Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Experimental Methods
2.2.1. Extraction Experiments of Lead Minerals Using EDTA
2.2.2. Cementation Experiments of the Extracted Pb2+
2.2.3. Flotation Experiments
3. Results and Discussion
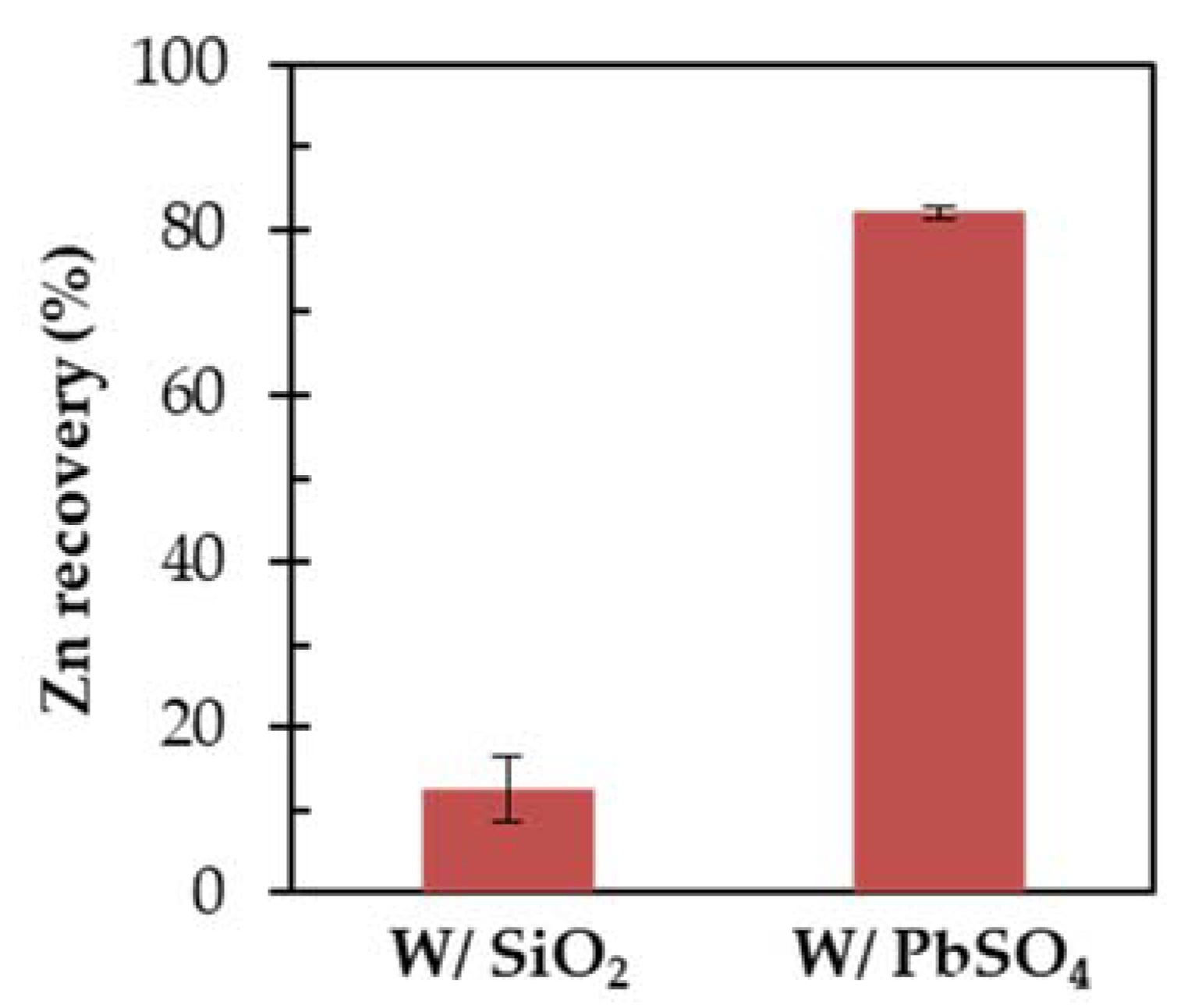
3.1. Effects of PbSO4 on ZnS Floatability
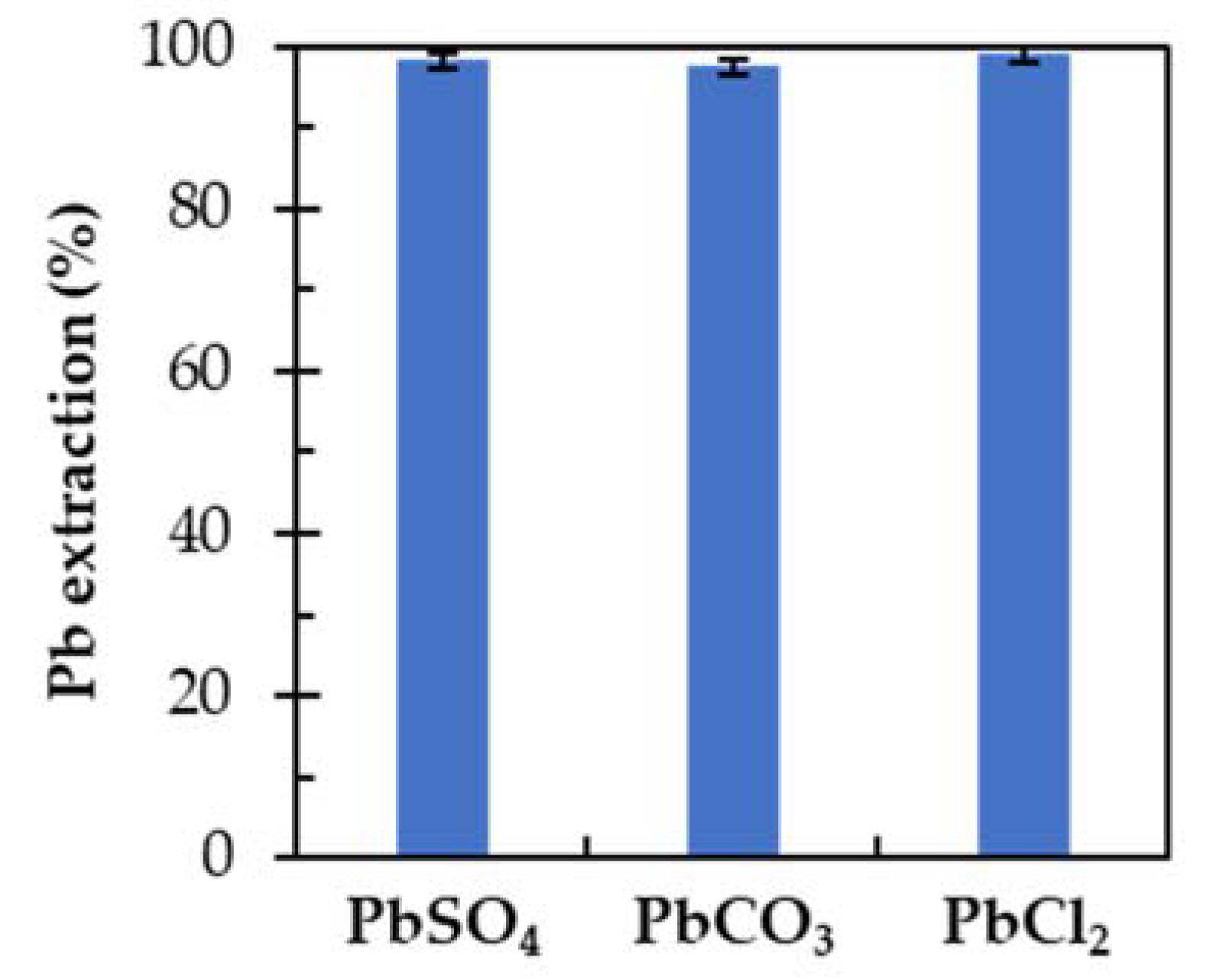
3.2. Extraction of PbSO4 Using EDTA
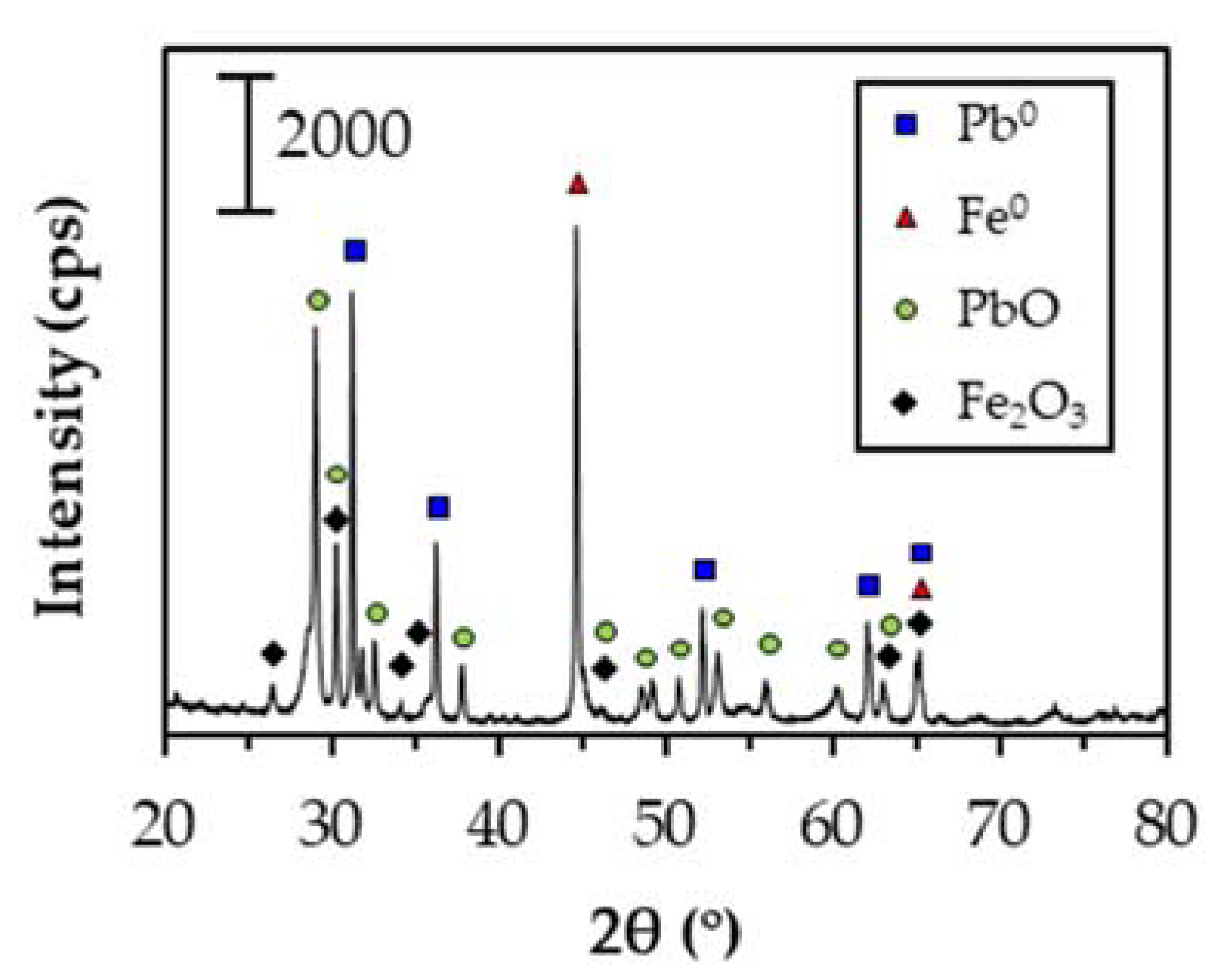
3.3. Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation
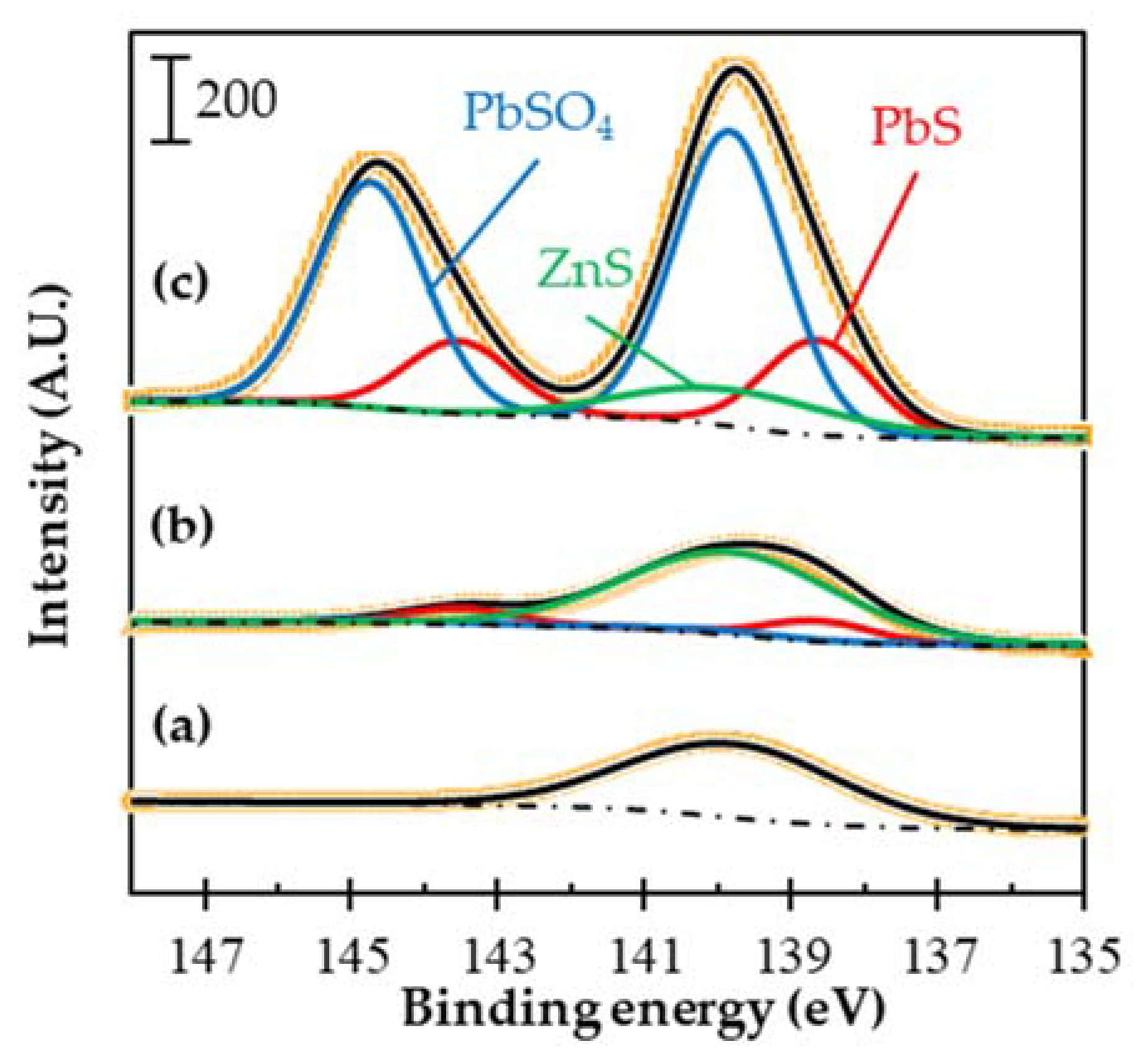
3.4. Effects of EDTA Pretreatment on ZnS Depression in Flotation
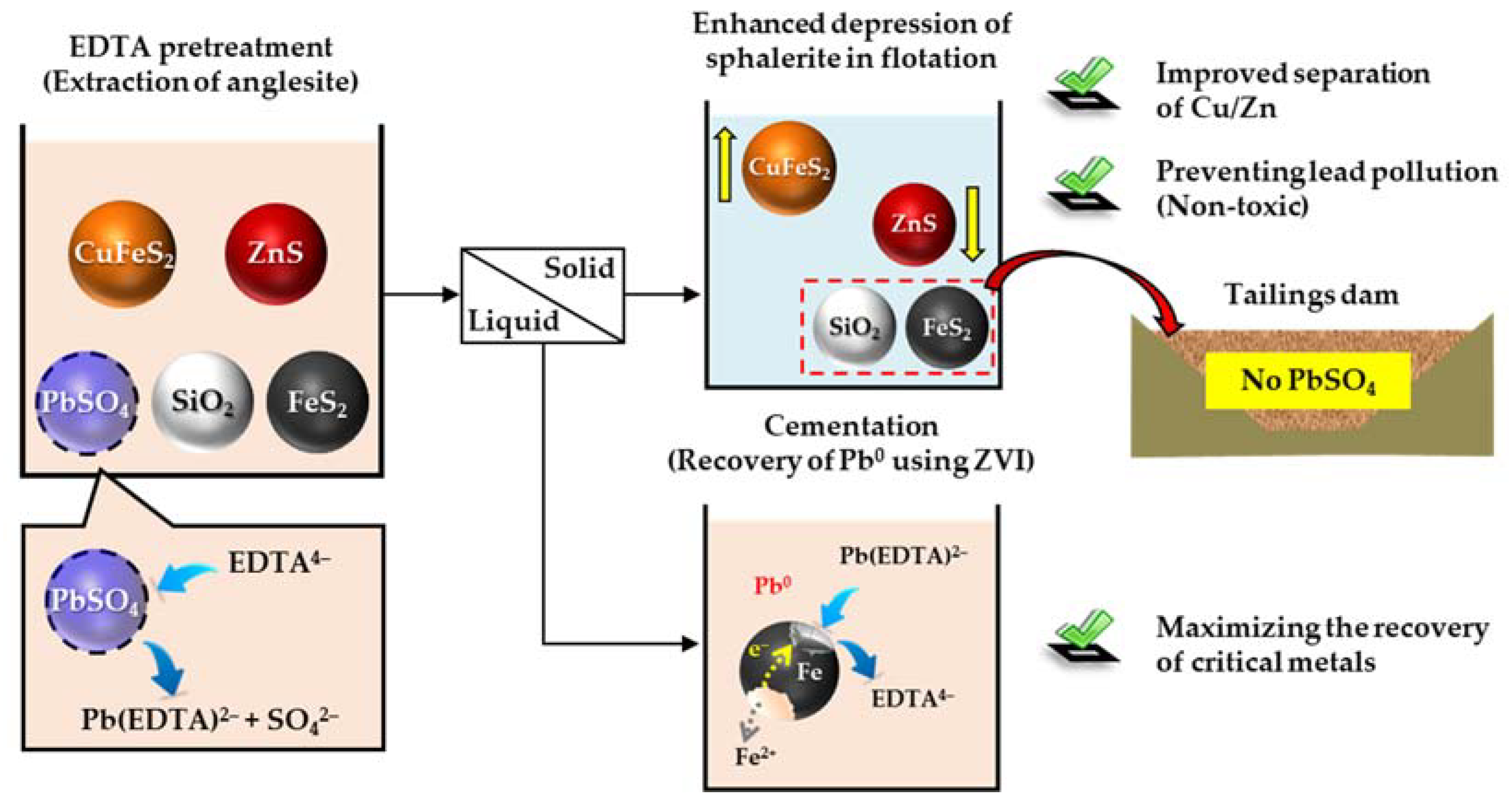
3.5. Implication of This Study
4. Conclusions
- ZnS floatability increased in the presence of PbSO4.
