Micro-Mechanisms and Implications of Continental Red Beds
Abstract
:1. Introduction
2. Red Substance
2.1. Color-Rendering Characteristics
2.2. Distribution and Existence of Hematite
3. The Source and Formation of Hematite
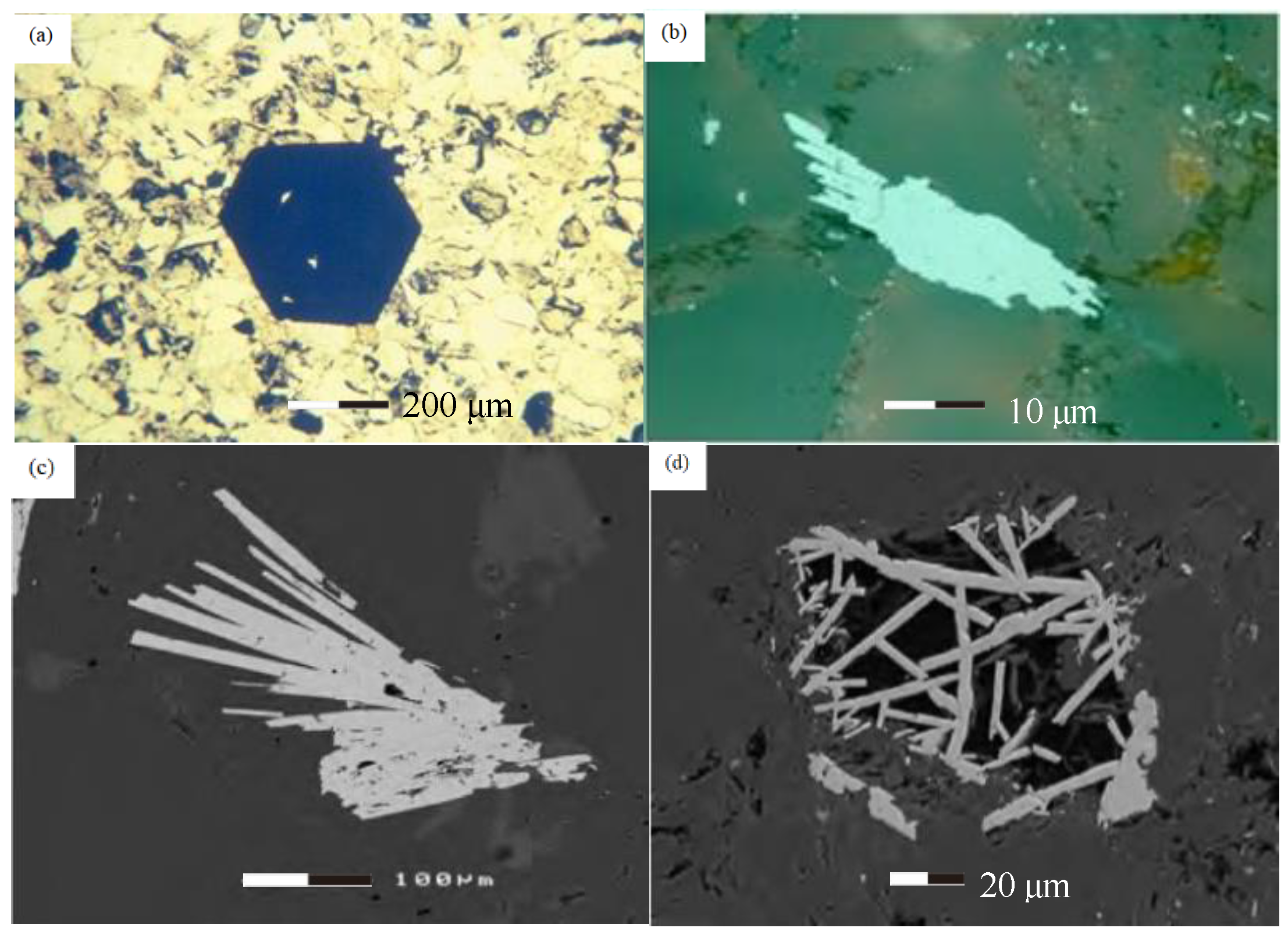
3.1. Source
3.2. Hematite Formation
4. Reduction and Leaching
4.1. Reduction Spots
4.2. Reduction Strips
4.3. Reduction Areas
5. Conclusions and Future Work
- (1)
- Distinguishing the difference and connection between hematite formed by alteration of minerals such as iron silicates or oxides and hematite formed by iron-bearing clay to explore the influence of provenance on the color of red beds;
- (2)
- Combining the thermodynamic behavior of iron oxide in the red beds, as well as the microscopic mechanism of formation of hematite and iron oxyhydroxide, to reveal the microscopic kinetic process of red sediments;
- (3)
- Detailed quantifying of hematite and other iron oxyhydroxides in red beds to provide a more accurate scientific basis for the definition of red beds and to clarify the similarities and differences between red beds and other sedimentary strata;
- (4)
- Exploring the relationships among the size, shape, and diagenetic environment of hematite crystals in the red beds;
- (5)
- Studying the color fading phenomena to further deepen understanding of the history and kinetic process leading to red-bed color formation.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.; Hu, X. Cretaceous world and oceanic red beds. Earth Sci. Front. 2005, 12, 11–21. (In Chinese) [Google Scholar]
- Hu, X. Distribution, Types and Origins of Phanerozoic Marine Red Beds. Bull. Mineral. Petrol. Geochem. 2013, 32, 335–342. (In Chinese) [Google Scholar]
- Lyu, X.; Liu, Z. Distribution, compositions and significance of oceanic red beds. Adv. Earth Sci. 2017, 32, 1307–1318. (In Chinese) [Google Scholar]
- Peng, H.; Pan, Z.; Yan, L.; Scott, S. A review of the research on red beds and Danxia landform. Acta Geogr. Sin. 2013, 68, 1170–1181. (In Chinese) [Google Scholar]
- Russell, I.C. Subaerial Decay of Rocks and Origin of the Red Color of Certain Formations; US Government Printing Office: Washington, DC, USA, 1889; pp. 537–597.
- Dorsey, G.E. The origin of the color of red beds. J. Geol. 1926, 34, 131–143. [Google Scholar] [CrossRef]
