Mineralogy, Geochemistry, and Stable Isotopes (C, O, S) of Hot Spring Waters and Associated Travertines near Tamiahua Lagoon, Veracruz, Gulf of Mexico (Mexico)
Abstract
:1. Introduction
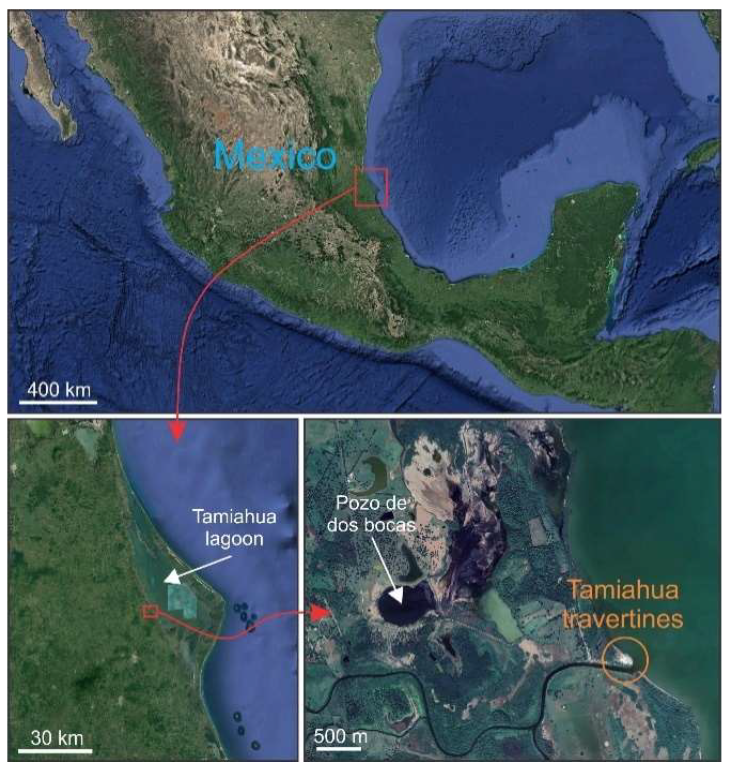
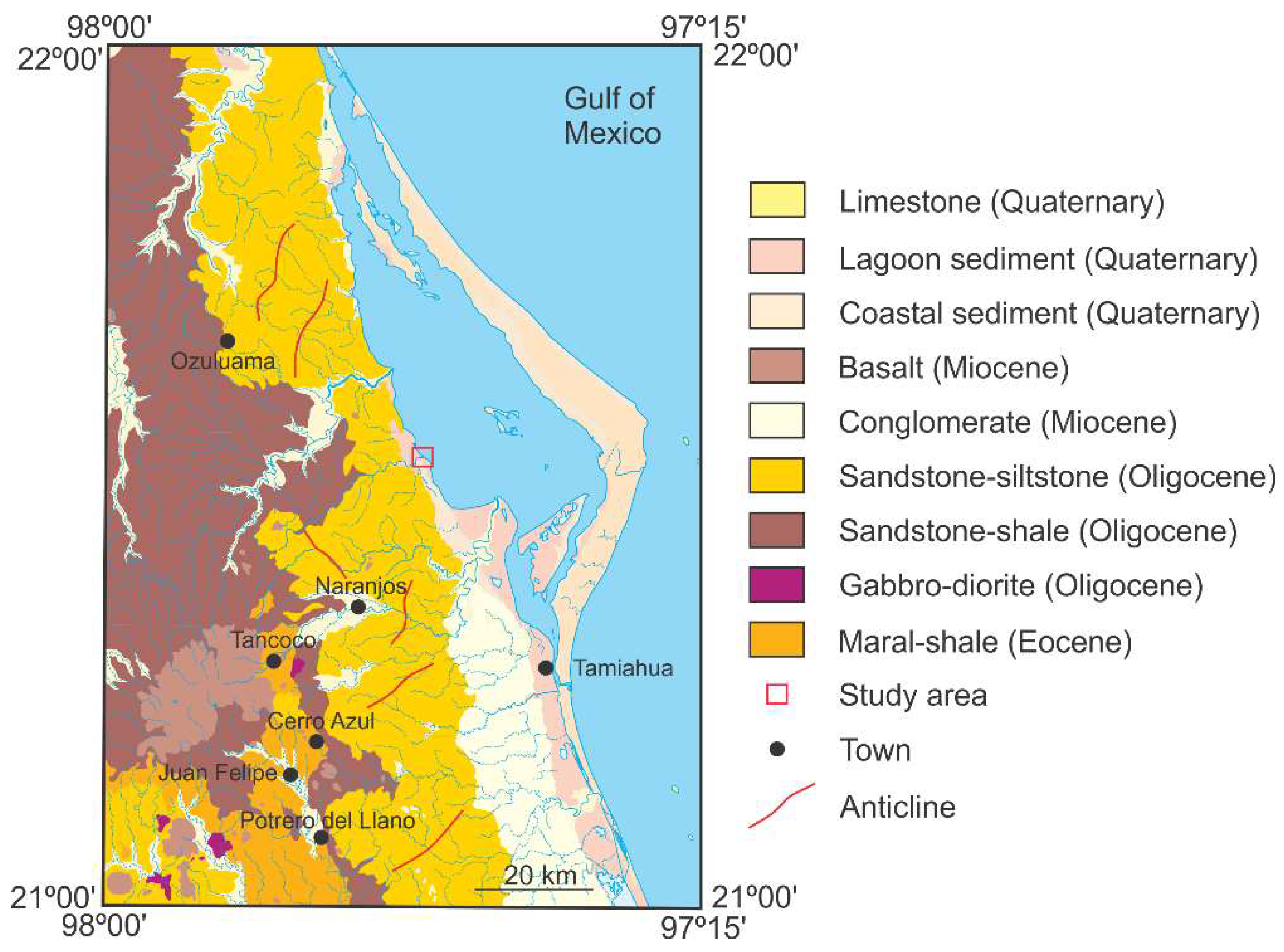
2. Geological Context
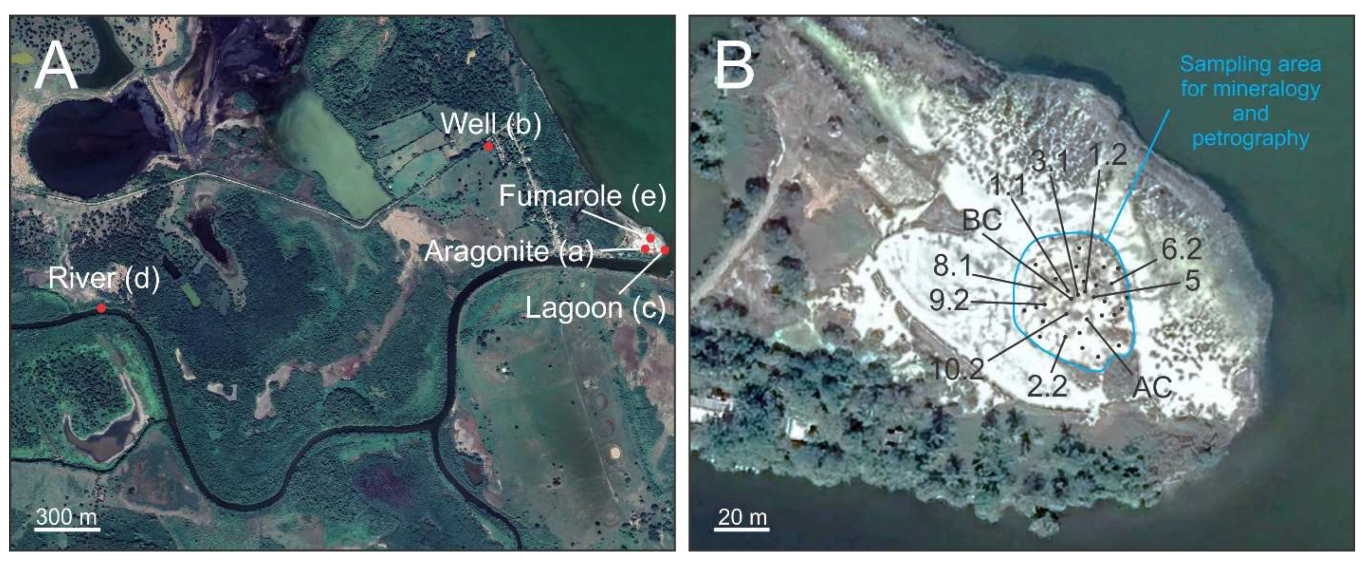
Outcrop Description
3. Materials and Methods
3.1. Rock System
3.2. Hydrothermal System near Tamiahua Lagoon
4. Results
4.1. Rock System
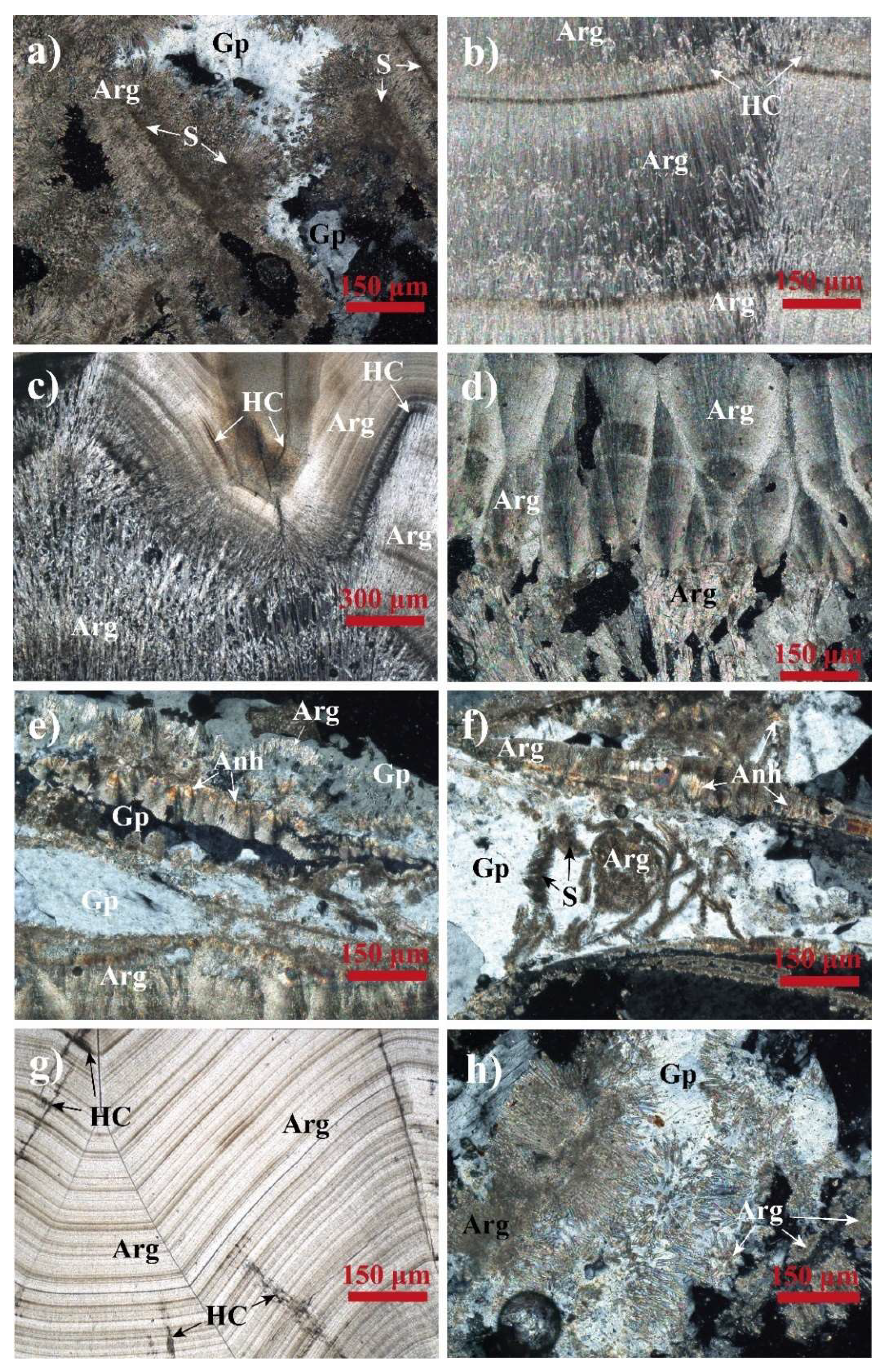
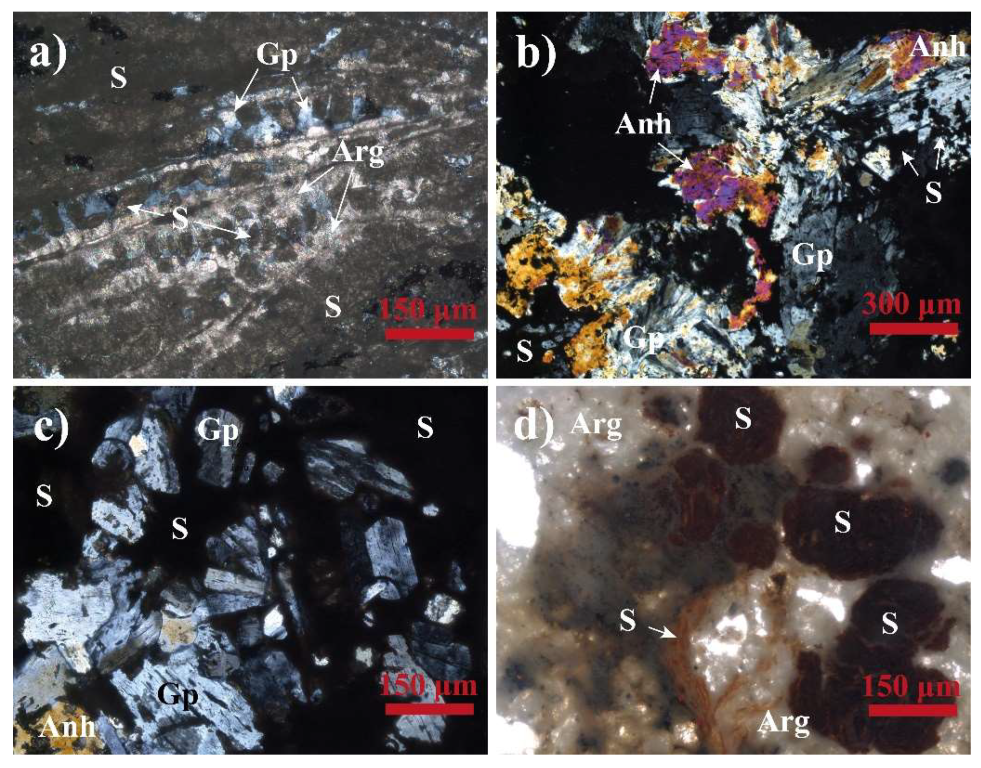
4.1.1. Mesofabric
4.1.2. Microfabric
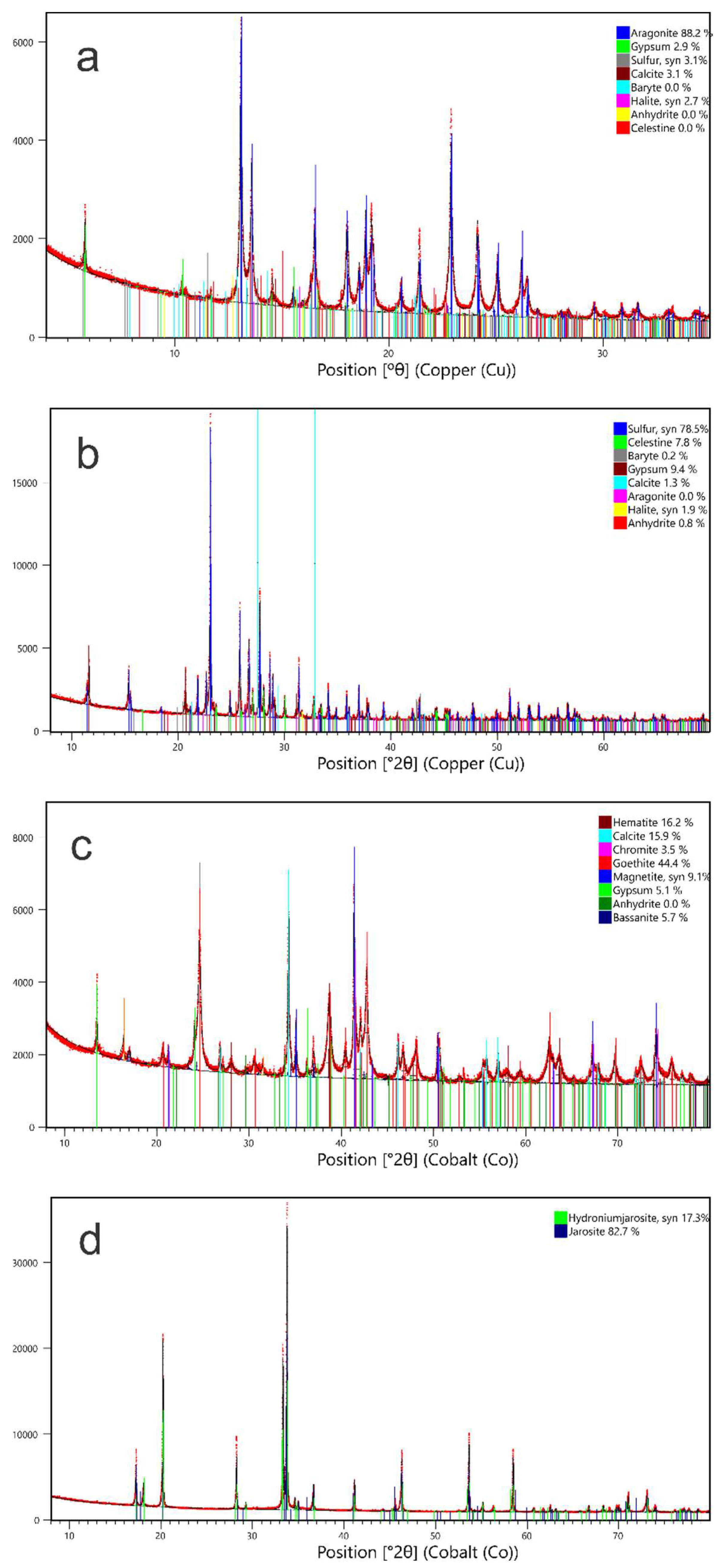
4.1.3. Mineralogy
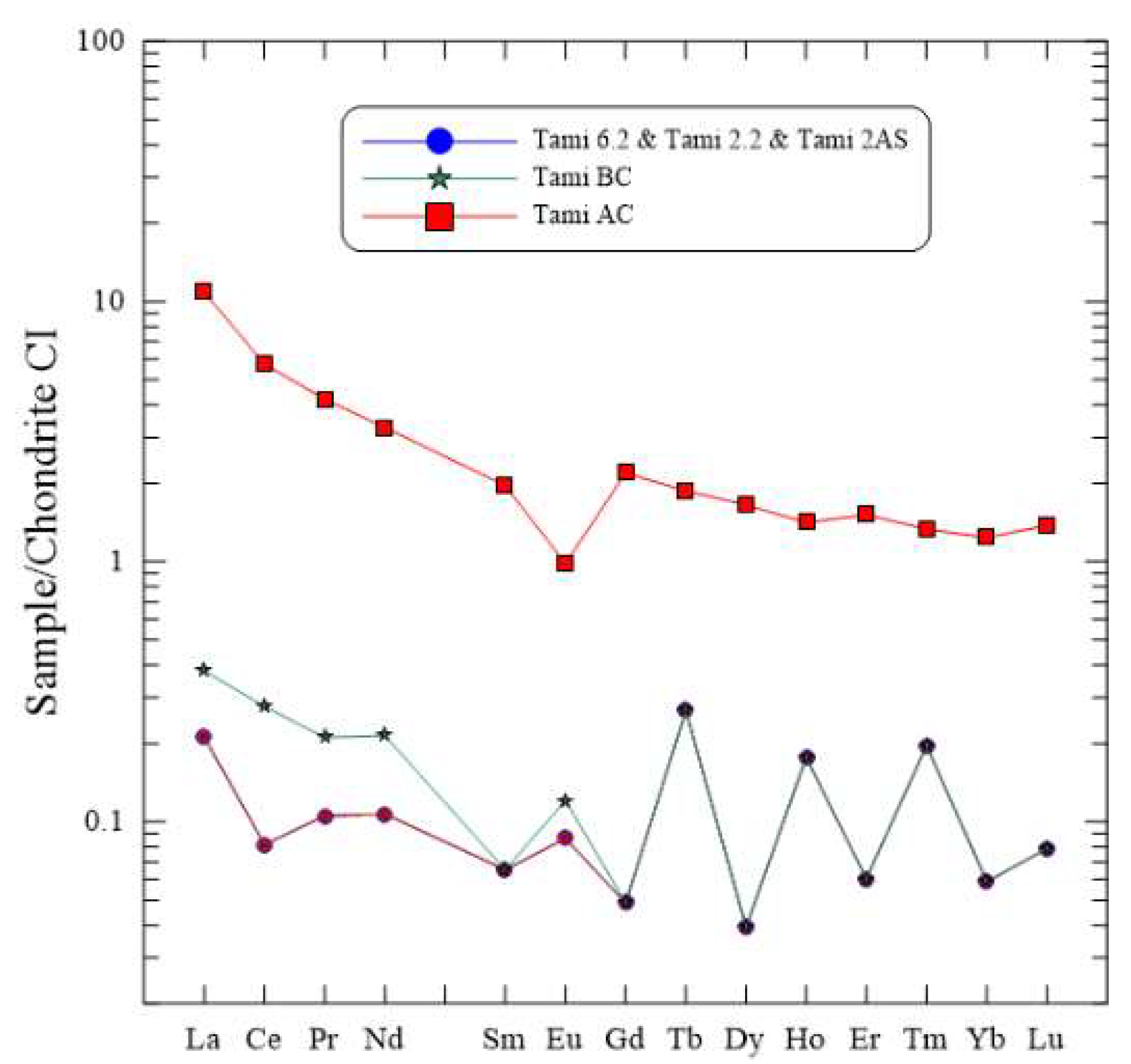
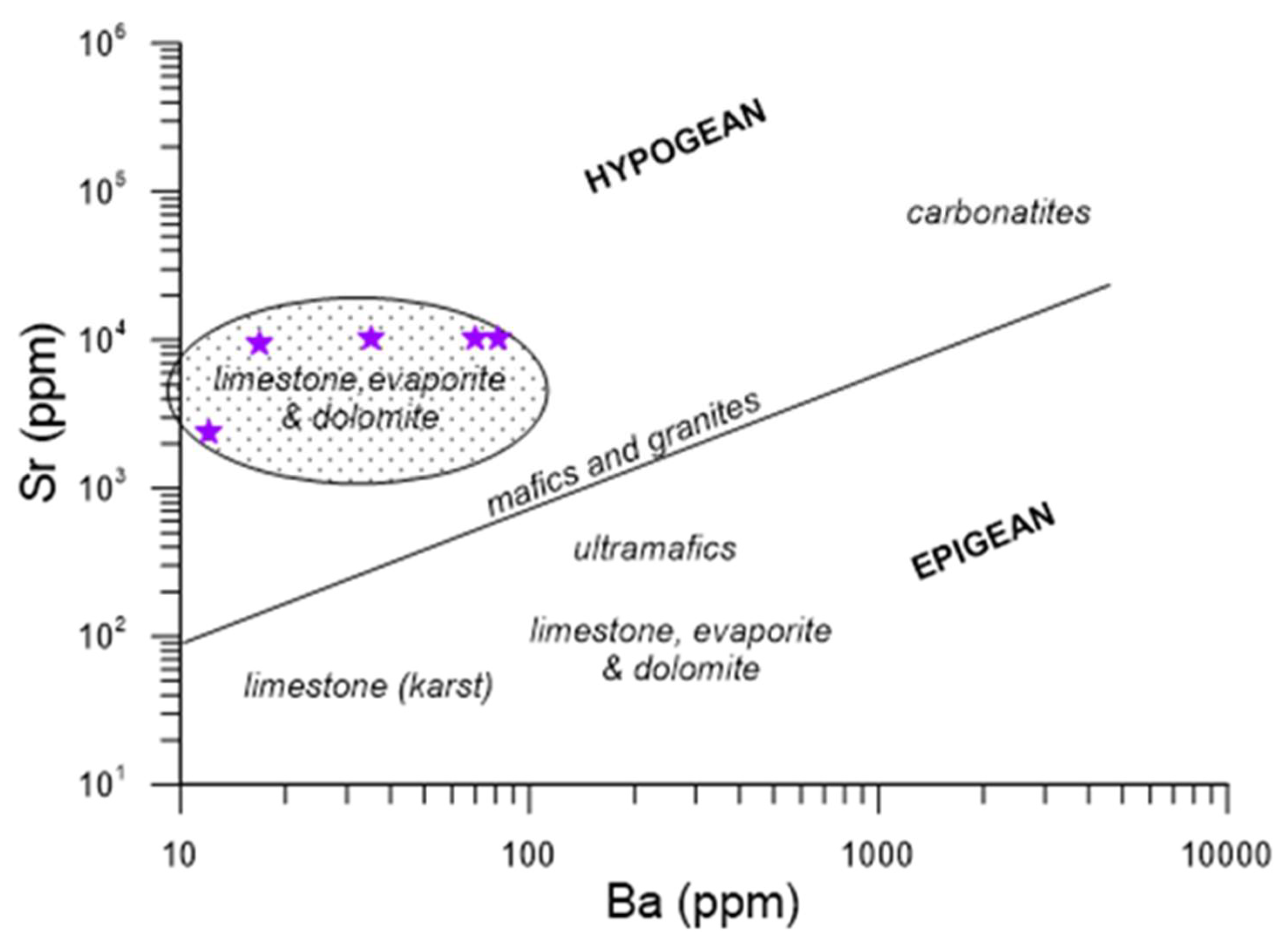
4.1.4. Geochemistry and Isotopic Composition of the Rock
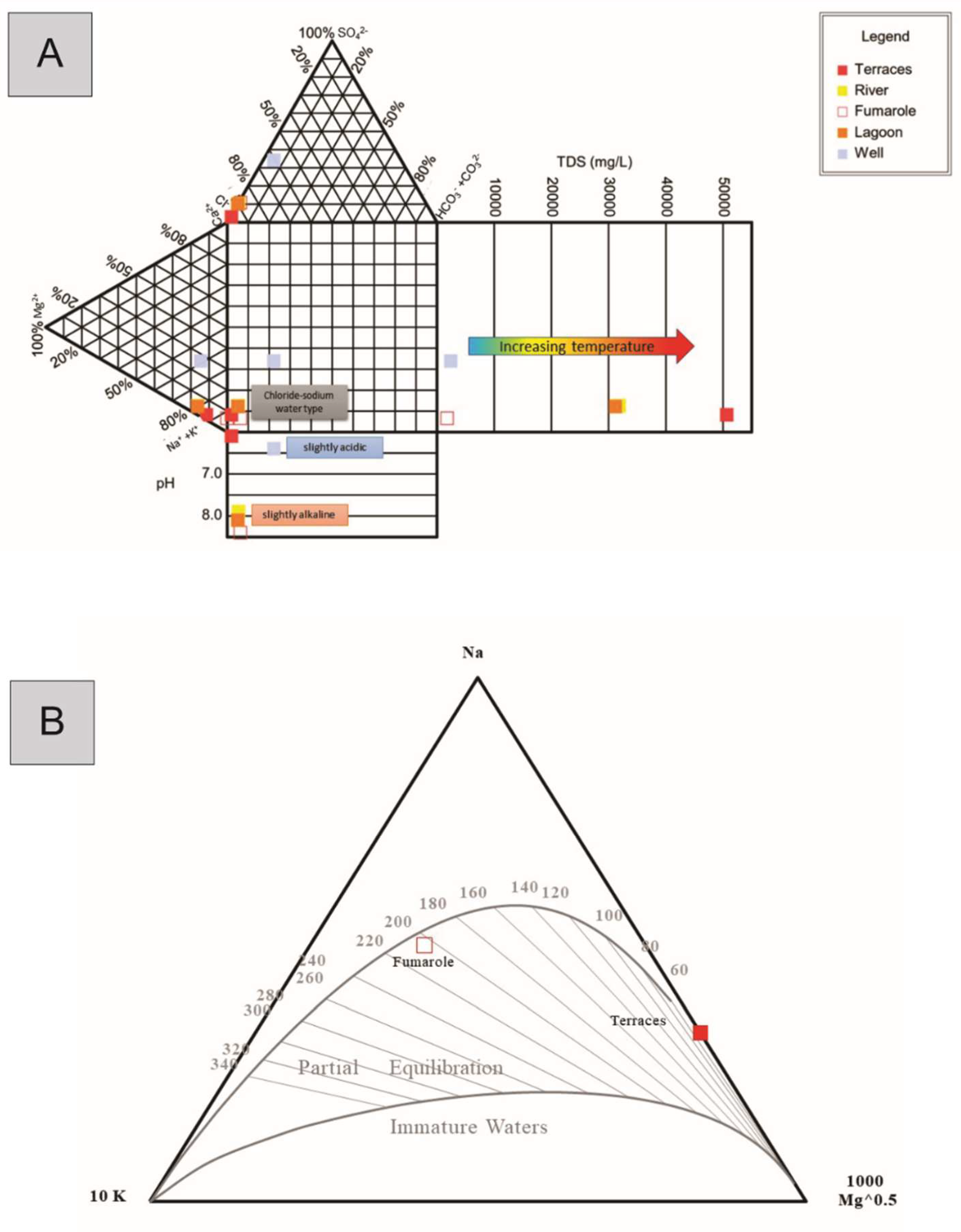
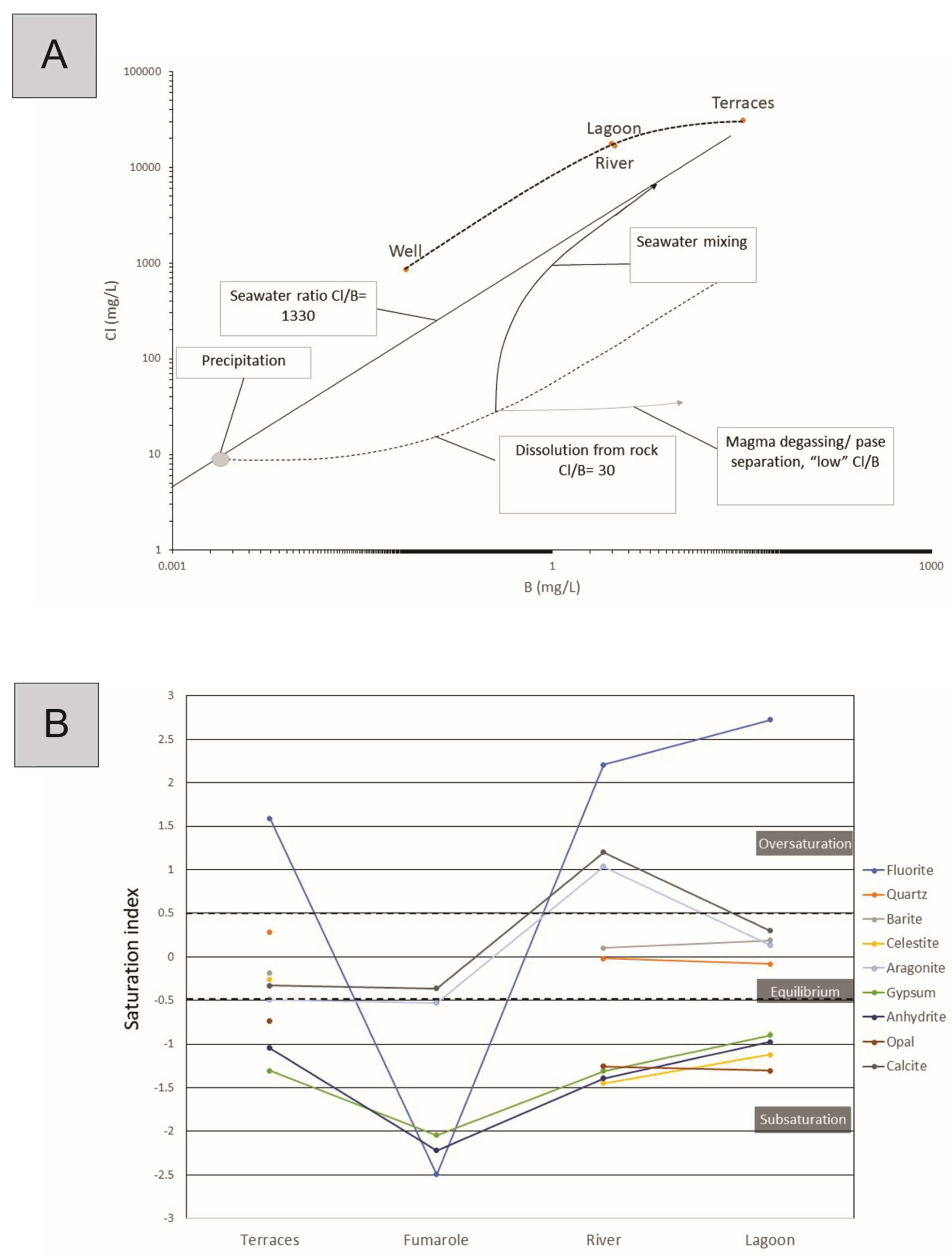
4.2. Water System
5. Discussion
5.1. Origin of the Hydrothermal System
5.2. Origin and Classification of Mineral Travertine System
5.2.1. Aragonite Precipitation and Its Typologies
5.2.2. Geochemistry, Isotopic Composition, and Travertine Classification
5.3. Geotectonic Context of Formation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ford, T.D.; Pedley, H.M. A review of tufa and travertine deposits of the world. Earth Sci. Rev. 1996, 41, 117–175. [Google Scholar] [CrossRef]
- Gandin, A.; Capezzuoli, E. Travertine versus calcareous tufa: Distinctive petrologic features and stable isotopes signatures. Quat. Ital. J. Quat. Sci. 2008, 21, 125–136. [Google Scholar]
- Gandin, A.; Capezzuoli, E. Travertine: Distinctive depositional fabrics of carbonates from thermal spring systems. Sedimentology 2014, 61, 264–290. [Google Scholar] [CrossRef]
- Capezzuoli, E.; Gandin, A.; Pedley, M. Decoding tufa and travertine (freshwater carbonates) in the sedimentary record: The state of the art. Sedimentology 2014, 61, 1–21. [Google Scholar] [CrossRef]
- Matera, P.F.; Ventruti, G.; Zucchi, M.; Brogi, A.; Capezzuoli, E.; Liotta, D.; Yu, T.-L.; Shen, C.-C.; Huntington, K.W.; Rinyu, L.; et al. Geothermal Fluid Variation Recorded by Banded Ca-Carbonate Veins in a Fault-Related, Fissure Ridge-Type Travertine Depositional System (Iano, southern Tuscany, Italy). Geofluids 2021, 2021, 8817487. [Google Scholar] [CrossRef]
- Pentecost, A. Travertine; Springer: Berlin/Heidelberg, Germany, 2005; pp. 11–240. [Google Scholar]
- Kano, A.; Okumura, T.; Takashima, C.; Shiraishi, F. Geomicrobiological Properties and Processes of Travertine with a Focus on Japanese Sites; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Luo, L.; Wen, H.; Li, Y.; You, Y.; Luo, X. Mineralogical, crystal morphological, and isotopic characteristics of smooth slope travertine deposits at Reshuitang, Tengchong, China. Sediment. Geol. 2019, 381, 29–45. [Google Scholar] [CrossRef]
- Pentecost, A.; Viles, H.A. A review and reassessment of travertine classification. Géogr. Phys. Quat. 1994, 48, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.; Peng, X. Signatures of biologically influenced CaCO3 and Mg–Fe silicate precipitation in hot springs: Case study from the Ruidian geothermal area, western Yunnan Province, China. Sedimentology 2014, 61, 56–89. [Google Scholar] [CrossRef]
- Rodríguez-Berriguete, A.; Alonso-Zarza, A.M. Controlling factors and implications for travertine and tufa deposition in a volcanic setting. Sediment. Geol. 2019, 381, 13–28. [Google Scholar] [CrossRef]
