Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Solution System
Abstract
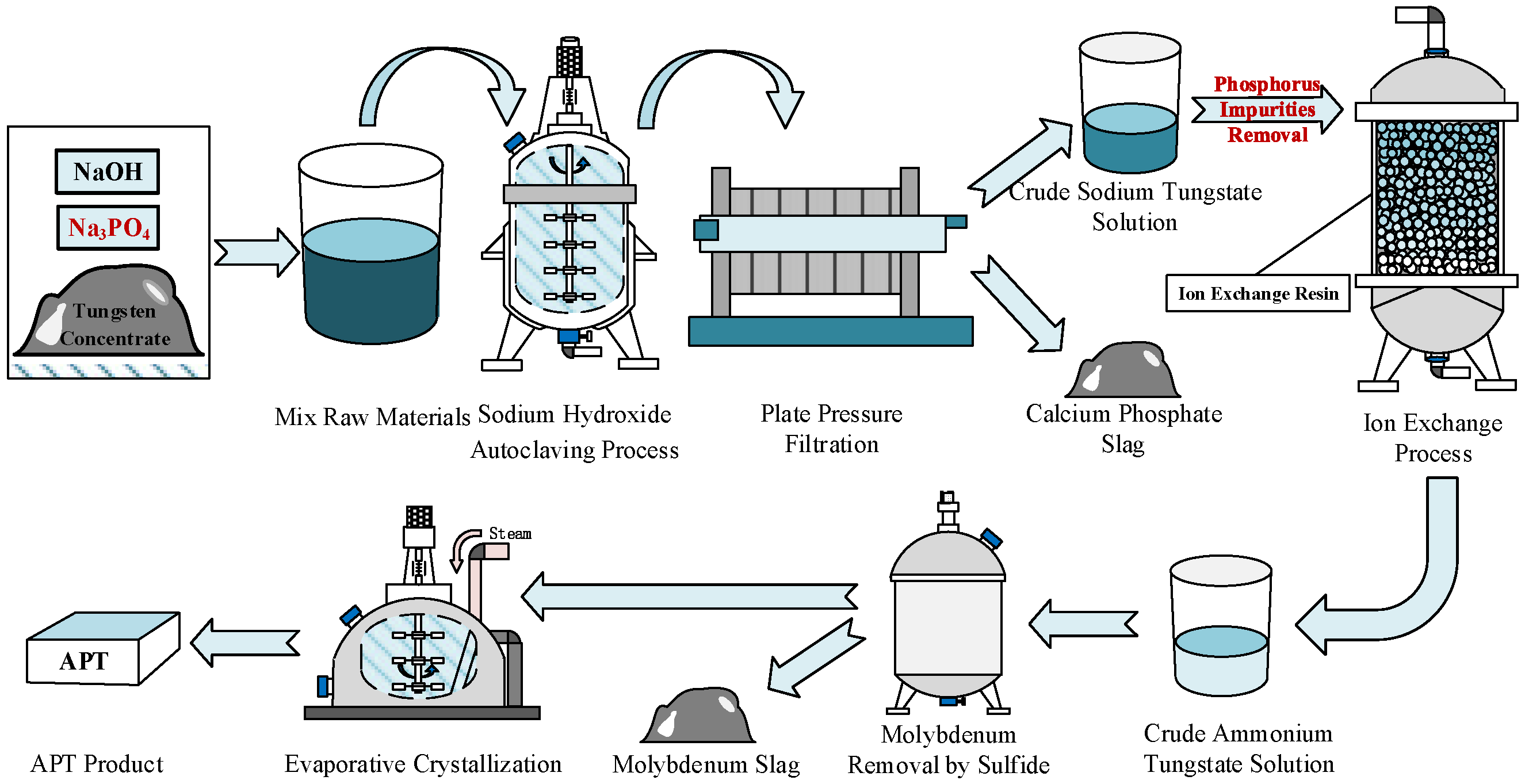
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus and Procedures
3. Results and Discussion
3.1. Dissolution Behavior of Sodium Phosphate in a Binary Na3PO4–Na2WO4 Solution System
3.2. Dissolution Behavior of Sodium Phosphate in a Binary Na3PO4–NaOH Solution System
3.3. Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Ternary Solution System
4. Conclusions
- The addition of sodium hydroxide/sodium tungstate into the sodium phosphate solution will produce the common-ion effect and the salt effect, both of which will affect the saturated phosphorus concentration in the solution. The common-ion effect decreases the saturated phosphorus concentration, while the salt effect increases the saturated phosphorus concentration. In most cases, the common-ion effect is stronger than the salt effect.
- In binary or ternary solution systems containing sodium phosphate, sodium tungstate, and sodium hydroxide, increasing the concentration of sodium hydroxide or sodium tungstate and decreasing the solution temperature can significantly decrease the saturated phosphorus concentration in the solution.
