CO2-Degassing Carbonate Conduits in Early Pleistocene Marine Clayey Deposits in Southwestern Umbria (Central Italy)
Abstract
:1. Introduction
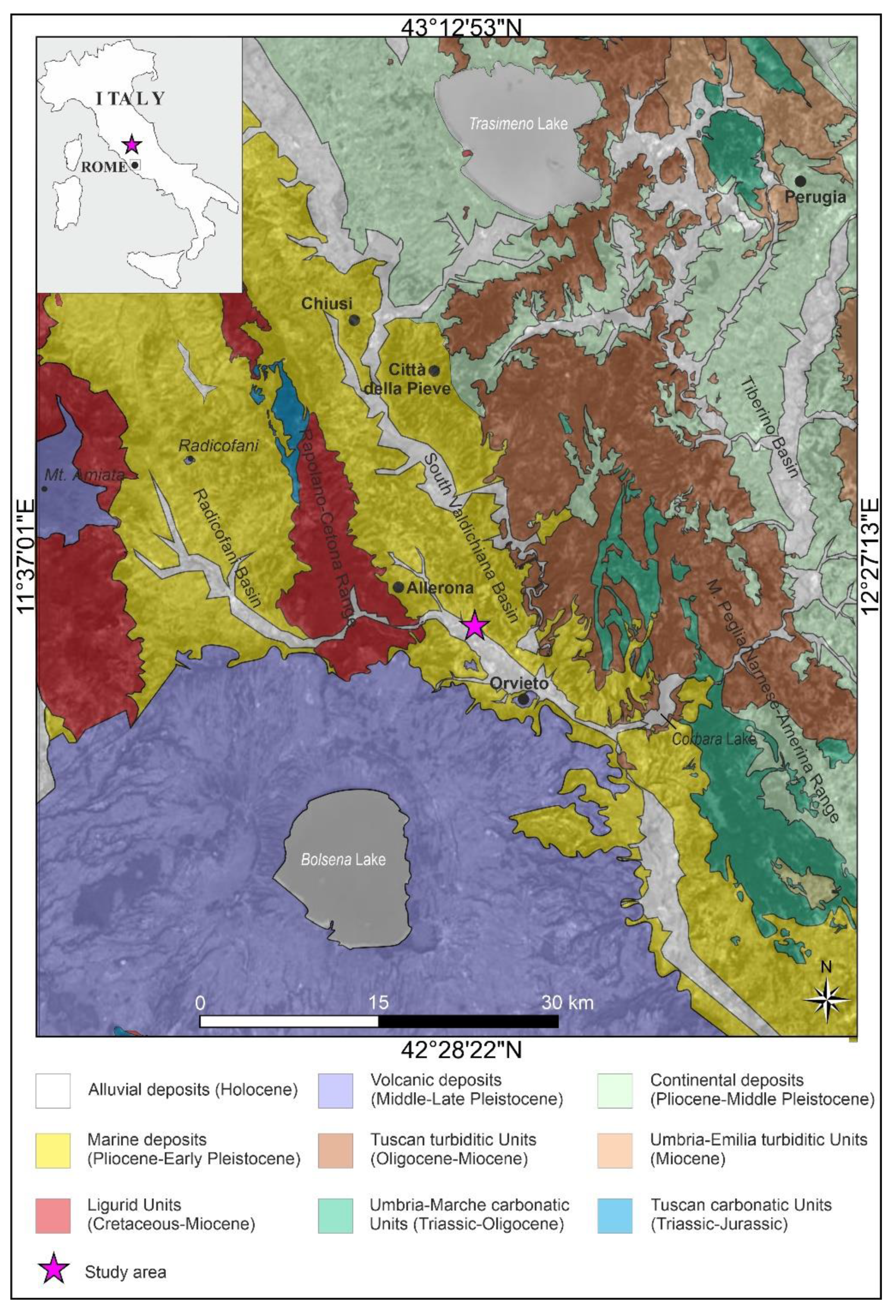
2. Geological Setting
- -
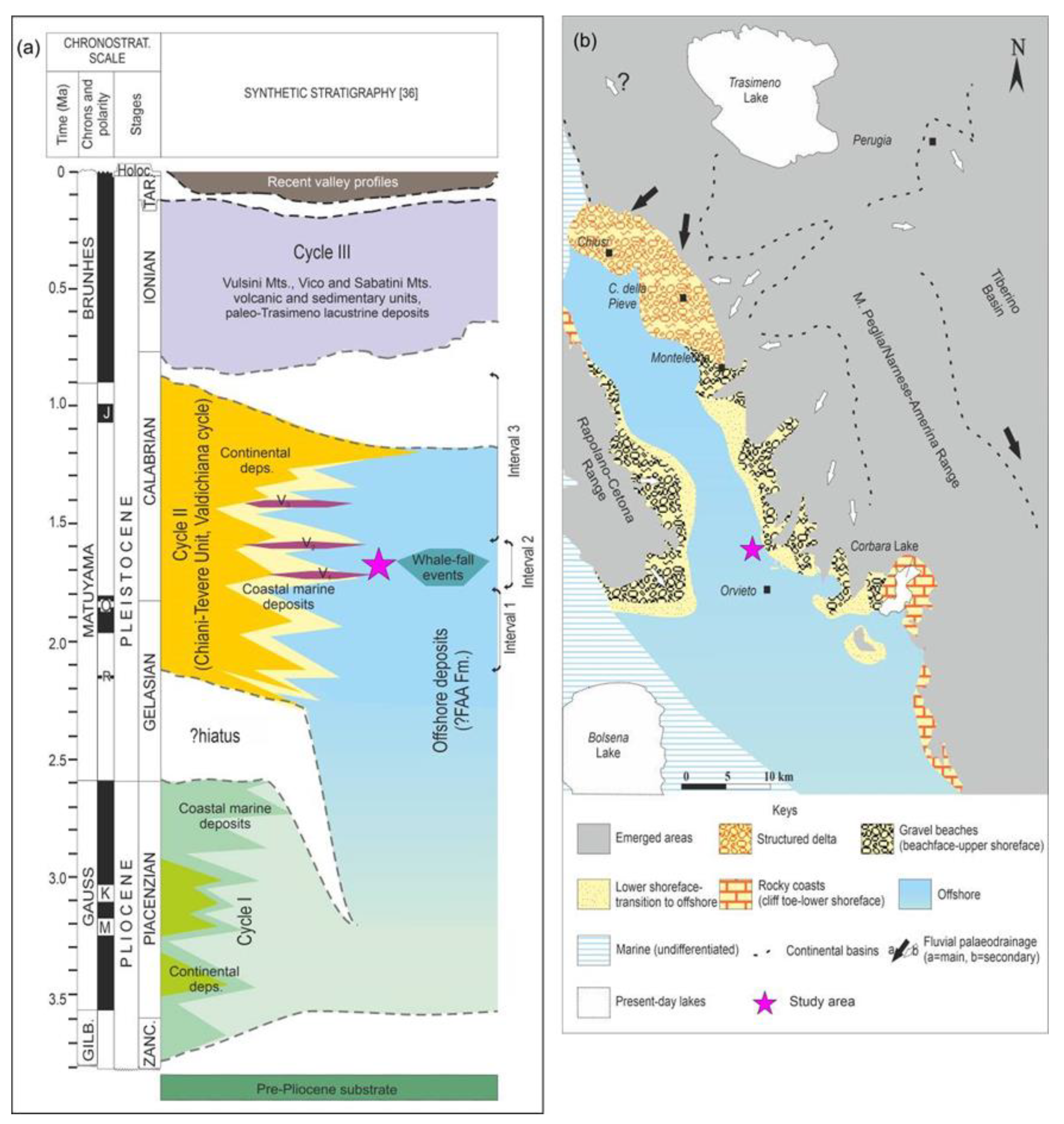
- the “Pliocene” Cycle, widely documented in neighbouring basins [28,30,31], is suspected but not yet recognised in the study area. Although the deposits are age-equivalent to the FAA Fm (Formazione delle Argille Azzurre [28,31]), they are reported as Cycle I (Zanclean–Piacenzian hypothetic evolution of coastal areas) and offshore deposits (continuous/paraconformable distal marine sedimentation) [28].