- Brogi, A.; Alçiçek, M.C.; Liotta, D.; Capezuoli, E.; Zucchi, M.; Matera, P.F. Step-over fault zones controlling geothermal fluid-flow and travertine formation (Denizli Basin, Turkey). Geothermics 2021, 89, 101941. [Google Scholar] [CrossRef]
- Teboult, P.-A.; Durlet, C.; Gaucher, E.C.; Virgone, A.; Girard, J.-P.; Curie, J.; Lopez, B.; Camoin, G.F. Origins of elements building travertine and tufa: New perspectives provided by isotopic and geochemical tracers. Sediment. Geol. 2016, 334, 97–114. [Google Scholar] [CrossRef]
- Pola, M.; Gandin, A.; Tuccimei, P.; Soligio, M.; Deianas, R.; Fabbri, P.; Zampieri, D. A multidisciplinary approach to understanding carbonate deposition under tectonically controlled hydrothermal circulation: A case study from a recent travertine mound in the Euganean hydrothermal system, northern Italy. Sedimentology 2014, 61, 172–199. [Google Scholar] [CrossRef]
- Bougeault, C.; Vennin, E.; Durlet, C.; Muller, E.; Mercuzot, M.; Chavez, M.; Gérard, E.; Ader, M.; Virgone, A.; Gaucher, E.C. Biotic-abiotic influences on modern Ca-Si-rich hydrothermal spring mounds of the Pastos Grandes volcanic caldera (Bolivia). Minerals 2019, 9, 380. [Google Scholar] [CrossRef] [Green Version]
- Chafetz, H.S.; Patrick, F.R.; Utech, N.M. Microenvironmental controls on mineralogy and habit of CaCO3 precipitates: An example from an active travertine system. Sedimentology 1991, 38, 107–126. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Guidry, S.A. Bacterial shrubs, crystal shrubs, and ray-crystal shrubs: Bacterial vs. abiotic precipitation. Sediment. Geol. 1999, 126, 57–74. [Google Scholar] [CrossRef]
- Koban, C.G.; Schweigert, G. Microbial Origin of Travertine Fabrics: Two Examples from Southern Germany (Pleistocene Stuttgart Travertines and Miocene Riedöschingen Travertine). Facies 1993, 29, 251–264. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Nishida, S.; Yukimura, K.; Naganuma, T.; Koike, H.; Arp, G.; Kano, A. Microbial Processes Forming Daily Lamination in an Aragonite Travertine, Nagano-yu Hot Spring, Southwest Japan. Geomicrobiol. J. 2011, 28, 135–148. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Akmaluddin; Kano, A. Textural transition in an aragonite travertine formed under various flow conditions at Pancuran Pitu, Central Java, Indonesia. Sediment. Geol. 2012, 265–266, 195–209. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Shiraishi, F.; Nishida, S.; Kano, A. Processes forming daily lamination in a microbe-rich travertine under low flow condition at the Nagano-yu hot spring, southwestern Japan. Geomicrobiol. J. 2013, 30, 910–927. [Google Scholar] [CrossRef]
- Okumura, T.; Takashima, C.; Kano, A. Textures and processes of laminated travertines formed by unicellular cyanobacteria in Myoken hot spring, southwestern Japan. Isl. Arc 2013, 22, 410–426. [Google Scholar] [CrossRef]
- Shiraishi, F.; Eno, Y.; Nakamura, Y.; Hanzawa, Y.; Asada, J.; Bahniuk, A.M. Relative influence of biotic and abiotic processes on travertine fabrics, Satono-yu hot spring, Japan. Sedimentology 2019, 66, 459–479. [Google Scholar] [CrossRef]
- Takashima, C.; Kano, A. Microbial processes forming daily lamination in a stromatolitic travertine. Sediment. Geol. 2008, 208, 114–119. [Google Scholar] [CrossRef]
- Takashima, C.; Okumura, T.; Nishida, S.; Koike, H.; Kano, A. Bacterial symbiosis forming laminated iron-rich deposits in Okuoku-hachikurou hot spring, Akita Prefecture Japan. Isl. Arc 2011, 20, 294–304. [Google Scholar] [CrossRef]
- Tatarinov, A.V.; Yalovik, L.I.; Kashkak, E.S.; Danilova, E.V.; Khromova, E.A.; Khakhinov, V.V.; Namsaraev, B.B. Mineralogical and geochemical features of bacterial mats and travertines of the Khoito-Gol thermal spring (East Sayan). Russ. Geol. Geophys. 2017, 58, 47–58. [Google Scholar] [CrossRef]
