Application of High-Temperature Copper Diffusion in Surface Recoloring of Faceted Labradorites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
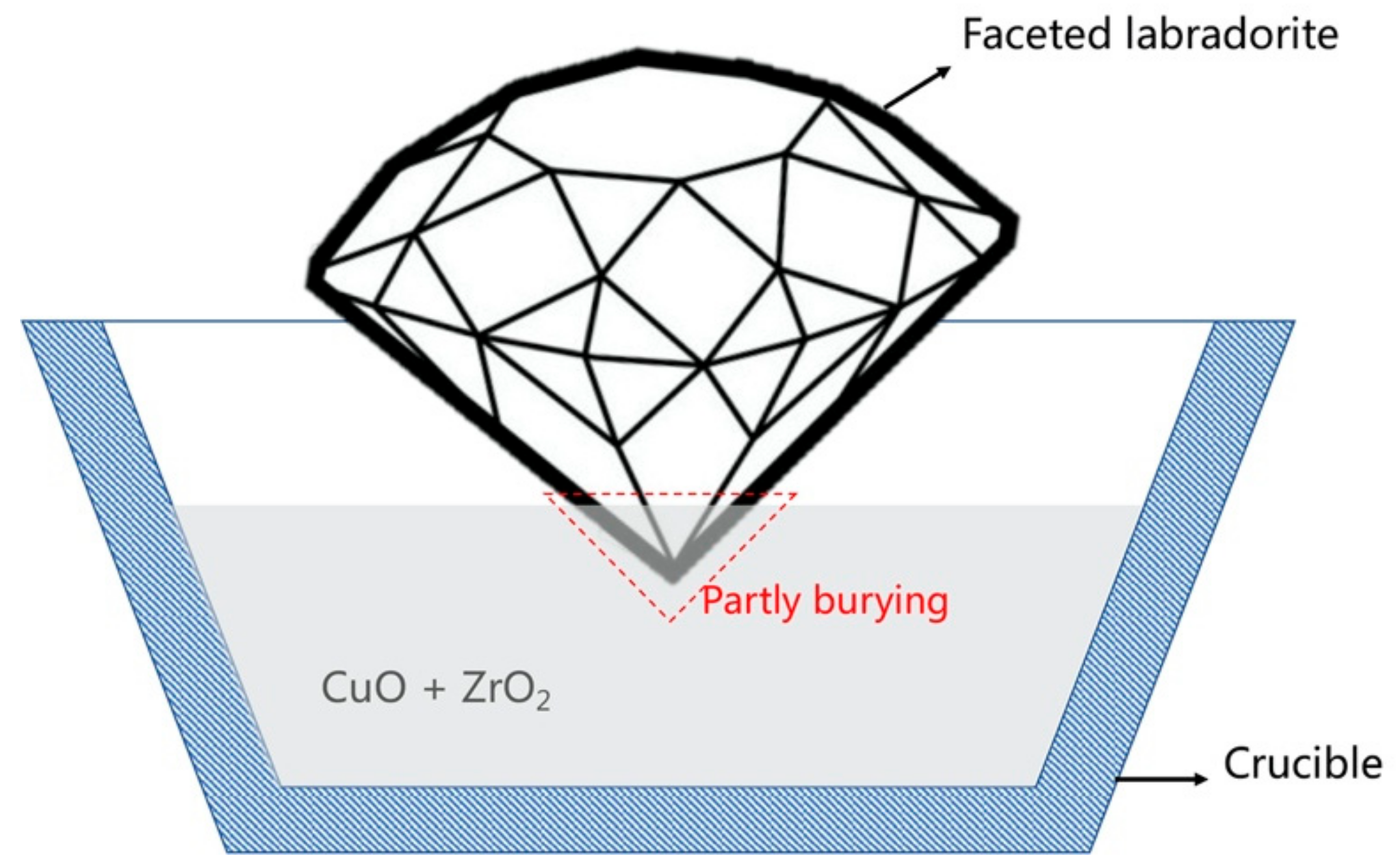
2.2.1. High-Temperature Copper Diffusion of Faceted Labradorites
2.2.2. Characterizations and Measurements
3. Results and Discussion
3.1. High-Temperature Copper Diffusion of Faceted Labradorites
3.2. Faceted Labradorites before Copper Diffusion
3.2.1. Gemological Characteristics
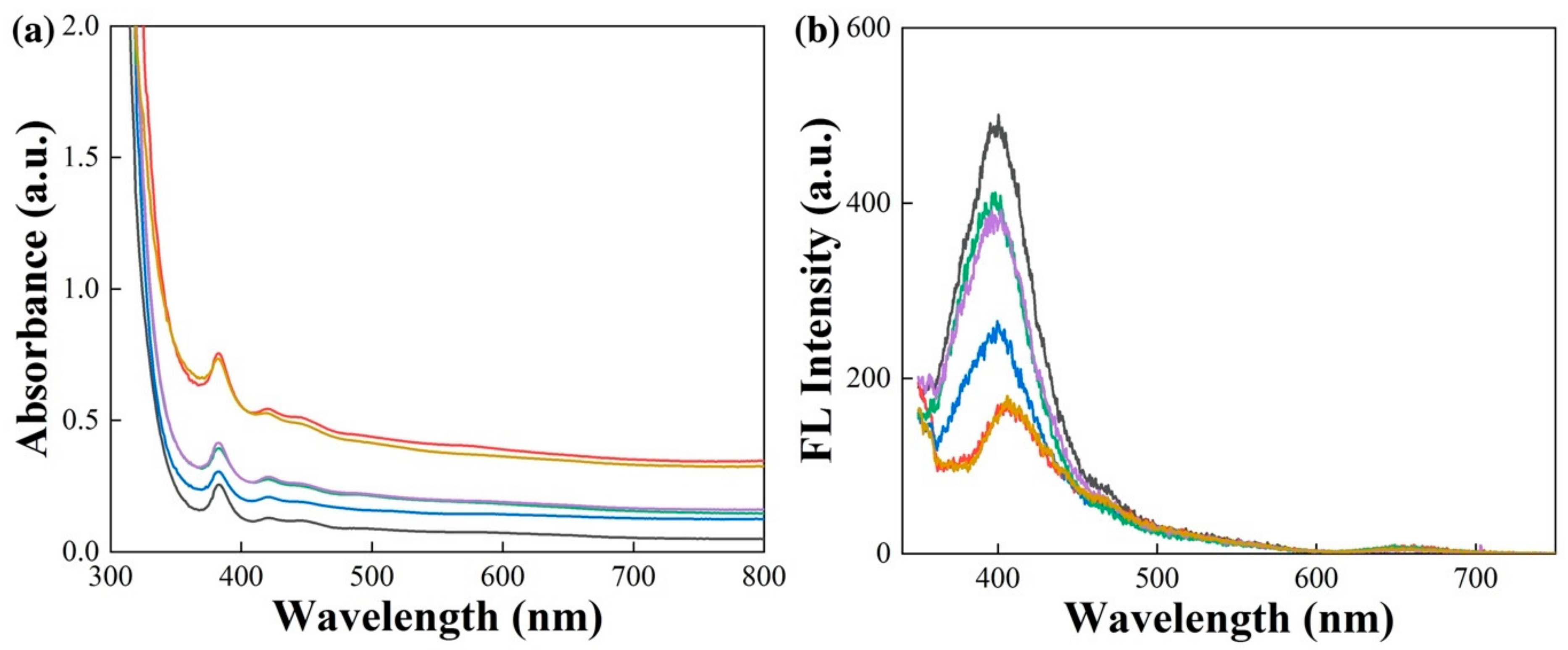
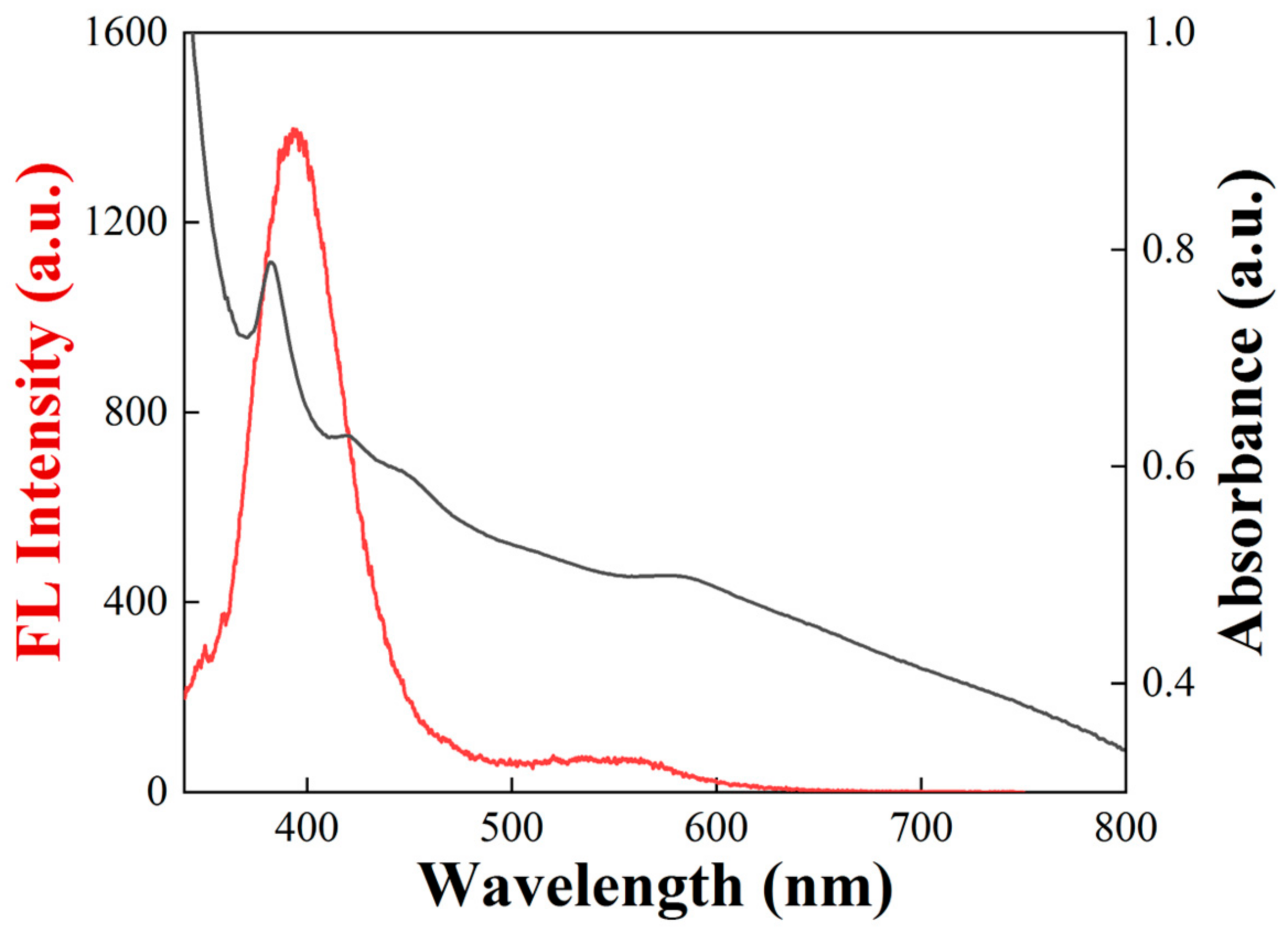
3.2.2. Spectroscopic Characteristics
3.3. Recolored Faceted Labradorites by High-Temperature Copper Diffusion
3.3.1. The Parameters of High-Temperature Copper Diffusion
3.3.2. Gemological Characteristics of the Recolored Faceted Labradorites
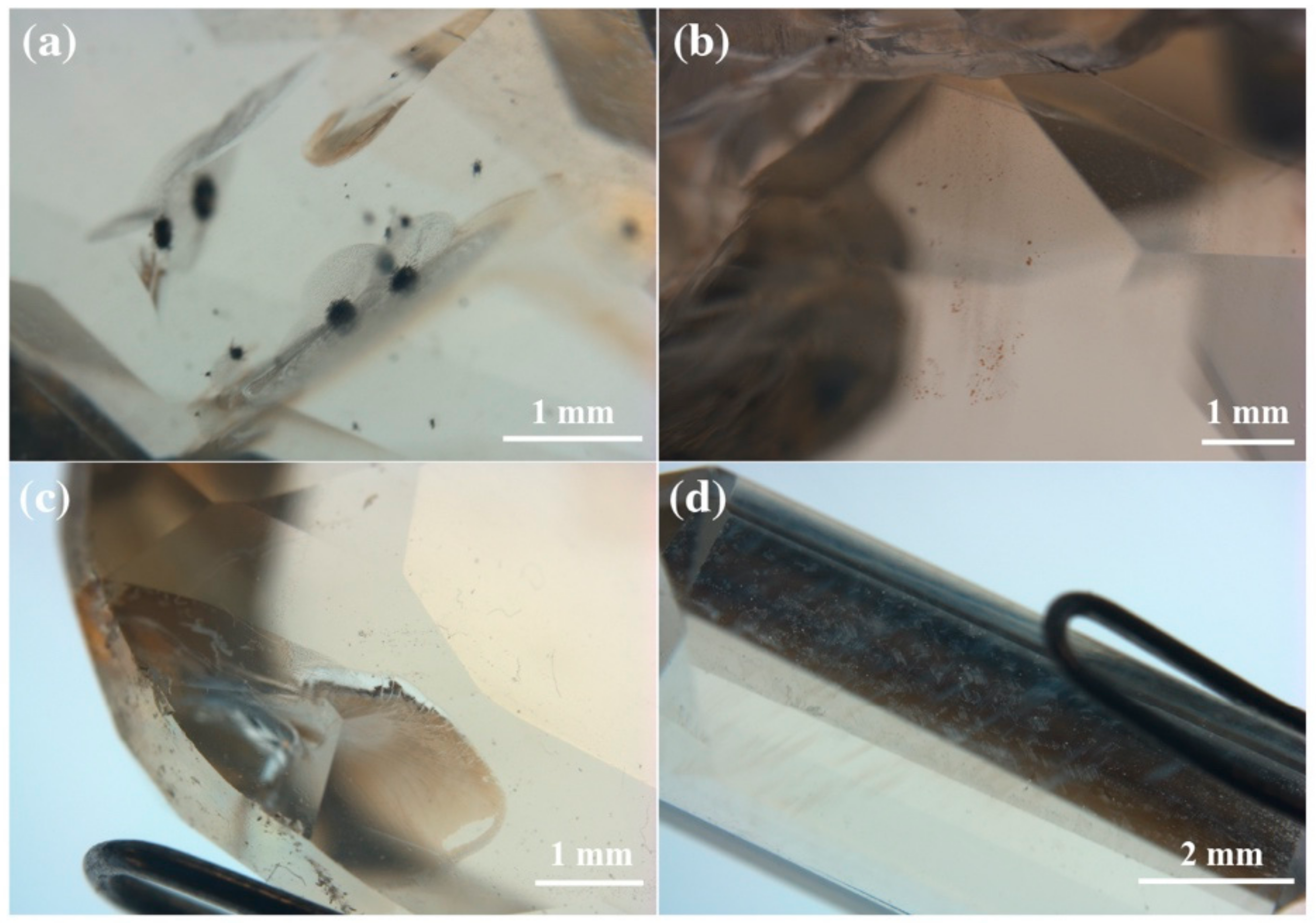
3.3.3. Optical Microscopic Characteristics of the Recolored Faceted Labradorites
3.3.4. Spectroscopic Characteristics of the Recolored Faceted Labradorite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnston, C.L.; Gunter, M.E.; Knowles, C.R. Sunstone labradorite from the Ponderosa mine, Oregon. Gems Gemol. 1991, 27, 220–233. [Google Scholar] [CrossRef] [Green Version]
