The Gold–Palladium Ozernoe Occurrence (Polar Urals, Russia): Mineralogy, Conditions of Formation, Sources of Ore Matter and Fluid
Abstract
:1. Introduction
2. Brief Description of the Study Area
3. Samples and Research Methods
4. Results
4.1. Mineralogy, Mineral Association, and Sequence of Mineral Formation at the Ozernoe Occurrence
4.1.1. Primary (Early) Rock-Forming Minerals of Clinopyroxenites
- Clinopyroxene
- Primary clinopyroxene with grain sizes from 0.1 to 10 mm in the samples of all groups of clinopyroxenites belongs to the diopside-hedenbergite with a varying ferrosilite mineral (Figure 3a). In clinopyroxenites I and II, it corresponds to diopside with an elevated content of FeO (4.47–7.04 wt.%) and Al2O3 (0.79–2.74 wt.%) (Table 2). It contains minor Ti, Mn, Cr, and Na in amounts less than 1 wt.%. Clinopyroxene in clinopyroxenite III is also represented by diopside but with lower contents of FeO (0.66–2.17 wt.%), Al2O3 (less than 1.7 wt.%), and other trace elements. This clinopyroxene also contains the thinnest plates of iron oxide, supposedly magnetite, which develop along cleavage cracks (Figure 4a). These plates seem to be the result of the decomposition of the solid solution or oxidation of Fe2+.
- In spite of considerable variations in the composition of primary clinopyroxene, clinopyroxenites I–III have a direct correlation with the contents of FeO and Al2O3 (Figure 3b). An inverse correlation dependence was revealed only for clinopyroxene grains with the highest iron content (Figure 3b, sign 4) (Table 2, No. 9–12). We attributed this clinopyroxene to a later generation. Along with sulfides and chlorite, it fills interstices in the aggregates of early primary pyroxene grains (Figure 4b).
| No | Sample # | SiO2 | TiO2 | Al2O3 | Cr2O3 | FeO * | MnO | MgO | CaO | Na2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1545-2-3 | 52.68 | 0.14 | 1.36 | b.d. | 5.44 | 0.25 | 14.78 | 25.17 | 0.14 | 99.96 |
| 2 | 1545-2-12 | 52.13 | 0.16 | 1.78 | b.d. | 6.2 | 0.2 | 14.44 | 24.73 | 0.06 | 99.70 |
| 3 | 1545-2-14 | 51.02 | 0.14 | 1.67 | 0.12 | 6.11 | 0.21 | 14.43 | 23.87 | 0.09 | 97.66 |
| 4 | 1545-2-18 | 52.83 | 0.04 | 1.1 | 0.02 | 4.47 | 0.16 | 15.36 | 25.6 | 0.07 | 99.65 |
| 5 | 1545-1-35 | 52.31 | 0.15 | 2.1 | 0.06 | 7.04 | 0.11 | 14.37 | 23.73 | 0.06 | 99.93 |
| 6 | 1545-1-45 | 51.85 | 0.1 | 1.83 | b.d. | 6.45 | 0.16 | 14.11 | 23.88 | 0.16 | 98.54 |
| 7 | 1546-3-8 | 53.4 | 0.1 | 0.87 | 0.13 | 5.34 | 0.16 | 14.29 | 25.16 | 0.11 | 99.56 |
| 8 | 1546-3-13 | 53.45 | 0.11 | 1.45 | b.d. | 5.84 | 0.26 | 14.44 | 24.46 | 0.05 | 100.06 |
| 9 | 1546-3-5 | 52.1 | 0.01 | b.d. | 0.01 | 13.77 | 1.25 | 9.21 | 24.31 | 0.03 | 100.69 |
| 10 | 1546-3-7 | 52.08 | 0.04 | 0.08 | 0.04 | 14.51 | 0.62 | 8.78 | 24.16 | 0.04 | 100.35 |
| 11 | 1546-3-6 | 52.91 | 0.01 | 0.31 | 0.09 | 9.56 | 0.26 | 11.92 | 24.58 | 0.17 | 99.81 |
| 12 | 1545-1-41 | 52.59 | 0.09 | 0.36 | 0.03 | 9.67 | 0.32 | 11.98 | 23.36 | 1.16 | 99.56 |
| 13 | 101013-24 | 51.74 | 0.18 | 2.74 | 0.12 | 6.77 | 0.22 | 14.8 | 21.97 | 0.34 | 98.88 |
| 14 | 101013-27 | 53.45 | 0.09 | 1.36 | 0.04 | 6.02 | 0.15 | 15.41 | 23.4 | 0.08 | 100.00 |
| 15 | 101013-33 | 54.27 | 0.02 | 0.79 | b.d. | 4.98 | 0.12 | 15.2 | 24.79 | 0.04 | 100.21 |
| 16 | 101012-39 | 53.34 | 0.16 | 2.1 | 0.07 | 5.98 | 0.11 | 15.07 | 23.52 | 0.1 | 100.45 |
| 17 | 101012-48 | 51.6 | 0.27 | 2.4 | 0.1 | 6.77 | 0.16 | 14.2 | 22.67 | 0.1 | 98.27 |
| 18 | 101012-57 | 52.4 | 0.09 | 1.81 | 0.01 | 5.42 | 0.25 | 14.72 | 23.38 | 0.09 | 98.17 |
| 19 | 211015-1_3 | 55.5 | b.d. | b.d. | b.d. | 1.04 | b.d. | 18.68 | 25.57 | b.d. | 100.79 |
| 20 | 211015-2_6 | 55.21 | b.d. | b.d. | b.d. | 0.66 | b.d. | 18.35 | 25.86 | b.d. | 100.08 |
| 21 | 211015-3_3 | 54.85 | b.d. | 0.32 | b.d. | 0.74 | b.d. | 19.19 | 24.48 | b.d. | 99.58 |
| 22 | 211015-4_1 | 54.31 | b.d. | 0.46 | b.d. | 0.93 | b.d. | 18.61 | 25.62 | b.d. | 99.93 |
| 23 | 211015-6_3 | 54.05 | b.d. | 1.56 | b.d. | 2.17 | b.d. | 17.61 | 24.47 | b.d. | 99.86 |
| 24 | 211014-2_2 | 55.17 | b.d. | b.d. | b.d. | 1.11 | b.d. | 18 | 26.15 | b.d. | 100.43 |
| 25 | 211014-3_4 | 55.3 | b.d. | b.d. | b.d. | 0.85 | b.d. | 18.23 | 26.02 | b.d. | 100.4 |
- 2.
- Olivine
- Magnetite
4.1.2. Secondary (Late) Minerals in Clinopyroxenites
- Secondary clinopyroxene
- Amphibole
- Chlorite
- Serpentine
- Secondary magnetite
- Sulfides
- Noble metal minerals
4.2. Sulfur Isotopic Composition of Sulfides
5. Discussion
5.1. Sequence of Mineral Formation at Ozernoe Occurrence
5.2. Specific Features of the Evolution of Mineral Formation in Clinopyroxenites I–III
5.3. Association of Noble Metal Minerals at Ozernoe Occurrence
5.4. Features of Native Gold Composition
5.5. Reconstruction of Physico-Chemical Parameters of Ore Formation
5.6. Model of Formation of Gold–Palladium Mineralization at Ozernoe Occurrence
6. Conclusions
- (1)
- The primary magmatic layering of rocks from the Dzelyatyshor massif, manifested in different quantitative ratios of clinopyroxene and olivine in them, controls the local trends of the variability in the chemistry of the mineral-forming medium and the concentrations of ore components, including noble metals, and sulfur in each separate layer on cooling.