- Folk, R.L.; Chafetz, H.S.; Tiezzi, P.A. Bizarre forms of depositional and diagenetic calcite in hotspring travertines, central Italy. In Carbonate Cements; Special Publication, 36, Scheidemann, N., Harris, P., Eds.; Society of Economic Paleontologists and Mineralogists: Tulsa, OK, USA, 1985; pp. 349–369. [Google Scholar]
- Fabré, G.; Fiche, J.L. Les concretionnements de l’Aqueduct Romain de Nîmes. Mediterranée 1986, 1–2, 129–130. [Google Scholar]
- Riding, R. (Ed.) Classification of Microbial Carbonates. In Calcareous Algae and Stromatolites; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar] [CrossRef]
- Prol-Ledesma, R.M.; Morán-Zenteno, D.J. Heat flow and geothermal provinces in Mexico. Geothermics 2019, 78, 183–200. [Google Scholar] [CrossRef]
- Ferrari, L.; Tagami, T.; Eguchi, M.; Orozco-Esquivel, M.T.; Petrone, C.M.; Jacobo-Albarrán, J.; López-Martínez, M. Geology, geochronology and tectonic setting of late Cenozoic volcanism along the southwestern Gulf of Mexico: The Eastern Alkaline Province revisited. J. Volcanol. Geotherm. Res. 2005, 146, 284–306. [Google Scholar] [CrossRef]
- Maldonado Lee, J.M.; Rosales Franco, E.; Hernández Loredo, A.; Serrano Gómez, S.J. Carta Geológico Minera Tamiahua; F14-9, Veracruz, 1:250,000; Servicio Geológico Mexicano: Pachuca, Mexico, 2004. [Google Scholar]
- Santos-Llorente, J.; Uribe Cruz, M.; Benítez-Juárez, M.; Zavala, R.; Olvera Rivera, A. El petróleo en Veracruz. In Episodios Petroleros; 50 Aniversario; Petróleos Mexicanos: Mexico City, Mexico, 1988. [Google Scholar]
- Révész, K.M.; Landwehr, J.M.; Keybl, J. Measurement of δ13C and δ18O Isotopic Ratios of CaCO3 Using a Thermoquest-Finnigan GasBench II Delta Plus XL Continuous Flow Isotope Ratio Mass Spectrometer with Application to Devils Hole Core DH-11 Calcite; U.S. Geological Survey, Open-File Report; U.S. Geological Survey: Reston, VA, USA, 2001; pp. 1–257. [Google Scholar]
- Révész, K.M.; Landwehr, J.M. δ13C and δ18O isotopic composition of CaCO3 measured by continuous flow isotope ratio mass spectrometry statistical evaluation and verification by application to Devils Hole Core DH-11 Calcite. Rapid Commun. Mass Spectrom. 2002, 16, 2102–2114. [Google Scholar] [CrossRef]
- Coplen, T. Normalization of oxygen and hydrogen isotope data. Chem. Geol. Isot. Geosci. Sect. 1988, 72, 293–297. [Google Scholar] [CrossRef]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer-Harro, A.J.; Toman, B.; Verkouteren, R.M. New Guidelines for δ13C Measurements: Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef] [Green Version]
- Grinenko, V.A. Preparation of sulfur dioxide for isotopic analysis. Z. Neorgan. Khim. 1962, 7, 2478–2483. [Google Scholar]
- Altunel, E.; Hancock, P.L. Morphology and structural setting of Quaternary travertines at Pamukkale, Turkey. Geol. J. 1993, 28, 335–346. [Google Scholar] [CrossRef]
- Altunel, E.; Hancock, P.L. Structural attributes of Travertine-filled Extnsional Fissures and the Pamukkale Plateau, western Turkey. Int. Geol. Rev. 1996, 38, 768–777. [Google Scholar] [CrossRef]
- Özkul, M.; Varol, B.; Alcicek, M.C. Depositional environments and petrography of the Denizli travertines. Bull. Miner. Res. Explor. 2002, 125, 13–29. [Google Scholar]
- Chafetz, H.S.; Folk, R.L. Travertines: Depositional morphology and the bacterially constructed constituents. J. Sediment. Res. 1984, 54, 289–316. [Google Scholar]