- Kiefert, L.; Wang, C.; Sintayehu, T.; Link, K. Sunstone labradorite-bytownite from Ethiopia. J. Gemmol. 2019, 36, 694–696. [Google Scholar] [CrossRef]
- Sun, Z.; Renfro, N.D.; Palke, A.C.; Breitzmann, H.; Muyal, J.; Hand, D.; Rossman, G.R. Sunstone plagioclase feldspar from Ethiopia. Gems Gemol. 2020, 56, 184–188. [Google Scholar]
- Farfan, G.; Xu, H. Pleochroism in Calcific Labradorite from Oregon: Effects from Size and Orientation of Nano-and Micro-Precipitates of Copper and Pyroxene. Chem. Geol. 2008, 161, 59–88. [Google Scholar]
- Hofmeister, A.M.; Rossman, G.R. Exsolution of Metallic Copper from Lake County Labradorite. Geology 1985, 13, 644–647. [Google Scholar] [CrossRef]
- Nishida, N.; Kimata, M. Identification of microinclusions in the nanometer dimension by electron microprobe analysis. The chromonophores of colored sunstones. Jpn. Mag. Mineral. Petrol. Sci. 2002, 31, 268–274. (In Japanese) [Google Scholar]
- Wang, C.S.; Shen, A.H.; Palke, A.C. Color origin of the Oregon sunstone. In Proceedings of the 36th International Gemmological Conference IGC, Nantes, France, 27–31 August 2019; pp. 71–74. [Google Scholar]
- Fritsch, E. Gem News International: Red andesine feldspar from Congo. Gems Gemol. 2002, 38, 94–95. [Google Scholar]
- Krzemnicki, M.S. Red and green labradorite feldspar from Congo. J. Gemmol. 2004, 29, 15–23. [Google Scholar] [CrossRef]