- The conventional depressants for ZnS, zinc sulfate and sodium sulfite, were not effective in depressing the floatability of ZnS when PbSO4 was present.
- Almost of all PbSO4 (>97%) was extracted using EDTA, and >97% of the extracted Pb2+ could be recovered as Pb0 by cementation using ZVI.
- A pretreatment of flotation extracting PbSO4 using EDTA was effective in depressing ZnS floatability.
- The proposed method for complex sulfide ores containing PbSO4, a combination of extraction of PbSO4 using EDTA and recovery of extracted Pb2+ as zero-valent Pb by cementation using ZVI, could achieve enhanced selective flotation of complex sulfide ores (depression of ZnS floatability) while preventing lead pollution to the surrounding environment of tailings dams (removal of toxic PbSO4 before flotation) and maximizing the recovery of critical elements by cementation (recovery of Pb0 using ZVI).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hák, T.; Janoušková, S.; Moldan, B. Sustainable Development Goals: A Need for Relevant Indicators. Ecol. Indic. 2016, 60, 565–573. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and Critical Metals Production from Porphyry Ores and E-Wastes: A Review of Resource Availability, Processing/Recycling Challenges, Socio-Environmental Aspects, and Sustainability Issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- Park, I.; Kanazawa, Y.; Sato, N.; Galtchandmani, P.; Jha, M.K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Beneficiation of Low-grade Rare Earth Ore from Khalzan Buregtei Deposit (Mongolia) by Magnetic Separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Goal 13 | Department of Economic and Social Affairs. Available online: https://sdgs.un.org/goals/goal13 (accessed on 26 April 2022).
- Nansai, K.; Nakajima, K.; Kagawa, S.; Kondo, Y.; Suh, S.; Shigetomi, Y.; Oshita, Y. Global Flows of Critical Metals Necessary for Low-Carbon Technologies: The Case of Neodymium, Cobalt, and Platinum. Environ. Sci. Technol. 2014, 48, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- WBG Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition. Available online: https://pubdocs.worldbank.org/en/961711588875536384/Minerals-for-Climate-Action-The-Mineral-Intensity-of-the-Clean-Energy-Transition.pdf (accessed on 25 April 2022).
