Co–Mn Mineralisations in the Ni Laterite Deposits of Loma Caribe (Dominican Republic) and Loma de Hierro (Venezuela)
Abstract
:1. Introduction
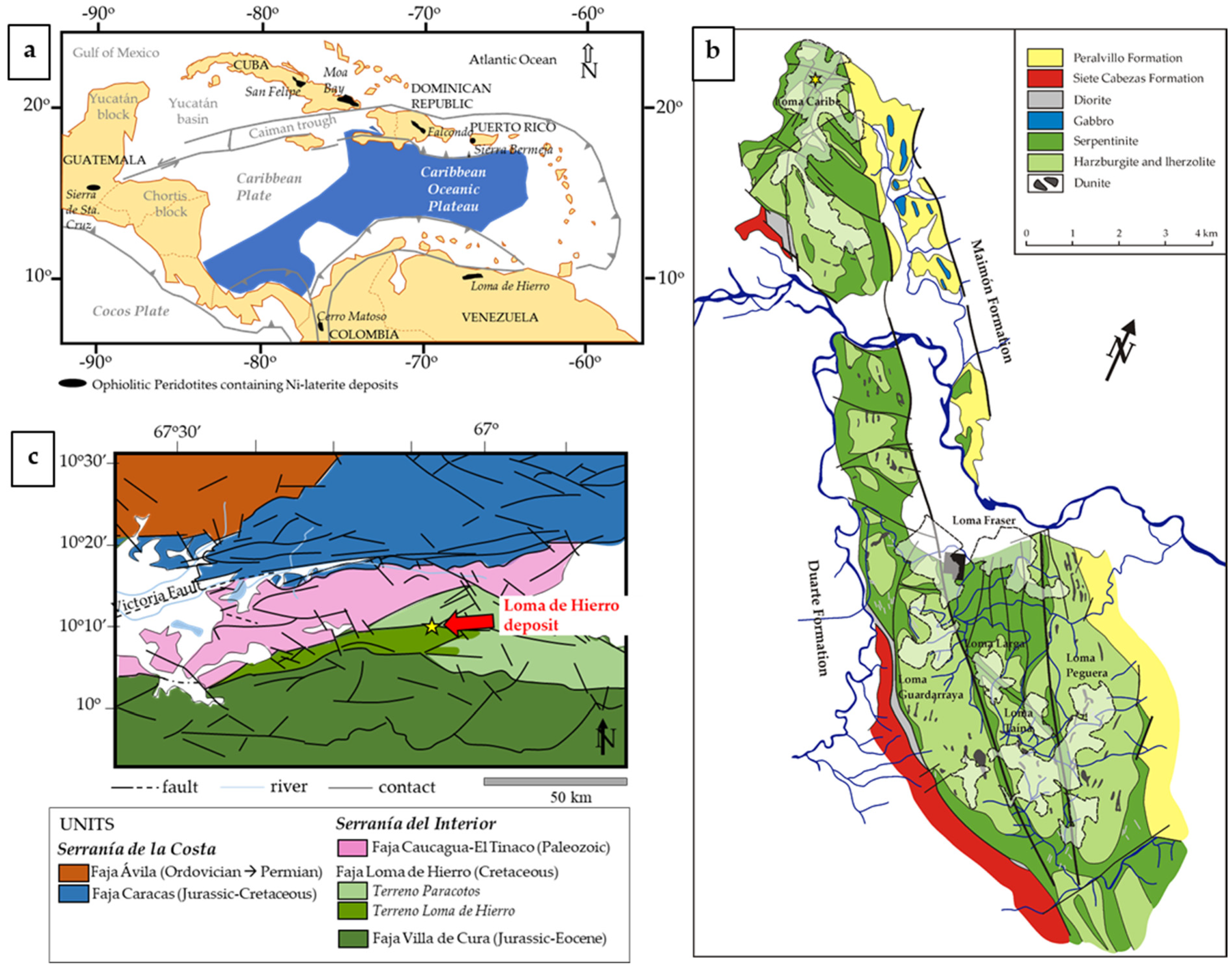
2. Geological Setting
3. Materials and Methods
3.1. Sampling and Sample Preparation
3.2. Analythical Methods
4. Results
4.1. Mineralogy and Petrography
4.2. Co-Mn Mineralisations
4.2.1. Mn-Oxyhydroxides
4.2.2. Mn-Bearing Phyllosilicates
5. Discussion
5.1. Co-Mn Bearing Minerals in Loma Caribe and Loma de Hierro
5.2. Comparison with Other Co-Mn Mineralisations in Lateritic Systems
5.3. Formation of Co-Mn Bearing Minerals in Laterite Deposits
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474 (accessed on 15 September 2021).