- (2)
- The deposition of native gold in parageneses with PGM and Cu-Fe sulfides at the Ozernoe occurrence proceeded during the replacement of earlier rock-forming minerals by chlorite. This process terminated mineral formation at the deposit and occurred at temperatures 150–250 °C and high activity of S, Te, Sb, and As of the fluid. The calculated variability in the conditions of mineral deposition during chloritization is reflected in the presence of several sulfide parageneses, in the replacement of native-sulfide forms by arsenide–antominide forms, in the considerable variability in the iron content of chlorite (XFe = 0.04–0.43), and in the sulfur isotopic composition of sulfides.
- (3)
- Palladium content in native gold increases from Au-Ag solid solution (<1.5 wt.% Pd) to Au-Cu intermetallides—Au-Cu solid solution (to 6 wt.% Pd) and Cu,Pd,Au solid solutions (16.2–16.9 wt.% Pd)
- (4)
- The sulfur isotopic composition of pyrite, chalcopyrite, and bornite (ẟ34S = −2.1…−2.9‰) just a little lighter relative to the zero mark of deep-seated magmatic sulfur. The deep-seated magmatic basic melt is assumed to be the source of fluid, ore components, and sulfur.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoloev, K.K.; Volchenko, Y.A.; Koroteev, V.A.; Malakhov, I.A.; Mardirosyan, A.N.; Khrypov, V.N. Platinum-Metal Mineralization in the Geological Complexes of the Urals; Department of Natural Resources for the Ural Region: Yekaterinburg, Russia, 2001; 199p. (In Russian) [Google Scholar]
- Maier, W.D.; Barnes, S.-J.; Gartz, V.; Andrews, G. Pt-Pd reefs in magnetitites of the Stella layered intrusion, South Africa: A world of new exploration opportunities for platinum group elements. Geology 2003, 31, 885–888. [Google Scholar] [CrossRef]
- Sluzhenikin, S.F.; Yudovskaya, M.A.; Barnes, S.J.; Abramova, V.D.; Le Vaillant, M.; Petrenko, D.B.; Brovchenko, V.D. Low-sulfide platinum group element ores of the Norilsk-Talnakh camp. Econ. Geol. 2020, 115, 1267–1303. [Google Scholar] [CrossRef]
- Mansur, E.; Barnes, S.-J.; Ferreira Filno, C.F. The effects of post-cumulus alteration on the distribution of chalcophile elements in magmatic sulfide deposits and implications for the formation of low-S-high-PGE zones: The Luanga deposit, Carajas mineral province, Brazil. Can. Mineral. 2021, 59, 1453–1484. [Google Scholar] [CrossRef]
- Zaccarini, F.; Anikina, E.; Pushkarev, E.; Rusin, I.; Garuti, G. Palladium and gold minerals from the Baronskoe-Kluevsky ore deposit (Volkovsky complex, Central Urals, Russia) Mineral. Petrol. 2004, 82, 137–156. [Google Scholar] [CrossRef]
- Anikina, E.V.; Malitch, K.N.; Pushkarev, E.V.; Shmelev, V.R. The Nizhny Tagil and Volkovsky massifs of the Uralian Platinum Belt, and related deposits. In Field Trip Guidebook. 12th International Platinum Symposium; IGG UB RAS: Yekaterinburg, Russia, 2014; 48p. [Google Scholar]
- Anikina, E.V.; Alekseev, A.V. Mineral-geochemical characteristic of gold-palladium mineralization in the Volkovsky gabbro massif (Platiniferous Urals Belt). Litosphere 2010, 5, 75–100. (In Russian) [Google Scholar]
- Kuznetsov, S.K.; Kotelnikov, V.G.; Onishchenko, S.A.; Filippov, V.N. Copper-gold-palladium mineralization in ultramafic rocks of the Voikaro-Synya massif in the Polar Urals. Bull. Inst. Geol. Komi Sci. Cent. Ural. Branch Russ. Acad. Sci. 2004, 5, 2–4. (In Russian) [Google Scholar]
- Kuznetsov, S.K.; Filippov, V.N.; Onishchenko, S.A.; Kotel'nikov, V.G. Copper-gold-palladium mineralization in ultrabasic rocks of the Polar Urals. Dokl. Earth Sci. 2007, 414, 501–503. [Google Scholar] [CrossRef]
- Pystin, A.M.; Potapov, I.L.; Pystina, Y.I.; Generalov, V.I.; Onishchenko, S.A.; Filippov, V.N.; Shloma, A.A.; Tereshko, V.V. Low-Sulfide Platinum-Metal Mineralization in the Polar Urals; Ural Branch of the Russian Academy of Sciences: Yekaterinburg, Russia, 2011; 150p. (In Russian) [Google Scholar]