- Fouke, B.W.; Farmer, J.D.; Des Marais, D.J.; Pratt, L.; Sturchio, N.C.; Burns, P.C.; Discipulo, M.K. Depositional facies and aqueous-solid geochemistry of travertine-depositing hot springs (Angel Terrace, Mammoth Hot Springs, Yellowstone National Park, U.S.A.). J. Sediment. Res. 2000, 70, 565–585. [Google Scholar] [CrossRef]
- Guo, L.; Riding, R. Aragonite laminae in hot water travertine crusts, Rapolano Terme, Italy. Sedimentology 1992, 39, 1067–1079. [Google Scholar] [CrossRef]
- Guo, L.; Riding, R. Hot-Spring travertine facies and sequences, Late Pleistocene, Rapolano Terme, Ilaty. Sedimentology 1998, 45, 163–180. [Google Scholar] [CrossRef]
- Jones, B.; Renaut, R.W. Calcareous spring deposits in continental settings. In Carbonates in Continental Settings. Facies Environments and Processes; Alonso-Zarza, A.M., Tanner, L.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 177–224. [Google Scholar]
- Liu, Z.; Li, H.; You, C.; Wan, N.; Sun, H. Thickness and stable isotopic characteristics of modern seasonal climate-controlled sub-annual travertine laminas in a travertine-depositing stream at Baishuitai, SW China: Implications for paleoclimate reconstruction. Environ. Geol. 2006, 51, 257–265. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, X.; Zhang, Y.; Wang, M.; Tan, M.; Hai, K.; Yu, M.; Huo, D. Hydrochemical characteristics of travertine-depositing hot springs in western of Yunnan, China. Quat. Int. 2020, 54, 63–74. [Google Scholar] [CrossRef]
- Pentecost, A. The formation of travertine shrubs: Mammoth Hot springs Wyoming. Geol. Mag. 1990, 127, 159–168. [Google Scholar] [CrossRef]
- Allen, E.T.; Day, A.L. Hot Springs of Yellowstone National Park; Publication 466; Carnegie Institute of Washington: Washington, DC, USA, 1935; pp. 1–525. [Google Scholar]
- Bargar, K.E. Geology and Thermal History of Mammoth Hot Springs, Yellowstone National Park, Wyoming; Bulletin 1444; U.S. Geological Survey: Reston, VA, USA, 1978; pp. 1–55. [Google Scholar]
- Wasson, J.T.; Kallemeyn, G.W. Composition of chondrites. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1988, 325, 533–544. [Google Scholar]
- Krouse, R.H.; Viau, C.A.; Eluik, L.S.; Ueda, A.; Halas, S. Chemical and isotopic evidence of thermochemical sulfate reduction by light hydrocarbon gases in deep carbonate reservoirs. Nature 1988, 333, 415–419. [Google Scholar] [CrossRef]
- Durov, S.A. Natural waters and graphic representations of their composition. Dokl. Akad. Nauk SSSR 1948, 59, 87–90. [Google Scholar]
- Kim, S.T.; O’Neil, J.R.; Hillaire-Marcel, C.; Mucci, A. Oxygen isotope fractionation between synthetic aragonite and water: Influence of temperature and Mg2+ concentration. Geochim. Cosmochim. Acta 2007, 71, 4704–4715. [Google Scholar] [CrossRef]
- Horita, J.; Clayton, R.N. Comment on the studies of oxygen isotope fractionation between calcium carbonates and water at low temperatures by Zhou and Zheng (2003; 2005). Geochim. Cosmochim. Acta 2005, 71, 3131–3135. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Powell, T.; Cumming, W. Spreadsheets for Geothermal Water and Gas Geochemistry. In Proceedings of the 35th Workshop on Geothermal Reservoir Engineering, Stanford, CA, USA, 1–3 February 2010. [Google Scholar]
- Arnórsson, S.; Andrésdóttir, A. Processes controlling the distribution of boron and chlorine in natural waters in Iceland. Geochim. Cosmochim. Acta 1995, 59, 4125–4146. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Water-Supply Paper 2254; US Geological Survey: Reston, VA, USA, 1985; 263p. [Google Scholar]