- Turner, P. Continental Red Beds; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Migoń, P. Geomorphology of conglomerate terrains—Global overview. Earth-Sci. Rev. 2020, 208, 103302. [Google Scholar] [CrossRef]
- Zhu, C.; Wu, L.; Zhu, T.; Hou, R.; Hu, Z.; Tan, Y.; Sun, W.; Jia, T.; Peng, H. Experimental studies on the Danxia landscape morphogenesis in Mt. Danxiashan, South China. J. Geogr. Sci. 2015, 25, 943–966. [Google Scholar] [CrossRef]
- Dill, H.G.; Kadirov, O.; Tsoy, Y.; Usmanov, A. Palaeogeography of Neogene red bed sequences along the Aksa-Ata River in the Parkent-Nurekata intermontane basin (Tien Shan Mountains, Uzbekistan): With special reference to the magnetic susceptibility of siliciclastic rocks. J. Asian Earth Sci. 2007, 29, 960–977. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Dekkers, M.J.; Zhao, X.; Roberts, A.P.; Yang, Z.; Jin, C.; Liu, J. Remagnetization mechanisms in Triassic red beds from South China. Earth Planet. Sci. Lett. 2017, 479, 219–230. [Google Scholar] [CrossRef]
- Thibal, J.; Etchecopar, A.; Pozzi, J.P.; Barthes, V.; Pocachard, J. Comparison of magnetic and gamma ray logging for correlations in chronology and lithology; example from the Aquitanian Basin (France). Geophys. J. Int. 1999, 137, 839–846. [Google Scholar] [CrossRef]
- Sheldon, N.D. Do red beds indicate paleoclimatic conditions? A Permian case study. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2005, 228, 305–319. [Google Scholar] [CrossRef]
- Bai, H.; Kuang, H.; Liu, Y.; Peng, N.; Chen, X.; Wang, Y. Marinoan-aged red beds at Shennongjia, South China: Evidence against global-scale glaciation during the Cryogenian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 559, 109967. [Google Scholar] [CrossRef]
- Gao, R.; Xue, C.; Zhao, X.; Chen, X.; Li, Z.; Symons, D. Source and possible leaching process of ore metals in the Uragen sandstone-hosted Zn-Pb deposit, Xinjiang, China: Constraints from lead isotopes and rare earth elements geochemistry. Ore Geol. Rev. 2019, 106, 56–78. [Google Scholar] [CrossRef]
- Folk, R.L. Reddening of desert sands: Simpson Desert, N. T., Australia. J. Sediment. Petrol. 1976, 46, 604–615. [Google Scholar] [CrossRef]
- Krynine, P.D. The origin of red beds. Trans. N. Y. Acad. Sci. 1949, 11, 60–68. [Google Scholar] [CrossRef]
- Krynine, P.D. Petrology, Stratigraphy, and Origin of the Triassic Sedimentary Rocks of Connecticut; State Geological and Natural History Survey: Berkeley, CA, USA, 1950; p. 247.