- -
- the Valdichiana Cycle (Gelasian–Calabrian), mainly consisting of the Chiani–Tevere unit and encompassing the higher lateral facies heterotopy and paleoenvironmental complexity of the area (Figure 2b), varying (from north to south and from coastal areas to the inner basin) from alluvial plains, deltas, river-fed beaches, rocky coasts, and shallow marine areas (shoreface to offshore transition), to open marine areas (~120–150 m deep [32,33]). This cycle is divided into three intervals by means of an integrated event stratigraphy (Figure 2a), and they are dated to Gelasian p.p. (Interval 1), Gelasian p.p.–Calabrian p.p. (Interval 2), and Calabrian p.p. (Interval 3) [28].
- -
- the Middle–Late Pleistocene evolution (Cycle III [28]), which is mainly represented by Paleo–Trasimeno lacustrine deposits northwards and in the southern part of the basin by the volcanic and sedimentary units of the Vulsini Mts., Vico Mts., and Sabatini Mts. This latest cycle precedes the onset of the present-day valleys (Late Pleistocene–Holocene, Figure 2a).
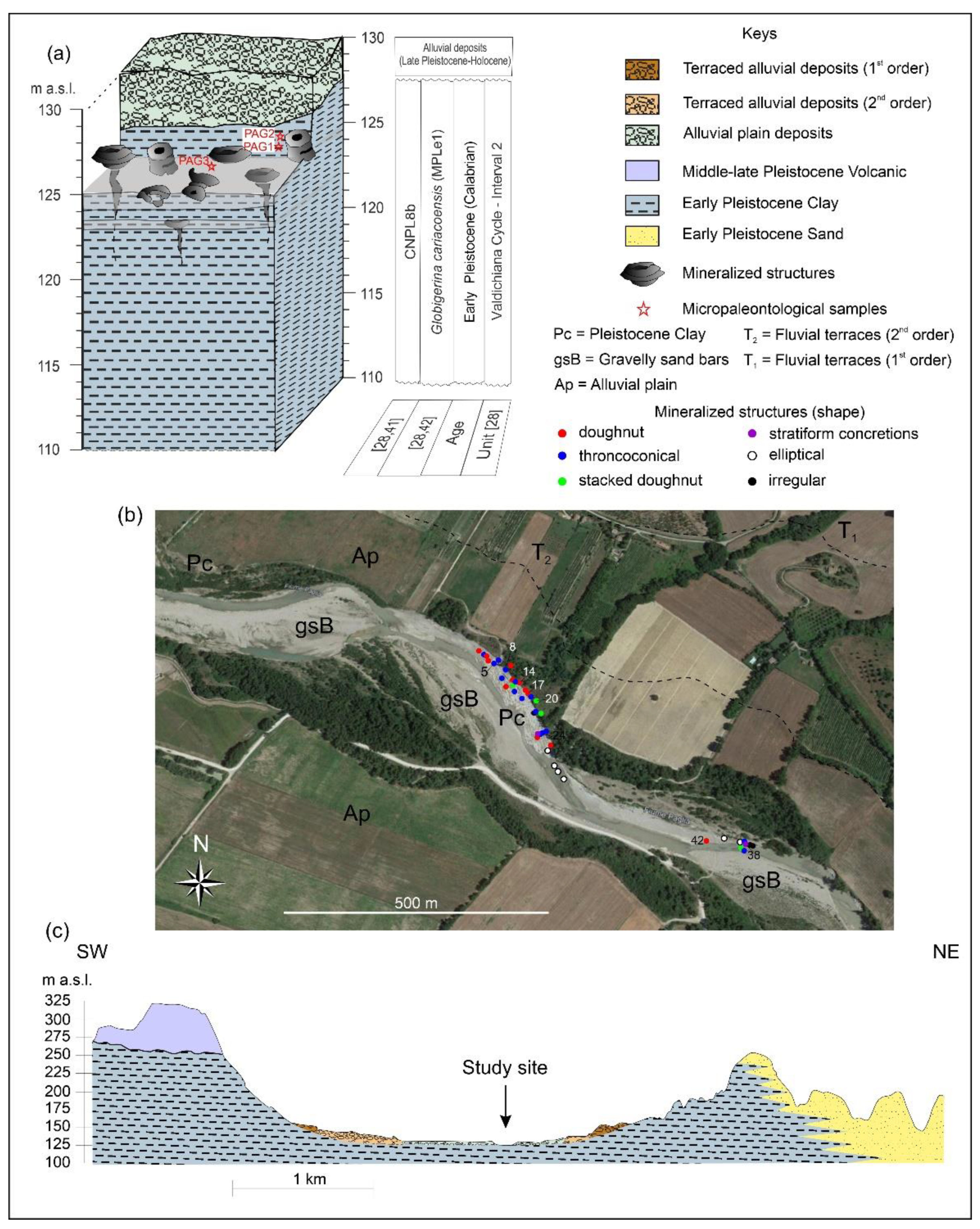
3. Materials and Methods
| Conduit Concretions | Morphology | Diameters (Max, min) | Height | Coordinates | |
|---|---|---|---|---|---|
| 1 | doughnut | 60 cm | 10 cm | 42°45′27.99″ N | 12°4′55.19″ E |
| 2 | troncoconical | 30 cm | 20–25 cm | 42°45′27.69″ N | 12°4′55.72″ E |
| 3 | doughnut | M 68 cm; m 55 cm | 18 cm | 42°45′27.62″ N | 12°4′55.92″ E |
| 4 | doughnut | M 120 cm; m 62 cm | 30 cm | 42°45′27.27″ N | 12°4′56.11″ E |
| 5 | troncoconical | M 78 cm; m 60–76 | 30 cm | 42°45′27.04″ N | 12°4′56.65″ E |
| 6 | troncoconical | M 107 cm; m 92 cm | 40 cm | 42°45′27.35″ N | 12°4′57.01″ E |
| 7 | doughnut | M 360 cm; m 340 cm | 80 cm | 42°45′26.93″ N | 12°4′58.22″ E |
| 8 | troncoconical with 2 mouths | M 132 cm; m 125 cm | 72–73 cm | 42°45′26.56″ N | 12°4′57.77″ E |
| 9 | troncoconical | not measured/on the river flow | 42°45′25.95″ N | 12°4′57.56″ E | |
| 10 | doughnut | M 195 cm; m 165 cm | 85 cm | 42°45′25.89″ N | 12°4′58.50″ E |
| 11 | doughnut | not measured/on the river flow | not measured | 42°45′25.37″ N | 12°4′57.97″ E |
| 12 | stacked doughnut | M 380 cm; m 330 cm | not measured | 42°45′25.46″ N | 12°4′58.58″ E |
| 13 | troncoconical with two mouths | M 190 cm; m 90 cm | 75 cm | 42°45′25.75″ N | 12°4′58.73″ E |
| 14 | ring doughnut with large central mouth | M 185 cm; m 170 cm | 27 cm | 42°45′25.67″ N | 12°4′59.03″ E |
| 15 | troncoconical | not measured/on the river flow | 42°45′25.07″ N | 12°4′58.79″ E | |
| 16 | ring doughnut | M 190 cm; m 190 cm | 45 cm | 42°45′25.12″ N | 12°4′59.83″ E |
| 17 | ring doughnut | M 290 cm; m 190 cm | 70 cm | 42°45′24.81″ N | 12°4′59.89″ E |
| 18 | troncoconical with central mouth | M 80 cm; m 72 cm | 30 cm | 42°45′24.56″ N | 12°4′59.52″ E |
| 19 | troncoconical | M 88 cm; m 73 cm | 24 cm | 42°45′24.65″ N | 12°5′0.26″ E |
| 20 | stacked doughnut | M 200 cm; m 170 cm | 95 cm | 42°45′24.37″ N | 12°5′0.75″ E |