- Bilal, M.; Ito, M.; Koike, K.; Hornn, V.; Ul Hassan, F.; Jeon, S.; Park, I.; Hiroyoshi, N. Effects of Coarse Chalcopyrite on Flotation Behavior of Fine Chalcopyrite. Miner. Eng. 2021, 163, 106776. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Akishino, R.; Bu, X.; Ul Hassan, F.; Park, I.; Jeon, S.; Aikawa, K.; Hiroyoshi, N. Heterogenous Carrier Flotation Technique for Recovering Finely Ground Chalcopyrite Particles Using Coarse Pyrite Particles as a Carrier. Miner. Eng. 2022, 180, 107518. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Yamazawa, R.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Kinetic Analysis for Agglomeration-Flotation of Finely Ground Chalcopyrite: Comparison of First Order Kinetic Model and Experimental Results. Mater. Trans. 2020, 61, 1940–1948. [Google Scholar] [CrossRef]
- Hornn, V.; Park, I.; Ito, M.; Shimada, H.; Suto, T.; Tabelin, C.B.; Jeon, S.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite Using Surfactant-Stabilized Oil Emulsions: Effects of Co-Existing Minerals and Ions. Miner. Eng. 2021, 171, 107076. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A Review of Recent Advances in Depression Techniques for Flotation Separation of Cu–Mo Sulfides in Porphyry Copper Deposits. Metals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part II. Flotation. Metals 2021, 11, 439. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Makino, Y.; Chea, M.; Phengsaart, T.; Park, I.; Hiroyoshi, N.; Ito, M. Improvement of Flotation and Suppression of Pyrite Oxidation Using Phosphate-Enhanced Galvanic Microencapsulation (GME) in a Ball Mill with Steel Ball Media. Miner. Eng. 2019, 143, 105931. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Kusano, A.; Park, I.; Oki, T.; Takahashi, T.; Furuya, H.; Hiroyoshi, N. Flotation of Seafloor Massive Sulfide Ores: Combination of Surface Cleaning and Deactivation of Lead-Activated Sphalerite to Improve the Separation Efficiency of Chalcopyrite and Sphalerite. Metals 2021, 11, 253. [Google Scholar] [CrossRef]
- Ministry of Economic, Trade and Industry (METI) and Japan Oil, Gas and Metals National Corporation (JOGMEC). Summary Report on Comprehensive Evaluations of Development Plan of Seafloor Massive Sulfide Deposits. Available online: http://www.jogmec.go.jp/content/300359550.pdf (accessed on 26 February 2021).
- Zeng, Z.; Chen, Z.; Qi, H. Two Processes of Anglesite Formation and a Model of Secondary Supergene Enrichment of Bi and Ag in Seafloor Hydrothermal Sulfide Deposits. J. Mar. Sci. Eng. 2021, 10, 35. [Google Scholar] [CrossRef]
- Woodcock, J.T.; Sparrow, G.J.; Bruckard, W.J.; Johnson, N.W.; Dunne, R. Plant Practice: Sulfide Minerals and Precious Metals. InFroth Flotation a Century of Innovation; Fuerstenau, M., Jameson, G., Yoon, R.H., Eds.; SME: Littleton, CO, USA, 2007; pp. 781–843. ISBN 978-0-873-35252-9. [Google Scholar]
- Wills, B.A.; Finch, J.A. Froth flotation. In Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Elsevier: Oxford, UK, 2016; pp. 265–380. ISBN 9780080970530. [Google Scholar]
- Ball, J.W.; Nordstrom, D.K. User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Text Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Waters; USGS Numbered Series Open-File Report 91-183; U.S. Geological Survey: Reston, VA, USA, 1991.
