Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
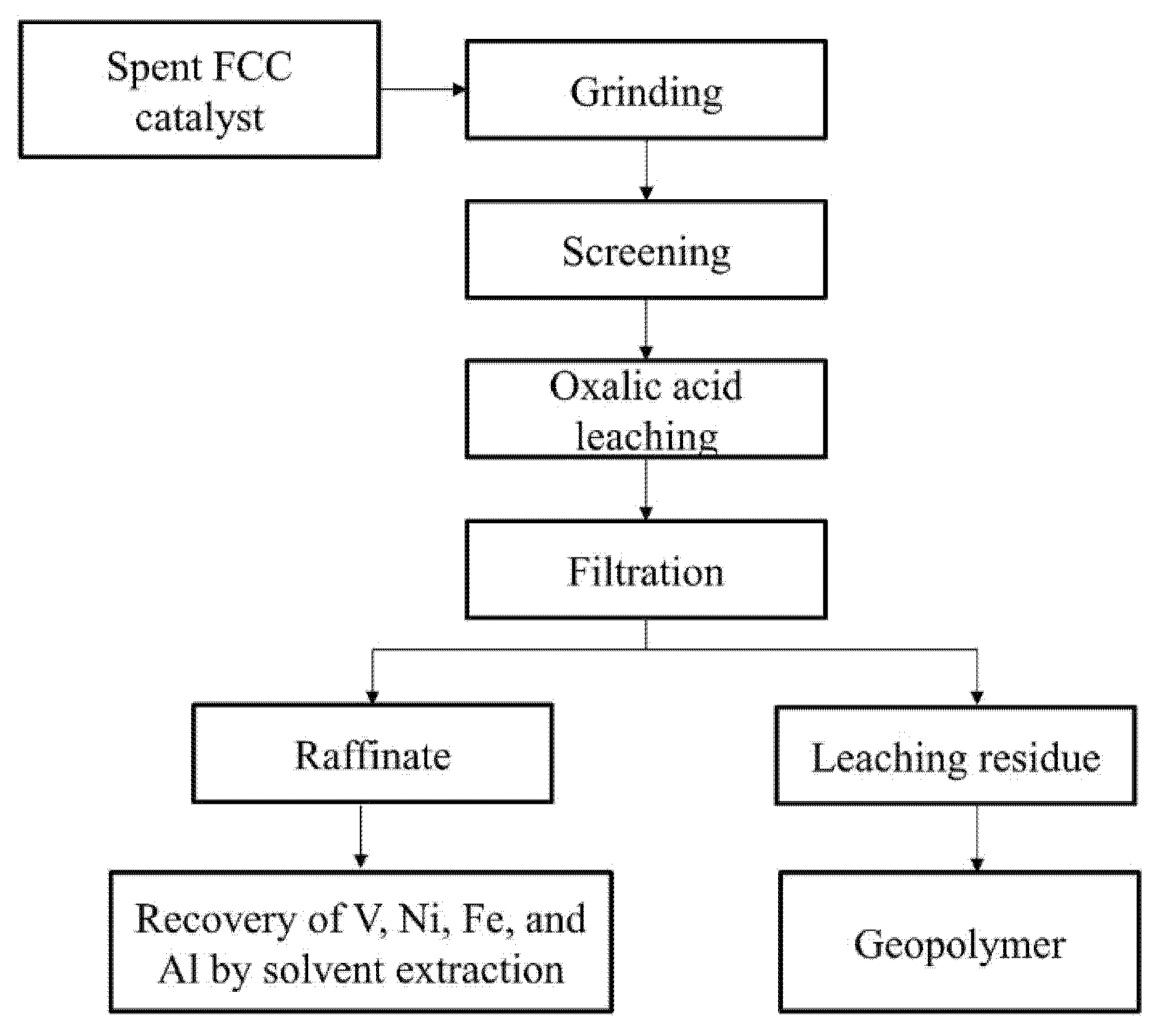
2.2. Experimental Procedure
2.3. Characterization
3. Results
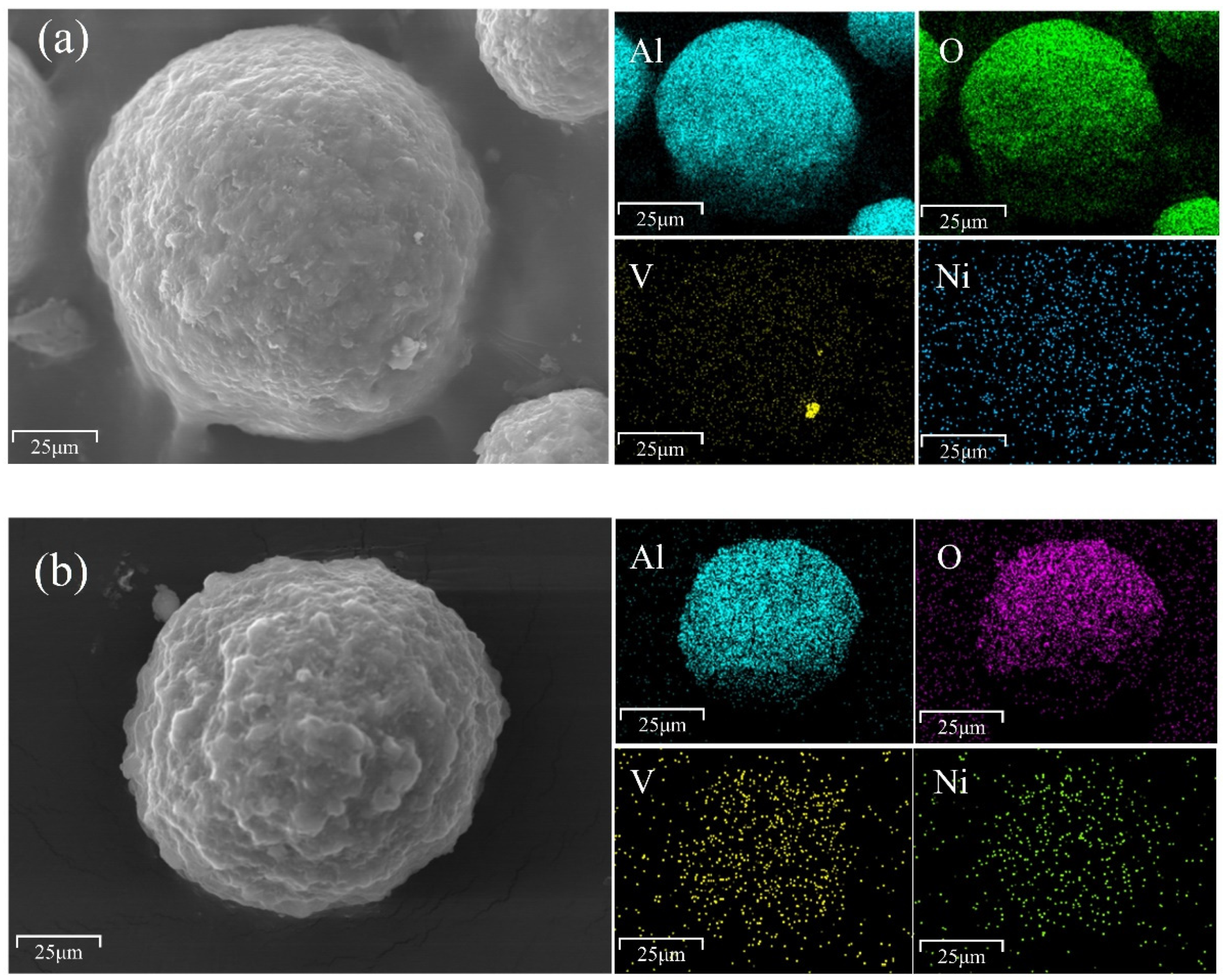
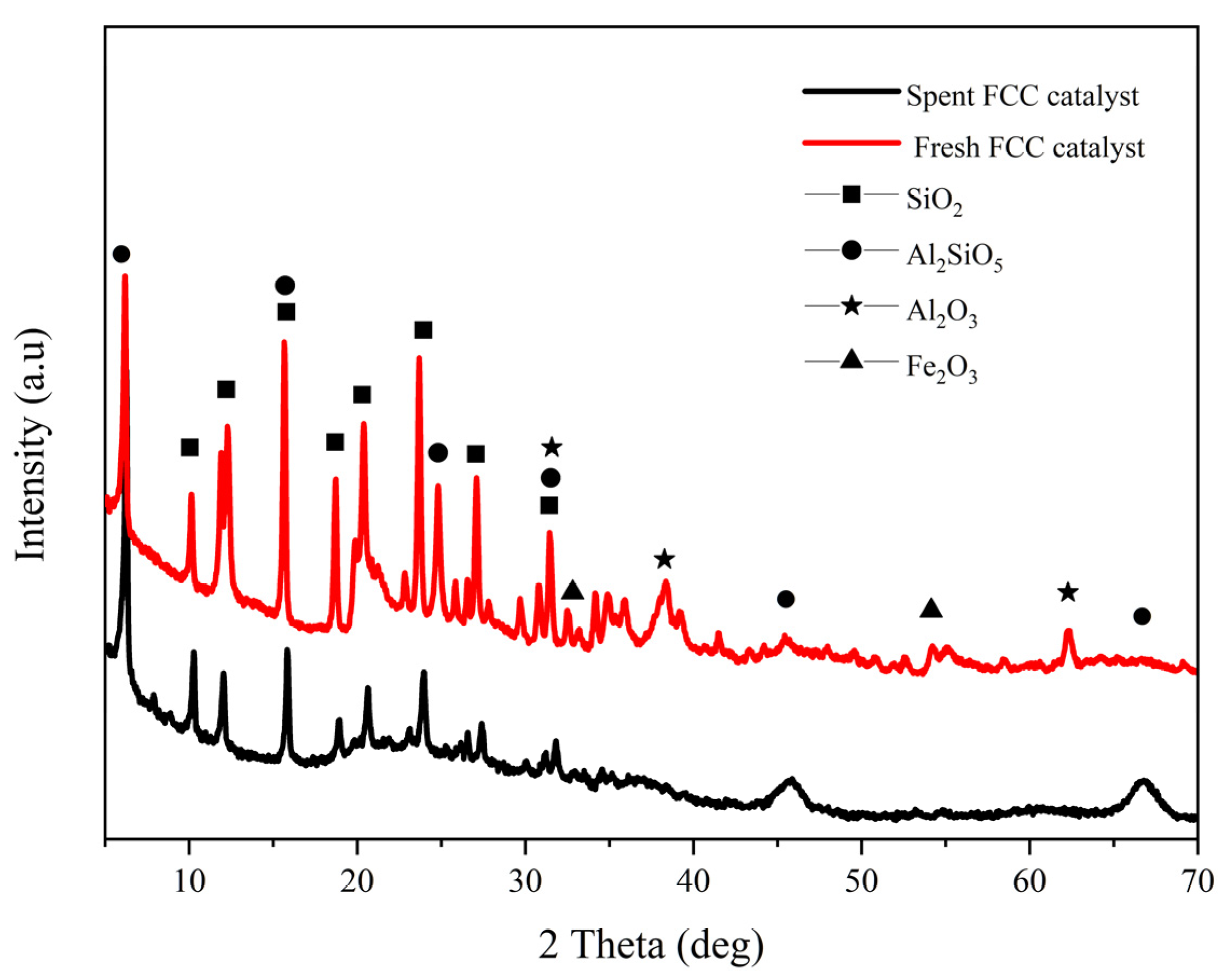
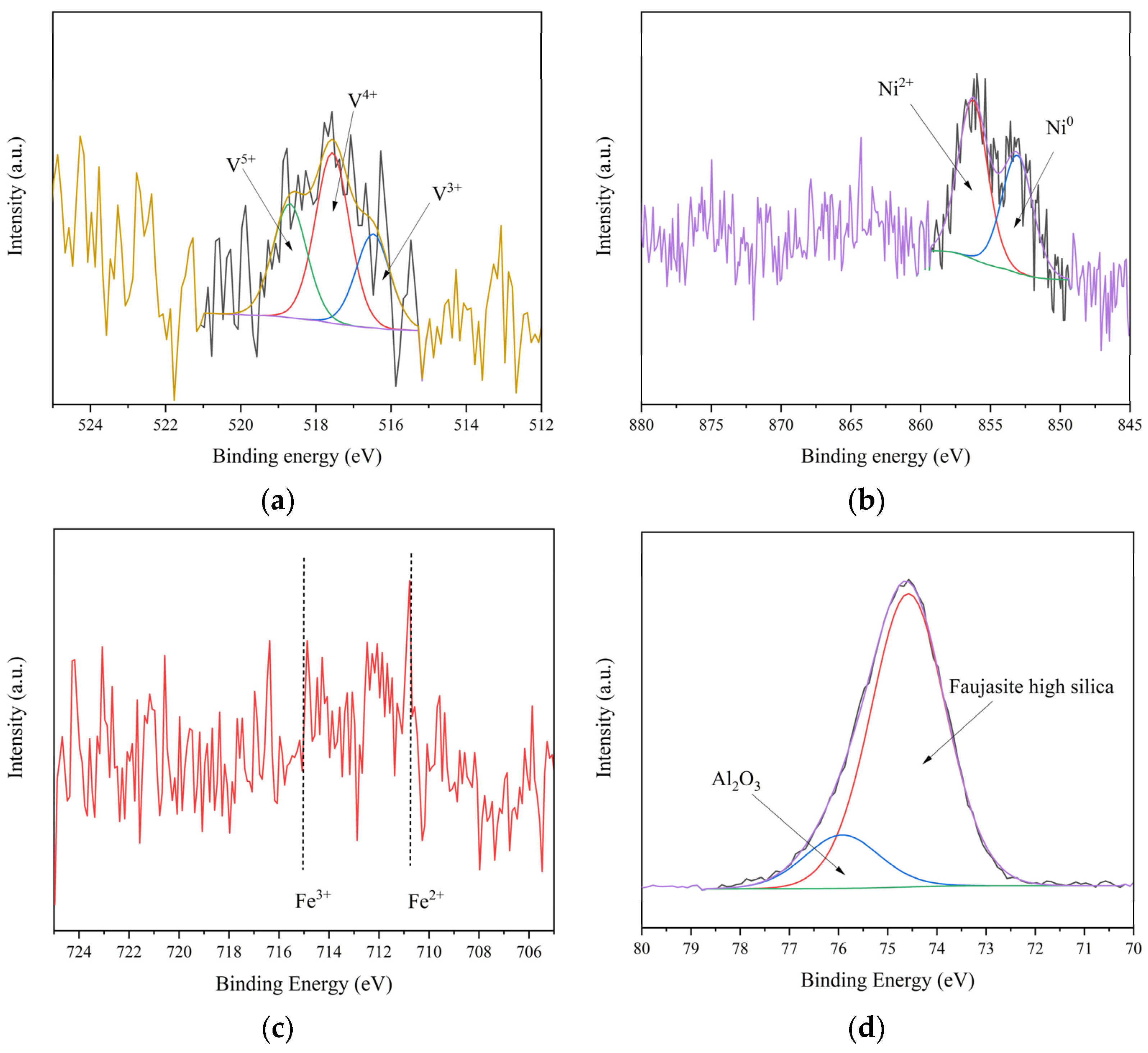
3.1. Analysis of Catalyst Samples
3.1.1. Catalyst Composition
3.1.2. Physical and Chemical Properties of Sample Catalyst
3.2. Leaching Test
3.2.1. Effects of Particle Size
3.2.2. Effects of Leaching Time
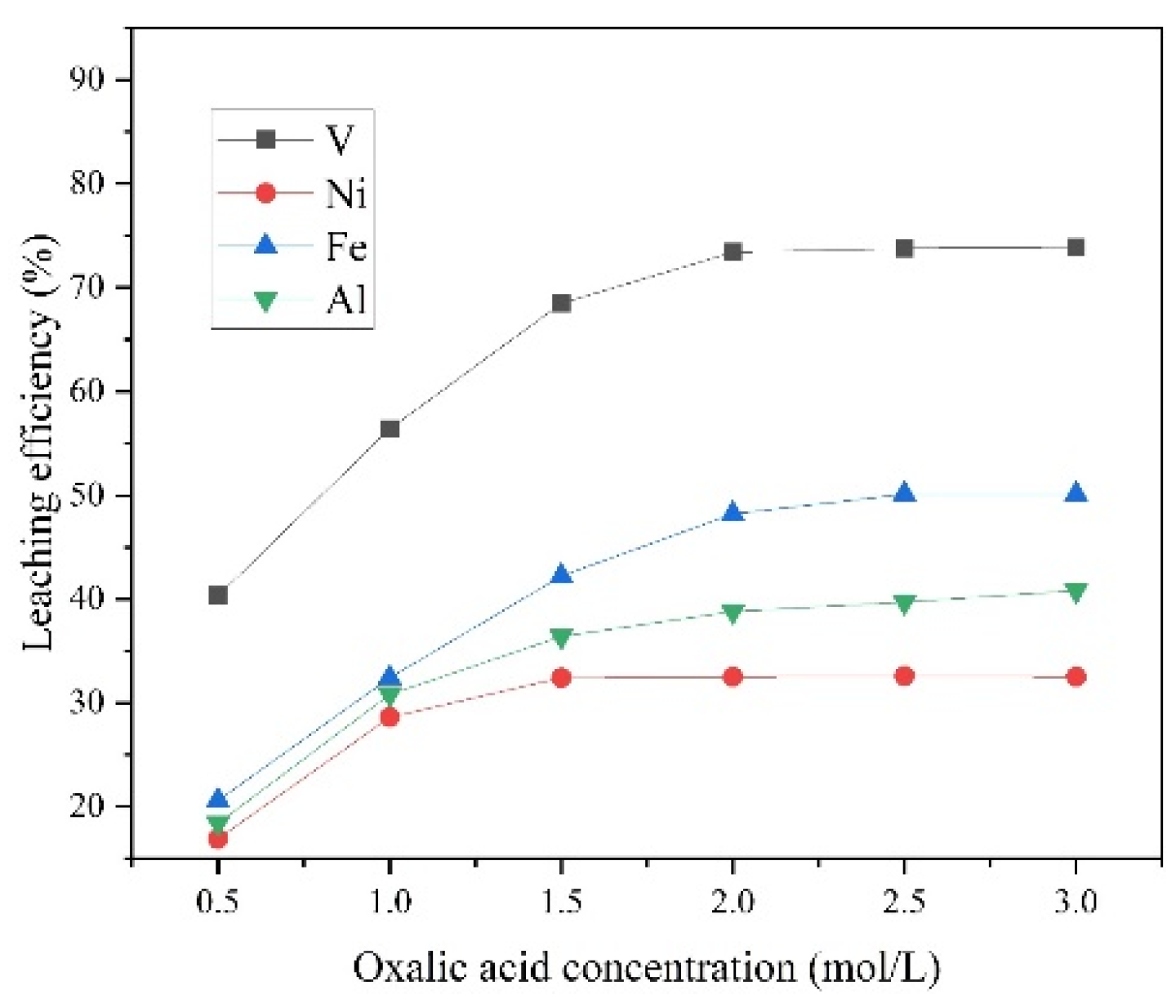
3.2.3. Effects of Oxalic Acid Concentration
3.2.4. Effects of Leaching Temperature
4. Discussion
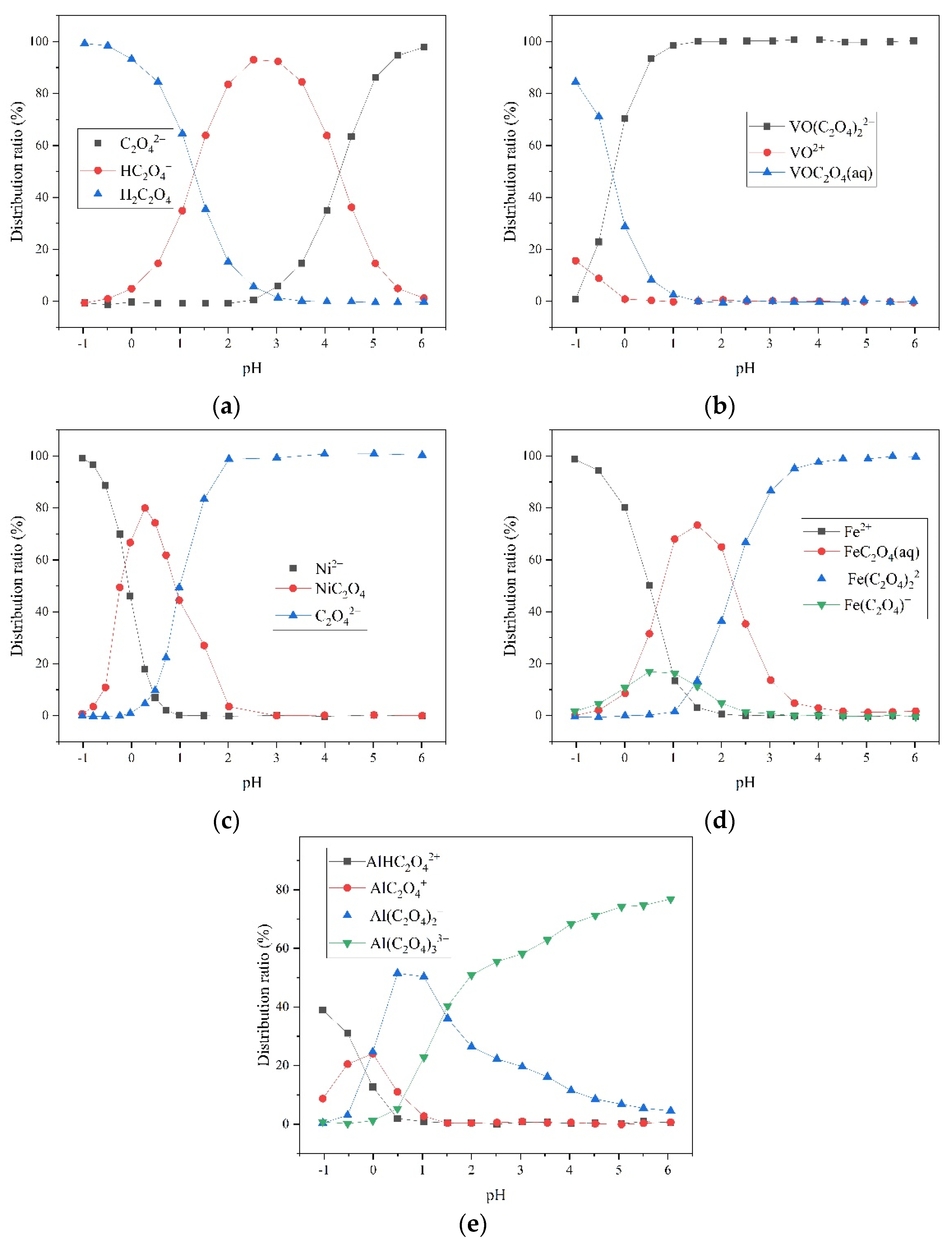
4.1. Oxalic-Acid-Based SFCC Leachate
4.2. Preparation of Geopolymer from Leaching Residue