- Van Houten, F.B. Climatic significance of red beds. Descr. Paleoclimatology 1961, 21, 89–139. [Google Scholar]
- Van Houten, F.B. Origin of red beds—Some unsolved problems. In Problems in palaeoclimatology, Proceedings of the NATO Paleoclimate Conference, Newcastle upon Tyne, UK, 7–12 January 1963; Inter-Science Pub. Inc.: New York, NY, USA, 1964; pp. 647–660. [Google Scholar]
- van Houten, F.B. Iron oxides in red beds. Geol. Soc. Am. Bull. 1968, 79, 399–416. [Google Scholar] [CrossRef]
- Walker, T.R. Formation of red beds in modern and ancient deserts. Geol. Soc. Am. Bull. 1967, 78, 353–368. [Google Scholar] [CrossRef]
- Walker, T.R. Color of recent sediments in tropical Mexico: A contribution to the origin of red beds. Geol. Soc. Am. Bull. 1967, 78, 917–920. [Google Scholar] [CrossRef]
- Walker, T.R. Formation of red beds in moist tropical climates: A hypothesis. Geol. Soc. Am. Bull. 1974, 85, 633–638. [Google Scholar] [CrossRef]
- Walker, T.R. Diagenetic origin of continental red beds. In The Continental Permain in Central, West, and South Europe; Springer: Berlin/Heidelberg, Germany, 1976; pp. 240–282. [Google Scholar]
- Hund, F. Inorganic Pigments: Bases for Colored, Uncolored, and Transparent Products. Angew. Chem. Int. Ed. Engl. 1981, 20, 723–730. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U. Influence of hematite on the color of red beds. J. Sediment. Petrol. 1987, 57, 682–686. [Google Scholar] [CrossRef]
- Chan, M.A.; Beitler, B.; Parry, W.T.; Ormo, J.; Komatsu, G. A possible terrestrial analogue for haematite concretions on Mars. Nature 2004, 429, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.A.; Bowen, B.B.; Parry, W.; Ormö, J.; Komatsu, G. Red rock and red planet diagenesis. GSA Today 2005, 15, 4–10. [Google Scholar] [CrossRef]
- Chen, S.A.; Heaney, P.J.; Post, J.E.; Fischer, T.B.; Eng, P.J.; Stubbs, J.E. Superhydrous hematite and goethite: A potential water reservoir in the red dust of Mars? Geology 2021, 49, 1343–1347. [Google Scholar] [CrossRef]
- McBride, E.F. Significance of color in red, green, purple, olive, brown, and gray beds of Difunta Group, northeastern Mexico. J. Sediment. Petrol. 1974, 44, 760–773. [Google Scholar] [CrossRef]
- Blodgett, R.H.; Crabaugh, J.P.; McBride, E.F. The color of red beds—A geologic perspective. Soil Color 1993, 31, 127–159. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences, and Uses; Wiley-vch Weinheim: Weinheim, Germany, 2003; Volume 2. [Google Scholar]
- Scheinost, A.C.; Schwertmann, U. Color identification of iron oxides and hydroxysulfates: Use and limitations. Soil Sci. Soc. Am. J. 1999, 63, 1463–1471. [Google Scholar] [CrossRef]
- Barrón, V.; Torrent, J. Use of the Kubelka—Munk theory to study the influence of iron oxides on soil colour. J. Soil Sci. 1986, 37, 499–510. [Google Scholar] [CrossRef]
- Fuller, C.W. Iron oxides, synthetics. In Chemical and Process Technology Encyclopedia; Considine, D.M., Ed.; McGraw-Hill Book Company: New York, NY, USA, 1974; p. 645. [Google Scholar]