- McArthur, J.M.; Ravenscroft, P.; Safiullah, S.; Thirlwall, M.F. Arsenic in groundwater: Testing pollution mechanism for aquifers in Bangladesh. Water Resour. Res. 2001, 37, 109–117. [Google Scholar] [CrossRef]
- Barth, S. Application of boron isotopes for tracing sources of anthropogenic contamination in groundwater. Water Res. 1998, 32, 685–690. [Google Scholar] [CrossRef]
- Vengosh, A.; De Lange, D.J.; Starinsky, A. Boron isotope and geochemical evidence for the origin of Urania and Bannock brines at the eastern Mediterranean: Effect of water-rock interactions. Geochim. Cosmochim. Acta 1998, 62, 3221–3228. [Google Scholar] [CrossRef]
- Ravenscroft, P.; McArthur, J.M. Mechanism pollution of groundwater by boron: The examples of Bangladesh and Michigan, USA. Appl. Geochem. 2004, 19, 1413–1430. [Google Scholar] [CrossRef]
- Hermann, A.G.; Knate, D.; Schneider, J.; Peters, H. Geochemistry of modern seawater and brines from saltpans: Main components and bromine distribution. Contrib. Mineral. Petrol. 1973, 40, 1–24. [Google Scholar] [CrossRef]
- Martínez-Serrano, R.G. Caractérisaton Minéralogique, Géochimique et Isotopique du Champ Géothermique de Los Humeros, Méxique. Ph.D. Thesis, Institut National Polytechnique de Lorraine, Nancy, France, 1993; p. 232. [Google Scholar]
- Clayton, R.N.; Friedman, I.; Graf, D.L.; Mayeda, T.K.; Meents, W.F.; Shimp, N.F. The origin of saline formation Waters. J. Geophys. Res. 1996, 71, 3869–3882. [Google Scholar] [CrossRef]
- Cortés, A.; Durazo, J.; Farvolden, R.N. Studies of isotopic hydrology of the basin of Mexico and vicinity: Annotated bibliography and interpretation: J. Hydrol. 1996, 198, 346–376. [Google Scholar] [CrossRef]
- Sachse, D.; Sachs, J.P. Inverse relationship between D/H fractionation in cyanobacterial lipids and salinity in Christmas Island saline ponds. Geochim. Cosmochim. Acta 2008, 72, 793–806. [Google Scholar] [CrossRef]
- Sharp, Z. Principles of Stable Isotope Geochemistry, 2nd ed.; The University of New Mexico: Albuquerque, NM, USA, 2017. [Google Scholar] [CrossRef]
- Kitano, Y.; Kawasaki, N. Behaviour of strontium in the progress of calcium carbonate separation from a bicarbonate solution. J. Earth Sci. 1958, 6, 43–74. [Google Scholar]
- Kitano, Y.; Park, K.; Hood, D.W. Pure aragonite synthesis. J. Geophys. Res. 1962, 67, 4873–4874. [Google Scholar] [CrossRef]
- Busenberg, E.; Plummer, L.N. A Comparative Study of the Dissolution and Crystal Growth Kinetics of Calcite and Aragonite: Ln Studies in Diagenesis; United States Geological Survey Bulletin, 1578; Mumpton, F.A., Ed.; US Geological Survey: Reston, VA, USA, 1986; pp. 139–168. [Google Scholar]
- Folk, R.L. Interaction between bacteria, nannobacteria, and mineral precipitation in hot springs in central Italy. Geogr. Phys. Quat. 1994, 48, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Renault, R.W.; Jones, B. Control son aragonite and calcite precipitation in hot spring travertines at Chemurkeu, Lake Bogoria, Kenya. Can. J. Earth Sci. 1997, 34, 818–881. [Google Scholar]
- Hurlbut, C.S., Jr.; Klein, C. Manual of Mineralogy; Editorial Reverté: Barcelona, Spain, 1982; ISBN 978-84-291-4606-6. [Google Scholar]