- Slack, J.F.; Kimball, B.E.; Shedd, K.B. Cobalt. In Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey Professional Paper: Reston, VA, USA, 2017; Chapter F; pp. F1–F40. [Google Scholar] [CrossRef]
- US Geological Survey. Mineral Commodity Summaries 2020: US Geological Survey; US Geological Survey: Reston, VA, USA, 2020; 200p. [Google Scholar] [CrossRef] [Green Version]
- Alves Dias, P.; Blagoeva, D.; Pavel, C.; Arvanitidis, N. Cobalt: Demand-Supply Balances in the Transition to Electric Mobility; EUR 29381 EN; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-94311-9. [Google Scholar] [CrossRef]
- Seck, G.S.; Hache, E.; Barnet, C. Potential bottleneck in the energy transition: The case of cobalt in an accelerating electro-mobility world. Resour. Policy 2021, 75, 102516. [Google Scholar] [CrossRef]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Herrington, R.; Najorka, J.; Boni, M.; Dosbaba, M.; Maczurad, M.; Balassone, G. Quan-titative mineralogical evaluation of Ni-Co laterite ores through XRPD-QPA- and automated SEM-based approaches: The Wingellina (Western Australia) case study. J. Geochem. Explor. 2021, 223, 106695. [Google Scholar] [CrossRef]
- Newsome, L.; Arguedas, A.F.S.; Coker, V.; Boothman, C.; Lloyd, J. Manganese and cobalt redox cycling in laterites; Biogeochemical and bioprocessing implications. Chem. Geol. 2019, 531, 119330. [Google Scholar] [CrossRef]
- Butt, C.R.M.; Cluzel, D. Nickel Laterite Ore Deposits: Weathered Serpentinites. Elements 2013, 9, 123–128. [Google Scholar] [CrossRef]
- Freyssinet, P.; Butt, C.R.M.; Morris, R.C.; Piantone, P. Ore-Forming Processes Related to Lateritic Weathering. In One Hundredth Anniversary Volume; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 681–722. [Google Scholar] [CrossRef]
- Golightly, J.P. Nickeliferous Laterite Deposits; Society of Economic Geologists: Littleton, CO, USA, 1981; pp. 710–735. [Google Scholar] [CrossRef]
- Golightly, J.P.; Goldfarb, R.J.; Marsh, E.E.; Monecke, T. Progress in Understanding the Evolution of Nickel Laterites. In The Challenge of Finding New Mineral Resources: Global Metallogeny, Innovative Exploration, and New Discoveries; Society of Economic Geologists: Littleton, CO, USA, 2010; Volume 15, pp. 451–485. [Google Scholar] [CrossRef]
- Villanova-De-Benavent, C.; Domènech, C.; Tauler, E.; Galí, S.; Tassara, S.; Proenza, J.A. Fe–Ni-bearing serpentines from the saprolite horizon of Caribbean Ni-laterite deposits: New insights from thermodynamic calculations. Miner. Depos. 2016, 52, 979–992. [Google Scholar] [CrossRef]
- Dehaine, Q.; Tijsseling, L.T.; Glass, H.J.; Törmänen, T.; Butcher, A.R. Geometallurgy of cobalt ores: A review. Miner. Eng. 2020, 160, 106656. [Google Scholar] [CrossRef]
- Elias, M.; Donaldson, M.J.; Giorgetta, N.E. Geology, mineralogy, and chemistry of lateritic nickel-cobalt deposits near Kalgoorie, Western Australia. Econ. Geol. 1981, 76, 1775–1783. [Google Scholar] [CrossRef]
- Putzolu, F.; Balassone, G.; Boni, M.; Maczurad, M.; Mondillo, N.; Najorka, J.; Pirajno, F. Mineralogical association and Ni-Co deportment in the Wingellina oxide-type laterite deposit (Western Australia). Ore Geol. Rev. 2018, 97, 21–34. [Google Scholar] [CrossRef]
- Aiglsperger, T.; Proenza, J.A.; Lewis, J.F.; Labrador, M.; Svojtka, M.; Rojas-Purón, A.; Longo, F.; Ďurišová, J. Critical metals (REE, Sc, PGE) in Ni laterites from Cuba and the Dominican Republic. Ore Geol. Rev. 2016, 73, 127–147. [Google Scholar] [CrossRef]
- Domènech, C.; Galí, S.; Soler, J.M.; Ancco, M.P.A.; Meléndez, W.; Rondón, J.; Villanova-De-Benavent, C.; Proenza, J.A. The Loma de Hierro Ni-laterite deposit (Venezuela): Mineralogical and chemical composition. Boletín Soc. Geológica Mex. 2020, 72, A050620. [Google Scholar] [CrossRef]
- Villanova-de-Benavent, C.; Proenza, J.A.; Galí, S.; García-Casco, A.; Tauler, E.; Lewis, J.F.; Longo, F. Garnierites and garnierites: Textures, mineralogy and geochemistry of garnierites in the Falcondo Ni laterite deposit, Dominican Republic. Ore Geol. Rev. 2014, 58, 91–109. [Google Scholar] [CrossRef]
- Lewis, J.F.; Jiménez, J.G. Duarte complex in the La Vega–Jarabacoa–Janico Area, Central Hispaniola: Geological and geochemical features of the sea floor during the early stages of arc evolution. In Geologic and Tectonic Development of the North America–Caribbean Plate Boundary in Hispaniola; Mann, P., Draper, G., Lewis, J.F., Eds.; Geological Society of America Special Papers: Boulder, CO, USA, 1991; Volume 262, pp. 115–142. [Google Scholar]
- Hackley, P.C.; Urbani, F.; Karlsen, A.W.; Garrity, C.P. Mapa Geologico de Venezuela a Escala 1:750,000; USGS Publications Open-File Report 2006-1109 Warehouse: Virgina, VA, USA, 2006. [Google Scholar] [CrossRef]
- Giunta, G.; Beccaluva, L.; Coltorti, M.; Mortellaro, D.; Siena, F.; Cutrupia, D. The peri-Caribbean ophiolites: Structure, tectono-magmatic significance and geodynamic implications. Caribb. J. Earth Sci. 2000, 36, 1–20. [Google Scholar]
- Urbani, F. Una revisión de los terrenos geológicos del sistema montañoso del Caribe, Norte de Venezuela. Boletín Geología 2018, 23, 118–216. [Google Scholar]
- Marvin, B.; Valencia, V.; Grande, S.; Urbani, F.; Hurtado, R. Geocronología U–Pb en cristales de zircón de la metadiorita de la Guacamaya, gabro de la ofiolita de Loma de Hierro y gabro de El Chacao, estados Aragua y Guárico. In Proceedings of the V Simposio Venezolano de Geociencias de las Rocas Ígneas y Metamórficas, Caracas, Venezuela, 28–29 November 2013. [Google Scholar]
- Neill, I.; Kerr, A.C.; Chamberlain, K.R.; Schmitt, A.K.; Urbani, F.; Hastie, A.R.; Pindell, J.L.; Barry, T.L.; Millar, I.L. Vestiges of the proto-Caribbean seaway: Origin of the San Souci Volcanic Group, Trinidad. Tectonophysics 2014, 626, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Urbani, F.; Rodríguez, J.A. Atlas Geológico de la Cordillera de la Costa; Ediciones Fundación Geos y Funvisis: Caracas, Venezuela, 2004; 146p. [Google Scholar]
- Cheary, R.W.; Coelho, A.A. A fundamental parameters approach to X-ray line-profile fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS-Academic, version 4.2; TOPAS-Academic: Brisbaine, Australia, 2007. [Google Scholar]
- Rius, J.; Vallcorba, O.; Frontera, C.; Peral, I.; Crespi, A.; Miravitlles, C. Application of synchrotron through-the-substrate microdiffraction to crystals in polished thin sections. IUCrJ 2015, 2, 452–463. [Google Scholar] [CrossRef] [Green Version]
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data ex-ploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Nardi, A.; de Vries, L.M. GibbsStudio, version 3.1.1; Barcelona Science Technologies SL: Barcelona, Spain, 2017; Available online: https://gibbsstudio.io/ (accessed on 31 October 2020).