- Turner, P.; Archer, R. The role of biotite in the diagenesis of red beds from the Devonian of northern Scotland. Sediment. Geol. 1977, 19, 241–251. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Schulze, D.G.; Schwertmann, U. Diffuse reflectance spectra of Al substituted goethite: A ligand field approach. Clays Clay Miner. 1999, 47, 156–164. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Roberts, A.P.; Dekkers, M.J.; Barrón, V.; Torrent, J.; Li, S. The Magnetic and Color Reflectance Properties of Hematite: From Earth to Mars. Rev. Geophys. 2022, 60, e2020RG000698. [Google Scholar] [CrossRef]
- Rasmussen, B.; Muhling, J.R. Monazite begets monazite: Evidence for dissolution of detrital monazite and reprecipitation of syntectonic monazite during low-grade regional metamorphism. Contrib. Mineral. Petrol. 2007, 154, 675–689. [Google Scholar] [CrossRef]
- Eren, M.; Kadir, S.; Kapur, S.; Huggett, J.; Zucca, C. Colour origin of Tortonian red mudstones within the Mersin area, southern Turkey. Sediment. Geol. 2015, 318, 10–19. [Google Scholar] [CrossRef]
- Bankole, O.M.; El Albani, A.; Meunier, A.; Rouxel, O.J.; Gauthier-Lafaye, F.; Bekker, A. Origin of red beds in the Paleoproterozoic Franceville Basin, Gabon, and implications for sandstone-hosted uranium mineralization. Am. J. Sci. 2016, 316, 839–872. [Google Scholar] [CrossRef]
- Mahmud, S.A.; Hall, M.W.; Almalki, K.A. Mineralogy and spectroscopy of Owen Group sandstones, Australia: Implications for the provenance, diagenesis, and origin of coloration. Geosci. J. 2018, 22, 765–776. [Google Scholar] [CrossRef]
- EREN, M. Colour origin of red sandstone beds within the Hüdai Formation (Early Cambrian), Aydıncık (Mersin), southern Turkey. Turk. J. Earth Sci. 2013, 22, 563–573. [Google Scholar] [CrossRef]
- Rasmussen, B.; Muhling, J.R. Syn-tectonic hematite growth in Paleoproterozoic Stirling Range “red beds”, Albany-Fraser Orogen, Australia: Evidence for oxidation during late-stage orogenic uplift. Precambrian Res. 2019, 321, 54–63. [Google Scholar] [CrossRef]
- Luo, X.; Yang, Z.; Zhang, K.; Wang, H.; Du, H.; He, W. A Mineralogical study of red origin in Mount Danxia, Guangdong Province. Acta Mineral. Sin. 2021, 41, 1–10. (In Chinese) [Google Scholar]
- Tan, C.; Yu, B.; Yuan, X.; Liu, C.; Wang, T.; Zhu, X. Color Origin of the Lower Triassic Liujiagou and Heshanggou Formations Red Beds in the Ordos Basin. Geoscience 2020, 34, 769–783. (In Chinese) [Google Scholar] [CrossRef]
- Al Juboury, A.I.; Hussain, S.H.; McCann, T.; Aghwan, T.A. Clay mineral diagenesis and red bed colouration: A SEM study of the Gercus Formation (Middle Eocene), northern Iraq. Geol. J. 2020, 55, 7977–7997. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, Y.; Ding, H.; Li, Y.; Lu, A. The Fine Characterization and Potential Photocatalytic Effect of Semiconducting Metal Minerals in Danxia Landforms. Minerals-Basel 2018, 8, 554. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Awasthi, A.K.; Khanna, Y.; Kumari, A.; Singh, B.; Kumar, A.; Popli, C. Sediment colour as recorder of climate and tectonics: Cenozoic continental red beds of the Himalayan foreland basin in NW India. Catena 2021, 203, 105298. [Google Scholar] [CrossRef]
- Schindler, M.; Michel, S.; Batcheldor, D.; Hochella, M.F. A nanoscale study of the formation of Fe-(hydr)oxides in a volcanic regolith; implications for the understanding of soil forming processes on Earth and Mars. Geochim. Cosmochim. Acta 2019, 264, 43–66. [Google Scholar] [CrossRef]