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal Recovery Using Oxalate Chemistry: A Technical Review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential techno-economic challenges: A review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1–43. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Du, H.; Olayiwola, A.; Liu, B.; Gao, F.; Jia, M.-L.; Wang, M.-H.; Gao, M.-L.; Wang, X.-D.; Wang, S.-N. Recent advances in the recovery of transition metals from spent hydrodesulfurization catalysts. Tungsten 2021, 3, 305–328. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Etim, U.; Bai, P.; Liu, X.; Subhan, F.; Ullah, R.; Yan, Z. Vanadium and nickel deposition on FCC catalyst: Influence of residual catalyst acidity on catalytic products. Microporous Mesoporous Mater. 2018, 273, 276–285. [Google Scholar] [CrossRef]
- Ferella, F. A review on management and recycling of spent selective catalytic reduction catalysts. J. Clean. Prod. 2019, 246, 118990. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Yang, M.-H. Chemical catalysed recycling of polypropylene over a spent FCC catalyst and various commercial cracking catalysts using TGA. Thermochim. Acta 2008, 470, 52–59. [Google Scholar] [CrossRef]
- Alonso-Fariñas, B.; Rodríguez-Galán, M.; Arenas, C.; Torralvo, F.A.; Leiva, C. Sustainable management of spent fluid catalytic cracking catalyst from a circular economy approach. Waste Manag. 2020, 110, 10–19. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Bedelova, Z.; Anarbekov, K.; Baikonurova, A.; Tuncuk, A. Recovery of vanadium from spent catalysts of sulfuric acid plant by using inorganic and organic acids: Laboratory and semi-pilot tests. Waste Manag. 2016, 49, 455–461. [Google Scholar] [CrossRef]