| 21 | troncoconical irregular | M 145 cm; m 125 cm | 35 | 42°45′23.62″ N | 12°5′0.80″ E |
| 22 | irregular | on the river flow | 42°45′23.59″ N | 12°5′0.65″ E | |
| 23 | stacked doughnut with central mouth and accessory small holes | M 175 cm +50; m 175 cm | 65 cm | 42°45′23.48″ N | 12°5′1.23″ E |
| 24 | troncoconical with two mouths | M 200 cm; m 188 cm | 50 (15 + 25 + 10) | 42°45′22.24″ N | 12°5′1.75″ E |
| 25 | troncoconical with two mouths | M 95 cm; m 25 cm | 50 cm | 42°45′22.19″ N | 12°5′1.46″ E |
| 26 | troncoconical with central mouth | M 115 cm; m 100 cm | 60 cm | 42°45′22.13″ N | 12°5′1.36″ E |
| 27 | doughnut | M 240 cm; m 180 cm | 40 cm | 42°45′21.87″ N | 12°5′1.15″ E |
| 28 | doughnut | M 190 cm; m 160 cm | 50 cm | 42°45′21.36″ N | 12°5′2.28″ E |
| 29 | stratiform concretions | on the river flow | 42°45′22.10″ N | 12°5′1.09″ E | |
| 30 to 33 | elliptical | on the river flow | ? | ? | |
| 34 | elliptical spiralled | M 185 cm; m 160 cm | 15 cm | 42°45′15.85″ N | 12°5′16.05″ E |
| 35 | elliptical spiralled | M 200 cm; m 137 cm | 25–30 cm | 42°45′15.67″ N | 12°5′17.19″ E |
| 36 | very large stacked doughnut | not measured on the opposite riverbank | 42°45′15.31″ N | 12°5′17.29″ E | |
| 37 | crescent shape | on the river flow | 42°45′15.63″ N | 12°5′17.54″ E | |
| 38 | stratiform concretions | M 320 cm; m 280 cm | 135 cm | 42°45′15.51″ N | 12°5′17.66″ E |
| 39 | crescent shape | submerged | 42°45′15.20″ N | 12°5′17.53″ E | |
| 40 and 41 | irregular | submerged | 42°45′15.52″ N | 12°5′17.98″ E | |
| 42 | ring doughnut | M 60 cm; m 20 cm | 30 cm | 42°45′15.75″ N | 12°5′14.58″ E |
| Sample | Position | Structure Morphology |
|---|---|---|
| 5 top | periphery, upper surface | troncoconical |
| 5 bottom | periphery, ~40 cm below sample 5 top | |
| 5a | close to the mouth | |
| 5b | 10 cm from the mouth | |
| 8a | close to the mouth (a) | troncoconical, with two mouths |
| 8b | close to the mouth (b) | |
| 14 | periphery, upper ring | ring doughnut |
| 17 | periphery, upper ring | ring doughnut |
| 20 | periphery, upper ring | stacked doughnut |
| 24 | periphery, upper ring | stacked doughnut |
| 38 top | upper surface, close to the mouth | stratiform concretion |
| 38 bottom | 60 cm below sample 38 top | |
| 42 | periphery, external ring | ring doughnut |
| 42 rim | rim around the mouth |
4. Results
4.1. Conduit Concretion Morphology and Spatial Distribution
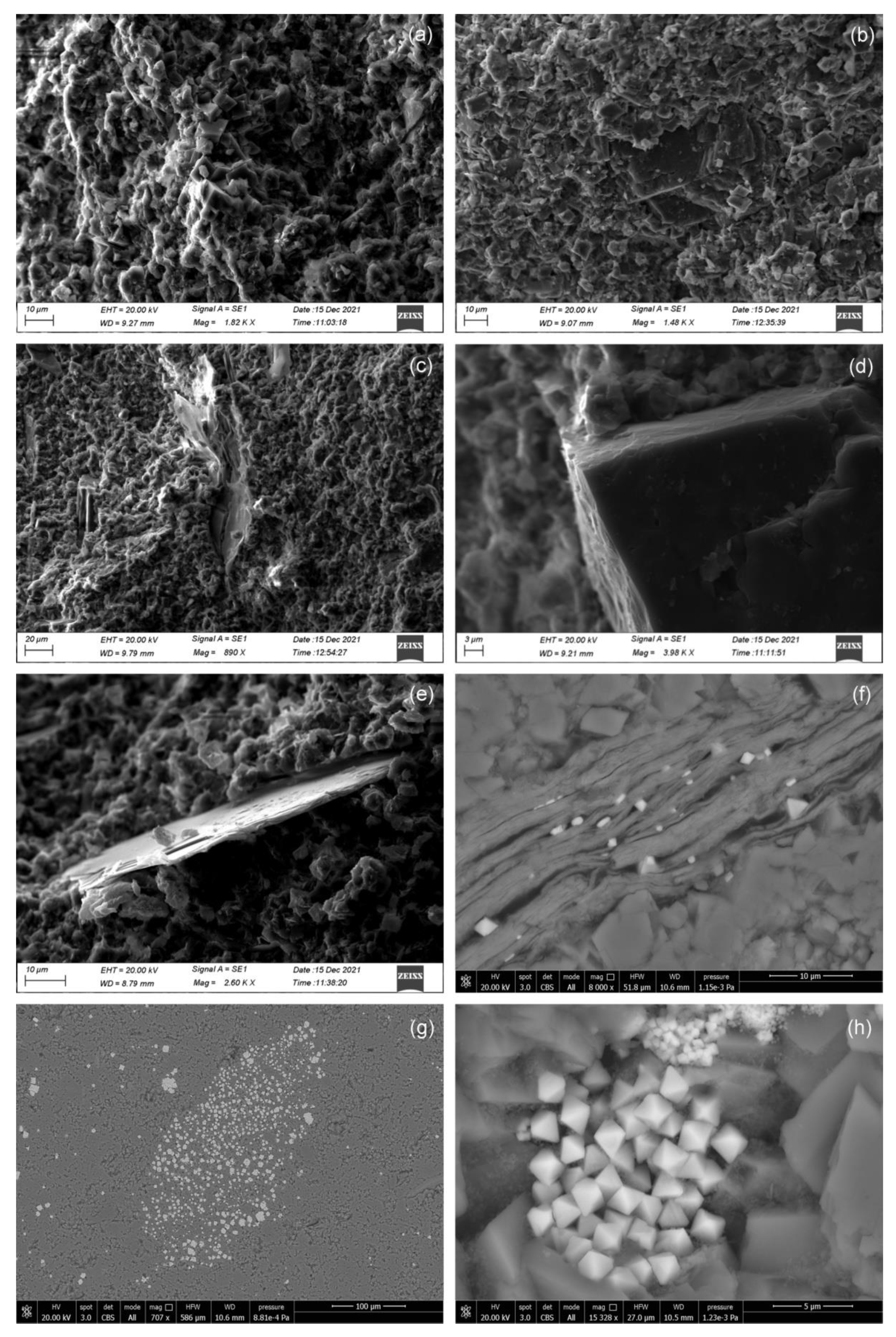
4.2. Mineralogical and Petrographic Features
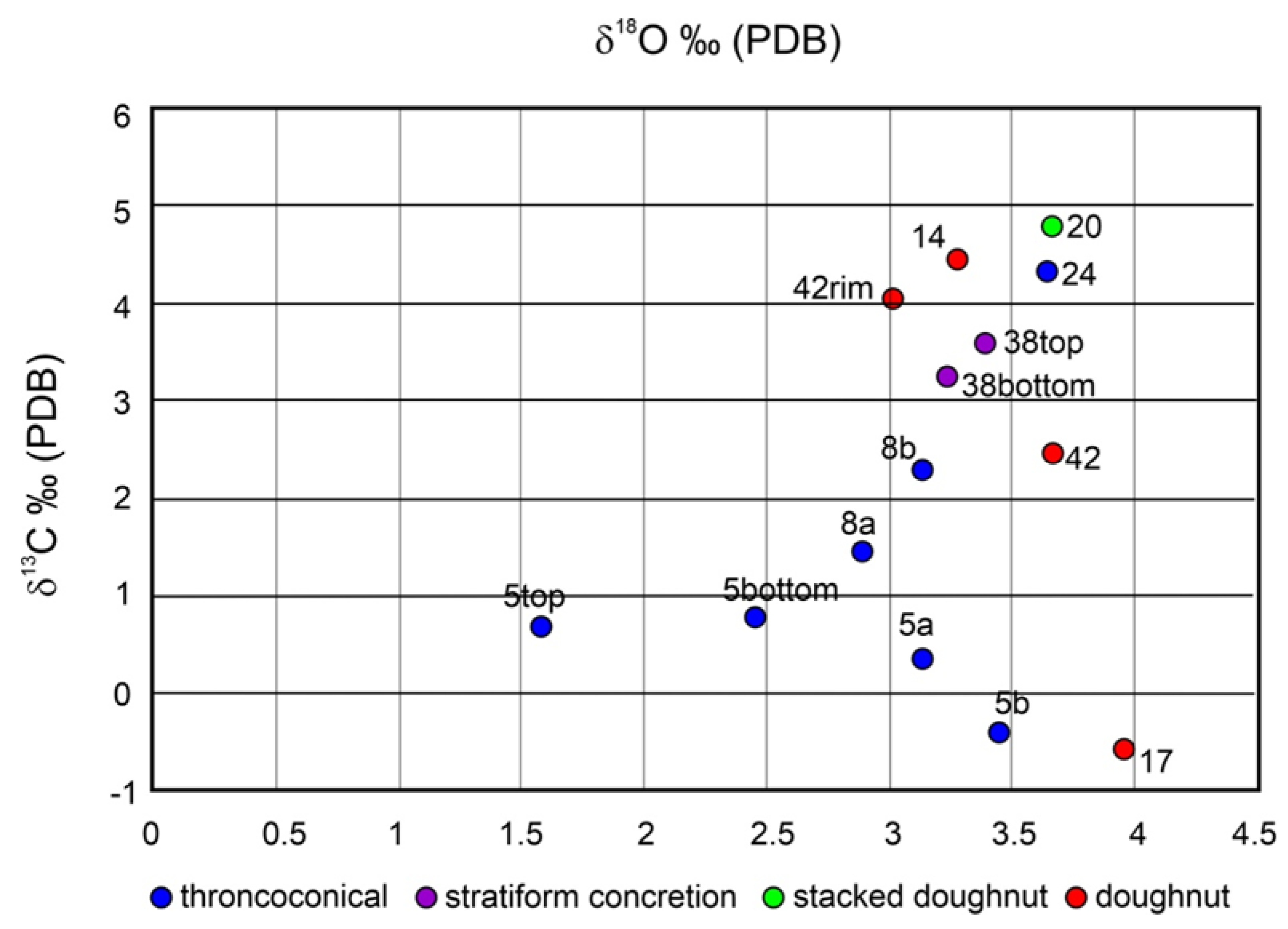
| Samples | Dolomite | Calcite | Quartz | Plagioclase | Mica | Kaolinite | Apatite | Pyrite | δ13C | δ18O | Z |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 top | XXX | X | X | tr | tr | tr | tr | tr | 0.68 | 1.58 | 129.47 |
| 5 bottom | XXX | tr | X | tr | tr | tr | tr | tr | 0.77 | 2.45 | 130.09 |
| 5a | XXX | X | tr | tr | tr | tr | tr | 0.36 | 3.13 | 129.59 | |