- Laurs, B.L. Gem News International: Gem plagioclase reportedly from Tibet. Gems Gemol. 2005, 41, 356–357. [Google Scholar]
- Lan, Y.; Chen, C.; Lu, T.J.; Wang, W.W. Characteristics of Surrounding Rocks and Surface Residues of “Red Feldspar from Tibet”. J. Gems Gemmol. 2011, 13, 1–5. (In Chinese) [Google Scholar]
- Wang, W.W.; Lan, Y.; Lu, T.J.; Jiang, W.; Chen, C.; Li, Q.; Chen, Z.; Xie, J. Documental Report of Geological Field Investigation on “Red Feldspar” in Tibet, China. J. Gems Gemmol. 2011, 13, 1–5. (In Chinese) [Google Scholar]
- Abduriyim, A. The characteristics of red andesine from the Himalaya Highland, Tibet. J. Gemmol. 2009, 31, 283–298. [Google Scholar] [CrossRef]
- Fontaine, G.H.; Hametner, K.; Peretti, A.; Günther, D. Authenticity and provenance studies of copper-bearing andesines using Cu isotope ratios and element analysis by fs-LA-MC-ICPMS and ns-LA-ICPMS. Anal. Bioanal. Chem. 2010, 398, 2915–2928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossman, G.R. The Chinese Red Feldspar Controversy: Chronology of Research Through July 2009. Gems Gemol. 2011, 47, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.C.; Wang, C.S.; Shen, A.H. The Fluorescence Characteristics and Identification of Copper Diffusion-Treated Red Feldspar. In Proceedings of the 38th International Gemmological Conference IGC, Online, 20–21 November 2021; pp. 32–33. [Google Scholar]
