of Yellow to Red Xuanhua-Type Agate and Its Indication to the Forming Condition. Minerals 2021, 11, 877. [Google Scholar] [CrossRef]
- Götze, J.; Möckel, R.; Vennemann, T.; Müller, A. Origin and geochemistry of agates in Permian volcanic rocks of the Sub-Erzgebirge basin, Saxony (Germany). Chem. Geol. 2016, 428, 77–91. [Google Scholar] [CrossRef]
- Dumanska-Slowik, M.; Natkaniec-Nowak, L.; Weselucha-Birczynska, A.; Gawel, A.; Lankosz, M.; Wrobel, P. Agates from Sidi Rahal, in the Atlas Mountains of Morocco: Gemological Characteristics and Proposed Origin. Gems Gemol. 2013, 49, 148–159. [Google Scholar] [CrossRef]
- Ruan, H.D.; Frost, R.L.; Kloprogge, J.T. The behavior of hydroxyl units of synthetic goethite and its dehydroxylated product hematite. Spectrochim. Acta A 2001, 57, 2575–2586. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Lin, C.; Jin, X.; Pan, Y. Identification of Beihong Agate and Nanhong Agate from China Based on Chromaticity and Raman Spectra. Spectrosc. Spectr. Anal. 2019, 39, 2153–2159. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Guo, Q. Color and Genesis of Beihong Agate and Its Spectroscopic Characteristics. Spectrosc. Spectr. Anal. 2020, 40, 2531–2537. [Google Scholar] [CrossRef]
- King, J.M.; Moses, T.M.; Shigley, J.E.; Liu, Y. Color Grading of Colored Diamonds in the GIA Gem Trade Laboratory. Gems Gemol. 1994, 30, 220–242. [Google Scholar] [CrossRef]
- King, J.M.; Geurts, R.H.; Gilbertson, A.M.; Shigley, J.E. Color Grading “D-to-Z” Diamonds at the GIA Laboratory. Gems Gemol. 2008, 44, 296–321. [Google Scholar] [CrossRef]
- Qiu, Y.; Guo, Y. Explaining Colour Change in Pyrope-Spessartine Garnets. Minerals 2021, 11, 865. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, Y.; Zhou, Y.; Li, X.; Liu, S. The Effects of Munsell Neutral Grey Backgrounds on the Colour of Chrysoprase and the Application of AP Clustering to Chrysoprase Colour Grading. Minerals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y. Measurement of Gem Colour Using a Computer Vision System: A Case Study with Jadeite-Jade. Minerals 2021, 11, 791. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y.; Shang, Y.; Yuan, B. Research on parameters optimization of digital imaging system in red-yellow jadeite color measurement. Sci. Rep. 2022, 12, 3619. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Y. The Effect of Munsell Neutral Value Scale on the Color of Yellow Jadeite and Comparison between AP and K-Means Clustering Color Grading Schemes. Crystals 2022, 12, 241. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Du, H.M. The foundation of a color-chip evaluation system of jadeite-jade green with color difference control of medical device. Multimed. Tools Appl. 2016, 75, 14491–14502. [Google Scholar] [CrossRef]
- Dubinsky, E.V.; Stone-Sundberg, J.; Emmett, J.L. A Quantitative Description of the Causes of Color in Corundum. Gems Gemol. 2020, 56, 2–28. [Google Scholar] [CrossRef]
- Yuan, B.; Guo, Y.; Liu, Z. The influence of light path length on the color of synthetic ruby. Sci. Rep. 2022, 12, 5943. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Guo, Y.; Xu, C. Color effect of light sources on peridot based on CIE1976L*a*b*color system androundRGB diagram system. Color Res. Appl. 2019, 44, 932–940. [Google Scholar] [CrossRef]
- Tang, J.; Guo, Y.; Xu, C. Metameric effects on peridot by changing background color. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2019, 36, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Guo, Y. Genesis and influencing factors of the colour of chrysoprase. Sci. Rep. 2021, 11, 9939. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Palke, A.C.; Renfro, N. Vanadium- and Chromium-Bearing Pink Pyrope Garnet: Characterization and Quantitative Colorimetric Analysis. Gems Gemol. 2015, 51, 348–369. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Guo, Y. Colour Quality Evaluation of Bluish-Green Serpentinite Based on the CIECAM16 Model. Minerals 2021, 12, 38. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y. The impact of trace metal cations and absorbed water on colour transition of turquoise. R. Soc. Open Sci. 2021, 8, 201110. [Google Scholar] [CrossRef]