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for specia-tion, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol. Surv. Tech. Methods 2013, 6, 497. Available online: https://pubs.usgs.gov/tm/06/a43/pdf/tm6-A43.pdf (accessed on 1 July 2022).
- Burlet, C.; Vanbrabant, Y. Study of the spectro-chemical signatures of cobalt-manganese layered oxides (asbolane-lithiophorite and their intermediates) by Raman spectroscopy. J. Raman Spectrosc. 2015, 46, 941–952. [Google Scholar] [CrossRef]
- Katsiapi, A.; Tsakiridis, P.; Oustadakis, P.; Agatzini-Leonardou, S. Cobalt recovery from mixed Co–Mn hydroxide precipitates by ammonia–ammonium carbonate leaching. Miner. Eng. 2010, 23, 643–651. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, S.; Zhang, Y.; Li, Z.; Ma, Y.; Zhang, Z. Mineralogical and thermal characteristics of low-grade Jinlong bauxite sourced from Guangxi Province, China. J. Therm. Anal. 2015, 122, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Gialanella, S.; Girardi, F.; Ischia, G.; Lonardelli, I.; Mattarelli, M.; Montagna, M. On the goethite to hematite phase transformation. J. Therm. Anal. Calorim. 2010, 102, 867–873. [Google Scholar] [CrossRef]
- De Aquino, T.F.; Riella, H.; Bernardin, A.M. Mineralogical and Physical–Chemical Characterization of a Bauxite Ore from Lages, Santa Catarina, Brazil, for Refractory Production. Miner. Process. Extr. Met. Rev. 2011, 32, 137–149. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional Papers of the Geological Institute of Hungary; Geological Institute of Hungary: Budapest, Hungary, 2011; ISBN 978-963-671-288-4. [Google Scholar]
- Llorca, S.; Monchoux, P. Supergene cobalt minerals from New Caledonia. Can. Mineral. 1991, 29, 149–161. [Google Scholar]
- Ribeiro, P.P.M.; de Souza, L.C.M.; Neumann, R.; dos Santos, I.D.; Dutra, A.J.B. Nickel and cobalt losses from laterite ore after the sulfation-roasting-leaching processing. J. Mater. Res. Technol. 2020, 9, 12404–12415. [Google Scholar] [CrossRef]
- Palchik, N.A.; Moroz, T.N.; Grigorieva, T.N.; Miroshnichenko, L.V. Manganese minerals from the Miassovo freshwater lake: Composition and structure. Russ. J. Inorg. Chem. 2014, 59, 511–518. [Google Scholar] [CrossRef]
- White, W.N.; Vito, C.; Scheetz, B.E. The mineralogy and trace element chemistry of black manganese oxide deposits from caves. J. Cave Karst Stud. 2009, 271, 136–143. [Google Scholar]
- Ling, F.T.; Post, J.E.; Heaney, P.J.; Kubicki, J.D.; Santelli, C.M. Fourier-transform infrared spectroscopy (FTIR) analysis of triclinic and hexagonal birnessites. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Chukanov, N.V. Infrared Spectra of Mineral Species: Extended Library; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Potter, R.M.; Rossman, G.R. The tetravalent manganese oxides: Identification, hydration, and structural relationships by infrared spectroscopy. Am. Mineral. 1979, 64, 1199–1218. [Google Scholar]
- Carmichael, S.K.; Doctor, D.H.; Wilson, C.G.; Feierstein, J.; McAleer, R. New insight into the origin of manganese oxide ore deposits in the Appalachian Valley and Ridge of northeastern Tennessee and northern Virginia, USA. GSA Bull. 2017, 129, 1158–1180. [Google Scholar] [CrossRef]
- Tauler, E.; Lewis, J.F.; Villanova-De-Benavent, C.; Aiglsperger, T.; Proenza, J.A.; Domènech, C.; Gallardo, T.; Longo, F.; Galí, S. Discovery of Ni-smectite-rich saprolite at Loma Ortega, Falcondo mining district (Dominican Republic): Geochemistry and mineralogy of an unusual case of “hybrid hydrous Mg silicate—Clay silicate” type Ni-laterite. Miner. Depos. 2017, 52, 1011–1030. [Google Scholar] [CrossRef]