- Emmett, J.; Douthit, T. Copper Diffusion of Plagioclase: GIA, News from Research. Available online: www.gia.edu/research-resources/news-from-research (accessed on 10 September 2009).
- Zhou, Q.C.; Wang, C.S.; Shen, A.H. Copper Nanoparticles Embedded in Natural Plagioclase Mineral Crystals: In Situ Formation and Third-Order Nonlinearity. J. Phys. Chem. C 2022, 126, 387–395. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Gao, S.; Günther, D.; Xu, J.; Gao, C.; Chen, H. In situ analysis of major and trace elements of anhydrous minerals by LA-ICP-MS without applying an internal standard. Chem. Geol. 2008, 257, 34–43. [Google Scholar] [CrossRef]
- Rakhshani, A.E. Preparation, Characteristics and Photovoltaic Properties of Cuprous Oxide—A Review. Solid-State Electron. 1986, 29, 7–17. [Google Scholar] [CrossRef]
- Boutinaud, P.; Parent, C.; Le Flem, G.; Pedrini, C.; Moine, B. Spectroscopic Investigation of the Copper (I)-Rich Phosphate CuZr2(PO4)3. J. Phys. Condens. Matter 1992, 4, 3031. [Google Scholar] [CrossRef]
- Boutinaud, P.; Garcia, D.; Parent, C.; Faucher, M.; Le Flem, G. Energy Levels of Cu+ in the Oxide Insulators CuLaO2 and CuZr2(PO4)3. J. Phys. Chem. Solids 1995, 56, 1147–1154. [Google Scholar] [CrossRef]

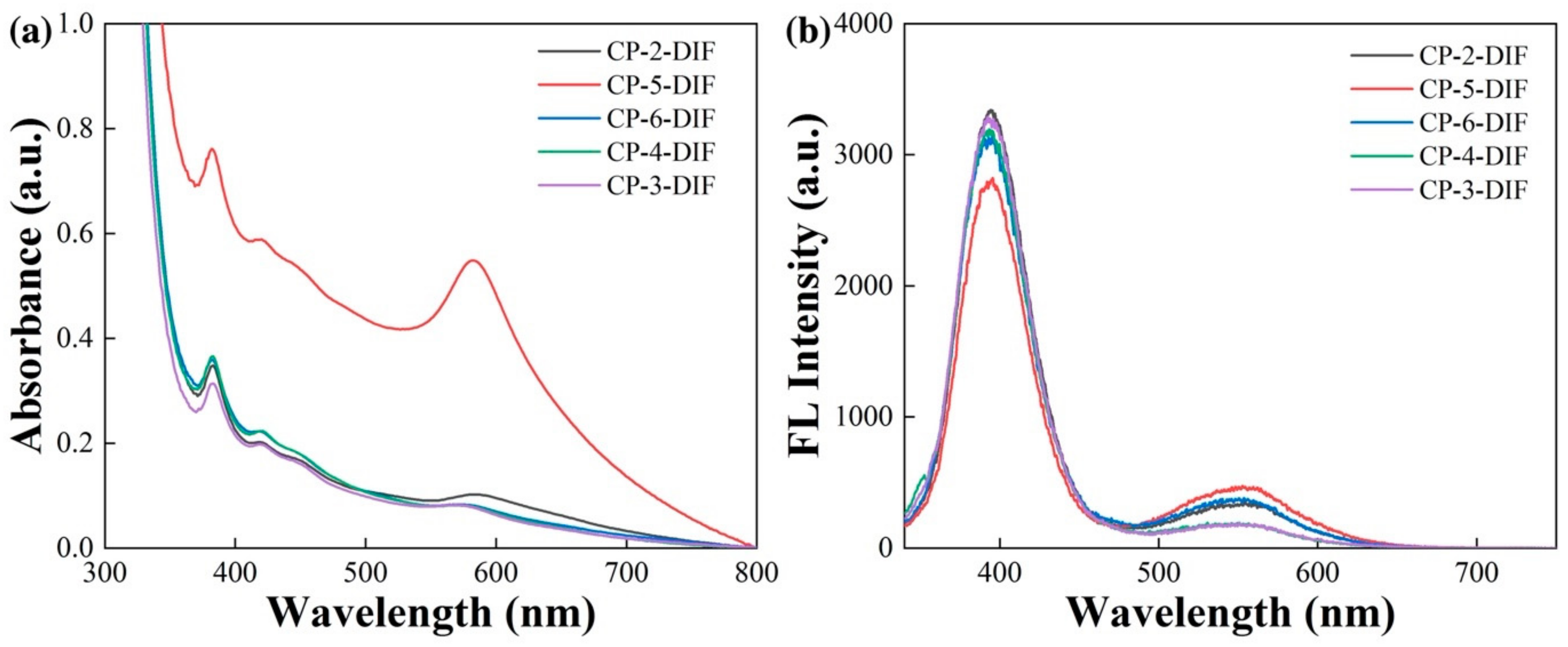
| Sample Number and Description | Images before Copper Diffusion | Images after Copper Diffusion |
|---|---|---|
| CP-1 oval cut, 2.08 ct 10.5 × 7.2 × 4.3 mm |  pale yellow |  gray black (partly red) |
| CP-2 oval cut, 2.09 ct 10.4 × 7.6 × 4.1 mm |  pale yellow |  light red |
| CP-3 emerald cut, 1.94 ct 11.7 × 5.5 × 3.9 mm |  pale yellow |  light red |
| CP-4 pear cut, 2.55 ct 11.3 × 8.2 × 5.2 mm |  pale yellow |  light red |
| CP-5 pear cut, 2.77 ct 12.6 × 7.4 × 5.7 mm |  pale yellow | 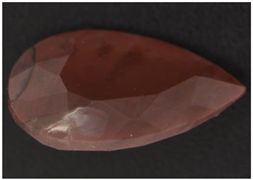 red |
| CP-6 cushion cut, 1.93 ct 8.9 × 7.0 × 4.0 mm |  pale yellow | 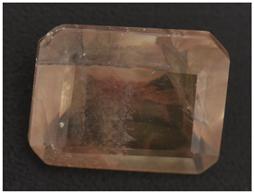 light red |
| Treated Labradorite from Oregon | Sunstone from Oregon | Andesine from Congo | Labradorite from Congo | Andesine from Tibet | |
|---|---|---|---|---|---|
| this paper | Krzemnicki, 2004 [9] | Fritsch, 2002 [8] | Krzemnicki, 2004 [9] | Abduriyim, 2009 [13] | |
| RI | RIα 1.561–1.563 RIβ 1.570–1.572 | RIα 1.560–1.565 RIβ 1.570–1.572 | RIα 1.551 RIβ 1.560 | RIα 1.553–1.555 RIβ 1.562–1.563 | RIα 1.550–1.552 RIβ 1.555–1.557 |