| 5b | XXX | X | tr | tr | tr | tr | tr | −0.41 | 3.44 | 128.17 | |
| 17 | XXX | X | tr | tr | tr | tr | tr | −0.57 | 4.07 | 128.10 | |
| 8a | XXX | X | tr | tr | tr | tr | tr | 1.45 | 2.89 | 131.70 | |
| 8b | XXX | X | tr | tr | tr | tr | tr | 2.30 | 3.13 | 133.56 | |
| 14 | XXX | X | tr | tr | tr | tr | tr | 4.45 | 3.27 | 138.04 | |
| 20 | XXX | X | tr | tr | tr | tr | tr | 4.79 | 3.66 | 138.93 | |
| 24 | XXX | X | tr | tr | tr | tr | tr | 4.33 | 3.64 | 137.98 | |
| 38 top | XXX | X | tr | tr | tr | tr | tr | 3.60 | 3.39 | 136.36 | |
| 38 bottom | XXX | X | tr | tr | tr | tr | tr | 3.23 | 3.23 | 135.52 | |
| 42 | XXX | X | tr | tr | tr | tr | tr | 2.46 | 3.67 | 134.17 | |
| 42 rim | XXX | X | tr | tr | tr | tr | tr | 4.05 | 3.01 | 137.09 |
4.3. Organic Geochemical Biomarkers
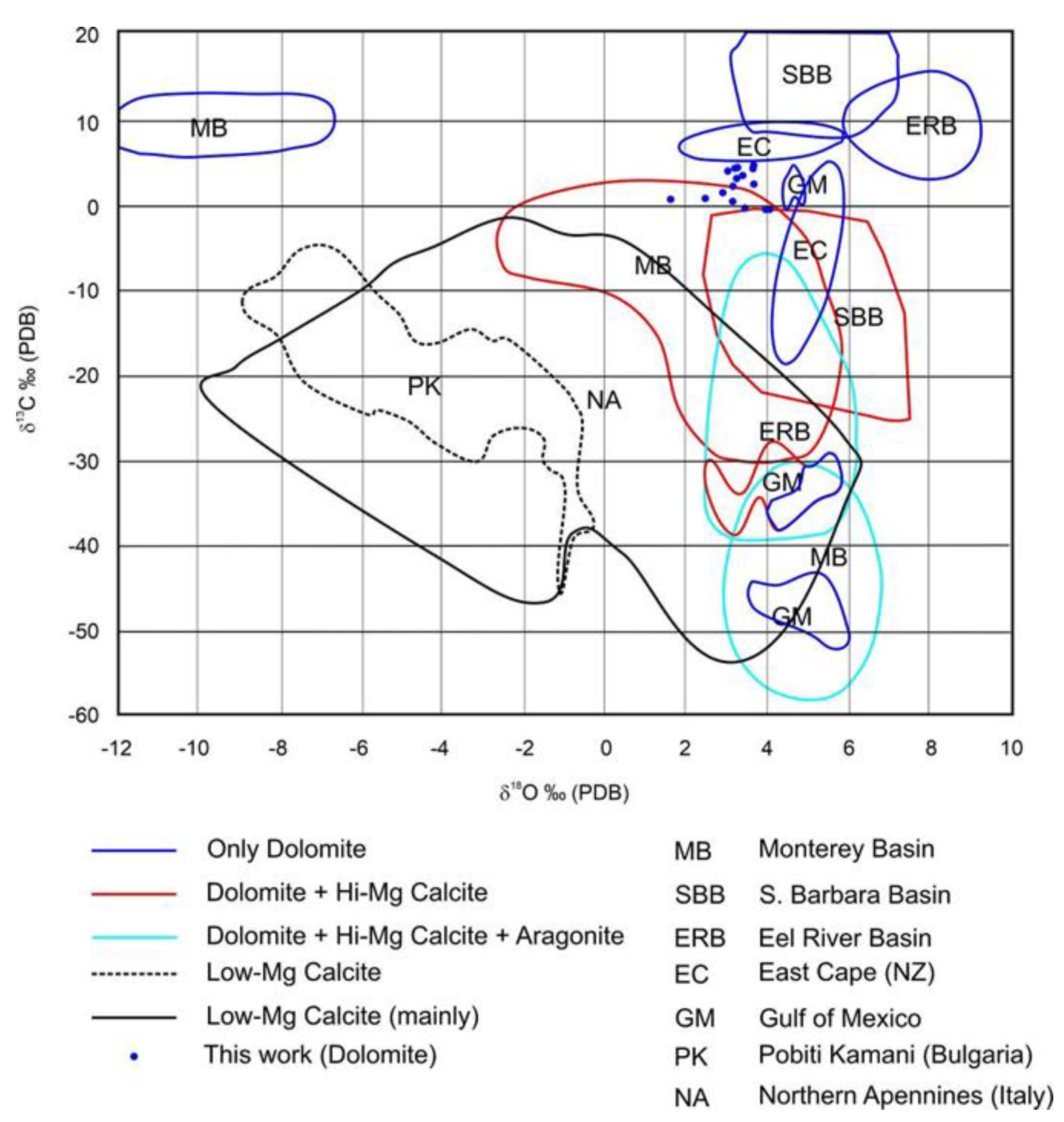
4.4. Stable Isotopes
4.5. Hosting Sediments
5. Discussion
5.1. Origin of the Fluids
5.2. Formation of Dolomite Conduits
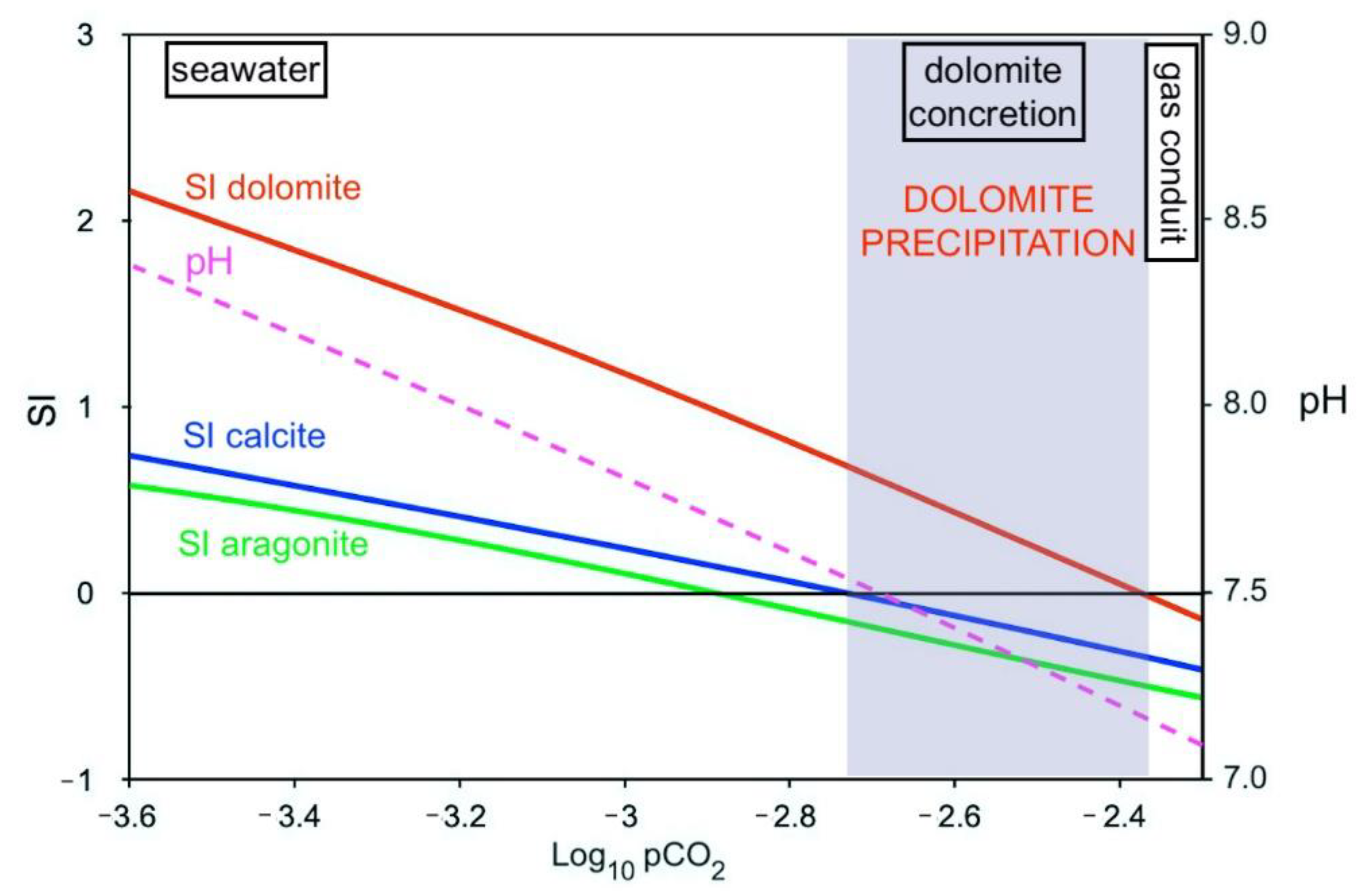
5.3. CO2 Flux and Dolomite Stability
5.4. Effects of CO2 Flow in the Marine Environment and Inhabitants
5.5. Geological and Palaeoenvironmental Implications
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capozzi, R.; Negri, A.; Reitner, J.; Taviani, M. Carbonate conduits linked to hydrocarbon-enriched fluid escape. Mar. Petrol. Geol. 2015, 66, 497–500. [Google Scholar] [CrossRef]
- Nelson, C.S.; Nyman, S.L.; Campbell, K.A.; Rowland, J.R. Influence of faulting on the distribution and development of cold seep-related dolomitic conduit concretions at East Cape, New Zealand. N. Z. J. Geol. Geop. 2017, 60, 478–496. [Google Scholar] [CrossRef]
- Judd, A.G.; Hovland, M. Seabed Fluid Flow—The Impact on Geology, Biology and the Marine Environment; Cambridge University Press: Cambridge, UK, 2007; pp. 1–475. [Google Scholar]
- Aloisi, G.; Pierre, C.; Rouchy, J.-M.; Foucher, J.-P.; Woodside, J. Methane-related authigenic carbonates of eastern Mediterranean Sea mud volcanoes and their possible relation to gas hydrate destabilisation. Earth Planet. Sci. Lett. 2000, 184, 321–338. [Google Scholar] [CrossRef]
- Pearson, M.J.; Grosjean, E.; Nelson, C.S.; Nyman, S.L.; Logan, G.A. Tubular concretions in New Zealand petroliferous basins: Lipid biomarker evidence for mineralisation around proposed Miocene hydrocarbon seep conduits. J. Petrol. Geol. 2010, 33, 205–220. [Google Scholar] [CrossRef]
- Nyman, S.L.; Nelson, C.S.; Campbell, K.A. Miocene tubular concretions in East Coast Basin, New Zealand: Analogue for the subsurface plumbing of cold seeps. Mar. Geol. 2010, 272, 319–336. [Google Scholar] [CrossRef]
- Campbell, K.A.; Francis, D.A.; Collins, M.; Gregory, M.R.; Nelson, C.S.; Greinert, J.; Aharon, P. Hydrocarbon seep-carbonates of a Miocene forearc (East Coast Basin), North Island, New Zealand. Sediment. Geol. 2008, 204, 83–105. [Google Scholar] [CrossRef]