- Walker, T.R. Red beds in the western interior of the United States. US Geol. Surv. Prof. Pap. 1975, 853, 49–56. [Google Scholar]
- Miki, T.; Matsueda, H.; Yim, W.W.S. Petrography and geochemistry of Cretaceous (?) red beds in Hong Kong. J. Southeast Asian Earth Sci. 1990, 4, 99–106. [Google Scholar] [CrossRef]
- Aehnelt, M.; Hilse, U.; Pudlo, D.; Heide, K.; Gaupp, R. On the origin of bleaching phenomena in red bed sediments of Triassic Buntsandstein deposits in Central Germany. Geochemistry 2021, 81, 125736. [Google Scholar] [CrossRef]
- Weibel, R. Diagenesis in oxidising and locally reducing conditions—An example from the Triassic Skagerrak Formation, Denmark. Sediment. Geol. 1998, 121, 259–276. [Google Scholar] [CrossRef]
- Walker, T.R.; Waugh, B.; Grone, A.J. Diagenesis in first-cycle desert alluvium of Cenozoic age, southwestern United States and northwestern Mexico. Geol. Soc. Am. Bull. 1978, 89, 19–32. [Google Scholar] [CrossRef]
- MüCke, A. Part i. postdiagenetic ferruginization of sedimentary rocks (sandstones, oolitic ironstones, kaolins and bauxites)—Including a comparative study of the reddening of red beds. In Developments in Sedimentology; Elsevier: Amsterdam, The Netherlands, 1994; Volume 51, pp. 361–395. [Google Scholar]
- Muchez, P.; Viaene, W.; Dusar, M. Diagenetic control on secondary porosity in flood plain deposits: An example of the Lower Triassic of northeastern Belgium. Sediment. Geol. 1992, 78, 285–298. [Google Scholar] [CrossRef]
- Bensing, J.P.; Mozley, P.S.; Dunbar, N.W. Importance of Clay in Iron Transport and Sediment Reddening: Evidence from Reduction Features of the Abo Formation, New Mexico, U.S.A. J. Sediment. Res. 2005, 75, 562–571. [Google Scholar] [CrossRef]
- Matlack, K.S.; Houseknecht, D.W.; Applin, K.R. Emplacement of clay into sand by infiltration. J. Sediment. Petrol. 1989, 59, 77–87. [Google Scholar] [CrossRef]
- Johnson, S.A.; Glover, B.W.; Turner, P. Multiphase reddening and weathering events in Upper Carboniferous red beds from the English West Midlands. J. Geol. Soc. Lond. 1997, 154, 735–745. [Google Scholar] [CrossRef]
- Chukhrov, F.V. On mineralogical and geochemical criteria in the genesis of red beds. Chem. Geol. 1973, 12, 67–75. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Thermodynamic modelling of nanomorphologies of hematite and goethite. J. Mater. Chem. 2011, 21, 11566–11577. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Roberts, A.P.; Barrón, V.; Torrent, J.; Zhang, Q. A new model for transformation of ferrihydrite to hematite in soils and sediments. Geology 2018, 46, 987–990. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawi, Y. Origin of red color in the gercus formation (eocene), Northeastern Iraq. Sediment. Geol. 1983, 35, 177–192. [Google Scholar] [CrossRef]
- Stel, H. Diagenetic crystallization and oxidation of siderite in red bed (Buntsandstein) sediments from the Central Iberian Chain, Spain. Sediment. Geol. 2009, 213, 89–96. [Google Scholar] [CrossRef]
- Weibel, R.; Grobety, B. Pseudomorphous transformation of goethite needles into hematite in sediments of the Triassic Skagerrak Formation, Denmark. Clay Miner. 1999, 34, 657. [Google Scholar] [CrossRef]