| DR | 0.009–0.010 | 0.007–0.010 | 0.009 | 0.007–0.011 | 0.009–0.010 |
| color | pale red and red | pale red and pale green | red | red and green | reddish orange, orange-red, and deep red |
| pleochroism | very weak | very weak (red sample) to distinct (green sample) | very weak | very weak (red sample) to distinct (green sample) | weak |
| transparency | transparent | transparent | transparent | transparent | transparent to translucent |
| LW-UV | weak to orange | none | none | weak to distinct orange | weak chalky orange |
| observations | “fire cloud” like inclusions, tiny particles with metallic luster | slight “schiller” effect | “schiller” effect | milky turbidity green labradorite: red under incandescent light | color zoning, twin lamellae, turbid milky clouds and particles, lath-like hollow channels, pipe-like growth tubes, irregular dislocations, fractures, uncommon tiny native copper grains or platelets |
| Sample Number | The Area in Contact with the Diffusant | Inclusions on the Shallow Surface |
|---|---|---|
| CP-1 |  Many black spots, the contamination is serious |  Dense micron-scale black inclusions |
| CP-2 |  White attachments, the contamination is minor | 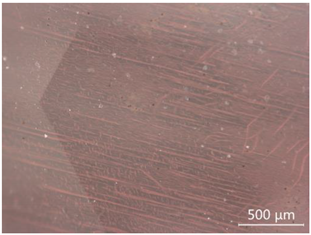 “Fire cloud” like inclusions |