- Metz, C.L. Tectonic controls on the genesis and distribution of late cretaceous, western interior basin hydrocarbon-seep mounds (tepee buttes) of North America. J. Geol. 2010, 118, 201–213. [Google Scholar] [CrossRef]
- Majima, R.; Nobuhara, T.; Kitazaki, T. Review of fossil chemosynthetic assemblages in Japan. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 227, 86–123. [Google Scholar] [CrossRef]
- Bojanowski, M.U. Oligocene cold-seep carbonates from the Carpathyans and their inferred relation to gas hydrates. Facies 2007, 53, 347–360. [Google Scholar] [CrossRef]
- Angeletti, L.; Canese, S.; Franchi, F.; Montagna, P.; Reitner, J.; Walliser, E.O.; Taviani, M. The “chimney forest” of the deep Montenegrin margin, South-Eastern Adriatic Sea. Mar. Petrol. Geol. 2015, 66, 542–554. [Google Scholar] [CrossRef]
- De Boever, E.; Swennen, R.; Dimitrov, L. Lower Eocene carbonate cemented chimneys (Varna, NE Bulgaria): Formation mechanisms and the (a)biological mediation of chimney growth? Sediment. Geol. 2006, 185, 159–173. [Google Scholar] [CrossRef]
- De Boever, E.; Huysmans, M.; Muchez, P.; Dimitrov, L.; Swennen, R. Controlling factors on the morphology and spatial distribution of methane-related tubular—Case study of an early Eocene seep system. Mar. Petrol. Geol. 2009, 26, 1580–1591. [Google Scholar] [CrossRef]
- Böttner, C.; Callow, B.J.; Schramm, B.; Gross, F.; Geersen, J.; Schmidt, M.; Vasilev, A.; Petsinski, P.; Berndt, C. Focused methane migration formed pipe structures in permeable sandstones: Insights from uncrewed aerial vehicle-based digital outcrop analysis in Varna, Bulgaria. Sedimentology 2021, 68, 2765–2782. [Google Scholar] [CrossRef]
- Conti, S.; Argentino, C.; Fioroni, C.; Salocchi, A.C.; Fontana, D. Miocene seep-carbonates of the Northern Apennines (Emilia to Umbria, Italy): An overview. Geosciences 2021, 11, 53. [Google Scholar] [CrossRef]
- Barbieri, R.; Cavalazzi, B. Microbial fabrics from Neogene cold seep carbonates, Northern Apennine, Italy. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 227, 143–155. [Google Scholar] [CrossRef]
- Taviani, M.; Roveri, M.; Aharon, P.; Zibrowius, H. A Pliocene Deepwater Cold Seep (Stirone River, N. Italy). In Cold-E-Vent. Hydrocarbon Seepage and Chemosynthesis in Tethyan Relic Basins: Products, Processes and Causes, Proceedings of the International Field Workshop to Be Held in Bologna and Nearby Apennines, Bologna, Italy, 23–26 June 1997; Vai, G.B., Taviani, M., Conti, S., Aharon, P., Eds.; Abstract with Program, 20; ISMAR: Bologna, Italy, 1997. [Google Scholar]
- Cau, S.; Franchi, F.; Roveri, M.; Taviani, M. The Pliocene-age Stirone River hydrocarbon chemoherm complex (Northern Apennines, Italy). Mar. Petrol. Geol. 2015, 66, 582–595. [Google Scholar] [CrossRef]
- Oppo, D.; Capozzi, R.; Picotti, V.; Ponza, A. A genetic model of hydrocarbon-derived carbonate chimneys in shelfal fine-grained sediments: The Enza River field, Northern Apennines (Italy). Mar. Petrol. Geol. 2015, 66, 555–565. [Google Scholar] [CrossRef]