- Berner, R.A. Goethite stability and the origin of red beds. Geochim. Cosmochim. Acta 1969, 33, 267–273. [Google Scholar] [CrossRef]
- Langmuir, D. Particle size effect on the reaction goethite = hematite + water. Am. J. Sci. 1971, 271, 147–156. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Pring, A. Mechanism and kinetics of hydrothermal replacement of magnetite by hematite. Geosci. Front. 2019, 10, 29–41. [Google Scholar] [CrossRef]
- García-Rivas, J.; Suárez, M.; Torres, T.; Sánchez-Palencia, Y.; García-Romero, E.; Ortiz, J. Geochemistry and Biomarker Analysis of the Bentonites from Esquivias (Toledo, Spain). Minerals-Basel 2018, 8, 291. [Google Scholar] [CrossRef] [Green Version]
- Chen, G. A solution to the origin of white spots in red rock formations. Geol. Rev. 1941, Z3, 393–396. (In Chinese) [Google Scholar]
- Hofmann, R.A. Mineralogy and geochemistry of reduction spheroids in red beds. Mineral. Petrol. 1991, 44, 107–124. [Google Scholar] [CrossRef]
- Spinks, S.C.; Parnell, J.; Bowden, S.A. Reduction spots in the Mesoproterozoic age: Implications for life in the early terrestrial record. Int. J. Astrobiol. 2010, 9, 209–216. [Google Scholar] [CrossRef]
- Fox, D.C.M.; Spinks, S.C.; Thorne, R.L.; Barham, M.; Aspandiar, M.; Armstrong, J.G.T.; Uysal, T.; Timms, N.E.; Pearce, M.A.; Verrall, M.; et al. Mineralogy and geochemistry of atypical reduction spheroids from the Tumblagooda Sandstone, Western Australia. Sedimentology 2019, 67, 677–698. [Google Scholar] [CrossRef]
- Hartmann, M. Einige geochemische Untersuchungen an Sandsteinen aus Perm und Trias. Geochim. Cosmochim. Acta 1963, 27, 459–499. [Google Scholar] [CrossRef]
- Durrance, E.M.; Meads, R.E.; Ballard, R.; Walsh, J.N. Oxidation state of iron in the Littleham Mudstone Formation of the New Red Sandstone Series (Permian-Triassic) of southeast Devon, England. Geol. Soc. Am. Bull. 1978, 89, 1231–1240. [Google Scholar] [CrossRef]
- Mykura, H.; Hampton, B.P. On the mechanism of formation of reduction spots in the Carboniferous/Permian red beds of Warwickshire. Geol. Mag. 1984, 121, 71–74. [Google Scholar] [CrossRef]
- Hofmann, B.A. Reduction spheroids from northern Switzerland: Mineralogy, geochemistry and genetic models. Chem. Geol. 1990, 81, 55–81. [Google Scholar] [CrossRef]
- Picard, M.D. Iron oxides and fine-grained rocks of Red Peak and Crow Mountain Members, Chugwater (Triassic) Formation, Wyoming. J. Sediment. Res. 1965, 35, 464–479. [Google Scholar] [CrossRef]
- Parnell, J.; Brolly, C.; Spinks, S.; Bowden, S. Metalliferous biosignatures for deep subsurface microbial activity. Orig. Life Evol. Biosph. 2016, 46, 107–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parnell, J.; Still, J.; Spinks, S.; Bellis, D. Gold in Devono-Carboniferous red beds of northern Britain. J. Geol. Soc. Lond. 2016, 173, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Spinks, S.C.; Parnell, J.; Still, J.W. Redox-controlled selenide mineralization in the Upper Old Red Sandstone. Scott. J. Geol. 2014, 50, 173–182. [Google Scholar] [CrossRef]
- Taylor, B.; Beaudoin, G. Sulphur Isotope Stratigraphy of the Sullivan Pb-Zn-Ag Deposit, B. C.: Evidence for Hydrothermal Sulphur, and Bacterial and Thermochemical Sulphate Reduction; Mineral Deposits Division of the Geological Association of Canada: Ottawa, ON, Canada, 2000; Volume 1, pp. 696–719. [Google Scholar]