- Roqué-Rosell, J.; Mosselmans, F.; Proenza, J.A.; Labrador, M.; Galí, S.; Atkinson, K.D.; Quinn, P.D. Sorption of Ni by “lithi-ophorite-asbolane” intermediates in Moa Bay lateritic deposits, eastern Cuba. Chem. Geol. 2010, 275, 9–18. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Dublet, G.; Juillot, F.; Brest, J.; Noël, V.; Fritsch, E.; Proux, O.; Olivi, L.; Ploquin, F.; Morin, G. Vertical changes of the Co and Mn speciation along a lateritic regolith developed on peridotites (New Caledonia). Geochim. Cosmochim. Acta 2017, 217, 1–15. [Google Scholar] [CrossRef]
- Putzolu, F.; Abad, I.; Balassone, G.; Boni, M.; Mondillo, N. Ni-bearing smectites in the Wingellina laterite deposit (Western Australia) at nanoscale: TEM-HRTEM evidences of the formation mechanisms. Appl. Clay Sci. 2020, 196, 105753. [Google Scholar] [CrossRef]
- Maurizot, P.; Sevin, B.; Lesimple, S.; Bailly, L.; Iseppi, M.; Robineau, B. Chapter 10 Mineral resources and prospectivity of the ultramafic rocks of New Caledonia. In New Caledonia: Geology, Geodynamic Evolution and Mineral Resource; Maurizot, P., Mortimer, N., Eds.; Geological Society: London, UK, 2020; Volume 51, pp. 247–277. [Google Scholar] [CrossRef]
- Llorca, S.M. Metallogeny of supergene cobalt mineralization, New Caledonia. Aust. J. Earth Sci. 1993, 40, 377–385. [Google Scholar] [CrossRef]
- Manceau, A.; Llorca, S.; Calas, G. Crystal chemistry of cobalt and nickel in lithiphorite and asbolane from New Caledonia. Geoch. Cosmoch. Acta 1987, 51, 105–113. [Google Scholar] [CrossRef]
- Ploquin, F.; Fritsch, E.; Guigner, J.M.; Esteve, I.; Delbes, L.; Dublet, G.; Juillot, F. Phyllomanganate vein-infillings in faulted and Al-poor regoliths of the New Caledonian ophiolite: Periodic and sequential crystallization of Ni–asbolane, Alk–birnessite and H–birnessite. Eur. J. Mineral. 2019, 31, 335–352. [Google Scholar] [CrossRef]
- Ulrich, M.; Cathelineau, M.; Muñoz, M.; Boiron, M.-C.; Teitler, Y.; Karpoff, A.M. The relative distribution of critical (Sc, REE) and transition metals (Ni, Co, Cr, Mn, V) in some Ni-laterite deposits of New Caledonia. J. Geochem. Explor. 2018, 197, 93–113. [Google Scholar] [CrossRef]
- Dzemua, G.L.; Gleeson, S.A.; Schofield, P.F. Mineralogical characterization of the Nkamouna Co–Mn laterite ore, southeast Cameroon. Miner. Depos. 2012, 48, 155–171. [Google Scholar] [CrossRef]
- Tupaz, C.A.J.; Watanabe, Y.; Sanematsu, K.; Echigo, T. Mineralogy and geochemistry of the Berong Ni-Co laterite deposit, Palawan, Philippines. Ore Geol. Rev. 2020, 125, 103686. [Google Scholar] [CrossRef]
- Chukhrov, F.V.; Gorshkov, A.I.; Vitovskaya, I.V.; Drits, V.A.; Sivtsov, A.V. On the Nature of Co-Ni Asbolane; a Component of Some Supergene Ores. In Ore Genesis: The State of the Art; Amstutz, G.C., El Goresy, A., Frenzel, G., Kluth, C., Moh, G., Wauschkuhn, A., Zimmermann, R.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 230–239. [Google Scholar] [CrossRef]
- Gorshkov, A.I.; Bogdanov, Y.A.; Sivtsov, A.V.; Mokhov, S.V. A new Mg-AI-Ni asbolane. Doklady Akad. Nauk 1995, 342, 781–784. (In Russian) [Google Scholar]
- Marsh, E.; Anderson, E.; Gray, F. Chapter H of Mineral deposit models for resource assessment. In Nickel-Cobalt Laterites—A Deposit Model; 2010–5070–H; Paper for U.S. Geological Survey Scientific Investigations: Denver, CO, USA, 2013; 38p. Available online: https://pubs.usgs.gov/sir/2010/5070/h/ (accessed on 28 June 2022).
- Burns, R.G.; Burns, V.M. Manganese oxides. In Marine Minerals; Ribbe, P.H., Ed.; Mineralogical Society of America: Washington, DC, USA, 1979. [Google Scholar]
- Quantin, C.; Becquer, T.; Berthelin, J. Mn-oxide: A major source of easily mobilizable Co and Ni under reducing conditions in New Caledonia Ferralsols. Comptes Rendus Geosci. 2002, 334, 273–278. [Google Scholar] [CrossRef]