- Viola, I.; Oppo, D.; Franchi, F.; Capozzi, R.; Dinelli, E.; Liverani, B.; Taviani, M. Mineralogy, geochemistry and petrography of methane-derived authigenic carbonates from Enza River, Northern Apennines (Italy). Mar. Petrol. Geol. 2015, 66, 566–581. [Google Scholar] [CrossRef]
- Blumenberg, M.; Walliser, E.O.; Taviani, M.; Seifert, R.; Reitner, J. Authigenic carbonate formation and its impact on the biomarker inventory at hydrocarbon seeps—A case study from the Holocene Black Sea and the Plio-Pleistocene Northern Apennines (Italy). Mar. Petrol. Geol. 2015, 66, 532–541. [Google Scholar] [CrossRef]
- Cavagna, S.; Clari, P.; De la Pierre, F.; Martire, L.; Natalicchio, M. Sluggish and steady focussed flows through fine grained sediments: The methane-derived cylindrical concretions of the Tertiary Piedmont Basin (NW Italy). Mar. Petrol. Geol. 2015, 66, 596–605. [Google Scholar] [CrossRef]
- Dela Pierre, F.; Martire, L.; Natalicchio, M.; Clari, P.; Petrea, C. Authigenic carbonates in upper Miocene sediments of the Tertiary Piedmont Basin (NW Italy): Vestiges of an ancient gas hydrate stability zone? Geol. Soc. Am. Bull. 2010, 122, 994–1010. [Google Scholar] [CrossRef] [Green Version]
- Greinert, J.; Bohrmann, G.; Suess, E. Methane-Venting and Gas Hydrate-Related Carbonates at the Hydrate Ridge: Their Classification, Distribution and Origin. In Natural Gas Hydrates: Occurrence, Distribution, and Detection; Paull, C.K., Dillon, W.P., Eds.; Geophysical Monograph 124; American Geophysical Union: Washington, DC, USA, 2001; pp. 99–113. [Google Scholar]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Peckmann, J.; Goedert, J.L.; Thiel, V.; Michaelis, W.; Reitner, J. A comprehensive approach to the study of methane-seep deposits from the Lincoln Creek Formation, Western Washington State, USA. Sedimentology 2002, 49, 855–873. [Google Scholar] [CrossRef]
- Campbell, K.A.; Farmer, J.D.; Des Marais, D. Ancient hydrocarbon seeps from the Mesozoic convergent margin of California: Carbonate geochemistry, fluids and palaeoenvironments. Geofluids 2002, 2, 63–94. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, R.; Baldanza, A. Integrated stratigraphy of the marine early Pleistocene in Umbria. Geosciences 2020, 10, 371 . [Google Scholar] [CrossRef]
- Martini, I.P.; Sagri, M. Tectono-sedimentary characteristics of late Miocene-Quaternary extensional basins of the North Apennines, Italy. Earth Sci. Rev. 1993, 34, 197–223. [Google Scholar] [CrossRef]
- Conti, P.; Cornamusini, G.; Carmignani, L. An outline of the geology of the Northern Apennines (Italy), with geological map at 1:250,000 scale. Ital. J. Geosci. 2020, 139, 149–194. [Google Scholar] [CrossRef]
- ISPRA. Carta Geologica D’italia 1:50.000. Catalogo delle Formazioni. I Quaderni Serie III 2007, 7(VII), 318–330. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/periodici-tecnici/i-quaderni-serie-iii-del-sgi/carta-geologica-ditalia-1-50-000-catalogo-delle-3 (accessed on 3 August 2020).