- Dill, H.G.; Berner, Z.A. Sedimentological and structural processes operative along a metalliferous catena from sandstone-hosted to unconformity-related Pb-Cu-Zn deposits in an epicontinental basin, SE Germany. Ore Geol. Rev. 2014, 63, 91–114. [Google Scholar] [CrossRef]
- Parry, W.T.; Chan, M.A.; Beitler, B. Chemical bleaching indicates episodes of fluid flow in deformation bands in sandstone. AAPG Bull. 2004, 88, 175–191. [Google Scholar] [CrossRef]
- Xie, D.; Yao, S.; Cao, J.; Hu, W.; Wang, X.; Zhu, N. Diagenetic alteration and geochemical evolution during sandstones bleaching of deep red-bed induced by methane migration in petroliferous basins. Mar. Pet. Geol. 2021, 127, 104940. [Google Scholar] [CrossRef]
- Chan, M.A.; Parry, W.T.; Bowman, J.R. Diagenetic hematite and manganese oxides and fault-related fluid flow in Jurassic sandstones, southeastern Utah. AAPG Bull. 2000, 84, 1281–1310. [Google Scholar]
- Beitler, B.; Chan, M.A.; Parry, W.T. Bleaching of Jurassic Navajo Sandstone on Colorado Plateau Laramide highs; evidence of exhumed hydrocarbon supergiants? Geology (Boulder) 2003, 31, 1041–1044. [Google Scholar] [CrossRef]
- Rainoldi, A.L.; Franchini, M.; Beaufort, D.; Patrier, P.; Giusiano, A.; Impiccini, A.; Pons, J. Large-Scale Bleaching of Red Beds Related To Upward Migration of Hydrocarbons: Los Chihuidos High, Neuquen Basin, Argentina. J. Sediment. Res. 2014, 84, 373–393. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Lei, K. Green altered sandstone related to hydrocarbon migration from the uranium deposits in the northern Ordos Basin, China. Ore Geol. Rev. 2019, 109, 482–493. [Google Scholar] [CrossRef]
- Rushton, J.C.; Wagner, D.; Pearce, J.M.; Rochelle, C.A.; Purser, G. Red-bed bleaching in a CO2 storage analogue: Insights from Entrada Sandstone fracture-hosted mineralization. J. Sediment. Res. 2020, 90, 48–66. [Google Scholar] [CrossRef] [Green Version]
- Mortezaei, K.; Amirlatifi, A.; Ghazanfari, E.; Vahedifard, F. Potential CO2 leakage from geological storage sites: Advances and challenges. Environ. Geotech. 2021, 8, 3–27. [Google Scholar] [CrossRef]
- Dill, H.G.; Wehner, H.; Blum, N. The origin of sulfide mineralization in arenaceous rocks beneath carbonaceous horizons in fluvial depositions of late Paleozoic through Cenozoic age (SE Germany). Chem. Geol. 1993, 104, 159–173. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Kappler, A.; Roden, E.E. Iron in microbial metabolisms. Elements 2011, 7, 89–93. [Google Scholar] [CrossRef]
- Garden, I.R.; Guscott, S.C.; Burley, S.D.; Foxford, K.A.; Walsh, J.J.; Marshall, J. An exhumed palaeo-hydrocarbon migration fairway in a faulted carrier system, Entrada Sandstone of SE Utah, USA. Geofluids 2001, 1, 195–213. [Google Scholar] [CrossRef]
- Petrovic, A.; Khan, S.D.; Chafetz, H.S. Remote detection and geochemical studies for finding hydrocarbon-induced alterations in Lisbon Valley, Utah. Mar. Pet. Geol. 2008, 25, 696–705. [Google Scholar] [CrossRef]
- Eichhubl, P.; Davatz, N.C.; Becker, S.P. Structural and diagenetic control of fluid migration and cementation along the Moab fault, Utah. AAPG Bull. 2009, 93, 653–681. [Google Scholar] [CrossRef] [Green Version]