- Rashchi, F.; Sui, C.; Finch, J.A. Sphalerite Activation and Surface Pb Ion Concentration. Int. J. Miner. Process. 2002, 67, 43–58. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; Zhan, Y. Sphalerite Activation: Flotation and Electrokinetic Studies. Miner. Eng. 1997, 10, 787–802. [Google Scholar] [CrossRef]
- Houot, R.; Raveneau, P. Activation of Sphalerite Flotation in the Presence of Lead Ions. Int. J. Miner. Process. 1992, 35, 253–271. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of Lead-Activated Sphalerite by Pyrite via Galvanic Interactions: Implications to the Selective Flotation of Complex Sulfide Ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Trahar, W.J.; Senior, G.D.; Heyes, G.W.; Creed, M.D. The Activation of Sphalerite by Lead—A Flotation Perspective. Int. J. Miner. Process. 1997, 49, 121–148. [Google Scholar] [CrossRef]
- Rashchi, F.; Dashti, A.; Arabpour-Yazdi, M.; Abdizadeh, H. Anglesite Flotation: A Study for Lead Recovery from Zinc Leach Residue. Miner. Eng. 2005, 18, 205–212. [Google Scholar] [CrossRef]
- Fuerstenau, M.C.; Olivas, S.A.; Herrera-Urbina, R.; Han, K.N. The Surface Characteristics and Flotation Behavior of Anglesite and Cerussite. Int. J. Miner. Process. 1987, 20, 73–85. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Alkaline Leaching and Concurrent Cementation of Dissolved Pb and Zn from Zinc Plant Leach Residues. Minerals 2022, 12, 393. [Google Scholar] [CrossRef]
- Schindler, M.; Santosh, M.; Dotto, G.; Silva, L.F.O.; Hochella, M.F. A Review on Pb-Bearing Nanoparticles, Particulate Matter and Colloids Released from Mining and Smelting Activities. Gondwana Res. 2021. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-Microencapsulation of Arsenopyrite Using Al-Catecholate Complex: Nature of Oxidation Products, Effects on Anodic and Cathodic Reactions, and Coating Stability under Simulated Weathering Conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirley, D.A. High-Resolution X-ray Photoemission Spectrum of the Valence Bands of Gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Tabelin, C.B.; Corpuz, R.D.; Igarashi, T.; Villacorte-Tabelin, M.; Ito, M.; Hiroyoshi, N. Hematite-Catalysed Scorodite Formation as a Novel Arsenic Immobilisation Strategy under Ambient Conditions. Chemosphere 2019, 233, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Zoleta, J.B.; Itao, G.B.; Resabal, V.J.T.; Lubguban, A.A.; Corpuz, R.D.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B. Improved Pyrolysis Behavior of Ammonium Polyphosphate-Melamine-Expandable (APP-MEL-EG) Intumescent Fire Retardant Coating System Using Ceria and Dolomite as Additives for I-Beam Steel Application. Heliyon 2020, 6, e03119. [Google Scholar] [CrossRef] [Green Version]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Hashizume, R.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; et al. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation Using Zero-Valent Aluminum in Chloride Medium. Metals 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Bicak, O. A Technique to Determine Ore Variability in a Sulphide Ore. Miner. Eng. 2019, 142, 105927. [Google Scholar] [CrossRef]