- Bizzarri, R.; Rosso, A.; Famiani, F.; Baldanza, A. Lunulite bryozoans from early Pleistocene deposits of SW Umbria (Italy), sedimentological and paleoecological inferences. Facies 2015, 61, 420. [Google Scholar] [CrossRef]
- Monaco, P.; Baldanza, A.; Bizzarri, R.; Famiani, F.; Lezzerini, M.; Sciuto, F. Ambergris cololites of Pleistocene sperm whales from central Italy and description of the new ichnogenus and ichnospecies Ambergrisichnus Alleronae. Palaeontol. Electron. 2014, 17, 17.2.29A. [Google Scholar] [CrossRef]
- Peccerillo, A.; Frezzotti, M.L. Magmatism, mantle evolution and geodynamics at the converging plate margins of Italy. J. Geol. Soc. 2015, 172, 407–427. [Google Scholar] [CrossRef] [Green Version]
- Petrelli, M.; Bizzarri, R.; Morgavi, D.; Baldanza, A.; Perugini, D. Combining machine learning techniques, microanalyses and large geochemical datasets for tephrochronological studies in complex volcanic areas: New age constraints for the Pleistocene magmatism of Central Italy. Quat. Geochronol. 2017, 40, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, R.; Baldanza, A. Plio-Pleistocene deltaic deposits in the Città della Pieve area (Western Umbria, Central Italy), facies analysis and inferred relations with the South Chiana Valley fluvial deposits. Quaternario 2009, 22, 127–138. [Google Scholar]
- Baldanza, A.; Bizzarri, R.; Famiani, F.; Garassino, A.; Pasini, G.; Cherin, M.; Rosatini, F. The early Pleistocene whale-fall community of Bargiano (Umbria, Central Italy): Paleoecological insights from benthic foraminifera and brachyuran crabs. Palaeontol. Electron. 2018, 21, 21.1.11A. [Google Scholar] [CrossRef]
- Baldanza, A.; Bizzarri, R.; Famiani, F.; Monaco, P.; Pellegrino, R.; Sassi, P. Enigmatic, biogenically induced structures in Pleistocene marine deposits, a first record of fossil ambergris. Geology 2013, 41, 1075–1078. [Google Scholar] [CrossRef]
- Brigante, R.; Cencetti, C.; De Rosa, P.; Fredduzzi, A.; Radicioni, F.; Stoppini, A. Use of aerial multispectral images for spatial analysis of flooded riverbed-alluvial plain systems: The case study of the Paglia River (Central Italy). Geomat. Nat. Haz. Risk 2017, 8, 1126–1143. [Google Scholar] [CrossRef] [Green Version]
- Cencetti, C.; De Rosa, P.; Fredduzzi, A. Geoinformatics in morphological study of River Paglia, Tiber River basin, Central Italy. Environ. Earth Sci. 2017, 76, 128. [Google Scholar] [CrossRef]
- Backman, J.; Raffi, I.; Rio, D.; Fornaciari, E.; Pälike, H. Biozonation and biochronology of Miocene through Pleistocene calcareous nannofossils from low and middle latitudes. Newsl. Stratigr. 2012, 45, 221–244. [Google Scholar] [CrossRef]
- Iaccarino, S.; Premoli Silva, I.; Biolzi, M.; Foresi, L.M.; Lirer, F.; Turco, E.; Petrizzo, M.R. Practical Manual of Neogene Planktonic Foraminifera. In Proceedings of the International School on Planktonic Foraminifera 6th Course, Perugia, Italy, 19–23 February 2007; University of Perugia: Perugia, Italy, 2007; pp. 1–181. [Google Scholar]
- Kontnik, R.; Boask, T.; Butcher, R.A.; Brocks, J.J.; Losick, R.; Clardy, J.; Pearson, A. Sporulenes, heptaprenyl metabolites from Bacillus subtilis spores. Org. Lett. 2008, 10, 3551–3554. [Google Scholar] [CrossRef]
- Bosak, T.; Losick, R.M.; Pearson, A. A polycyclic terpenoid that alleviates oxidative stress. Proc. Natl. Acad. Sci. USA 2008, 105, 6725–6729. [Google Scholar] [CrossRef] [Green Version]
- Bray, E.E.; Evans, E.D. Distribution of n-paraffins as a clue to recognition of source beds. Geochem. Cosmochim. Acta 1961, 22, 2–15. [Google Scholar] [CrossRef]
- Scanlan, R.S.; Smith, J.E. An improved measure of odd-to-even predominance in the normal alkanes of sediment extracts and petroleum. Geochem. Cosmochim. Acta 1970, 34, 611–620. [Google Scholar] [CrossRef]
- McDuffee, K.E.; Eglinton, T.I.; Sessions, A.L.; Sylva, S.; Wagner, T.; Hayes, J.M. Rapid analysis of 13C in plant-wax n-alkanes for reconstruction of terrestrial vegetation signals from aquatic sediments. Geochem. Geophys. Geosyst. 2004, 5, Q10004. [Google Scholar] [CrossRef]
- Elvert, M.; Suess, E.; Greinert, J.; Whiticar, M.J. Archaea mediating anaerobic methane oxidation in deep-sea sediments at cold seeps of the Eastern Aleutiansubduction zone. Org. Geochem. 2000, 31, 1175–1187. [Google Scholar] [CrossRef]
- Robson, J.N.; Rowland, S.J. Synthesis, chromatographic and spectral characterisation of 2,6,11,15-tetramethylhexadecane (crocetane) and 2,6,9,13-tetramethyltetradecane; reference acyclic isoprenoids for geochemical studies. Org. Geochem. 1992, 20, 1093–1098. [Google Scholar] [CrossRef]
- Rowland, S.J.; Lamb, N.A.; Wilkinson, C.F.; Maxwell, J.R. Confirmation of 2,6,10,15,19-pentamethyleicosane in methanogenic bacteria and sediments. Tetrahedron Lett. 1982, 23, 101–104. [Google Scholar] [CrossRef]
- Elvert, M.; Greinert, J.; Suess, E.; Whiticar, M.J. Carbon Isotopes of Biomarkers Derived from Methane-Oxidizing Microbes at Hydrate Ridge, Cascadia Convergent Margin. In Natural Gas Hydrates: Occurrence, Distribution, and Dynamics; Paull, C.K., Dillon, W.P., Eds.; Geophysical Monograph Series 124; American Geophysical Union: Washington, DC, USA, 2001; pp. 115–129. [Google Scholar] [CrossRef]
- Valentine, D.L. Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: A review. Antonie Van Leeuwenhoek J. Microb. 2002, 81, 271–282. [Google Scholar] [CrossRef]
- Gough, M.; Rowland, S. Characterization of unresolved complex mixtures of hydrocarbons in petroleum. Nature 1990, 344, 648–650. [Google Scholar] [CrossRef]
- Middleditch, B.S. Analytical Artifacts: GC, MS, HPLC, TLC and PC, 1st ed.; Journal of Chromatography Library 44; Elsevier Science: Amsterdam, The Netherlands, 1989; pp. 1–103. [Google Scholar]
- Keith, M.L.; Weber, J.N. Carbon and oxygen isotopic composition of selected limestone and fossils. Geochim. Cosmochim. Acta 1964, 28, 1787–1816. [Google Scholar] [CrossRef]
- Rosenthal, Y.; Morley, A.; Barras, C.; Katz, M.E.; Jorissen, F.; Reichart, G.-J.; Oppo, D.W.; Linsley, B.K. Temperature calibration of Mg/Ca ratios in the intermediate water benthic foraminifer Hyalinea balthica. Geochem. Geophys. Geosyst. 2011, 12, Q04003. [Google Scholar] [CrossRef] [Green Version]