- Pospiech, B.; Warzecha, M. Application of oxalic acid as an efficient leaching agent of aluminum from industrial waste. Physicochem. Probl. Miner. Process. 2020, 56, 264–270. [Google Scholar]
- Mazurek, K. Recovery of vanadium, potassium and iron from a spent vanadium catalyst by oxalic acid solution leaching, precipitation and ion exchange processes. Hydrometallurgy 2013, 134–135, 26–31. [Google Scholar] [CrossRef]
- Le, M.N.; Lee, M.S. Selective dissolution of vanadium(V) from spent petroleum catalysts by oxalic acid solution. J. Min. Met. Sect. B Met. 2020, 56, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Muddanna, M.H.; Baral, S.S. A comparative study of the extraction of metals from the spent fluid catalytic cracking catalyst using chemical leaching and bioleaching by Aspergillus niger. J. Environ. Chem. Eng. 2019, 7, 103335. [Google Scholar] [CrossRef]
- Etim, U.J.; Wu, P.; Bai, P.; Xing, W.; Ullah, R.; Subhan, F.; Yan, Z. Location and Surface Species of Fluid Catalytic Cracking Catalyst Contaminants: Implications for Alleviating Catalyst Deactivation. Energy Fuels 2016, 30, 10371–10382. [Google Scholar] [CrossRef]
- Bai, P.; Etim, U.J.; Yan, Z.; Mintova, S.; Zhang, Z.; Zhong, Z.; Gao, X. Fluid catalytic cracking technology: Current status and recent discoveries on catalyst contamination. Catal. Rev. 2018, 61, 333–405. [Google Scholar] [CrossRef]