- Pystin, A.M.; Potapov, I.L.; Pystina, Y.I. The low-sulfide gold-platinum ore occurrence at the Polar Urals. Zap. RMO 2012, 141, 60–63, (In Russian with English abstract). [Google Scholar]
- Remizov, D.N.; Petrov, S.Y.; Kos'yanov, A.O.; Nosikov, M.V.; Sergeev, S.A.; Grigoriev, S.I. New age datings of gabbroides of the kershor complex (Polar Urals). Dokl. Earth Sci. 2010, 434, 1235–1239. [Google Scholar] [CrossRef]
- Puchkov, V.N. General features relating to the occurrence of mineral deposits in the Urals: What, where, when and why. Ore Geol. Rev. 2017, 85, 4–29. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Ignatiev, A.V.; Velivetskaya, T.A.; Budnitskiy, S.Y.; Yakovenko, V.V.; Vysotskiy, S.V.; Levitskii, V.I. Precision analysis of multisulfur isotopes in sulfides by femtosecond laser ablation GC-IRMS at high spatial resolution. Chem. Geol. 2018, 493, 316–326. [Google Scholar] [CrossRef]
- Velivetskaya, T.A.; Ignatiev, A.V.; Yakovenko, V.V.; Vysotskiy, S.V. An improved femtosecond laser-ablation fluorination method for measurements of sulfur isotopic anomalies (∆33S and ∆36S) in sulfides with high precision. Rapid Commun. Mass Spectrom. 2019, 33, 1722–1729. [Google Scholar] [CrossRef]
- Leake, B.E.; Woolley, A.R.; Birch, W.D.; Burke, E.A.J.; Ferraris, G.; Grice, J.D.; Hawthorne, F.C.; Kisch, H.J.; Krivovichev, V.G.; Schumacher, J.C.; et al. Nomenclature of amphiboles: Additions and revisions to the International Mineralogical Association’s amphibole nomenclature. Am. Miner. 2004, 89, 883–887. [Google Scholar]
- Morimoto, N.C. Nomenclature of pyroxenes. Am. Miner. 1988, 73, 1123–1133. [Google Scholar]
- Cathelineau, M. Cation site occupancy in chlorites and illites as a function of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Hey, M.H. A new review of chlorites. Miner. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Fershtater, G.B.; Pushkarev, E.V. Magmatic clinopyroxenites of the Urals and their evolution. Proc. Acad. Sci. USSR Ser. Geol. 1987, 3, 13–23. [Google Scholar]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Smagunov, N.; Tauson, V.; Lipko, S.; Babkin, D.; Pastushkova, T.; Belozerova, O.; Bryansky, N. Partitioning and Surficial Segregation of Trace Elements in Iron Oxides in Hydrothermal Fluid Systems. Minerals 2021, 11, 57. [Google Scholar] [CrossRef]
- Fleet, M.E. Phase equilibria at high temperatures. Rev. Mineral. Geochem. 2006, 61, 365–419. [Google Scholar] [CrossRef]
- Palyanova, G.A.; Sazonov, A.M.; Zhuravkova, T.V.; Silyanov, S.A. Composition of pyrrhotite as an indicator of gold ore formation conditions at the Sovetskoe deposit (Yenisei Ridge, Russia). Russ. Geol. Geophys. 2019, 60, 735–751. [Google Scholar] [CrossRef]
- Sorokhtina, N.V.; Zaitsev, V.A.; Petrov, S.V.; Kononkova, N.N. Estimation of formation temperature of the noble metal mineralization of the Kovdor alkaline-ultrabasic massif (Kola Peninsula). Geochem. Int. 2021, 59, 474–490. [Google Scholar] [CrossRef]
- Lambert, J.M.; Simkovich, J.R.; Walker, P.L. The kinetics and mechanism of the pyrite-to-pyrrhotite transformation. Metall. Mater. Trans. B 1998, 29B, 385–396. [Google Scholar] [CrossRef]
- Chareev, D.A.; Osadchii, E.G. Pyrrhotite-pyrite equilibria in the Ag–Fe–S system et 245 to 310 °C and standard pressure. Geochem. Miner. Petrol. Bulgar. Acad. Sci. 2005, 43, 41–46. [Google Scholar]
- Sinyakova, E.; Karmanov, N.; Kosyakov, V.; Distler, V. Behavior of Pt, Pd, and Au during crystallization of Cu-rich magmatic sulfide minerals. Can. Mineral. 2016, 54, 491–509. [Google Scholar] [CrossRef]