- Tong, H.; Feng, D.; Peckmann, J.; Roberts, H.H.; Chen, L.; Bian, Y.; Chen, D. Environments favouring dolomite formation at cold seeps: A case study from the Gulf of Mexico. Chem. Geol. 2019, 518, 9–18. [Google Scholar] [CrossRef]
- Naehr, T.H.; Eichhubl, P.; Orphan, V.J.; Hovland, M.; Paull, C.K.; Ussler, W., III; Lorenson, T.D.; Green, H.G. Authigenic carbonate formation at hydrocarbon seeps in continental margin sediments: A comparative study. Deep-Sea Res. II 2007, 54, 1268–1291. [Google Scholar] [CrossRef]
- Bizzarri, R.; Baldanza, A.; Petrelli, M.; Famiani, F.; Peccerillo, A. Early Pleistocene distal pyroclastic-fallout material in continental and marine deposits of western Umbria (Italy): Chemical composition, provenance and correlation potential. Quaternario 2010, 23, 245–250. [Google Scholar]
- Chiodini, G.; Frondini, F.; Cardellini, C.; Parello, F.; Peruzzi, L. Rate of diffuse carbon dioxide Earth degassing estimated from carbon balance of regional aquifers: The case of central Apennine, Italy. J. Geophys. Res. 2000, 105, 8423–8434. [Google Scholar] [CrossRef]
- Chiodini, G.; Caliro, S.; Aiuppa, A.; Avino, A.; Granieri, D.; Moretti, R.; Parello, F. First 13C/12C isotopic characterisation of volcanic plume CO2. Bull. Volcanol. 2011, 73, 531–542. [Google Scholar] [CrossRef]
- Chiodini, G.; Pappalardo, L.; Aiuppa, A.; Caliro, S. The geological CO2 degassing history of a long-lived caldera. Geology 2015, 43, 767–770. [Google Scholar] [CrossRef]
- Caliro, S.; Chiodini, G.; Avino, R.; Cardellini, C.; Frondini, F. Volcanic degassing at Somma–Vesuvio (Italy) inferred by chemical and isotopic signatures of groundwater. Appl. Geochem. 2005, 20, 1060–1076. [Google Scholar] [CrossRef]
- Fritz, P.; Smith, D.G.W. The isotopic composition of secondary dolomites. Geochim. Cosmochim. Acta 1970, 34, 1161–1173. [Google Scholar] [CrossRef]
- Chiodini, G.; Baldini, A.; Barberi, F.; Carapezza, M.L.; Cardellini, C.; Frondini, F.; Granieri, D.; Ranaldi, M. Carbon dioxide degassing at Latera caldera (Italy): Evidence of geothermal reservoir and evaluation of its potential energy. J. Geophys. Res. 2007, 112, B12204. [Google Scholar] [CrossRef]
- Carapezza, M.L.; Ranaldi, M.; Gattuso, A.; Pagliuca, N.M.; Tarchini, L. The sealing capacity of the cap rock above the Torre Alfina geothermal reservoir (Central Italy) revealed by soil CO2 flux investigations. J. Volcanol. Geotherm. Res. 2015, 291, 25–34. [Google Scholar] [CrossRef]
- Frondini, F. Geochemistry of regional aquifer systems hosted by carbonate-evaporite formations in Umbria and southern Tuscany (central Italy). Appl. Geochem. 2008, 23, 2091–2104. [Google Scholar] [CrossRef]
- Chiodini, G.; Cardellini, C.; Bini, G.; Frondini, F.; Caliro, S.; Ricci, L.; Lucidi, B. The carbon dioxide emission as indicator of the geothermal heat flow: Review of local and regional applications with a special focus on Italy. Energies 2021, 14, 6590. [Google Scholar] [CrossRef]
- Aiuppa, A.; Hall-Spencer, J.; Milazzo, M.; Turco, G.; Caliro, S.; Di Napoli, R. Volcanic CO2 seep geochemistry and use in understanding ocean acidification. Biogeochemistry 2021, 152, 93–115. [Google Scholar] [CrossRef]
- Turekian, K.K. Oceans; Foundations of Earth Science Series; Prentice-Hall: Englewood, NJ, USA, 1968; pp. 1–120. [Google Scholar]
- Parkhurst, D.L. User’s Guide to PHREEQC—A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations; U.S. Geological Survey Water-Resources Investigations Report 95-4227; USGS: Reston, VA, USA, 1995; pp. 1–143. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In US Geological Survey Techniques and Methods; Book 6, Chapter A43; USGS: Reston, VA, USA, 2013; p. 497. Available online: http://pubs.usgs.gov/tm/06/a43 (accessed on 6 January 2021).
- Wolery, T.J.; Daveler, S.A. EQ6, A Computer Program for Reaction Path Modeling of Aqueous Geochemical Systems: Theoretical Manual, User’s Guide, and Related Documentation (Version 7.0); Lawrence Livermore National Laboratory, University of California: Livermore, CA, USA, 1992; pp. 1–359. [Google Scholar]
- Nemoto, T.; Okiyama, M.; Iwasaki, N.; Kikuchi, T. Squid as Predators on Krill (Euphausia superba) and Prey for Sperm Whales in the Southern Ocean. In Antarctic Ocean and Resources Variability; Sahrhage, D., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 292–296. [Google Scholar] [CrossRef]
- Díaz-del-Río, V.; Somoza, L.; Martínez-Frias, J.; Mata, M.P.; Delgado, A.; Hernandez-Molina, F.J.; Lunar, R.; Martín-Rubí, J.A.; Maestro, A.; Fernández-Puga, M.C.; et al. Vast fields of hydrocarbon-derived carbonate chimneys related to the accretionary wedge/olistostrome of the Gulf of Cádiz. Mar. Geol. 2003, 195, 177–200. [Google Scholar] [CrossRef]
- Aiello, I.W.; Garrison, R.E.; Moore, J.C.; Kastner, M.; Stakes, D.S. Anatomy and origin of carbonate structures in a Miocene cold-seep field. Geology 2001, 29, 1111–1114. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldanza, A.; Bizzarri, R.; Boschi, C.; Famiani, F.; Frondini, F.; Lezzerini, M.; Rowland, S.; Sutton, P.A. CO2-Degassing Carbonate Conduits in Early Pleistocene Marine Clayey Deposits in Southwestern Umbria (Central Italy). Minerals 2022, 12, 819. https://doi.org/10.3390/min12070819
Baldanza A, Bizzarri R, Boschi C, Famiani F, Frondini F, Lezzerini M, Rowland S, Sutton PA. CO2-Degassing Carbonate Conduits in Early Pleistocene Marine Clayey Deposits in Southwestern Umbria (Central Italy). Minerals. 2022; 12(7):819. https://doi.org/10.3390/min12070819
Chicago/Turabian StyleBaldanza, Angela, Roberto Bizzarri, Chiara Boschi, Federico Famiani, Francesco Frondini, Marco Lezzerini, Steven Rowland, and Paul A. Sutton. 2022. "CO2-Degassing Carbonate Conduits in Early Pleistocene Marine Clayey Deposits in Southwestern Umbria (Central Italy)" Minerals 12, no. 7: 819. https://doi.org/10.3390/min12070819
APA StyleBaldanza, A., Bizzarri, R., Boschi, C., Famiani, F., Frondini, F., Lezzerini, M., Rowland, S., & Sutton, P. A. (2022). CO2-Degassing Carbonate Conduits in Early Pleistocene Marine Clayey Deposits in Southwestern Umbria (Central Italy). Minerals, 12(7), 819. https://doi.org/10.3390/min12070819