- Etim, U.J.; Xu, B.; Bai, P.; Ullah, R.; Subhan, F.; Yan, Z. Role of nickel on vanadium poisoned FCC catalyst: A study of physiochemical properties. J. Energy Chem. 2016, 25, 667–676. [Google Scholar] [CrossRef]
- Zhou, Q.; Qi, Y.; Liu, Q.; Ren, F.; Chen, Z.; Zhu, Y.; Etschmann, B.; Zhang, L. A detailed speciation of iron on FCC catalysts based on an integrated use of advanced characterisation methods and thermodynamic equilibrium simulation. Appl. Catal. A Gen. 2020, 599, 117597. [Google Scholar] [CrossRef]
- Amaya, Á.A.; González, C.A.; Martínez, O.F. The behavior of the surface Si/(Si+Al) ratio in the FCC catalyst deactivation. J. Electron. Spectrosc. Relat. Phenom. 2020, 239, 146932. [Google Scholar] [CrossRef]
- Tavakoli, M.; Dreisinger, D. The kinetics of oxidative leaching of vanadium trioxide. Hydrometallurgy 2014, 147–148, 83–89. [Google Scholar] [CrossRef]
- Wu, W.; Wang, C.; Bao, W.; Li, H. Selective reduction leaching of vanadium and iron by oxalic acid from spent V2O5-WO3/TiO2 catalyst. Hydrometallurgy 2018, 179, 52–59. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Liu, T.; Huang, J.; Yuan, Y.; Xue, N. Source separation of vanadium over iron from roasted vanadium-bearing shale during acid leaching via ferric fluoride surface coating. J. Clean. Prod. 2018, 181, 399–407. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Studies on rejuvenation of spent residue hydroprocessing catalysts by leaching of metal foulants. J. Mol. Catal. A Chem. 2003, 202, 117–125. [Google Scholar] [CrossRef]
- ISO 6353/1-1982; Regents for Chemical Analysis—Part 1: General Test Methods. International Organization for Standardization: Geneva, Switzerland, 1982.