- Grano, S.R.; Ralston, J.; Johnson, N.W. Characterization and Treatment of Heavy Medium Slimes in the Mt. Isa Mines Lead-Zinc Concentrator. Miner. Eng. 1988, 1, 137–150. [Google Scholar] [CrossRef]
- Kant, C.; Rao, S.R.; Finch, J.A. Distribution of Surface Metal Ions among the Products of Chalcopyrite Flotation. Miner. Eng. 1994, 7, 905–916. [Google Scholar] [CrossRef]
- Rumball, J.A.; Richmond, G.D. Measurement of Oxidation in a Base Metal Flotation Circuit by Selective Leaching with EDTA. Int. J. Miner. Process. 1996, 48, 1–20. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Addition of Fe3O4 as Electron Mediator for Enhanced Cementation of Cd2+ and Zn2+ on Aluminum Powder from Sulfate Solutions and Magnetic Separation to Concentrate Cemented Metals from Cementation Products. J. Environ. Chem. Eng. 2021, 9, 106699. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. The Effects of Coexisting Copper, Iron, Cobalt, Nickel, and Zinc Ions on Gold Recovery by Enhanced Cementation via Galvanic Interactions between Zero-Valent Aluminum and Activated Carbon in Ammonium Thiosulfate Systems. Metals 2021, 11, 1352. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals 2021, 11, 248. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium Thiosulfate Extraction of Gold from Printed Circuit Boards (PCBs) of End-of-Life Mobile Phones and Its Recovery from Pregnant Leach Solution by Cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Gold via Galvanic Interactions Using Activated Carbon and Zero-Valent Aluminum: A Novel Approach to Recover Gold Ions from Ammonium Thiosulfate Medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Kuze, A.; Ito, M.; Hiroyoshi, N. A Kinetic Study on Enhanced Cementation of Gold Ions by Galvanic Interactions between Aluminum (Al) as an Electron Donor and Activated Carbon (AC) as an Electron Mediator in Ammonium Thiosulfate System. Minerals 2022, 12, 91. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. A Simple and Efficient Recovery Technique for Gold Ions from Ammonium Thiosulfate Medium by Galvanic Interactions of Zero-Valent Aluminum and Activated Carbon: A Parametric and Mechanistic Study of Cementation. Hydrometallurgy 2022, 208, 105815. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, K.; Alorro, R.D.; Tabelin, C.B. Cementation of Co Ion in Leach Solution Using Zn Powder Followed by Magnetic Separation of Cementation-Precipitate for Recovery of Unreacted Zn Powder. Miner. Eng. 2020, 145, 106061. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Cd2+, Co2+, Ni2+, and Zn2+ on Al from Sulfate Solutions by Activated Carbon Addition. Hydrometallurgy 2021, 201, 105580. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Tabelin, C.B.; Park, I.; Jeon, S.; Takada, M.; Kubo, Y.; Hokari, N.; Tsunekawa, M.; Hiroyoshi, N. Simultaneous Extraction and Recovery of Lead Using Citrate and Micro-Scale Zero-Valent Iron for Decontamination of Polluted Shooting Range Soils. Environ. Adv. 2021, 5, 100115. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Reduction and Adsorption of Pb2+ in Aqueous Solution by Nano-Zero-Valent Iron—A SEM, TEM and XPS Study. Mater. Res. Bull. 2010, 45, 1361–1367. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; Perry, D.L. An X-ray Photoelectron and Electron Energy Loss Study of the Oxidation of Lead. J. Vac. Sci. Technol. A Vac. Surf. Film. 1998, 2, 771. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of Lead-Bearing Zinc Plant Leach Residues from Kabwe, Zambia by Coupled Extraction-Cementation Method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- El-Shall, H.E.; Elgillani, D.A.; Abdel-Khalek, N.A. Role of Zinc Sulfate in Depression of Lead-Activated Sphalerite. Int. J. Miner. Process. 2000, 58, 67–75. [Google Scholar] [CrossRef]
- Basilio, C.I.; Kartio, I.J.; Yoon, R.-H. Lead Activation of Sphalerite during Galena Flotation. Miner. Eng. 1996, 9, 869–879. [Google Scholar] [CrossRef]
- Helgeson, H.C. Thermodynamics of Hydrothermal Systems at Elevated Temperatures and Pressures. Am. J. Sci. 1969, 267, 729–804. [Google Scholar] [CrossRef]
- Latimer, W.M. The Oxidation States of the Elements and Their Potentials in Aqueous Solutions, 2nd ed.; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 1952; pp. 72, 152, 169. ISBN 978-0-758-15876-5. [Google Scholar]
- Leckie, J.O.; James, R.O. Aqueous Environmental Chemistry of Metals; Rubin, A.J., Ed.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1974; pp. 1–76. ISBN 978-0-250-40060-7. [Google Scholar]
- Liu, J.; Ejtemaei, M.; Nguyen, A.V.; Wen, S.; Zeng, Y. Surface Chemistry of Pb-Activated Sphalerite. Miner. Eng. 2020, 145, 106058. [Google Scholar] [CrossRef]
- Hernan, L.; Morales, J.; Sanchez, L.; Tirado, J.L.; Espinos, J.P.; Gonzalez Elipe, A.R. Diffraction and XPS Studies of Misfit Layer Chalcogenides Intercalated with Cobaltocene. Chem. Mater. 1995, 7, 1576–1582. [Google Scholar] [CrossRef]
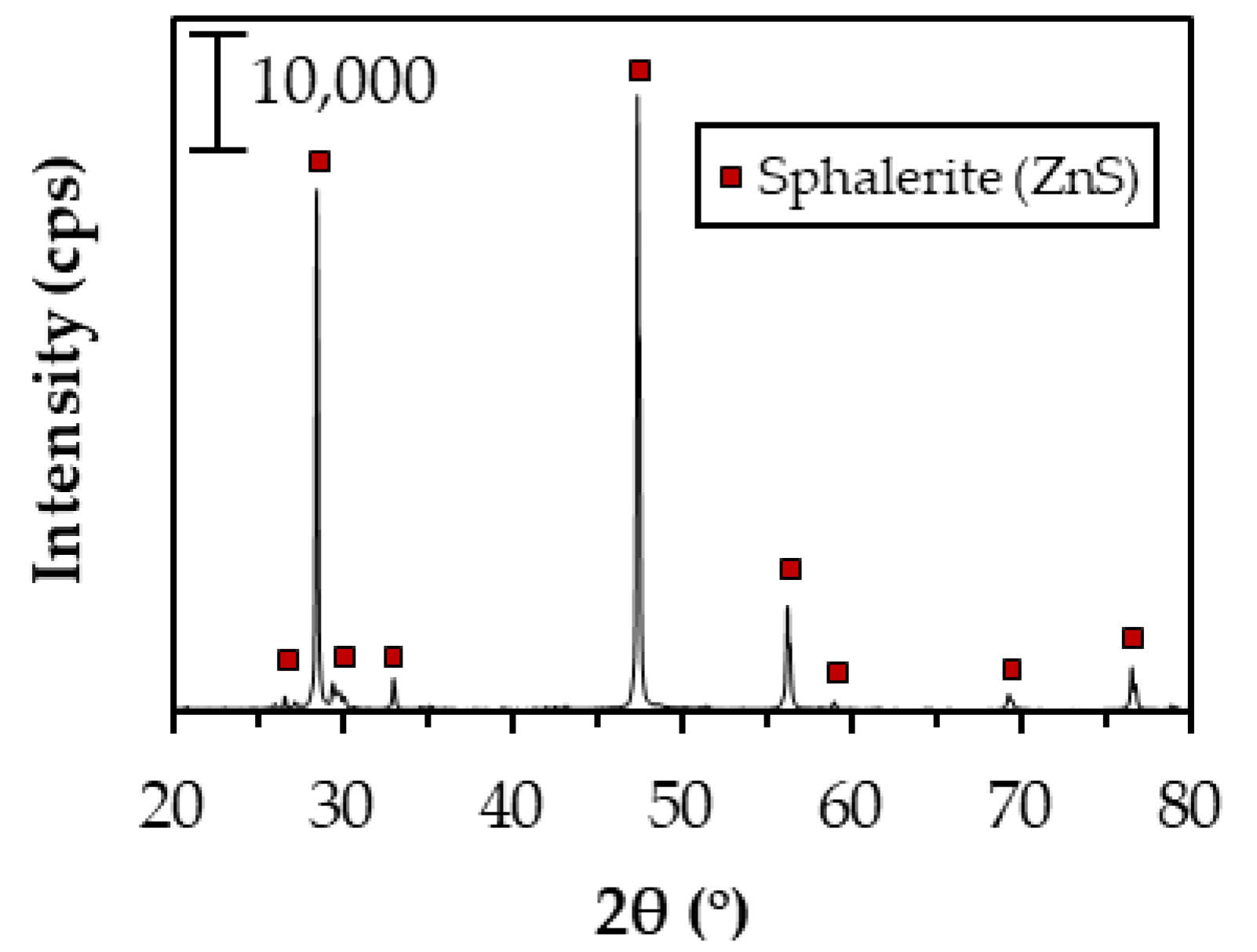


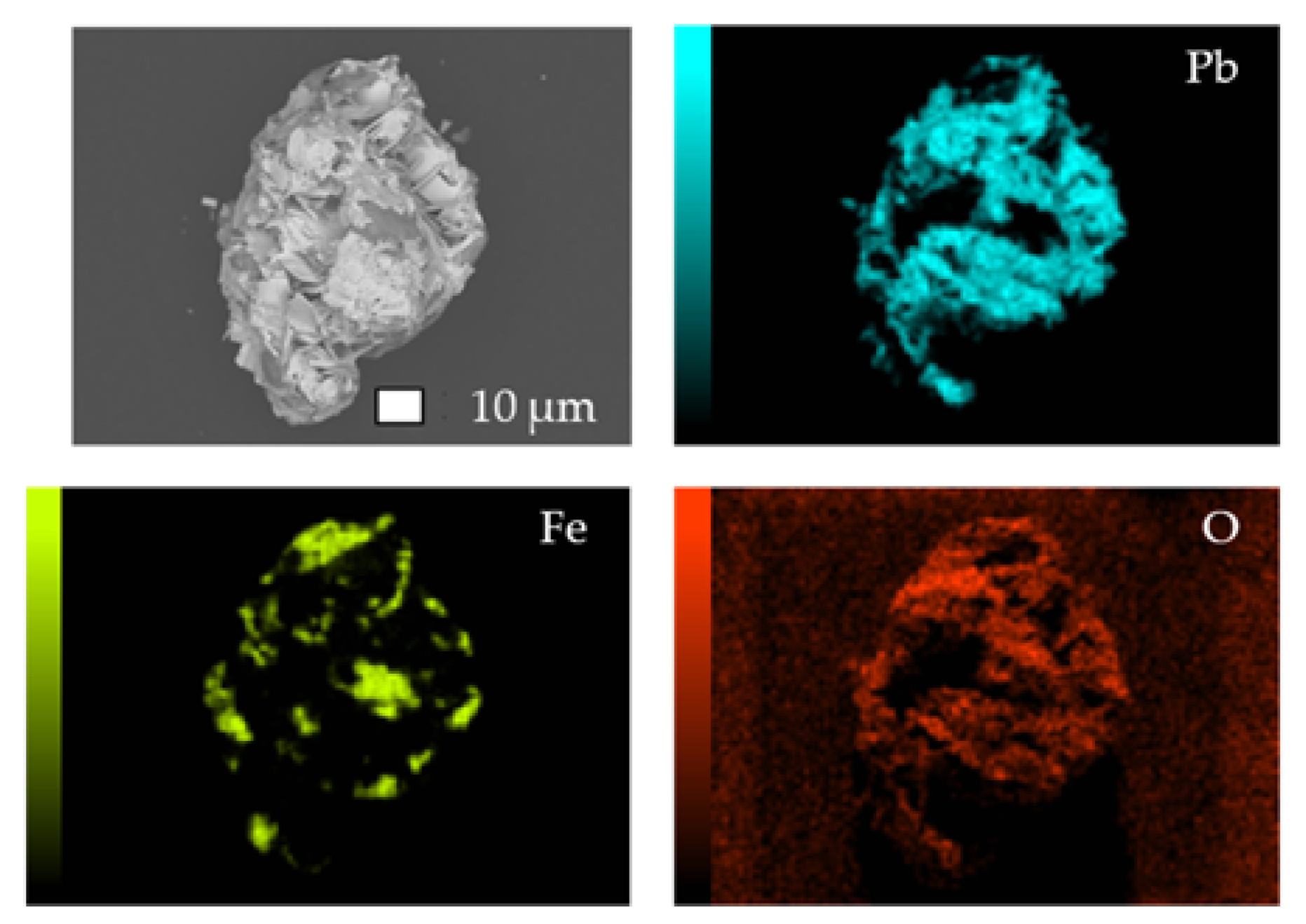

| Elements | Zn | Fe | Pb | S | Si |
|---|---|---|---|---|---|
| Mass fraction (%) | 55.7 | 6.2 | 0.2 | 21.3 | 8.4 |
| K | ΔG (kJ/mol) Without EDTA Pretreatment | ΔG (kJ/mol) With EDTA Pretreatment |
|---|---|---|
| 1000 a | −6.9 | 1.8 |
| 704 b | −6.1 | 2.7 |
| 1059 c | −7.1 | 1.7 |
| 1127 d | −7.2 | 1.5 |
| Binding Energy (eV) | FWHM | Assignments | Contents (at.%) | ||
|---|---|---|---|---|---|
| Untreated a | W/ EDTA b Pretreatment | W/o EDTA a Pretreatment | |||
| 138.6 ± 0.05 | 1.7 | PbS | 0 | 17.1 | 21.5 |
| 143.5 ± 0.05 | |||||
| 139.8 ± 0.05 | 1.7 | PbSO4 | 0 | 8.8 | 48.4 |
| 144.7 ± 0.05 | |||||
| 139.8 ± 0.05 | 3.4 | ZnS | 100 | 80.5 | 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aikawa, K.; Ito, M.; Kusano, A.; Jeon, S.; Park, I.; Hiroyoshi, N. Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals 2022, 12, 723. https://doi.org/10.3390/min12060723
Aikawa K, Ito M, Kusano A, Jeon S, Park I, Hiroyoshi N. Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals. 2022; 12(6):723. https://doi.org/10.3390/min12060723
Chicago/Turabian StyleAikawa, Kosei, Mayumi Ito, Atsuhiro Kusano, Sanghee Jeon, Ilhwan Park, and Naoki Hiroyoshi. 2022. "Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe" Minerals 12, no. 6: 723. https://doi.org/10.3390/min12060723
APA StyleAikawa, K., Ito, M., Kusano, A., Jeon, S., Park, I., & Hiroyoshi, N. (2022). Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals, 12(6), 723. https://doi.org/10.3390/min12060723