- Wigley, M.; Kampman, N.; Dubacq, B.; Bickle, M. Fluid-mineral reactions and trace metal mobilization in an exhumed natural CO2 reservoir, Green River, Utah. Geology (Boulder) 2012, 40, 555–558. [Google Scholar] [CrossRef]
- Jung, N.; Han, W.S.; Watson, Z.T.; Graham, J.P.; Kim, K. Fault-controlled CO2 leakage from natural reservoirs in the Colorado Plateau, East-Central Utah. Earth Planet. Sci. Lett. 2014, 403, 358–367. [Google Scholar] [CrossRef]
- Parnell, J.; Spinks, S.; Brolly, C. Tellurium and selenium in Mesoproterozoic red beds. Precambrian Res. 2018, 305, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Parnell, J.; Wang, X.; Raab, A.; Feldmann, J.; Brolly, C.; Michie, R.; Armstrong, J. Metal Flux from Dissolution of Iron Oxide Grain Coatings in Sandstones. Geofluids 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth-Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Castillo-Oliver, M.; Melgarejo, J.C.; Torró, L.; Villanova-de-Benavent, C.; Campeny, M.; Díaz-Acha, Y.; Amores-Casals, S.; Xu, J.; Proenza, J.; Tauler, E. Sandstone-Hosted Uranium Deposits as a Possible Source for Critical Elements: The Eureka Mine Case, Castell-Estaó, Catalonia. Minerals 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, O.; Dunlop, D.J. Intermediate magnetite formation during dehydration of goethite. Earth Planet. Sci. Lett. 2000, 177, 59–67. [Google Scholar] [CrossRef]
- Prasad, P.S.R.; Shiva Prasad, K.; Krishna Chaitanya, V.; Babu, E.V.S.S.; Sreedhar, B.; Ramana Murthy, S. In situ FTIR study on the dehydration of natural goethite. J. Asian Earth Sci. 2006, 27, 503–511. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, C.; Feng, G.; Li, Y.; Wang, J.; Jiao, H. Dehydration of goethite to hematite from molecular dynamics simulation. J. Mol. Struct. THEOCHEM 2010, 950, 20–26. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]



| N | Hue | Value | Chroma | |
|---|---|---|---|---|
| Hematite | 59 | 1.2 YR | 3.6 | 5.2 |
| 3.5 R–4.1 YR | 2.4–4.4 | 1.5–7.9 | ||
| Goethite | 82 | 0.4 Y | 6.0 | 6.9 |
| 7.3 YR–1.6 Y | 4.0–6.8 | 6.0–7.9 | ||
| Lepidocrocite | 32 | 6.8 YR | 5.5 | 8.2 |
| 4.9 YR–7.9 YR | 4.6–5.9 | 7.1–9.9 | ||
| Ferrihydrite | 59 | 6.6 YR | 4.9 | 6.3 |
| 2.8 YR–9.2 YR | 2.3–6.3 | 1.9–7.3 | ||
| Akaganéite | 8 | 5.2 YR | 3.8 | 5.8 |
| 1.2 YR–6.8 YR | 2.8–4.3 | 4.4–7.3 | ||
| Schwertmannite | 16 | 8.5 YR | 5.9 | 6.9 |
| 6.2 YR–0.3 Y | 4.7–6.7 | 4.0–9.1 | ||
| Feroxyhyte | 10 | 4.2 YR | 3.8 | 6.0 |
| 3.7 YR–5.4 YR | 3.4–4.7 | 5.5–7.0 | ||
| Maghemite | 7 | 8.3 YR | 3.1 | 3.2 |
| 6.2 YR–9.4 YR | 2.5–3.6 | 2.5–4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Yang, Z.; Du, H.; Hu, J.; Zhang, K.; Hou, W.; Li, H. Micro-Mechanisms and Implications of Continental Red Beds. Minerals 2022, 12, 934. https://doi.org/10.3390/min12080934
He W, Yang Z, Du H, Hu J, Zhang K, Hou W, Li H. Micro-Mechanisms and Implications of Continental Red Beds. Minerals. 2022; 12(8):934. https://doi.org/10.3390/min12080934
Chicago/Turabian StyleHe, Wang, Zhijun Yang, Hengheng Du, Jintao Hu, Ke Zhang, Weisheng Hou, and Hongwei Li. 2022. "Micro-Mechanisms and Implications of Continental Red Beds" Minerals 12, no. 8: 934. https://doi.org/10.3390/min12080934