- Regarding the actual situation of tungsten smelting, the phosphorus concentration in the solution can be reduced by increasing the sodium hydroxide concentration and lowering the temperature in the ternary Na3PO4–Na2WO4–NaOH solution system. In this way, no additional impurities are introduced, and the phosphorus removal effect is significant, meeting the requirements of industrial production. Furthermore, the recovered sodium phosphate can be recycled as an additive for subsequent scheelite decomposition processes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Liu, X.; Zhao, Z. Leaching kinetics of scheelite with sodium phytate. Hydrometallurgy 2019, 186, 83–90. [Google Scholar] [CrossRef]
- Li, H.G.; Zhao, Z.W. The technology progress in China tungsten metallurgy—To the 100th anniversary of China tungsten industry. China Tungsten Ind. 2007, 7–10. [Google Scholar]
- Mishra, D.; Sinha, S.; Sahu, K.K.; Agrawal, A.; Kumar, R. Recycling of Secondary Tungsten Resources. Trans. Indian Inst. Met. 2017, 70, 479–485. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.S.; Liu, L.X.; Liu, Q.Y.; Sun, L.N.; Zhao, M. Analysis of the tungsten resources reserve plan in China. China Min. Mag. 2016, 25, 15–18. [Google Scholar]
- Zhang, C.M. To promote the healthy and sustainable development of tungsten industry by rising to the challenges of the economic norm of China. China Tungsten Ind. 2016, 31, 3–8. [Google Scholar]
- Zhang, W.; Li, J.; Zhao, Z. Leaching kinetics of scheelite with nitric acid and phosphoric acid. Int. J. Refract. Met. Hard Mater. 2015, 52, 78–84. [Google Scholar] [CrossRef]
- Fang, Q. Decomposition of Scheelite with NaOH in Autoclaving. China Tungsten Ind. 2001, 16, 80–81. [Google Scholar]
- Li, H.G.; Yang, J.G.; Li, K.D. Tungsten Metallurgy; Central South University Press: Changsha, China, 2010; p. 357. [Google Scholar]
- Zhao, Z.; Li, J.; Wang, S.; Li, H.; Liu, M.; Sun, P.; Li, Y. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Li, H. Kinetics of sodium hydroxide leaching of scheelite. Int. J. Refract. Met. Hard Mater. 2011, 29, 289–292. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Liu, X.; Chen, A.; Li, H. Sodium hydroxide digestion of scheelite by reactive extrusion. Int. J. Refract. Met. Hard Mater. 2011, 29, 739–742. [Google Scholar] [CrossRef]
- Zhongwei, Z.; Fenglong, S.; Jinhong, Y.; Qi, F.; Wenwei, J.; Xuheng, L.; Xingyu, C.; Jiangtao, L. Status and prospect for tungsten resources, technologies and industrial development in China. Chin. J. Nonferrous Met. 2019, 29, 1902–1916. [Google Scholar] [CrossRef]
- Zhongwei, Z.; Jiangtao, L.; Xingyu, C.; Xuheng, L. Technology status and development of scheelite metallurgy in China. Nonferrous Met. Sci. Eng. 2013, 4, 11–14. [Google Scholar] [CrossRef]
- He, L.H.; Cao, C.F.; Zhao, Z.W.; Chen, X.Y.; Chen, A.L.; Liu, X.H.; He, X.H. Thermodynamic analysis on decomposition of scheelite using sodium hydroxide. Mater. Sci. Eng. Powder Metall. 2013, 18, 368–372. [Google Scholar]
- Liu, L.; Xue, J.; Liu, K.; Zhu, J.; Wang, Z. Complex Leaching Process of Scheelite in Hydrochloric and Phosphoric Solutions. JOM 2016, 68, 2455–2462. [Google Scholar] [CrossRef]
- Ji, L.; Yin, C.; Chen, X.; Liu, X.; Zhao, Z. Hydrogen peroxide coordination-calcium salt precipitation for deep phosphorus removal from crude sodium tungstate solution. Hydrometallurgy 2020, 191, 105189. [Google Scholar] [CrossRef]
- Jupp, A.R.; Beijer, S.; Narain, G.C.; Schipper, W.; Slootweg, J.C. Phosphorus recovery and recycling—Closing the loop. Chem. Soc. Rev. 2021, 50, 87–101. [Google Scholar] [CrossRef]
- Carrillo, V.; Fuentes, B.; Gómez, G.; Vidal, G. Characterization and recovery of phosphorus from wastewater by combined technologies. Rev. Environ. Sci. Bio/Technol. 2020, 19, 389–418. [Google Scholar] [CrossRef]