- Aoshima, M.; Tani, Y.; Fujita, R.; Tanaka, K.; Miyata, N.; Umezawa, K. Simultaneous Sequestration of Co2+ and Mn2+ by Fungal Manganese Oxide through Asbolane Formation. Minerals 2022, 12, 358. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Trace Metal Retention on Biogenic Manganese Oxide Nanoparticles. Elements 2005, 1, 223–226. [Google Scholar] [CrossRef]
- Giffaut, E.; Grivé, M.; Blanc, P.; Vieillard, P.; Colàs, E.; Gailhanou, H.; Gaboreau, S.; Marty, N.; Madé, B.; Duro, L. Andra thermodynamic database for performance assessment: ThermoChimie. Appl. Geochem. 2014, 49, 225–236. [Google Scholar] [CrossRef]
- Plyasunova, N.V.; Zhang, Y.; Muhammed, M. Critical evaluation of thermodynamic of complex formation of metals ions in aqueous solution: IV hydrolysis and hydroxo-complex of Ni2+ at 298.15K. Hydrometallurgy 1998, 48, 153–169. [Google Scholar] [CrossRef]
- Cui, H.; You, L.; Feng, X.; Tan, W.; Qiu, G.; Liu, F. Factors Governing the Formation of Lithiophorite at Atmospheric Pressure. Clays Clay Miner. 2009, 57, 353–360. [Google Scholar] [CrossRef]
- Rao, D.; Nayak, B.; Acharya, B. Cobalt-rich lithiophorite from the Precambrian Eastern Ghats manganese ore deposit of Nishikhal, south Orissa, India. Mineralogia 2010, 41, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Farré-De-Pablo, J.; Proenza, J.A.; González-Jiménez, J.M.; Aiglsperger, T.; Garcia-Casco, A.; Escuder-Viruete, J.; Colás, V.; Longo, F. Ophiolite hosted chromitite formed by supra-subduction zone peridotite–plume interaction. Geosci. Front. 2020, 11, 2083–2102. [Google Scholar] [CrossRef]
- Marchesi, C.; Garrido, C.J.; Proenza, J.A.; Hidas, K.; Varas-Reus, M.I.; Butjosa, L.; Lewis, J.F. Geochemical record of subduction initiation in the sub-arc mantle: Insights from the Loma Caribe peridotite (Dominican Republic). Lithos 2016, 252-253, 1–15. [Google Scholar] [CrossRef]
- Decrée, S.; Pourret, O.; Baele, J.-M. Rare earth element fractionation in heterogenite (CoOOH): Implication for cobalt oxidized ore in the Katanga Copperbelt (Democratic Republic of Congo). J. Geochem. Explor. 2015, 159, 290–301. [Google Scholar] [CrossRef]
- Nicholson, K. Contrasting mineralogical-geochemical signatures of manganese oxides; guides to metallogenesis. Econ. Geol. 1992, 87, 1253–1264. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic Manganese Oxides: Properties and Mechanisms of Formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Lanson, B.; Feng, X.; Yin, H.; Tan, W.; He, F.; Liu, F. Transformation of the phyllomanganate vernadite to tectomanganates with small tunnel sizes: Favorable geochemical conditions and fate of associated Co. Geochim. Cosmochim. Acta 2021, 295, 224–236. [Google Scholar] [CrossRef]
- Beak, D.G.; Kirby, J.K.; Hettiarachchi, G.M.; Wendling, L.; McLaughlin, M.J.; Khatiwada, R. Cobalt Distribution and Speciation: Effect of Aging, Intermittent Submergence, In Situ Rice Roots. J. Environ. Qual. 2011, 40, 679–695. [Google Scholar] [CrossRef]













| Label | Texture | SiO2 | Al2O3 | CaO | K2O | Na2O | MgO | NiO | CoO | FeO | MnO | Cr2O3 | BaO | TiO2 | V2O3 | Sc2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LC-6_01 | F | 6.24 | 8.37 | 0.04 | <0.01 | 0.01 | 0.37 | 17.22 | 6.73 | 6.84 | 26.01 | <0.01 | 0.01 | n.a | n.a | n.a | 71.84 |
| LC-6_02 | F | 3.01 | 8.18 | 0.05 | 0.04 | 0.09 | 0.37 | 18.82 | 6.96 | 7.82 | 27.90 | <d.l. | 0.11 | n.a | n.a | n.a | 73.34 |
| LC-6_03 | F | 3.99 | 8.71 | 0.03 | 0.03 | 0.01 | 0.12 | 20.60 | 8.52 | 8.72 | 28.63 | 0.03 | <d.l. | n.a | n.a | n.a | 79.38 |
| LC-6_04 | F | 4.14 | 9.47 | 0.02 | 0.02 | 0.03 | 0.24 | 14.97 | 6.89 | 9.32 | 23.57 | 0.05 | 0.04 | n.a | n.a | n.a | 68.75 |