- Alsheeha, H.; Marafi, M.; Raghavan, V.; Rana, M.S. Recycling and Recovery Routes for Spent Hydroprocessing Catalyst Waste. Ind. Eng. Chem. Res. 2013, 52, 12794–12801. [Google Scholar] [CrossRef]
- Szymczycha-Madeja, A. Kinetics of Mo, Ni, V and Al leaching from a spent hydrodesulphurization catalyst in a solution containing oxalic acid and hydrogen peroxide. J. Hazard Mater. 2011, 186, 2157–2161. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M. V and Ni recovery from a vanadium-rich power plant residual ash using acid producing fungi: Aspergillus niger and Penicillium simplicissimum. RSC Adv. 2016, 6, 9139–9151. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Zheng, Q. Separation and recovery of vanadium and iron from oxalic-acid-based shale leachate by coextraction and stepwise stripping. Sep. Purif. Technol. 2020, 244, 58–69. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Luo, D. Separation and recovery of vanadium and aluminum from oxalic acid leachate of shale by solvent extraction with Aliquat 336. Sep. Purif. Technol. 2020, 249, 116867. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Zhang, Y.; Liu, T.; Hu, P.; Liu, H.; Zheng, Q. Separation and recovery of iron impurities from a complex oxalic acid solution containing vanadium by K3Fe(C2O4)3·3H2O crystallization. Sep. Purif. Technol. 2019, 232, 115970. [Google Scholar] [CrossRef]
- Wei, B.; Zhang, Y.; Bao, S. Preparation of geopolymers from vanadium tailings by mechanical activation. Constr. Build. Mater. 2017, 145, 236–242. [Google Scholar] [CrossRef]
- Meng, R.; Liu, T.; Zhang, Y.; Huang, J.; Yuan, Y.; Hu, P. Synchronous activation of Si and Al in vanadium-bearing shale leaching residue via sodium carbonate additive. Constr. Build. Mater. 2018, 170, 20–25. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Liu, T.; Wan, Q.; Zheng, D. Immobilization of vanadium and nickel in spent fluid catalytic cracking (SFCC) catalysts-based geopolymer. J. Clean. Prod. 2021, 332, 130112. [Google Scholar] [CrossRef]



| Sample | Constituents (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Fe | Ca | K | V | Ni | La | Ce | |
| Fresh | 32.22 | 16.09 | 0.19 | 0.16 | 0.13 | 0.01 | 0.01 | 1.95 | 0.01 |
| Spent | 29.26 | 17.59 | 0.48 | 0.40 | 0.16 | 0.44 | 0.53 | 1.67 | 0.68 |
| Constituent (wt.%) | Al | Si | Fe | Ca | K | V | Ni | La | Ce |
|---|---|---|---|---|---|---|---|---|---|
| Leaching residue | 14.60 | 23.76 | 0.03 | 0.55 | 0.01 | 0.06 | 0.03 | 0.23 | 0.31 |
| Element | Metal Concentration in Extraction Fluid (mg/L) | |||
|---|---|---|---|---|
| Spent FCC | Leaching Residue | Regulatory Levels (U.S. EPA) | Regulatory Levels (National Hazardous Waste List, China) | |
| Al | 1.45 ± 0.07 | 0.91 ± 0.01 | — | — |
| Fe | 0.24 ± 0.03 | 0.14 ± 0.02 | — | — |
| V | 7.13 ± 0.15 | 0.33 ± 0.02 | 1.6 | 3.6 |
| Ni | 2.45 ± 0.02 | 0.21 ± 0.01 | 11 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Zhang, Y.; Liu, T.; Huang, J.; Cai, Z.; Zhang, R. Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid. Minerals 2022, 12, 748. https://doi.org/10.3390/min12060748
Zheng D, Zhang Y, Liu T, Huang J, Cai Z, Zhang R. Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid. Minerals. 2022; 12(6):748. https://doi.org/10.3390/min12060748
Chicago/Turabian StyleZheng, Dalong, Yimin Zhang, Tao Liu, Jing Huang, Zhenlei Cai, and Ruobing Zhang. 2022. "Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid" Minerals 12, no. 6: 748. https://doi.org/10.3390/min12060748
APA StyleZheng, D., Zhang, Y., Liu, T., Huang, J., Cai, Z., & Zhang, R. (2022). Selective Leaching of Valuable Metals from Spent Fluid Catalytic Cracking Catalyst with Oxalic Acid. Minerals, 12(6), 748. https://doi.org/10.3390/min12060748