- Cheng, R.; Guo, Y. Study on the effect of heat treatment on amethyst color and the cause of coloration. Sci. Rep. 2020, 10, 14927. [Google Scholar] [CrossRef]
- Nagano, T. The Use of Color to Quantify the Effects of pH and Temperature on the Crystallization Kinetics of Goethite Under Highly Alkaline Conditions. Clays Clay Miner. 1994, 42, 226–234. [Google Scholar] [CrossRef]
- Komadel, P.; Grygar, T.; Mehner, H. Reductive dissolution and Mossbauer spectroscopic study of Fe forms in the fine fractions of Slovak Fe-rich bentonites. Clay Min. 1998, 33, 593–599. [Google Scholar] [CrossRef]
- Poulton, S.W.; Canfield, D.E. Development of a sequential extraction procedure for iron: Implications for iron partitioning in continentally derived particulates. Chem. Geol. 2005, 214, 209–221. [Google Scholar] [CrossRef]
- Spinola, D.N.; Portes, R.d.C.; Srivastava, P.; Torrent, J.; Barrón, V.; Kühn, P. Diagenetic reddening of Early Eocene paleosols on King George Island, Antarctica. Geoderma 2018, 315, 149–159. [Google Scholar] [CrossRef]
- Deaton, B.C.; Balsam, W.L. Visible Spectroscopy—A Rapid Method for Determining Hematite and Goethite Concentration in Geological-Materials. J. Sediment. Pet. 1991, 61, 628–632. [Google Scholar] [CrossRef]
- Balsam, W.; Ji, J.; Renock, D.; Deaton, B.C.; Williams, E. Determining hematite content from NUV/Vis/NIR spectra: Limits of detection. Am. Mineral. 2014, 99, 2280–2291. [Google Scholar] [CrossRef]
- Kingma, K.J.; Hemley, R.J. Raman-Spectroscopic Study of Microcrystalline Silica. Am. Mineral. 1994, 79, 269–273. [Google Scholar]
- Gotze, J.; Nasdala, L.; Kleeberg, R.; Wenzel, N. Occurrence and distribution of “moganite” in agate/chalcedony: A combined micro-Raman, Rietveld, and cathodoluminescence study. Contrib. Miner. Petrol. 1998, 133, 96–105. [Google Scholar] [CrossRef]
- Heaney, P.J.; Post, J.E. The Widespread Distribution of a Novel Silica Polymorph in Microcrystalline Quartz Varieties. Science 1992, 255, 441–443. [Google Scholar] [CrossRef]
- Gotze, J.; Plotze, M.; Fuchs, H.; Habermann, D. Defect structure and luminescence behaviour of agate—Results of electron paramagnetic resonance (EPR) and cathodoluminescence (CL) studies. Mineral. Mag. 1999, 63, 149. [Google Scholar] [CrossRef]
- Hatipoglu, M.; Ajo, D.; Kirikoglu, M.S. Cathodoluminescence (CL) features of the Anatolian agates, hydrothermally deposited in different volcanic hosts from Turkey. J. Lumin. 2011, 131, 1131–1139. [Google Scholar] [CrossRef]
- French, M.W.; Worden, R.H.; Lee, D.R. Electron backscatter diffraction investigation of length-fast chalcedony in agate: Implications for agate genesis and growth mechanisms. Geofluids 2013, 13, 32–44. [Google Scholar] [CrossRef]
- Heaney, P.J. Moganite as an Indicator for Vanished Evaporites—A Testament Reborn. J. Sediment. Res. A 1995, 65, 633–638. [Google Scholar]
- Gislason, S.; Heaney, P.; Oelkers, E. Kinetic and thermodynamic properties of moganite, a novel silica polymorph. Geochim. Cosmochim. Acta 1997, 61, 1193–1204. [Google Scholar] [CrossRef]
- Rodgers, K.A.; Cressey, G. The occurrence, detection and significance of moganite (SiO2) among some silica sinters. Mineral. Mag. 2001, 65, 157–167. [Google Scholar] [CrossRef]
- Moxon, T.; Ríos, S. Moganite and water content as a function of age in agate: An XRD and thermogravimetric study. Eur. J. Mineral. 2004, 16, 269–278. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Interact. 1998, 112, 59–65. [Google Scholar] [CrossRef]