| LC-6_05 | F | 2.09 | 9.50 | 0.07 | <0.01 | 0.02 | 0.13 | 19.23 | 6.38 | 6.21 | 23.09 | 0.03 | 0.02 | n.a | n.a | n.a | 66.76 |
| LC-7_01 | F | 0.49 | 12.47 | 0.02 | 0.17 | 0.61 | 0.64 | 11.82 | 9.51 | 4.02 | 31.08 | <d.l. | <d.l. | n.a | n.a | n.a | 70.82 |
| LC-6_06 | CA | 4.36 | 8.91 | 0.06 | <0.01 | 0.01 | 0.11 | 19.11 | 7.88 | 8.72 | 26.86 | <0.01 | <d.l. | n.a | n.a | n.a | 76.02 |
| LC-6_07 | CA | 6.65 | 10.47 | 0.04 | <0.01 | 0.06 | 0.08 | 16.32 | 6.12 | 7.47 | 20.68 | <d.l. | 0.09 | n.a | n.a | n.a | 67.98 |
| LC-6_08 | CA | 1.40 | 10.73 | 0.03 | 0.02 | 0.06 | 0.07 | 16.63 | 6.30 | 6.45 | 21.62 | 0.03 | 0.09 | n.a | n.a | n.a | 63.43 |
| LC-6_09 | CA | 7.70 | 10.91 | 0.05 | 0.02 | 0.18 | 0.15 | 13.95 | 6.64 | 5.79 | 22.35 | 0.02 | 0.08 | n.a | n.a | n.a | 67.86 |
| LC-7_02 | CA | 0.39 | 5.95 | 0.11 | 0.43 | 1.13 | 2.02 | 10.20 | 5.56 | 6.24 | 37.47 | 0.02 | 0.42 | n.a | n.a | n.a | 69.94 |
| LC-7_03 | CA | 1.09 | 11.39 | 0.04 | 0.16 | 0.46 | 0.35 | 15.70 | 8.11 | 3.72 | 30.91 | <0.01 | <d.l. | n.a | n.a | n.a | 71.93 |
| LC-7_04 | CA | 1.91 | 16.03 | 0.05 | 0.14 | 0.37 | 0.05 | 6.01 | 7.79 | 12.62 | 25.95 | 0.05 | <d.l. | n.a | n.a | n.a | 70.97 |
| LC-7_05 | CA | 0.50 | 19.67 | 0.04 | 0.05 | 0.18 | 0.02 | 3.85 | 9.12 | 4.08 | 33.27 | 0.10 | <d.l. | n.a | n.a | n.a | 70.87 |
| LC-6_10 | C | 0.39 | 0.24 | 0.03 | 0.03 | 0.04 | 7.78 | 3.36 | 0.30 | 0.63 | 55.20 | <d.l. | 0.66 | n.a | n.a | n.a | 68.66 |
| LC-6_11 | C | 0.61 | 0.76 | 0.04 | 0.05 | 0.04 | 6.65 | 4.76 | 0.46 | 0.91 | 52.85 | <d.l. | 0.57 | n.a | n.a | n.a | 67.71 |
| LC-6_12 | R | 11.27 | 10.38 | 0.09 | 0.03 | 0.16 | 0.11 | 11.43 | 5.88 | 5.13 | 19.96 | 0.02 | <d.l. | n.a | n.a | n.a | 64.46 |
| LC-6_13 | R | 1.52 | 9.69 | <d.l. | 0.03 | 0.06 | 0.07 | 10.41 | 4.83 | 7.46 | 17.20 | 0.04 | 0.06 | n.a | n.a | n.a | 51.38 |
| LC-6_14 | R | 2.11 | 8.16 | 0.10 | <0.01 | <d.l. | 0.21 | 15.53 | 6.81 | 8.13 | 23.74 | <d.l. | <d.l. | n.a | n.a | n.a | 64.79 |
| LC-6_15 | R | 1.56 | 13.53 | 0.04 | 0.03 | 0.08 | 0.11 | 13.74 | 7.99 | 4.58 | 25.72 | 0.02 | <d.l. | n.a | n.a | n.a | 67.40 |
| LC-6_16 | R | 7.92 | 12.20 | 0.18 | 0.04 | 0.19 | 0.11 | 14.23 | 6.80 | 6.49 | 22.84 | <d.l. | <d.l. | n.a | n.a | n.a | 71.00 |
| LC-6_17 | R | 1.34 | 11.85 | 0.09 | 0.04 | 0.04 | 0.25 | 16.23 | 8.13 | 4.91 | 26.05 | 0.02 | <d.l. | n.a | n.a | n.a | 68.95 |
| LC-7_06 | GS | 0.26 | 15.33 | 0.07 | 0.11 | 0.24 | 0.16 | 6.85 | 11.36 | 1.90 | 34.17 | 0.02 | 0.33 | n.a | n.a | n.a | 70.81 |
| LC-7_07 | GS | 3.40 | 12.39 | 0.31 | 0.05 | 0.49 | 0.24 | 5.60 | 8.71 | 11.08 | 22.74 | 0.65 | 0.06 | n.a | n.a | n.a | 65.71 |
| LC-7_08 | GS | 5.46 | 16.01 | 0.14 | 0.12 | 0.37 | 0.11 | 3.48 | 7.10 | 13.70 | 21.66 | 0.86 | 0.02 | n.a | n.a | n.a | 69.03 |
| LC-7_09 | GS | 2.63 | 14.82 | 0.13 | 0.07 | 0.18 | 0.08 | 3.90 | 10.04 | 8.30 | 25.64 | 0.26 | <d.l. | n.a | n.a | n.a | 66.05 |
| LC-7_10 | GS | 7.29 | 15.57 | 0.17 | 0.10 | 0.32 | 0.10 | 3.43 | 7.49 | 9.97 | 22.02 | 0.39 | <d.l. | n.a | n.a | n.a | 66.84 |
| LC-7_11 | GS | 1.73 | 19.73 | 0.06 | 0.05 | 0.13 | 0.05 | 3.58 | 8.29 | 6.69 | 30.43 | 0.19 | <d.l. | n.a | n.a | n.a | 70.92 |
| LC-7_12 | GS | 0.98 | 21.11 | 0.02 | <0.01 | 0.09 | 0.02 | 3.69 | 8.20 | 2.89 | 33.13 | 0.05 | 0.09 | n.a | n.a | n.a | 70.26 |
| LC-7_13 | GS | 1.69 | 21.26 | 0.02 | 0.01 | 0.09 | 0.04 | 2.85 | 5.70 | 10.58 | 28.71 | 0.18 | 0.10 | n.a | n.a | n.a | 71.23 |
| LC-7_14 | Ba | 0.30 | 3.91 | 0.04 | 0.51 | 0.36 | 0.05 | 0.70 | 0.96 | 2.75 | 58.51 | 0.08 | 9.87 | n.a | n.a | n.a | 78.04 |
| LC-7_15 | Ba | 0.25 | 3.20 | 0.05 | 0.51 | 0.29 | 0.02 | 0.74 | 1.04 | 3.16 | 57.82 | 0.10 | 10.51 | n.a | n.a | n.a | 77.70 |
| LC-7_16 | Ba | 0.27 | 3.81 | <0.01 | 0.41 | 0.27 | 0.03 | 0.71 | 1.04 | 2.79 | 57.63 | 0.07 | 10.56 | n.a | n.a | n.a | 77.59 |