- Sunawana, I.; Takahashi, Y.; Imai, H. Strontium and aragonite-calcite precipitation. J. Mineral. Petrol. Sci. 2007, 102, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, K.M.; Makhlouf, I.M.; El Naqah, A.R.; Al-Thawabteh, S.M. Geochemistry and stable isotopes of travertine from Jordan Valley and Dead Sea areas. Minerals 2017, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Berriguete, A.; Alonso-Zarza, A.M.; Cabrera, M.C.; Rodriguez-González, A. The Azuaje travertine: An example of aragonite deposition in a recent volcanic setting, N Gran Canaria Island, Spain. Sediment. Geol. 2012, 277–278, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Erthal, M.; Capezzuoli, E.; Mancini, A.; Claes, H.; Soete, J.; Swennen, R. Shrub morpho-types as indicator for the water flow energy—Tivoli travertine case (Central Italy). Sediment. Geol. 2017, 347, 79–99. [Google Scholar] [CrossRef]
- McLennan, S.M. Chapter 7: Rare earth elements in sedimentary rocks: Influence of provenance and sedimentary processes. In Geochemistry and Mineralogy of Rare Earth Elements; Lipin, B.R., McKay, G.A., Eds.; De Gruyter: Berlin, Germany, 2018; pp. 169–200. [Google Scholar] [CrossRef]
- Smrzka, D.; Zwicker, J.; Bach, W.; Feng, D.; Himmler, T.; Chen, D.; Peckmann, J. The behavior of trace elements in seawater, sedimentary pore water, and their incorporation into carbonate minerals: A review. Facies 2019, 65, 41. [Google Scholar] [CrossRef]
- Lavrushin, V.Y.; Kuleshov, V.N.; Kikvadz, O.E. Travertines of the Northern Caucasus. Lithol. Miner. Resour. 2006, 41, 137–164. [Google Scholar] [CrossRef]
- Desouky, H.E.; Soete, J.; Claes, H.; Özkul, M.; Vanhaecke, F.; Swennen, R. Novel applications of fluid inclusions and isotope geochemistry in unravelling the genesis of fossil travertine systems. Sedimentology 2015, 62, 27–56. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Lawrence, J.R. Stable isotopic variability within modern travertines. Geogr. Phys. Quat. 1994, 48, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.L.; Ward, W.C. Chapter 4: Early Cretaceous Carbonate Platforms of Northeastern and East-Central Mexico. Cretaceous Carbonate Platforms. AAPG Bull. 1993, 56, 35–49. [Google Scholar]
- Karimi, H.; Moore, F. The source and heating mechanism for Ahram, Mirahmad and Garu thermal springs, Zagros Mountains, Iran. Geothermics 2008, 37, 84–100. [Google Scholar] [CrossRef]
- Kempe, A.L.W.; Thode, H.G. The mechanism of bacterial reduction of sulfate and sulfite from isotope fractionation studies. Geochim. Cosmochim. Acta 1968, 32, 71–91. [Google Scholar] [CrossRef]
- Hill, C.A. Geology of Carlsbad Caverns and other caves of the Guadalupe Mountains, New Mexico and Texas; Bulletin 117; New Mexico Bureau Mines and Mineral Resources: Socorro, NM, USA, 1987; 150p. [Google Scholar]
- Kompani-Zare, M.; Moore, F. Chemical thermometry and origin of the Dalaki mineral springs, Boshehr Province, Iran. J. Hydrol. 2001, 40, 189–204. [Google Scholar]
- Kele, S.; Demény, A.; Siklósy, Z.; Németh, T.; Tóth, M.; Kovács, M.-B. Chemical and stable isotope composition of recent hot-water travertines and associated thermal waters, from Egerszalók, Hungary: Depositional facies and non-equilibrium fractionation. Sediment. Geol. 2008, 211, 53–57. [Google Scholar] [CrossRef]
- Morán-Zenteno, D.; Cerca, M.; Keppie, J.D. La evolución tectónica y magmática cenozoica del suroeste de México: Avances y problemas de interpretación. Bol. Soc. Geol. Mex. 2005, 57, 319–341. [Google Scholar] [CrossRef]
- Aguayo-Camargo, J.E.; Arellano-Gil, J.; Santillán-Piña, N. Prograding low-density turbidite systems and oil traps at the Lower Paleogene Chicontepec Foreland Basin, East-Central Mexico. Ing. Investig. Tecnol. 2018, 19, 1–12. [Google Scholar] [CrossRef]
- Rodríguez-Berriguete, A.; Alonso-Zarza, A.M.; Martín-García, R. Diagenesis of continental carbonate country rocks underlaying surficial travertine spring deposits. Quat. Int. 2017, 437, 4–14. [Google Scholar] [CrossRef]