- Sinyakova, E.F.; Borisenko, A.S.; Kosyakov, V.I. Effect of the presence of As, Bi and Te on the behavior of Pt metals during fractionation crystallization of sulfide magma. Dokl. Earth Sci. 2017, 477, 1422–1425. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the platinum group elements with applications to ore deposits. In Geology, Geochemistry, Mineralogy and Mineral Benefication of Platinum Group Element; Cabri, L., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum: Ottawa, ON, Canada, 2002; Special Volume 54, pp. 211–249. [Google Scholar]
- Holwell, D.; Adeyemi, Z.; Ward, L.A.; Smith, D.J.; Graham, S.D.; Mcdonald, I.; Smith, J.W. Low temperature alteration of magmatic Ni-Cu-PGE sulfides as a source for hydrothermal Ni and PGE ores: A quantitative approach using automated mineralogy. Ore Geol. Rev. 2017, 91, 718–740. [Google Scholar] [CrossRef] [Green Version]
- Poltavetc, Y.A.; Poltavetc, Z.I.; Nechkin, G.S. Volkovsky deposit of titanomagnetite and copper-titanomagnetite ores with accompanying noble-metal mineralization, the Central Urals, Russia). Geol. Ore Depos. 2011, 53, 126–139. [Google Scholar] [CrossRef]
- Murzin, V.V.; Palyanova, G.A.; Anikina, E.V.; Moloshag, V.P. Mineralogy of noble metals (Au, Ag, Pd, Pt) in Volkovskoe Cu-Fe-Ti-V deposit (Middle Urals, Russia). Lithosphere 2021, 21, 643–659. [Google Scholar] [CrossRef]
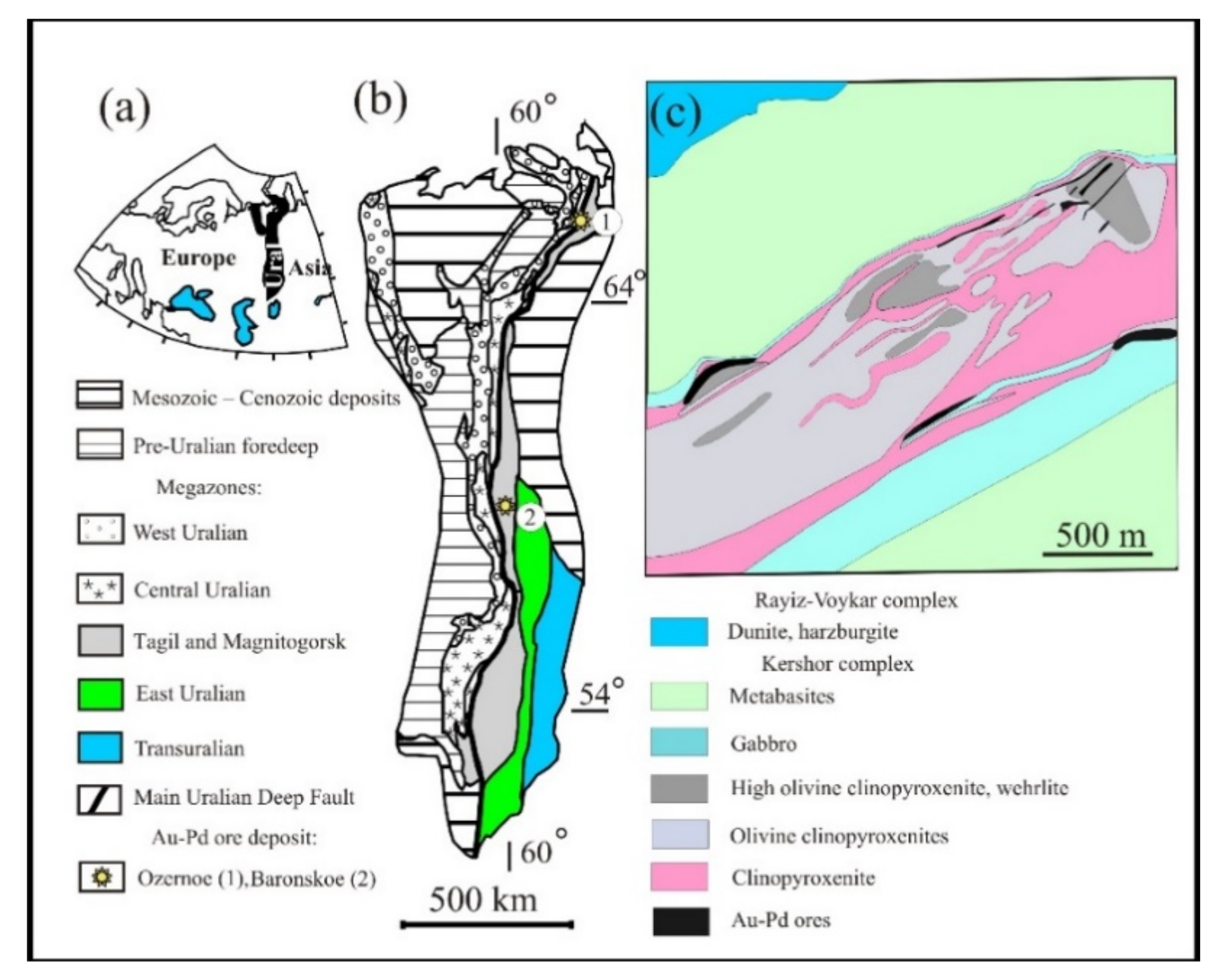













| Samples of Groups | No Sample | Trench Exploration Nor | Olivine Content, Vol.% | Sulfide Content, Vol.% | Sulfide Parageneses |
|---|---|---|---|---|---|
| clinopyroxenite I | 1545, 1546 | K-108, 126 | <5 | <0.1 | Py >> Pyh > Ccp |
| clinopyroxenite II | 101012, 101013 | K-101 | 5–10 | 0.1–0.5 | Bn-Ccp |
| clinopyroxenite III | 211014, 211015 | P2110, K-104 | >10–15 | <10 | Pyh > Ccp > Py |
| Clinopyroxenite I | II | III | |||||
|---|---|---|---|---|---|---|---|
| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Sample # | 1545-2-7 | 1545-2-30 | 101012-47 | 101013-26 | 211015/6 | 211015/8 | 211014/4 |
| SiO2 | 38.34 | 37.97 | 38.06 | 37.79 | 40.07 | 40.25 | 39.96 |
| TiO2 | b.d. | b.d. | b.d. | 0.03 | b.d. | b.d. | b.d. |
| Al2O3 | 0.01 | b.d. | b.d. | 0.04 | b.d. | b.d. | b.d. |
| Cr2O3 | b.d. | b.d. | b.d. | 0.07 | b.d. | b.d. | b.d. |
| FeO | 22.41 | 23.36 | 24.82 | 27.01 | 13.75 | 12.34 | 13.28 |
| MnO | 0.43 | 0.43 | 0.4 | 0.37 | 0.49 | 0.4 | 0.53 |
| MgO | 38.36 | 37.8 | 36.06 | 34.82 | 45.43 | 46.95 | 46.48 |
| CaO | 0.01 | 0.02 | b.d. | 0.01 | 0.01 | b.d. | b.d. |
| Na2O | 0.01 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| K2O | b.d. | b.d. | 0.01 | b.d. | b.d. | b.d. | b.d. |
| Σ | 99.57 | 99.58 | 99.35 | 100.14 | 99.75 | 99.94 | 100.25 |
| x(Fo) | 0.75 | 0.74 | 0.72 | 0.70 | 0.85 | 0.87 | 0.87 |
| x(Fa) | 0.25 | 0.26 | 0.28 | 0.30 | 0.15 | 0.13 | 0.13 |
| No | # Sample | No. Grain | FeO * | Cr2O3 | TiO2 | Al2O3 | MgO | MnO | V2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 1 | 1545-2-19 | 89.8 | 2.53 | 0.61 | 0.52 | 0.14 | 0.1 | 0.94 | 94.64 |
| 2 | 1545-2-26 | 89.16 | 2.21 | 0.77 | 0.11 | 0.44 | 0.16 | 0.85 | 93.70 | |
| 3 | 1545-1-36 | 87.25 | 1.57 | 2.46 | 1.35 | 0.25 | 0.15 | 0.94 | 93.97 | |
| II | 4 | 101013-22 | 87.21 | 2.89 | 2.16 | 1.64 | 0.16 | 0.21 | 0.74 | 95.01 |
| 5 | 101013-23 | 87.17 | 2.78 | 2.44 | 0.99 | 0.06 | 0.14 | 1.05 | 94.63 | |
| 6 | 101012-49 | 87.79 | 2.36 | 1.74 | 0.56 | 0.47 | 0.21 | 0.91 | 94.04 |
| No | Sample # | SiO2 | Al2O3 | Na2O | MgO | F | Cl | K2O | CaO | TiO2 | FeO * | MnO | Cr2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1546-3-9 | 50.54 | 6.1 | 0.96 | 16.38 | 0.07 | 0.03 | 0.03 | 13.16 | 0.17 | 9.59 | 0.16 | 0.13 | 97.31 |
| 2 | 1546-3-16 | 51.26 | 5.01 | 0.71 | 16.33 | 0.09 | 0.04 | 0.08 | 12.43 | 0.22 | 10.32 | 0.16 | 0.2 | 96.85 |
| 3 | 1546-3-19 | 50.85 | 5.84 | 1.05 | 17.52 | 0.03 | 0.33 | 0.01 | 12.53 | 0.36 | 8.01 | 0.2 | 0.16 | 96.91 |
| 4 | 1546-3-20 | 52.28 | 5.09 | 0.63 | 17.34 | 0.05 | 0.03 | 0.03 | 12.88 | 0.28 | 8.54 | 0.12 | 0.2 | 97.48 |
| 5 | 1546-3-21 | 53.83 | 4.23 | 0.39 | 18.77 | н.o | 0.02 | 0.01 | 13.00 | 0.18 | 6.94 | 0.14 | 0.06 | 97.57 |