| LC-7_17 | Ba | 0.47 | 4.21 | <d.l. | 0.43 | 0.26 | 0.02 | 0.71 | 0.91 | 6.33 | 54.30 | 0.17 | 10.51 | n.a | n.a | n.a | 78.32 |
| Label | Texture | SiO2 | Al2O3 | CaO | K2O | Na2O | MgO | NiO | CoO | FeO | MnO | Cr2O3 | BaO | TiO2 | V2O3 | Sc2O3 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LH-10_01 | F | 3.47 | 1.06 | 0.03 | 0.04 | 0.01 | 4.60 | 18.19 | 3.23 | 12.48 | 31.30 | 0.03 | 0.05 | n.a. | n.a. | n.a. | 74.50 |
| LH-10_02 | F | 3.93 | 1.81 | 0.08 | 0.02 | 0.04 | 6.29 | 20.40 | 3.71 | 3.87 | 35.48 | <d.l. | 0.24 | n.a. | n.a. | n.a. | 75.86 |
| LH-10_03 | F | 5.56 | 0.82 | 0.06 | 0.06 | <d.l. | 6.16 | 13.20 | 2.19 | 24.20 | 21.70 | <d.l. | n.a. | <0.01 | <d.l. | n.a. | 73.94 |
| LH-10_04 | F | 4.75 | 0.98 | 0.07 | 0.04 | 0.06 | 5.80 | 16.76 | 3.08 | 12.47 | 29.73 | <d.l. | n.a. | 0.02 | <0.01 | n.a. | 73.76 |
| LH-11_01 | CA | 0.12 | 2.01 | 0.08 | n.a. | n.a. | 3.12 | 18.77 | 3.78 | 9.96 | 40.03 | <d.l. | n.a. | 0.01 | 0.02 | <d.l. | 77.90 |
| LH-11_02 | CA | 0.20 | 2.26 | 0.05 | n.a. | n.a. | 3.48 | 18.15 | 4.83 | 10.12 | 39.41 | <d.l. | n.a. | <d.l. | <d.l. | <d.l. | 78.50 |
| LH-11_03 | CA | 0.13 | 2.68 | 0.06 | n.a. | n.a. | 2.07 | 20.02 | 5.17 | 8.90 | 38.13 | <d.l. | n.a. | <d.l. | <d.l. | <d.l. | 77.16 |
| LH-11_04 | CA | 0.16 | 2.98 | 0.04 | n.a. | n.a. | 4.26 | 13.52 | 8.95 | 8.96 | 37.55 | <d.l. | n.a. | <d.l. | 0.01 | <d.l. | 76.44 |
| LH-11_05 | CA | 0.37 | 6.35 | 0.04 | n.a. | n.a. | 3.76 | 16.23 | 7.37 | 4.21 | 40.69 | <d.l. | n.a. | <d.l. | n.a. | <d.l. | 79.02 |
| LH-11_06 | CA | 0.30 | 7.53 | 0.03 | n.a. | n.a. | 4.51 | 13.98 | 10.70 | 2.08 | 39.03 | <d.l. | n.a. | <d.l. | <0.01 | <d.l. | 78.16 |
| LHA_1 | C | 0.04 | 1.02 | 0.02 | 0.12 | <d.l. | <d.l. | 0.27 | 1.23 | 0.45 | 78.56 | <d.l. | 0.12 | n.a. | n.a. | n.a. | 81.82 |
| LHA_2 | C | 0.13 | 1.72 | <0.01 | 0.15 | 0.08 | 0.05 | 0.25 | 1.05 | 1.62 | 75.97 | 0.01 | 0.26 | n.a. | n.a. | n.a. | 81.30 |
| LHA_3 | C | 0.05 | 1.06 | <0.01 | 0.08 | <d.l. | <0.01 | 0.30 | 1.38 | 0.45 | 78.74 | 0.02 | 0.07 | n.a. | n.a. | n.a. | 82.15 |
| LHA_4 | R | 0.08 | 8.58 | <0.01 | 0.16 | 0.07 | 0.03 | 2.54 | 2.06 | 3.05 | 54.91 | 0.03 | 0.50 | n.a. | n.a. | n.a. | 72.02 |
| LHA_5 | R | 0.13 | 9.13 | 0.03 | 0.22 | <d.l. | 0.10 | 2.37 | 2.59 | 3.55 | 52.98 | <d.l. | 0.72 | n.a. | n.a. | n.a. | 71.81 |
| LHA_6 | R | 0.08 | 18.56 | <d.l. | 0.08 | 0.10 | 0.04 | 5.61 | 5.97 | 1.47 | 39.21 | <d.l. | 0.12 | n.a. | n.a. | n.a. | 71.24 |
| LHA_7 | R | 0.09 | 2.10 | 0.02 | 0.59 | 0.09 | 0.03 | 0.37 | 1.25 | 0.49 | 72.74 | <d.l. | 1.30 | n.a. | n.a. | n.a. | 79.07 |
| LHA_8 | R | 0.09 | 3.26 | 0.04 | 0.90 | 0.05 | 0.02 | 0.57 | 1.55 | 0.56 | 70.65 | 0.01 | 1.31 | n.a. | n.a. | n.a. | 79.01 |
| LH-11_07 | T | 1.41 | 5.25 | 0.04 | n.a. | n.a. | 2.50 | 10.43 | 7.42 | 24.66 | 26.30 | 0.80 | n.a. | 0.04 | 0.03 | <d.l. | 78.88 |
| LH-11_08 | T | 1.19 | 5.79 | 0.02 | n.a. | n.a. | 2.45 | 10.36 | 7.38 | 22.38 | 28.17 | 0.66 | n.a. | 0.02 | 0.03 | <d.l. | 78.44 |
| LH-11_09 | T | 0.92 | 6.43 | 0.02 | n.a. | n.a. | 2.72 | 14.72 | 6.72 | 17.50 | 33.08 | 0.46 | n.a. | 0.02 | 0.02 | <d.l. | 82.61 |
| LH-11_10 | T | 1.35 | 5.15 | 0.03 | n.a. | n.a. | 2.74 | 11.27 | 6.00 | 28.23 | 26.08 | 0.69 | n.a. | 0.04 | 0.04 | <d.l. | 81.62 |
| Label | Texture | SiO2 | Al2O3 | CaO | K2O | Na2O | MgO | NiO | CoO | FeO | MnO | Cr2O3 | BaO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LHA_10 | C | 34.35 | 28.60 | 0.06 | 0.06 | 0.00 | 0.08 | 0.30 | 1.54 | 1.13 | 5.78 | 0.04 | <d.l. | 71.95 |
| LHA_11 | C | 26.50 | 24.96 | 0.03 | 0.04 | 0.06 | 0.04 | 0.54 | 3.95 | 1.49 | 12.08 | <d.l. | 0.04 | 69.73 |
| LHA_12 | R | 19.91 | 24.12 | 0.04 | 0.13 | 0.02 | 0.01 | 0.86 | 5.93 | 3.29 | 18.22 | 0.02 | 0.02 | 72.57 |
