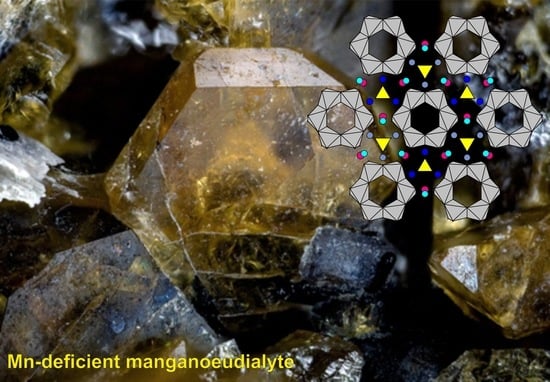
On the Isomorphism of Sodium at the M(2) Site in Eudialyte-Group Minerals: The Crystal Structure of Mn-Deficient Manganoeudialyte and the Problem of the Existence of the M(2)Na-Dominant Analogue of Eudialyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Description
2.2. Electron Probe Microanalysis
2.3. Infrared Spectroscopy
2.4. Single Crystal X-ray Diffraction Analysis
3. Results
3.1. Chemical Composition
3.2. Infrared Spectrum
3.3. Crystal Structure
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Johnsen, O.; Grice, J.D. The crystal chemistry of eudialyte group. Can. Mineral. 1999, 37, 865–891. [Google Scholar]
- Johnsen, O.; Ferraris, G.; Gault, R.A.; Grice, J.D.; Kampf, A.R.; Pekov, I.V. The nomenclature of eudialyte-group minerals. Can. Mineral. 2003, 41, 785–794. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. Classification of eudialyte-group minerals. Geol. Ore Depos. 2012, 54, 487–497. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Aksenov, S.M. Minerals of Eudialyte Group: Crystal Chemistry, Properties, Genesis; University of Nizhni Novgorod: Nizhniy Novgorod, Russia, 2012; ISBN 978-5-91326-207-3. [Google Scholar]
- Rastsvetaeva, R.K. Structural mineralogy of the eudialyte group: A review. Crystallogr. Rep. 2007, 52, 47–64. [Google Scholar] [CrossRef]
- Mikhailova, J.A.; Stepenshchikov, D.G.; Kalashnikov, A.O.; Aksenov, S.M. Who Is Who in the Eudialyte Group: A New Algorithm for the Express Allocation of a Mineral Name Based on the Chemical Composition. Minerals 2022, 12, 224. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Kabanova, N.A.; Chukanov, N.V.; Panikorovskii, T.L.; Blatov, V.A.; Krivovichev, S.V. The role of local heteropolyhedral substitutions in the stoichiometry, topological characteristics and ion-migration paths in the eudialyte-related structures: A quantitative analysis. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2022, 78, 80–90. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. New Data on the Isomorphism in Eudialyte-Group Minerals. 2. Crystal-Chemical Mechanisms of Blocky Isomorphism at the Key Sites. Minerals 2020, 10, 720. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Pekov, I.V.; Schäfer, C.; Van, K.V. New Data on the Isomorphism in Eudialyte-Group Minerals. 1. Crystal Chemistry of Eudialyte-Group Members with Na Incorporated into the Framework as a Marker of Hyperagpaitic Conditions. Minerals 2020, 10, 587. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V. New Data on the Isomorphism in Eudialyte-Group Minerals. X: Crystal Structure of the Intermediate Member of the Raslakite–Sergevanite Series. Crystallogr. Rep. 2021, 66, 120–125. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Möckel, S.; Dudka, A.P.; Aksenov, S.M. New Data on the Isomorphism in Eudialyte-Group Minerals. V: Crystal Structure of an Intermediate Member of the Manganoeudialyte–Ilyukhinite Isomorphous Series. Crystallogr. Rep. 2020, 65, 27–32. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Van, K.V. New Data on the Isomorphism in Eudialyte-Group Minerals. VII: Crystal Structure of the Eudialyte–Sergevanite Series Mineral from the Lovozero Alkaline Massif. Crystallogr. Rep. 2020, 65, 554–559. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Aksenov, S.M.; Rozenberg, K.A. Crystal structure and genesis of the hydrated analog of rastsvetaevite. Crystallogr. Rep. 2015, 60, 831–840. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Vigasina, M.F.; Rastsvetaeva, R.K.; Aksenov, S.M.; Mikhailova, J.A.; Pekov, I.V. The evidence of hydrated proton in eudialyte-group minerals based on Raman spectroscopy data. J. Raman Spectrosc. 2022, 53, 1188–1203. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Netschelyustov, G.N.; Rastsvetaeva, R.K. Alluaivite Na19(Ca,Mn)6(Ti,Nb)3Si26O74Cl·2H2O—A new titanosilicate of of eudialyte–like structure. Proc. Russ. Mineral. Soc. 1990, 119, 117–120. [Google Scholar]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Dualite, Na30(Ca,Na,Ce,Sr)12(Na,Mn,Fe,Ti)6Zr3Ti3MnSi51O144(OH,H2O,Cl)9, a new zircono-titanosilicate with a modular eudialyte-like structure from the Lovozero alkaline Pluton, Kola Peninsula, Russia. Geol. Ore Depos. 2008, 50, 574–582. [Google Scholar] [CrossRef]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastvetaeva, R.K. Labyrinthite (Na,K,Sr)35Ca12Fe3Zr6TiSi51O144(O,OH,H2O)9Cl3, a new mineral with the modular eudialyte-like structure from Khibiny alkaline massif, Kola Peninsula. Proc. Russ. Mineral. Soc. 2006, 135, 38–48. [Google Scholar]
- Chukanov, N.V.; Pekov, I.V.; Zadov, A.E.; Korovushkin, V.V.; Ekimenkova, I.A.; Rastsvetaeva, R.K. Ikranite, (Na,H3O)15(Ca,Mn,REE)6Fe3+2Zr3(□,Zr)(□,Si)Si24O66(O,OH)6Cl·nH2O and raslakite Na15Ca3Fe3(Na,Zr)3Zr3(Si,Nb)(Si25O73)(OH,H2O)3(Cl,OH)—The new eudialyte-group minerals from Lovozero massif, Kola peninsula. Proc. Russ. Mineral. Soc. 2003, 132, 22–33. [Google Scholar]
- Chukanov, N.V.; Aksenov, S.M.; Pekov, I.V.; Belakovskiy, D.I.; Vozchikova, S.A.; Britvin, S.N. Sergevanite, Na15(Ca3Mn3)(Na2Fe)Zr3Si26O72(OH)3·H2O, a new eudialyte-group mineral from the Lovozero alkaline massif, Kola Peninsula. Can. Mineral. 2020, 58, 421–436. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Pekov, I.V.; Varlamov, D.A.; Kazheva, O.N. New Data on the Isomorphism in Eudialyte-Group Minerals. XI: Crystal Structure of a Potentially New Mineral—A Case of Complete Substitution of Fe2+ with Na+2/3Zr4+1/3 in the E.eudialyte-type structures. Crystallogr. Rep. 2022, 67, 166–171. [Google Scholar] [CrossRef]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Bruker. SADABS; Bruker AXS Inc.: Madison, WI, USA, 2001. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Für Krist. 2014, 229, 345–352. [Google Scholar]
- Hawthorne, F.C.; Ungaretti, L.; Oberti, R. Site populations in minerals; terminology and presentation of results of crystal-structure refinement. Can. Mineral. 1995, 33, 907–911. [Google Scholar]
- Prince, E. (Ed.) Volume C: Mathematical, Physical and Chemical Tables. In International Tables for Crystallography, 3rd ed.; IUCr Editorial Office: Chester, UK, 2004; ISBN 978-0-470-71029-6. [Google Scholar]
- Brandenburg, K.; Putz, H. DIAMOND, version 3; Crystal Impact GbR: Bonn, Germany, 2005. [Google Scholar]
- Nomura, S.F.; Atencio, D.; Chukanov, N.V.; Rastsvetaeva, R.K.; Cutinho, J.M.V.; Karipidis, T.K. Manganoeudialyte, a new mineral from Poços de Caldas, Minas Gerais, Brazil. Proc. Russ. Mineral. Soc. 2010, 139, 35–47. [Google Scholar]
- Bosi, F.; Hatert, F.; Hålenius, U.; Pasero, M.; Miyawaki, R.; Mills, S.J. On the application of the IMA−CNMNC dominant-valency rule to complex mineral compositions. Mineral. Mag. 2019, 83, 627–632. [Google Scholar] [CrossRef] [Green Version]
- Khomyakov, A.P.; Nechelyustov, G.N.; Rastsvetaeva, R.K. Aqualite, (H3O)8(Na,K,Sr)5Ca6Zr3Si26O66(OH)9Cl, a new eudialyte-group mineral from Inagli alkaline massif (Sakha-Yakutia,Russia),and the problem of oxonium in hydrated eudialytes. Proc. Russ. Mineral. Soc. 2007, 136, 39–55. [Google Scholar]