| 6 | 101013-31 | 53.88 | 4.51 | 0.59 | 19.34 | н.o | 0.03 | 0.11 | 13.01 | 0.13 | 6.04 | 0.15 | н.o | 97.8 |
| 7 | 1546-3-15 | 47.67 | 8.12 | 0.9 | 15.28 | 0.15 | 0.08 | 0.14 | 11.48 | 0.45 | 12.68 | 0.24 | 0.24 | 97.45 |
| 8 | 101013-32 | 47.19 | 10.35 | 1.4 | 16.48 | 0.06 | 0.03 | 0.26 | 12.63 | 0.48 | 8.45 | 0.11 | 0.25 | 97.68 |
| 9 | 101012-45 | 49.05 | 9.59 | 1.37 | 16.82 | 0.03 | 0.01 | 0.01 | 12.21 | 0.52 | 7.11 | 0.08 | 0.14 | 96.95 |
| 10 | 101012-46 | 48.58 | 8.68 | 1.33 | 16.87 | 0.08 | 0.02 | н.o | 12.24 | 0.49 | 8.27 | 0.1 | 0.1 | 96.77 |
| 11 | 101013-25 | 51.11 | 7.35 | 1.08 | 17.89 | 0.07 | 0.02 | 0.05 | 12.28 | 0.28 | 7.47 | 0.1 | 0.05 | 97.76 |
| 12 | 101012-55 | 48.67 | 9.32 | 1.28 | 17.11 | 0.07 | 0.02 | 0.04 | 12.33 | 0.37 | 7.46 | 0.04 | 0.12 | 96.83 |
| 13 | 101012-56 | 48.5 | 9.82 | 1.53 | 17 | 0.04 | 0.03 | 0.03 | 12.35 | 0.38 | 7.63 | 0.1 | 0.05 | 97.45 |
| 14 | 1545-2-4 | 48.78 | 7.55 | 1.01 | 18.07 | н.o | 0.01 | 0.07 | 12.5 | 0.35 | 8.01 | 0.12 | 0.06 | 96.52 |
| 15 | 101012-42 | 57.72 | 0.02 | b.d. | 22.7 | 0.02 | н.o | 0.01 | 13.22 | 0.07 | 2.65 | 0.03 | н.o | 96.44 |
| 16 | 1545-2-5 | 56.1 | 1.14 | 0.19 | 22.22 | н.o | 0.01 | 0.02 | 12.96 | 0.08 | 3.82 | 0.17 | н.o | 96.7 |
| No Grain | 1545-2-2 | 1545-2-13 | 1545-2-17 | 1545-2-21 | 1545-2-22 | 1545-2-25 | 1545-1-34 | 1545-1-39 | 1545-1-44 | 1546-3-10 | 1546-3-17 | 101012-54 | 211015/4_2 | 211014/2_3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||||||||
| No | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| SiO2 | 30.67 | 31.63 | 31.59 | 30.71 | 35.12 | 28.65 | 34.51 | 27.18 | 30.96 | 28.81 | 30.61 | 33.82 | 34.87 | 35.94 |
| TiO2 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.23 |
| Al2O3 | 13.88 | 13.61 | 14.03 | 15.09 | 11.75 | 15.39 | 10.76 | 17.53 | 13.9 | 17.93 | 14.21 | 14.32 | 12.85 | 12.18 |
| Cr2O3 | 0.51 | 0.21 | 0.68 | 0.11 | 0.03 | 0.16 | 0.45 | 0.28 | 0.1 | 0.05 | 0.64 | 0.19 | 0.14 | b.d. |
| FeO | 16.53 | 13.63 | 12.13 | 14.84 | 6.02 | 21.29 | 11.76 | 23.09 | 16.35 | 16.36 | 17.69 | 5.05 | 2.69 | 3.11 |
| MnO | 0.1 | 0.12 | 0.16 | 0.26 | 0.08 | 0.17 | 0.41 | 0.21 | 0.21 | 0.25 | 0.2 | 0.03 | b.d. | b.d. |
| MgO | 24.33 | 25.69 | 26.69 | 24.63 | 32.25 | 20 | 28.24 | 17.07 | 24.02 | 22.38 | 22.4 | 31.15 | 34.04 | 32.97 |
| CaO | 0.03 | 0.11 | 0.07 | 0.05 | 0.06 | 0.1 | 0.14 | 0.08 | 0.02 | 0.12 | 0.1 | 0.06 | b.d. | b.d. |
| Na2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. |
| K2O | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 0.54 | 2.10 |
| ∑ | 86.05 | 85.00 | 85.35 | 85.69 | 85.31 | 85.76 | 86.27 | 85.44 | 85.56 | 85.90 | 85.85 | 84.62 | 85.13 | 86.53 |
| Crystallochemical coefficients in the chlorite formula (calculation for 20 cations) | ||||||||||||||
| Si | 6.29 | 6.47 | 6.40 | 6.27 | 6.87 | 6.05 | 6.91 | 5.84 | 6.38 | 5.92 | 6.36 | 6.66 | 6.72 | 6.86 |
| Ti | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 |
| Al | 3.35 | 3.28 | 3.35 | 3.63 | 2.71 | 3.83 | 2.54 | 4.44 | 3.38 | 4.34 | 3.48 | 3.32 | 2.92 | 2.74 |
| Cr | 0.08 | 0.03 | 0.11 | 0.02 | 0.00 | 0.03 | 0.07 | 0.05 | 0.02 | 0.01 | 0.11 | 0.03 | 0.02 | 0.00 |
| Fe‴ | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 |
| Fe″ | 2.83 | 2.33 | 1.98 | 2.53 | 0.98 | 3.63 | 1.97 | 4.14 | 2.81 | 2.81 | 3.05 | 0.83 | 0.43 | 0.50 |
| Mn | 0.02 | 0.02 | 0.03 | 0.04 | 0.01 | 0.03 | 0.07 | 0.04 | 0.04 | 0.04 | 0.04 | 0.01 | 0.00 | 0.00 |
| Mg | 7.43 | 7.83 | 8.05 | 7.49 | 9.40 | 6.29 | 8.42 | 5.47 | 7.37 | 6.85 | 6.93 | 9.14 | 9.77 | 9.37 |
| Ca | 0.01 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 | 0.00 | 0.03 | 0.02 | 0.01 | 0.00 | 0.00 |
| Na | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| K | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.51 |
| x(Mg) | 0.72 | 0.77 | 0.80 | 0.74 | 0.90 | 0.63 | 0.81 | 0.57 | 0.72 | 0.71 | 0.69 | 0.92 | 0.96 | 0.95 |