| LHA_13 | R | 18.76 | 23.36 | 0.03 | 0.06 | <d.l. | 0.02 | 0.79 | 6.53 | 3.41 | 18.95 | <d.l. | 0.05 | 71.96 |
| Mn-Bearing Minerals | Laterite Deposit | MnO2 (wt.%) | CoO (wt.%) | NiO (wt.%) | Reference |
|---|---|---|---|---|---|
| Asbolane | Lipovsk (Middle Urals, Russia) (1) | 94.2 | -- | 6.3 | [59] |
| Lipovsk (Middle Urals, Russia) (2) | 46.9–49.1 | 6.9 | 11.4 | [58] | |
| Urals (Russia) (3) | 55.1–66.7 | <6.9 | 11.4–21.6 | [38] | |
| New Caledonia (1) | 48.3 | 21.1 | 10.7 | [53] | |
| New Caledonia (2) | 48.3–66.7 | <21.1 | 10.7–17.9 | [38] | |
| New Caledonia (2) | 58.9–65.2 | 1.2–1.7 | 10.1–12.6 | [54] | |
| New Caledonia (3) | 40.1–66.7 | 0.9–5.1 | 17.9–20.1 | [52] | |
| Moa Bay (Cuba) (3) | 31.3–43.3 | 1.2–7.8 | 19.3–20.5 | [47] | |
| Birnessite | New Caledonia (3) | 85.1–91.9 | 0.8–1.5 | 0.1–1.0 | [54] |
| Cryptomelane | Nkamouna (Cameroon) (5) | 34.0–80.9 | 2.7–16.9 | 1.1–6.9 | [56] |
| Heterogenite | New Caledonia (1) | 0.2 | 69.3 | 4.7 | [38,52,53] |
| Shaba (Democratic Republic of the Congo) (1) | 0.5 | 76.4 | 1.9 | [38] | |
| Lithiophorite | New Caledonia (1) | 46.0 | 7.1 | 1.5 | [38,52] |
| Postmasburg (South Africa) (1) | 47.0 | -- | -- | [38] | |
| Nkamouna (Cameroon) (12) | 29.3–50.5 | 3.1–10.8 | 1.7–7.9 | [56] | |
| New Caledonia (2) | 44.1–46.0 | 7.1–8.9 | 1.3–1.4 | [53] | |
| Loma Ortega (Dominican Republic) (27) | 14.1–85.6 | 0.1–6.5 | 0.6–23.2 | [46] | |
| Lithiophorite–asbolane intermediate | Palawan (Philippines) (4) | 34.3–44.7 | 1.3–8.1 | 3.7–19.5 | [57] |
| New Caledonia (1) | 45.4 | 12.6 | 2.7 | [52] | |
| Wingellina (Australia) (8) | 33.7–55.1 | 0.1–2.5 | 10.5–14.1 | [15] | |
| Nkamouna (Cameroon) (5) | 20.2–43.8 | 2.3–17.0 | 0.7–7.5 | [56] | |
| Romanechite | Wingellina (Australia) (4) | 51.9–69.3 | 0.7–1.6 | 1.1–2.9 | [15] |
| Phyllomanganate | New Caledonia (4) | 74.6–88.7 | 1.2–1.5 | 1.4–6.2 | [53] |
| Pyrolusite | Nkamouna (Cameroon) (2) | 70.0–96.4 | 0.7–1.2 | 0.9–1.0 | [56] |
| Ramsdellite | New Caledonia (1) | 95.3 | 0.8 | -- | [52] |
| Todorokite | New Caledonia (1) | 61.9 | 0.9 | 0.4 | [52] |
| Co–Ni-bearing Mn-hydroxides | Wingellina (Australia) (6) | 54.1–90.8 | 0.2–3.0 | 2.3–14.1 | [15] |
| Mn-oxide | New Caledonia (1) | 38.7 | 11.9 | 5.8 | [53] |
| Unspecified | New Caledonia (9) | 13.6–38.8 | 4.2–10.1 | 15.9–22.6 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domènech, C.; Villanova-de-Benavent, C.; Proenza, J.A.; Tauler, E.; Lara, L.; Galí, S.; Soler, J.M.; Campeny, M.; Ibañez-Insa, J. Co–Mn Mineralisations in the Ni Laterite Deposits of Loma Caribe (Dominican Republic) and Loma de Hierro (Venezuela). Minerals 2022, 12, 927. https://doi.org/10.3390/min12080927
Domènech C, Villanova-de-Benavent C, Proenza JA, Tauler E, Lara L, Galí S, Soler JM, Campeny M, Ibañez-Insa J. Co–Mn Mineralisations in the Ni Laterite Deposits of Loma Caribe (Dominican Republic) and Loma de Hierro (Venezuela). Minerals. 2022; 12(8):927. https://doi.org/10.3390/min12080927
Chicago/Turabian StyleDomènech, Cristina, Cristina Villanova-de-Benavent, Joaquín A. Proenza, Esperança Tauler, Laura Lara, Salvador Galí, Josep M. Soler, Marc Campeny, and Jordi Ibañez-Insa. 2022. "Co–Mn Mineralisations in the Ni Laterite Deposits of Loma Caribe (Dominican Republic) and Loma de Hierro (Venezuela)" Minerals 12, no. 8: 927. https://doi.org/10.3390/min12080927
APA StyleDomènech, C., Villanova-de-Benavent, C., Proenza, J. A., Tauler, E., Lara, L., Galí, S., Soler, J. M., Campeny, M., & Ibañez-Insa, J. (2022). Co–Mn Mineralisations in the Ni Laterite Deposits of Loma Caribe (Dominican Republic) and Loma de Hierro (Venezuela). Minerals, 12(8), 927. https://doi.org/10.3390/min12080927