| Geometry | Bragg–Brentano |
|---|---|
| Goniometer radius | 240 mm |
| Radiation source | CoKα & CuKα |
| Generator | 45 kV, 40 mA |
| Tube | Fine Focus |
| Divergence Slit | ½° (fixed) |
| Soller Slits | 0.04 rad (incident and diffracted beam) |
| Incident beam optics | Parallel mirror |
| Filter | Iron filter & Nickel filter |
| Detector | PIXcel3D |
| Step Size | 0.002° |
| Integration Time | 40 s |
| Sample | V | Cr | Co | Ni | Cu | Zn | Ga | Ge | As | Rb | Sr | Y | Zr | Nb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DL | 5 | 20 | 1 | 20 | 10 | 30 | 1 | 0.5 | 5 | 1 | 2 | 0.5 | 1 | 0.2 | |
| TAMI 6.2 | <5 | <20 | <1 | <20 | <10 | <30 | <1 | <0.5 | <5 | <1 | >10000 | <0.5 | 1 | <0.2 | |
| TAMI 2.2 | <5 | <20 | <1 | 50 | <10 | <30 | <1 | <0.5 | <5 | <1 | 9350 | <0.5 | <1 | <0.2 | |
| TAMI 2AS | <5 | <20 | <1 | <20 | <10 | <30 | <1 | <0.5 | <5 | <1 | 2370 | <0.5 | <1 | <0.2 | |
| TAMI AC | <5 | <20 | 3 | <20 | 10 | <30 | <1 | <0.5 | <5 | <1 | >10,000 | 3.5 | 2 | <0.2 | |
| TAMI BC | <5 | <20 | 3 | 40 | <10 | <30 | <1 | <0.5 | <5 | <1 | >10,000 | <0.5 | <1 | <0.2 | |
| Sample | Mo | Ag | In | Sn | Sb | Cs | Ba | Hf | Ta | W | Tl | Pb | Bi | Th | U |
| DL | 2 | 0.5 | 0.1 | 1 | 0.2 | 0.1 | 3 | 0.1 | 0.01 | 0.5 | 0.05 | 5 | 0.1 | 0.05 | 0.01 |
| TAMI 6.2 | <2 | <0.5 | <0.1 | <1 | <0.2 | <0.1 | 35 | <0.1 | <0.01 | <0.5 | <0.05 | <5 | <0.1 | <0.05 | <0.01 |
| TAMI 2.2 | <2 | <0.5 | <0.1 | <1 | <0.2 | <0.1 | 17 | <0.1 | <0.01 | 0.8 | <0.05 | <5 | <0.1 | <0.05 | 0.01 |
| TAMI 2AS | <2 | <0.5 | <0.1 | <1 | <0.2 | <0.1 | 12 | <0.1 | <0.01 | <0.5 | <0.05 | <5 | <0.1 | <0.05 | <0.01 |
| TAMI AC | <2 | <0.5 | <0.1 | <1 | <0.2 | <0.1 | 70 | <0.1 | <0.01 | <0.5 | 0.06 | <5 | <0.1 | <0.05 | 1.33 |
| TAMI BC | <2 | <0.5 | <0.1 | <1 | <0.2 | <0.1 | 82 | <0.1 | 0.02 | 0.5 | <0.05 | <5 | 0.1 | <0.05 | 0.02 |
| Sample | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ΣREE |
| DL | 0.05 | 0.05 | 0.01 | 0.05 | 0.01 | 0.005 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.005 | 0.01 | 0.002 | |
| TAMI 6.2 | <0.05 | <0.05 | <0.01 | <0.05 | <0.01 | <0.005 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.005 | <0.01 | <0.002 | 0.01 |
| TAMI 2.2 | <0.05 | <0.05 | <0.01 | <0.05 | 0.01 | <0.005 | 0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.005 | <0.01 | <0.002 | 0.03 |
| TAMI AS | <0.05 | <0.05 | <0.01 | <0.05 | <0.01 | <0.005 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.005 | <0.01 | <0.002 | 0 |
| TAMI AC | 2.57 | 3.51 | 0.4 | 1.53 | 0.3 | 0.057 | 0.45 | 0.07 | 0.42 | 0.08 | 0.25 | 0.034 | 0.21 | 0.035 | 9.916 |
| TAMI BC | 0.09 | 0.17 | 0.02 | 0.1 | 0.01 | 0.007 | 0.01 | <0.01 | 0.01 | <0.01 | 0.01 | <0.005 | 0.01 | 0.002 | 0.439 |
| Carbon and Oxygen Isotopic Composition of Travertines | |||||
|---|---|---|---|---|---|
| Sample Number | Sample Code | XRD Mineralogy | δ13CVPDB (‰) | δ18OVPDB (‰) | δ18OVSMOW (‰) |
| 1 | Cima 1 | 31% Ar, 9% Cc, 60% Gy | +2.37 | −0.78 | +30.11 |
| 2 | Capa 2 | 100% Ar | +1.83 | −1.39 | +29.47 |
| 3 | Banda 3.1 | 78% Ar, 28% Gy | +1.93 | −1.52 | +29.34 |
| 4 | Banda 3.2 | 100% Ar | +1.83 | −1.60 | +29.26 |
| 5 | Banda 3.3 | 100% Ar | +2.02 | −1.46 | +29.40 |
| 6 | Tami 7 | 100% Ar | +1.75 | −1.70 | +29.16 |
| Sulfur Isotopic Composition of Travertines | |||||
| Sample Number | Sample Code | XRD Mineralogy | δ34S (‰) | ||
| 1 | Tamiahua 2 | Gypsum-anhydrite | −4.0 | ||
| 2 | Tamiahua 1 | Gypsum-anhydrite | −0.6 | ||
| 3 | Tamiahua 5 | Gypsum-anhydrite | −0.8 | ||