| Al(IV) | 1.71 | 1.53 | 1.60 | 1.73 | 1.13 | 1.95 | 1.09 | 2.16 | 1.62 | 2.08 | 1.64 | 1.34 | 1.28 | 1.14 |
| Al(VI) | 1.64 | 1.76 | 1.75 | 1.90 | 1.58 | 1.88 | 1.44 | 2.28 | 1.76 | 2.26 | 1.84 | 1.98 | 1.64 | 1.59 |
| x(Fe) | 0.28 | 0.23 | 0.21 | 0.26 | 0.10 | 0.38 | 0.19 | 0.43 | 0.28 | 0.29 | 0.31 | 0.08 | 0.04 | 0.05 |
| T, °C | 205 | 190 | 200 | 209 | 160 | 221 | 147 | 238 | 195 | 243 | 195 | 184 | 181 | 166 |
| No | # Grain | MgO | FeO | Al2O3 | SiO2 | Cr2O3 | MnO | NiO | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| I | 1 | 1545-2-55 | 37.65 | 4.63 | b.d. | 44.01 | B.d. | B.d. | B.d. | 86.29 |
| II | 2 | 101013-29 | 28.91 | 12.78 | 0.54 | 43.47 | 0.01 | 0.27 | 0.05 | 86.17 |
| 3 | 101013-30 | 31.43 | 7.34 | 0.01 | 50.52 | 0.07 | 0.06 | 0.04 | 89.84 | |
| 4 | 101012-43 | 32.86 | 5.71 | 0.03 | 49.76 | 0.03 | 0.12 | 0.04 | 88.83 | |
| 5 | 101012-44 | 34.38 | 6.02 | 0.02 | 45.39 | 0 | 0.08 | 0.05 | 86.17 | |
| III | 6 | 211015-3_1 | 39.52 | 2.15 | 0.49 | 44.89 | B.d. | B.d. | B.d. | 87.05 |
| 7 | 211015-3_2 | 39.37 | 1.18 | B.d. | 45.08 | B.d. | B.d. | B.d. | 85.63 | |
| 8 | 211015-6_2 | 40.64 | 1.12 | B.d. | 45.33 | B.d. | B.d. | B.d. | 87.1 | |
| 9 | 211015-8_2 | 40.3 | 1.25 | B.d. | 45.61 | B.d. | B.d. | B.d. | 87.16 | |
| 10 | 211014-4_2 | 39.63 | 1.48 | B.d. | 44.38 | B.d. | B.d. | B.d. | 85.5 |
| No | # Grain | FeO | Cr2O3 | TiO2 | Al2O3 | MgO | MnO | V2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1545-2-9 | 92.67 | b.d. | 0.32 | 0.02 | 0.15 | 0.02 | 0.1 | 93.28 |
| 2 | 101013-34 | 92.16 | 0.44 | 0.29 | 0.07 | 0.16 | 0.01 | 0.28 | 93.41 |
| 3 | 101012-37 | 90.14 | 0.63 | 0.65 | 0.02 | 0.1 | 0.12 | 0.54 | 92.20 |
| 4 | 211015_3_9 | 94.18 | b.d. | b.d. | b.d. | b.d. | b.d. | b.d. | 94.18 |
| 5 | 211015_5_1 | 89.89 | b.d. | b.d. | b.d. | b.d. | 0.37 | b.d. | 90.26 |
| No. | # Sample | Cu | Fe | Ni | Co | S | Ag | Total | Mineral |
|---|---|---|---|---|---|---|---|---|---|
| Pyrite-pyrrhotite-chalcopyrite paragenesis (clinopyroxenite I) | |||||||||
| 1 | 1545-1-33 | B.d. | 47.61 | 0.02 | 0.14 | 53.24 | B.d. | 101.01 | Py |
| 2 | 1545-1-43 | 0.01 | 47.09 | B.d. | 0.09 | 52.68 | B.d. | 99.87 | Py |
| 3 | 1546-3-3 | 0.18 | 45.43 | 0.05 | 1.01 | 51.86 | B.d. | 98.53 | Py |
| 4 | 1545-2-1 | B.d. | 62.68 | 0.05 | 0.13 | 37.77 | B.d. | 100.63 | Pyh |
| 5 | 1545-2-16 | 0.02 | 61.4 | B.d. | 0.13 | 38.11 | B.d. | 99.66 | Pyh |
| 6 | 1546-3-1 | 34.74 | 30.34 | 0.01 | 0.07 | 33.33 | B.d. | 98.49 | Ccp |
| 7 | 1546-3-2 | 34.36 | 30.72 | 0.03 | 0.05 | 33.31 | B.d. | 98.47 | Ccp |
| Bornite–chalcopyrite paragenesis (clinopyroxenite II) | |||||||||
| 8 | 101012-36 | 35.55 | 29.73 | B.d. | B.d. | 33.09 | B.d. | 98.37 | Ccp |
| 9 | 101012-59 | 35.71 | 29.29 | 0.02 | 0.06 | 33.03 | B.d. | 98.11 | Ccp |
| 10 | 101012-35 | 61.64 | 11.87 | B.d. | 0.03 | 26.14 | B.d. | 99.68 | Bn |
| 11 | 101012-40 | 62.25 | 11.49 | B.d. | 0.04 | 24.69 | B.d. | 98.47 | Bn |
| 12 | 101012-50 | 61.84 | 11.45 | B.d. | B.d. | 25.55 | B.d. | 98.84 | Bn |
| 13 | 101012-51 | 70.94 | 1.04 | 0.03 | B.d. | 25.66 | B.d. | 97.67 | Spi-Yar |
| 14 | 101012-52 | 68.87 | 0.99 | 0.02 | 0.01 | 29.72 | B.d. | 99.61 | Spi-Yar |
| 15 | 101012-60 | 68.9 | 1.24 | 0.05 | 0.02 | 29.84 | B.d. | 100.05 | Spi-Yar |
| Pyrrhotite–chalcopyrite–pyrite (clinopyroxenite III) | |||||||||
| 16 | 211014_1_1 | B.d. | 34.23 | 19.1 | B.d. | 32.71 | 14.72 | 100.76 | Apn |
| 17 | 211014_1_4 | B.d. | 31.49 | 34.39 | B.d. | 34.07 | B.d. | 99.95 | Pn |
| 18 | 211014_2_1 | B.d. | 60.91 | B.d. | B.d. | 39.34 | B.d. | 100.25 | Pyh |
| 19 | 211014_4_3 | B.d. | 61.33 | B.d. | B.d. | 38.85 | B.d. | 100.17 | Pyh |
| 20 | 211014_1_5 | 34.09 | 31.05 | B.d. | B.d. | 35.48 | B.d. | 100.63 | Ccp |
| No | # Grain | Pd | Cu | Fe | Ni | Pt | Au | Ag | As | Sb | S | ∑ | Mineral |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1546-2-65 | 71.57 | B.d. | 0.06 | B.d. | 0.04 | B.d. | B.d. | 2.95 | 25.07 | 0.02 | 99.71 | Met-I |