- Powolny, T.; Dumanska-Slowik, M.; Sikorska-Jaworowska, M.; Wojcik-Bania, M. Agate mineralization in spilitized Permian volcanics from “Borowno” quarry (Lower Silesia, Poland)—Microtextural, mineralogical, and geochemical constraints. Ore Geol. Rev. 2019, 114, 103130. [Google Scholar] [CrossRef]
- Legodi, M.; Dewaal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dyes Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Sandeep, K.; Shankar, R.; Warrier, A.K.; Balsam, W. Diffuse reflectance spectroscopy of a tropical southern Indian lake sediment core: A window to environmental change. Episodes 2017, 40, 47–56. [Google Scholar] [CrossRef]
- Wu, C.; Long, H.; Cheng, T.; Liu, L.; Qian, P.; Wang, H.; Ren, S.; Zhou, L.; Zheng, X. Quantitative estimations of iron oxide minerals in the Late Pleistocene paleosol of the Yangtze River Delta: Implications for the chemical weathering, sedimentary environment, and burial conditions. Catena 2021, 207, 105662. [Google Scholar] [CrossRef]
- Balsam, W.L.; Wolhart, R.J. Sediment Dispersal in the Argentine Basin—Evidence from Visible-Light Spectra. Deep-Sea Res. Pt. II 1993, 40, 1001–1031. [Google Scholar] [CrossRef]
- Balsam, W.; Damuth, J. Further investigations of shipboard vs. shore-based spectral data: Implications for interpreting Leg 164 sediment composition. Proc. Ocean Drill. Program Sci. Results 2000, 164, 313–324. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U.; Fechter, H.; Alferez, F. Quantitative Relationships between Soil Color and Hematite Content. Soil Sci. 1983, 136, 354–358. [Google Scholar] [CrossRef]
- Ji, J.F.; Balsam, W.; Chen, J.; Liu, L.W. Rapid and quantitative measurement of hematite and goethite in the Chinese loess-paleosol sequence by diffuse reflectance spectroscopy. Clays Clay Miner. 2002, 50, 208–216. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V.; Liu, Q. Magnetic enhancement is linked to and precedes hematite formation in aerobic soil. Geophys. Res. Lett. 2006, 33, L02401. [Google Scholar] [CrossRef] [Green Version]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides:Structure, Properties, Reactions, Occurences and Uses; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Zou, X.; Chen, T.; Liu, H.; Chen, D.; Zhang, P.; Xie, Q. Structural and Chromatic Evolution of Goethite by Thermal Treatment. J. Chin. Ceram. Soc. 2013, 41, 669–673. [Google Scholar] [CrossRef]
- Zhou, W.; Ji, J.; Balsam, W.; Chen, J. Determination of Goethite and Hematite in Red Clay by Diffuse Reflectance Spectroscopy. Geol. J. China Univ. 2007, 13, 730–736. [Google Scholar]







| Moganite | α-Quartz | |
|---|---|---|
| (cm−1) 1 | (cm−1) 1 | Mode Symmetry |
| 129 | 128 | E(LO + TO) |
| 141 | ||
| 220 | 206 | A1 |
| 265 | 263 | E(LO + TO) |
| 317 | ||
| 355 | A1 | |
| 370 | ||
| 377 | ||
| 398 | 396 | E(TO) |
| 401 | E(TO) | |
| 432 | ||
| 449 | 450 | E(TO) |
| 463 | 465 | A1 |
| 502 | ||
| 511 | E(LO) | |
| 693 | 696 | E(LO + TO) |
| 792 | ||
| 796 | E(TO) | |
| 808 | E(LO) | |
| 833 | ||
| (950) | ||
| (978) | ||
| 1058 | ||
| 1065 | E(TO) | |
| 1084 | 1085 | A1 |
| 1171 | 1162 | E(LO + TO) |
| 1177 | ||
| 1230 | E(LO) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Liu, Z.; Zhao, Z.; Guo, Y. Quantitative Study on Colour and Spectral Characteristics of Beihong Agate. Minerals 2022, 12, 677. https://doi.org/10.3390/min12060677
Zhou Y, Liu Z, Zhao Z, Guo Y. Quantitative Study on Colour and Spectral Characteristics of Beihong Agate. Minerals. 2022; 12(6):677. https://doi.org/10.3390/min12060677
Chicago/Turabian StyleZhou, Yufei, Ziyuan Liu, Zitong Zhao, and Ying Guo. 2022. "Quantitative Study on Colour and Spectral Characteristics of Beihong Agate" Minerals 12, no. 6: 677. https://doi.org/10.3390/min12060677
APA StyleZhou, Y., Liu, Z., Zhao, Z., & Guo, Y. (2022). Quantitative Study on Colour and Spectral Characteristics of Beihong Agate. Minerals, 12(6), 677. https://doi.org/10.3390/min12060677