| 4 | M 1.2 Cristal | Native sulfur | −0.1 | ||
| 5 | Tamiahua 2R Sup | Native sulfur | −3.7 | ||
| 6 | M 5 | Native sulfur | +1.2 | ||
| 7 | M 5.2 | Native sulfur | −2.3 | ||
| 8 | M6 | Native sulfur | −1.5 | ||
| 9 | M 8.2 Sup | Native sulfur | −0.6 | ||
| 10 | M 13 | Native sulfur | +0.3 | ||
| Sulfur Isotopic Composition of Hydrocarbons | |||||
| Sample Number | Sample Code | δ13CVPDB (‰) | % Sulfur | δ34S (‰) | |
| 1 | MX0242 | −26.85 | 2.16 | 7.64 | |
| 2 | MX0243 | −26.44 | 3.02 | 7.50 | |
| Sample | Description | Surficial Temperature (°C) | pH | Electrical Conductivity (mS/cm) | δ18OVSMOW2 | δ2HVSMOW2 |
|---|---|---|---|---|---|---|
| a | Terraces water | 70 | 6.1 | 118 | +7.94 | −5.39 |
| b | Well water | 27 | 6.4 | 3 | −3.82 | −18.87 |
| c | Lagoon water | 34 | 8.1 | 45 | +3.73 | +23.75 |
| d | River water | 32 | 7.9 | 0.06 | +3.58 | +22.49 |
| e | Fumarole water | Not measured | 8.4 | 3 | +3.53 | −9.54 |
| f | Duplicate of a | 70 | 6.1 | Not measured | +7.77 | −5.50 |
| g | Duplicate of b | 27 | 6.4 | Not measured | −3.76 | −18.85 |
| Sample | Al | As | B | Ba | Fe | Cs | Mn | SiO2 | Sr | V | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Terraces | 0.02 | 0.13 | 32.27 | 0.18 | <DL | 8 | <DL | 38 | 73 | <DL | |||||||
| River | 0.02 | ≤DL | 2.96 | 0.1 | <DL | 2.06 | 0.16 | 6.6 | 4.55 | <DL | |||||||
| Fumarole | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||||||
| Lagoon | 0.42 | ≤DL | 3.11 | 0.07 | 0.36 | 2.88 | 0.03 | 6.8 | 4.61 | <DL | |||||||
| Well | 0.04 | ≤DL | 0.07 | 0.08 | <DL | 1.13 | 0.5 | 67.4 | 0.87 | 0.01 | |||||||
| DL (mg/L) | NA | 0.08 | 0.03 | 0.003 | 0.002 | NA | 0.001 | 0.15 | NA | 0.002 | |||||||
| Sample | F- | Cl− | SO42− | HCO3− | Na+ | Mg2+ | Ca2+ | K+ | Li+ | ||||||||
| Terraces | 58 | 31,014 | 1288 | 730 | 15,953 | 1121 | 410 | 0.1 | 20 | ||||||||
| River | 59 | 17,830 | 2864 | 45 | 9236 | 1023 | 452 | 339 | 0.03 | ||||||||
| Fumarole | 0.2 | 907 | 151 | 15 | 634 | 0.1 | 40 | 44 | 5 | ||||||||
| Lagoon | 104 | 16,836 | 2548 | 45 | 9762 | 1080 | 446 | 347 | 0.03 | ||||||||
| Well | 13 | 864 | 655 | 231 | 366 | 49 | 147 | 15 | 0.03 | ||||||||
| DL (mg/L) | 0.2 | 0.3 | 0.3 | NA | 0.1 | 0.1 | 0.08 | 0.1 | 0.03 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porras-Toribio, I.; Pi-Puig, T.; Villanueva-Estrada, R.E.; Rubio-Ramos, M.A.; Solé, J. Mineralogy, Geochemistry, and Stable Isotopes (C, O, S) of Hot Spring Waters and Associated Travertines near Tamiahua Lagoon, Veracruz, Gulf of Mexico (Mexico). Minerals 2022, 12, 822. https://doi.org/10.3390/min12070822
Porras-Toribio I, Pi-Puig T, Villanueva-Estrada RE, Rubio-Ramos MA, Solé J. Mineralogy, Geochemistry, and Stable Isotopes (C, O, S) of Hot Spring Waters and Associated Travertines near Tamiahua Lagoon, Veracruz, Gulf of Mexico (Mexico). Minerals. 2022; 12(7):822. https://doi.org/10.3390/min12070822
Chicago/Turabian StylePorras-Toribio, Israel, Teresa Pi-Puig, Ruth Esther Villanueva-Estrada, Marco Antonio Rubio-Ramos, and Jesús Solé. 2022. "Mineralogy, Geochemistry, and Stable Isotopes (C, O, S) of Hot Spring Waters and Associated Travertines near Tamiahua Lagoon, Veracruz, Gulf of Mexico (Mexico)" Minerals 12, no. 7: 822. https://doi.org/10.3390/min12070822
APA StylePorras-Toribio, I., Pi-Puig, T., Villanueva-Estrada, R. E., Rubio-Ramos, M. A., & Solé, J. (2022). Mineralogy, Geochemistry, and Stable Isotopes (C, O, S) of Hot Spring Waters and Associated Travertines near Tamiahua Lagoon, Veracruz, Gulf of Mexico (Mexico). Minerals, 12(7), 822. https://doi.org/10.3390/min12070822