- Rozenberg, K.A.; Rastsvetaeva, R.K.; Khomyakov, A.P. Decationized and hydrated eudialytes. Oxonium problem. Eur. J. Mineral. 2006, 17, 875–882. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Lisitsin, D.V.; Van, K.V.; Viktorova, K.A. First find of a potentially new mineral, Na-dominant at M 2-site eudialyte analogue: Crystal structure and significance as a marker of ultraagpaitic environment. Vestn. Geosci. 2021, 14–20. [Google Scholar] [CrossRef]
- Rastsvetaeva, R.K.; Chukanov, N.V.; Schäfer, C. Na-dominant (at the M2 site) eudialyte analogue: Crystal structure and indicatory significance. Vestn. Geosci. 2020, 9, 19–25. [Google Scholar] [CrossRef]



| Constituents | Contents (wt.%) | |
|---|---|---|
| Mean | Ranges | |
| Na2O | 13.50 | 11.54–14.55 |
| K2O | 0.05 | bdl–0.16 |
| CaO | 9.52 | 9.10–10.08 |
| MnO | 3.14 | 2.89–3.54 |
| FeO | 0.88 | 0.56–1.19 |
| SrO | 2.04 | bdl–4.68 |
| Al2O3 | 0.12 | bdl–0.27 |
| La2O3 | 0.90 | 0.23–1.40 |
| Ce2O3 | 1.69 | 1.08–2.29 |
| Pr2O3 | 0.14 | bdl–0.52 |
| Nd2O3 | 0.86 | 0.29–1.30 |
| SiO2 | 50.11 | 47.85–51.79 |
| TiO2 | 0.66 | 0.26–1.09 |
| ZrO2 | 11.53 | 10.40–12.25 |
| HfO2 | 0.28 | bdl–1.14 |
| Nb2O5 | 1.18 | 0.61–1.71 |
| SO3 | 1.29 | 0.97–1.66 |
| Cl | 0.24 | 0.12–0.37 |
| H2O | 2.10 | |
| –O = Cl | −0.05 | |
| Total | 100.31 | |
| Crystal Data | |
|---|---|
| Simplified formula | Na14Ca6(Na2Mn)Zr3Si2[Si24O72](OH)2·2H2O |
| Formula weight, Mr (g) | 2985.87 |
| Crystal system, space group | Trigonal, R3m (#143) |
| Temperature (K) | 293 |
| a, c (Å) | 14.1848(2), 30.4726(3) |
| V (Å3) | 5309.90(11) |
| Z | 3 |
| Radiation type; λ | MoKα; 0.71073 |
| Absorption coefficient, μ (mm−1) | 2.653 |
| Crystal size (mm) | 0.05 × 0.08 × 0.12 |
| Data Collection | |
| Diffractometer | Bruker SMART APEX II |
| Absorption correction | Multi scan |
| No. of measured, independent and observed (I > 2σ(I)) reflections | 26,619, 3838, 3330 |
| Rint | 3.63 |
| Data range θ (°); h, k, l | 1.79–30.52; −19 < h < 20, −18 < k < 20, −43 < l < 43 |
| Refinement | |
| Refinement on | Full-matrix least squares on F2 |
| R(I > 2σ(I)), wR(I), S | 3.43, 4.34, 1.36 |
| Weight scheme | 1/(σ2F+ 0.0004F) |
| Δρmax, Δρmin (e Å−3) | −0.81/0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksenov, S.M.; Chukanov, N.V.; Pekov, I.V.; Nelyubina, Y.V.; Varlamov, D.A.; Kogarko, L.N. On the Isomorphism of Sodium at the M(2) Site in Eudialyte-Group Minerals: The Crystal Structure of Mn-Deficient Manganoeudialyte and the Problem of the Existence of the M(2)Na-Dominant Analogue of Eudialyte. Minerals 2022, 12, 949. https://doi.org/10.3390/min12080949
Aksenov SM, Chukanov NV, Pekov IV, Nelyubina YV, Varlamov DA, Kogarko LN. On the Isomorphism of Sodium at the M(2) Site in Eudialyte-Group Minerals: The Crystal Structure of Mn-Deficient Manganoeudialyte and the Problem of the Existence of the M(2)Na-Dominant Analogue of Eudialyte. Minerals. 2022; 12(8):949. https://doi.org/10.3390/min12080949
Chicago/Turabian StyleAksenov, Sergey M., Nikita V. Chukanov, Igor V. Pekov, Yulia V. Nelyubina, Dmitry A. Varlamov, and Lia N. Kogarko. 2022. "On the Isomorphism of Sodium at the M(2) Site in Eudialyte-Group Minerals: The Crystal Structure of Mn-Deficient Manganoeudialyte and the Problem of the Existence of the M(2)Na-Dominant Analogue of Eudialyte" Minerals 12, no. 8: 949. https://doi.org/10.3390/min12080949