- Thistleton, J.; Berry, T.A.; Pearce, P.; Parsons, S.A. Mechanisms of Chemical Phosphorus Removal II: Iron (III) Salts. Process Saf. Environ. Prot. 2002, 80, 265–269. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus removal and recovery technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Meng, S.L.; Qiu, L.P.; Chen, J.C.; Xu, P. The Research Process of Chemistry Precipitation Method in Phosphorus Removal in Wastewater. Chin. Agric. Sci. Bull. 2012, 28, 264–268. [Google Scholar]
- Wang, G.W.; Qiu, L.P.; Zhang, S.B. Development on phosphorus removement and recovery from wastewater. Technol. Water Treat. 2010, 36, 17–22. [Google Scholar]
- Yang, M.M.; Liu, S.Y.; Zheng, X.P.; Liu, Q.; Du, B. Present Situation and Prospects of Technology of Phosphorus Removal from Wastewater. J. Jinan Univ. (Sci. Technol.) 2008, 166–170. [Google Scholar]
- Xu, F.G.; Luo, J.Z.; Lin, D.X. Present and prospects of the removal of phosphorus from wastewater chemically. Ind. Water Treat. 2003, 23, 18–20. [Google Scholar]
- Yeoman, S.; Stephenson, T.; Lester, J.N.; Perry, R. The removal of phosphorus during wastewater treatment: A review. Environ. Pollut. 1988, 49, 183–233. [Google Scholar] [CrossRef]
- Li, X.-b.; Gao, C.-h.; Zhou, J.; Zhou, Q.-s.; Qi, T.-g.; Liu, G.-h.; Peng, Z.-h. Dissolving behavior of ammonium paratungstate in (NH4)2CO3–NH3·H2O–H2O system. Trans. Nonferrous Met. Soc. China 2018, 28, 1456–1464. [Google Scholar] [CrossRef]
- Lin-sheng, W.; Xian-wen, J.; Xing-ren, L.; Liang, Y.; Peng, L.; Yong, L. Research on the Solubility of APT in (NH4)2HPO4-NH3·H2O-H2O System. Rare Met. Cem. Carbides 2016, 44, 6–10. [Google Scholar]
- Linsheng, W.; Zhongning, S.; Xueyou, X.; Bangming, C.; Hui, W. Study on the Solubility of NH4Cl-NH3·H2O-H2O System APT. China Tungsten Ind. 2002, 35–38. [Google Scholar]
- Liang, Y.; Lin-sheng, W.; Liu-fei, S. Equilibrium solubility of (NH4)3PO4 in (NH4)3PO4-NH3·H2O-H2O system. Nonferrous Met. Sci. Eng. 2016, 7, 39–42, 109. [Google Scholar] [CrossRef]
- Shijie, L.; Qianchen, L.; Jiangtao, L. Precipitation behavior of impurity elements during evaporation crystallization of ammonium paratungstate. Nonferrous Met. Sci. Eng. 2021, 12, 19–26. [Google Scholar] [CrossRef]
- Wu, X.; He, W.; Guan, B.; Wu, Z. Solubility of Calcium Sulfate Dihydrate in Ca—Mg—K Chloride Salt Solution in the Range of (348.15 to 371.15) K. J. Chem. Eng. Data 2010, 55, 2100–2107. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Li, Z. A new industrial process of NaHCO3 and its crystallization kinetics by using the common ion effect of Na2CO3. Chem. Eng. J. 2019, 360, 740–749. [Google Scholar] [CrossRef]
- Wang, X.; Ge, Q. Separation and recovery of NaF from fluorine containing solution by the common ion effect of Na+. Heliyon 2018, 4, e01029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; Taylor and Francis: Abingdon, UK, 2016; p. 2670. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.-X.; Liang, Y.; Fan, Z.-K.; Xu, L.-W.; Liu, D.-G.; Xu, G.-Z. Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Solution System. Minerals 2022, 12, 732. https://doi.org/10.3390/min12060732
He B-X, Liang Y, Fan Z-K, Xu L-W, Liu D-G, Xu G-Z. Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Solution System. Minerals. 2022; 12(6):732. https://doi.org/10.3390/min12060732
Chicago/Turabian StyleHe, Bing-Xuan, Yong Liang, Ze-Kun Fan, Lue-Wei Xu, De-Gang Liu, and Guo-Zuan Xu. 2022. "Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Solution System" Minerals 12, no. 6: 732. https://doi.org/10.3390/min12060732
APA StyleHe, B.-X., Liang, Y., Fan, Z.-K., Xu, L.-W., Liu, D.-G., & Xu, G.-Z. (2022). Dissolution Behavior of Sodium Phosphate in a Na3PO4–Na2WO4–NaOH Solution System. Minerals, 12(6), 732. https://doi.org/10.3390/min12060732