| 2 | 1546-2-66 | 71.71 | B.d. | 0.1 | B.d. | 0.5 | B.d. | B.d. | 3.01 | 25.24 | 0.03 | 100.59 | Met-I |
| 3 | 1546-2-107 | 67.20 | 0.54 | 1.16 | B.d. | B.d. | B.d. | B.d. | 3.86 | 27.24 | B.d. | 100 | Stpdn |
| 4 | 1546-2-116 | 69.67 | B.d. | 0.72 | B.d. | B.d. | B.d. | B.d. | 4.3 | 25.30 | B.d. | 100 | Stpdn |
| 5 | 1546-2-121 | 67.7 | B.d. | 0.85 | B.d. | B.d. | B.d. | B.d. | 4.22 | 27.23 | B.d. | 100 | Stpdn |
| 6 | 1546-2-130 | 50.73 | 13.77 | 21.89 | B.d. | B.d. | B.d. | B.d. | 1.24 | 4.01 | 20.76 | 100 | Pd-Fe-S phase |
| 7 | 1546-3-16 | B.d. | B.d. | B.d. | B.d. | 51.65 | B.d. | B.d. | 45.34 | B.d. | 3.02 | 100 | Spy |
| 8 | 1546-3-18 | B.d. | B.d. | B.d. | B.d. | 51.58 | B.d. | B.d. | 44.91 | B.d. | 3.52 | 100 | Spy |
| 9 | 101013-93 | 57.38 | 30.41 | 2.99 | B.d. | 3.97 | 5.25 | B.d. | B.d. | B.d. | B.d. | 100 | Skg |
| 10 | 101013-99 | 46.52 | 31.51 | 8.24 | B.d. | 2.13 | 11.6 | B.d. | B.d. | B.d. | B.d. | 100 | Phase Cu > Pd >> Fe |
| 11 | 101013-100 | 83.53 | 9.98 | B.d. | 6.49 | B.d. | B.d. | B.d. | B.d. | B.d. | B.d. | 100 | Phase Pd3(Cu,Ni) |
| 12 | 101013-101 | 68.05 | 12.42 | 5.42 | B.d. | B.d. | B.d. | B.d. | B.d. | B.d. | 14.11 | 100 | (Pd,Cu,Fe)2S |
| 13 | 101012-154 | B.d. | B.d. | B.d. | B.d. | B.d. | 16.6 | 83.4 | B.d. | B.d. | B.d. | 100 | Ag |
| 14 | 211015_4-1 | B.d. | B.d. | B.d. | B.d. | B.d. | 70.36 | 27.53 | B.d. | B.d. | B.d. | 97.89 | Au |
| Deposit | Mineral (Number of Analyses) | ẟ34S (VCDT), ‰ |
|---|---|---|
| Ozernoe, clinopyroxenite II | Chalcopyrite (3) | −2.3…−2.6 |
| Bornite (1) | −2.1 | |
| Bornite, chalcopyrite (4) | −2.1…−2.6 | |
| Sulfur sulfides (spionkopite–yarrowite) (4) | −0.8…−2.7 | |
| Ozernoe, clinopyroxenite III | Pyrite (5) | −2.1…−2.5 |
| Chalcopyrite (5) | −2.5…−2.9 | |
| Baronsky | Chalcopyrite (7) | −1.2…−2.6 |
| Minerals | Clinopyroxenite I | Clinopyroxenite II | Clinopyroxenite III |
|---|---|---|---|
| Primary: | |||
| Clinopyroxene 1 | Diopside (4.5–7 wt.% FeO) | Diopside (5–6.8 wt.% FeO) | Diopside (0.7–2.2 wt.% FeO) |
| Olivine | x(Fa) = 0.25–0.26 | x(Fa) = 0.28–0.30 | x(Fa) = 0.13–0.15 |
| Magnetite 1 | Cr-Ti-V-bearing | Cr-Ti-V-bearing | Cr-Ti-V-bearing |
| Secondary: | |||
| Clinopyroxene 2 | Diopside (9.6–14.5 wt.% FeO) | ||
| Amphibole | Actinolite, magnesio-ferri-hornblende, tremolite | Magnesio-hornblende, tremolite | |
| Chlorite | Pennine, picnochlorite | Pennine | Pennine |
| Serpentine | Serpentine A | Serpentine A | Serpentine B |
| Magnetite 2 | 0.n wt.% TiO2 и MgO | <1 wt.% Cr2O3, MgO, V2O3 | 0.n wt.% MnO |
| Sulfides | Ccp >> Po > Py | (Bn-Ccp)ss >Ccp; ẟ34S = −2.1…−2.7‰ | Py > Ccp >> Po; ẟ34S = −2.1...−2.9‰ |
| Pyrrhotite | NFeS = 0.96–0.97 | NFeS = 0.94–0.95 | |
| Noble metal minerals | Antimonides Pd (mertieite, stibiopalladinite), sulfo-arsenide Pt (sperrylite-platarsite) | Native Pd-Cu and Pd-Cu-Ni phases, native silver (170–240‰). | Mertieite, stibiopalladinite, argentopentlandite, native gold (720 ‰) |
| Classes | Minerals Associations | |
|---|---|---|
| Cubanite-Pentlandite-Pyrrhotite- Chalcopyrite | Bornite-Chalcopyrite | |
| Native metals | Au-Ag solid solution, tetra-auricupride Au-Cu, auricupride Cu3Au, nielsenite Cu3Pd, native Pt | Au-Ag solid solution, auricupride, skaergaardite *, phase Cu > Pd >> Fe *, phase Pd3(Cu,Ni)* |
| Sulfides | Vysotskite PdS, braggite (Pt,Pd)S) | Phase (Pd,Cu,Fe)2S * |
| Arsenides | Sperrylite PtAs2, arsenopalladinite Pd8As2.5Sb0.5, atheneite (Pd,Hg)3As, isomertieite Pd11Sb2As2, majakite PdNiAs | Sperrylite PtAs2, atheneite (Pd,Hg)3As |
| Antimonides | Stibiopalladinite (Pd5+xSb)2−x | Stibiopalladinite (Pd5+xSb)2−x, mertieite Pd5Sb2 |
| Tellurides | Merenskyite PdTe2, moncheite PtTe2 | Kotulskite PdTe, hessite Ag2Te |
| Tellurobismuthides, bismuthides | Michenerite PdBiTe, froodite PdBi2 | Froodite PdBi2, sobolevskite PdBi |