| CP-3 |  White attachments, the contamination is minor |  “Fire cloud” like inclusions, tiny particles with metallic luster |
| CP-4 | 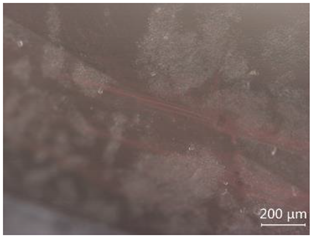 White attachments, the contamination is minor | 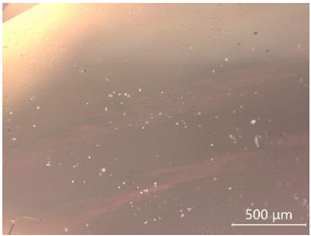 “Fire cloud” like inclusions |
| CP-5 |  Few white attachments, and the contamination is very slight | 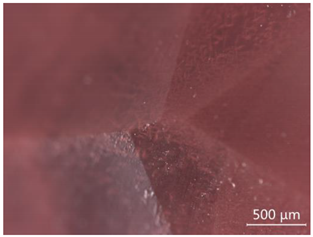 “Fire cloud” like inclusions |
| CP-6 |  White attachments, the contamination is minor | 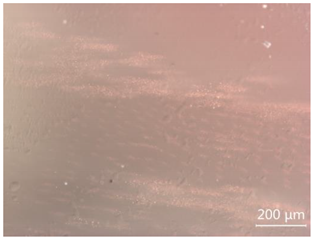 “Fire cloud” like inclusions, tiny particles with metallic luster |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Wang, C.; Shen, A.-H. Application of High-Temperature Copper Diffusion in Surface Recoloring of Faceted Labradorites. Minerals 2022, 12, 920. https://doi.org/10.3390/min12080920
Zhou Q, Wang C, Shen A-H. Application of High-Temperature Copper Diffusion in Surface Recoloring of Faceted Labradorites. Minerals. 2022; 12(8):920. https://doi.org/10.3390/min12080920
Chicago/Turabian StyleZhou, Qingchao, Chengsi Wang, and Andy-Hsitien Shen. 2022. "Application of High-Temperature Copper Diffusion in Surface Recoloring of Faceted Labradorites" Minerals 12, no. 8: 920. https://doi.org/10.3390/min12080920