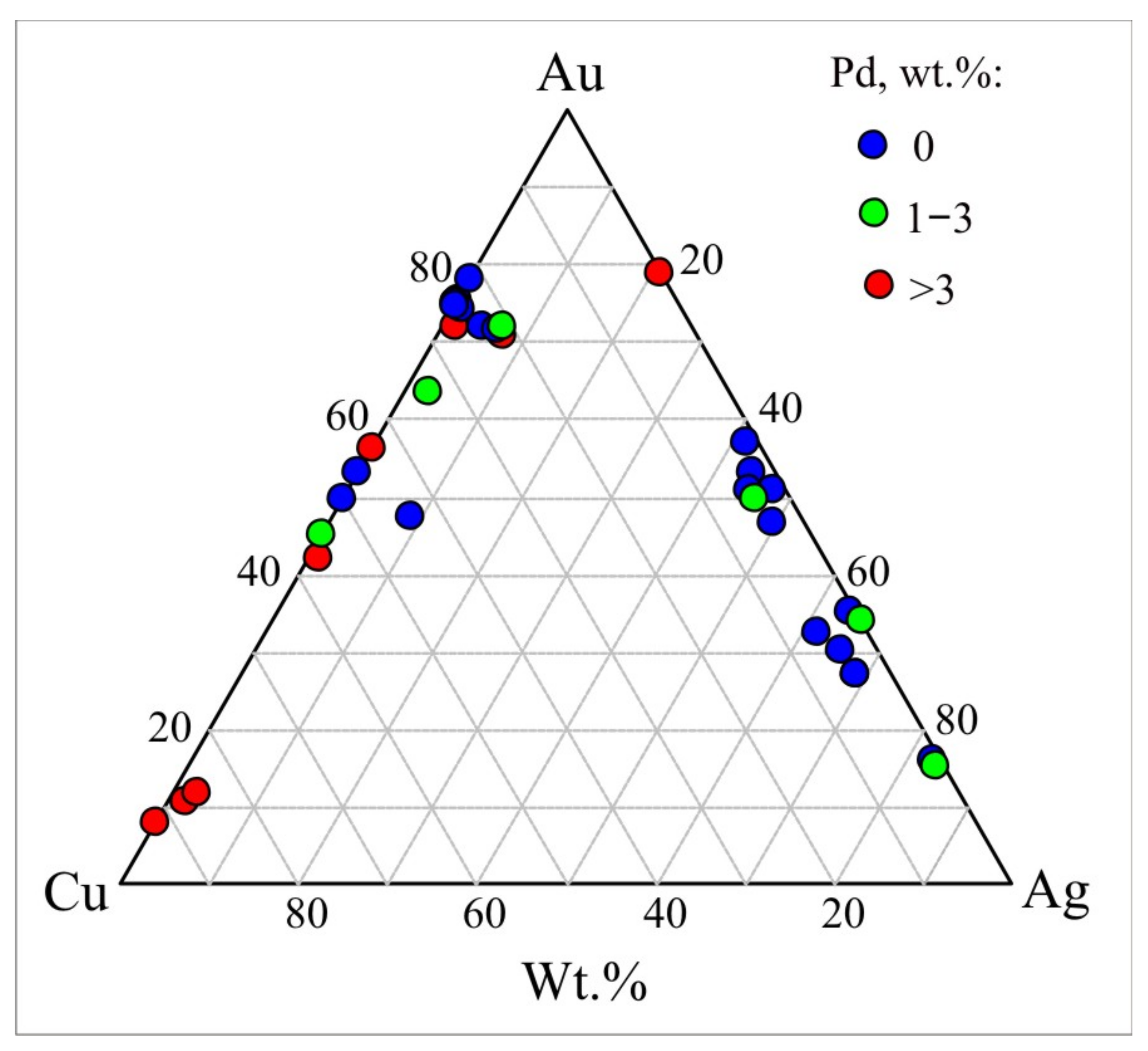
| # | Mineral | Au | Ag | Cu | Pd | Fe | ∑ | Fineness, ‰ |
|---|---|---|---|---|---|---|---|---|
| S (Bornite-chalcopyrite) | ||||||||
| 509019 | AuCu | 74.62 | 0 | 23.96 | 0 | 0.84 | 99.42 | 751 |
| 509019 | AuCu | 74.03 | 0.94 | 24.43 | 0 | 0.9 | 100.30 | 738 |
| 509019 | Cu3Au | 52.2 | 0 | 45.71 | 0 | 1.65 | 99.56 | 524 |
| 509019 | AuCu | 73.85 | 0 | 24.33 | 0 | 1.36 | 99.54 | 742 |
| 509019 | AuCu | 73.17 | 0 | 23.96 | 0 | 2.28 | 99.41 | 736 |
| 509019 | AuCu | 74.03 | 0 | 24.93 | 0 | 1.17 | 100.13 | 739 |
| 509019 | AuCu | 75.27 | 0 | 21.01 | 0 | 2.1 | 98.38 | 765 |
| 509019 | AuCu | 69.3 | 6.15 | 21.2 | 0 | 3.23 | 99.88 | 694 |
| 509019 | AuCu | 69.61 | 4.38 | 22.46 | 0 | 3.42 | 99.87 | 697 |
| 509019 | ss(Ag,Au,Cu) | 50.59 | 41.66 | 2.4 | 0 | 1.26 | 95.91 | 527 |
| 509019 | ss(Ag,Au,Cu) | 55.87 | 40.71 | 1.27 | 0 | 1.32 | 99.17 | 563 |
| 509019 | ss(Ag,Au,Cu) | 49.42 | 45.93 | 1.53 | 0 | 1.4 | 98.28 | 503 |
| 509019 | ss(Ag,Au,Cu) | 46.48 | 49.07 | 3.5 | 0 | 1.4 | 100.45 | 463 |
| 509019 | ss(Ag,Au,Cu) | 48.09 | 42.51 | 3.89 | 0 | 0.87 | 95.36 | 504 |
| 509019 | ss(Ag,Au,Cu) | 27.34 | 69.25 | 3.97 | 0 | 0.63 | 101.19 | 270 |
| 509019 | ss(Ag,Au,Cu) | 27.28 | 69.11 | 3.97 | 0 | 0.63 | 100.99 | 270 |
| 509019 | ss(Ag,Au,Cu) | 30.49 | 65.89 | 4.2 | 0 | 0.89 | 101.47 | 300 |
| 509019 | ss(Ag,Au,Cu) | 33.26 | 62.86 | 5.76 | 0 | 0.98 | 102.86 | 323 |
| S-Te-Sb (bornite, chalcopyrite, mertieite, merenskyite, moncheite, native Te) | ||||||||
| 1 | ss(Au,Cu,Ag,Pd) | 58.9 | 5.5 | 17.3 | 2.8 | 84.50 | 697 | |
| 2 | ss(Au,Cu,Ag,Pd) | 61.2 | 6.4 | 18.5 | 3.2 | 89.30 | 685 | |
| 3 | ss(Au,Cu,Ag,Pd) | 55.5 | 2.5 | 29.3 | 1.6 | 88.90 | 624 | |
| 4 | ss(Cu,Au,Pd) | 49.7 | 0 | 38.2 | 3.8 | 91.70 | 542 | |
| 5 | ss(Cu,Au,Ag) | 44.7 | 8.3 | 41.3 | 0 | 94.30 | 474 | |
| 6 | ss(Cu,Au,Pd) | 44.3 | 0 | 54 | 2.1 | 100.40 | 441 | |
| 7 | ss(Cu,Au,Pd,Ag) | 39.7 | 1.3 | 53.5 | 6 | 100.50 | 395 | |
| 8 | ss(Cu,Pd,Au,Ag) | 7.9 | 1.4 | 63.5 | 16.4 | 89.20 | 89 | |
| 9 | ss(Cu,Pd,Au) | 5.5 | 0 | 64.3 | 16.2 | 86.00 | 64 | |
| 10 | ss(Cu,Pd,Au,Ag) | 9.1 | 2.2 | 65.7 | 16.9 | 93.90 | 97 | |
| S-Te-As-Sb (bornite, chalcopyrite stibiopalladinite, kotulskite, braggite, moncheite, atheneite, majakite, mertieite) | ||||||||
| 145042 | AuCu | 66.63 | 1.54 | 24.48 | 4.73 | 1.58 | 98.96 | 673 |
| 126010 | ss(Au,Ag,Pd) | 63.84 | 17.02 | 0.00 | 13.80 | 1.60 | 96.26 | 663 |
| 145042 | ss(Ag,Au,Cu,Pd) | 47.83 | 44.06 | 3.84 | 1.25 | 1.26 | 98.24 | 487 |
| 510015 | ss(Ag,Au,Pd) | 33.49 | 64.84 | 0 | 1.4 | 0 | 99.73 | 336 |
| 11 | ss(Ag,Au,Cu,Pd) | 14.6 | 80.7 | 1.2 | 1 | 97.50 | 150 | 150 |
| 12 | ss(Ag,Au,Cu) | 15.9 | 83.3 | 0.9 | 0 | 100.10 | 159 | 159 |
| 13 | ss(Ag,Au,Cu) | 34.6 | 62.7 | 0.8 | 0 | 98.10 | 353 | 353 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzin, V.; Palyanova, G.; Mayorova, T.; Beliaeva, T. The Gold–Palladium Ozernoe Occurrence (Polar Urals, Russia): Mineralogy, Conditions of Formation, Sources of Ore Matter and Fluid. Minerals 2022, 12, 765. https://doi.org/10.3390/min12060765
Murzin V, Palyanova G, Mayorova T, Beliaeva T. The Gold–Palladium Ozernoe Occurrence (Polar Urals, Russia): Mineralogy, Conditions of Formation, Sources of Ore Matter and Fluid. Minerals. 2022; 12(6):765. https://doi.org/10.3390/min12060765
Chicago/Turabian StyleMurzin, Valery, Galina Palyanova, Tatiana Mayorova, and Tatiana Beliaeva. 2022. "The Gold–Palladium Ozernoe Occurrence (Polar Urals, Russia): Mineralogy, Conditions of Formation, Sources of Ore Matter and Fluid" Minerals 12, no. 6: 765. https://doi.org/10.3390/min12060765
APA StyleMurzin, V., Palyanova, G., Mayorova, T., & Beliaeva, T. (2022). The Gold–Palladium Ozernoe Occurrence (Polar Urals, Russia): Mineralogy, Conditions of Formation, Sources of Ore Matter and Fluid. Minerals, 12(6), 765. https://doi.org/10.3390/min12060765






