Authigenic Gypsum Precipitation in the ARAON Mounds, East Siberian Sea
Abstract
:1. Introduction
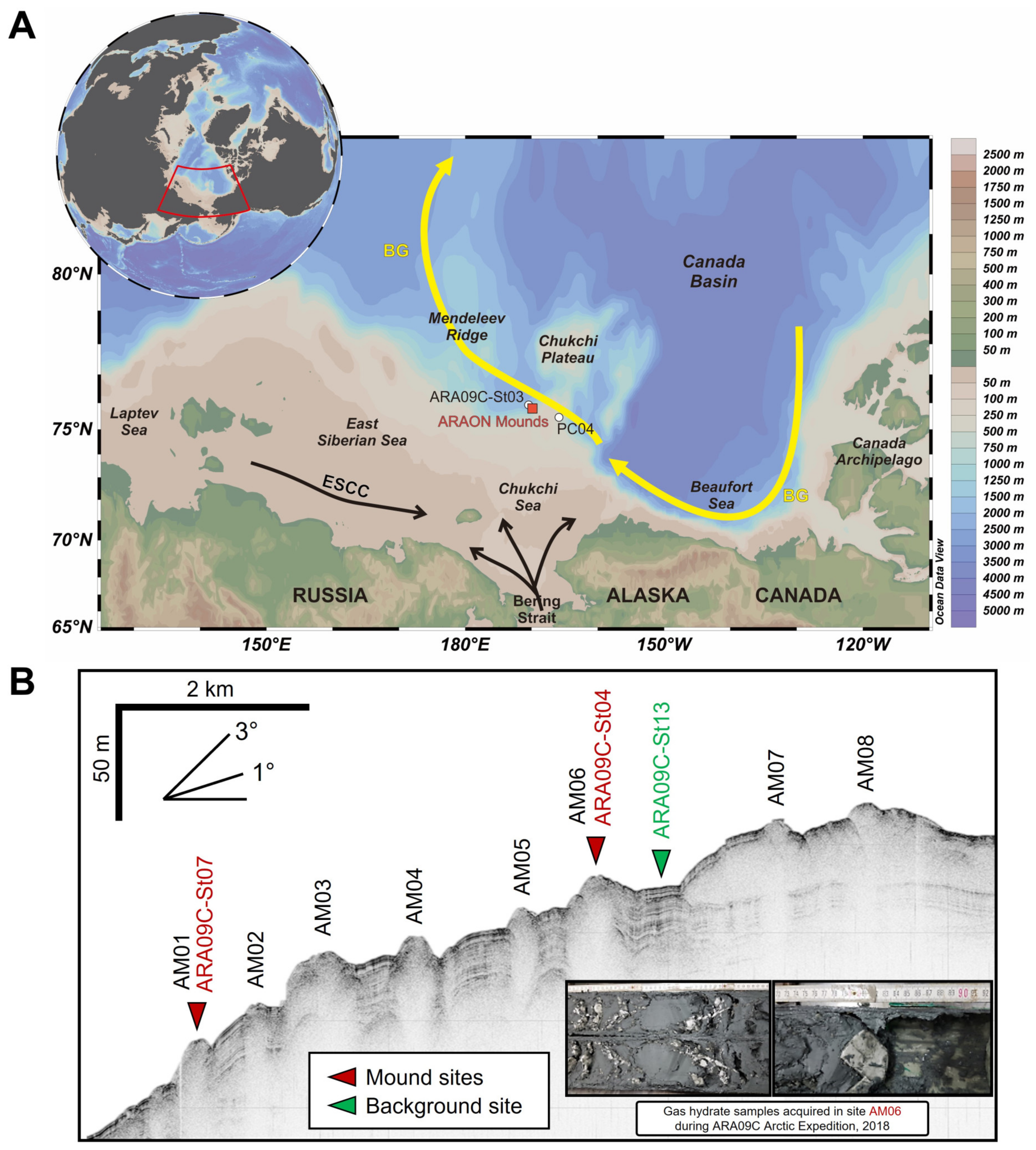
2. Materials and Methods
3. Results
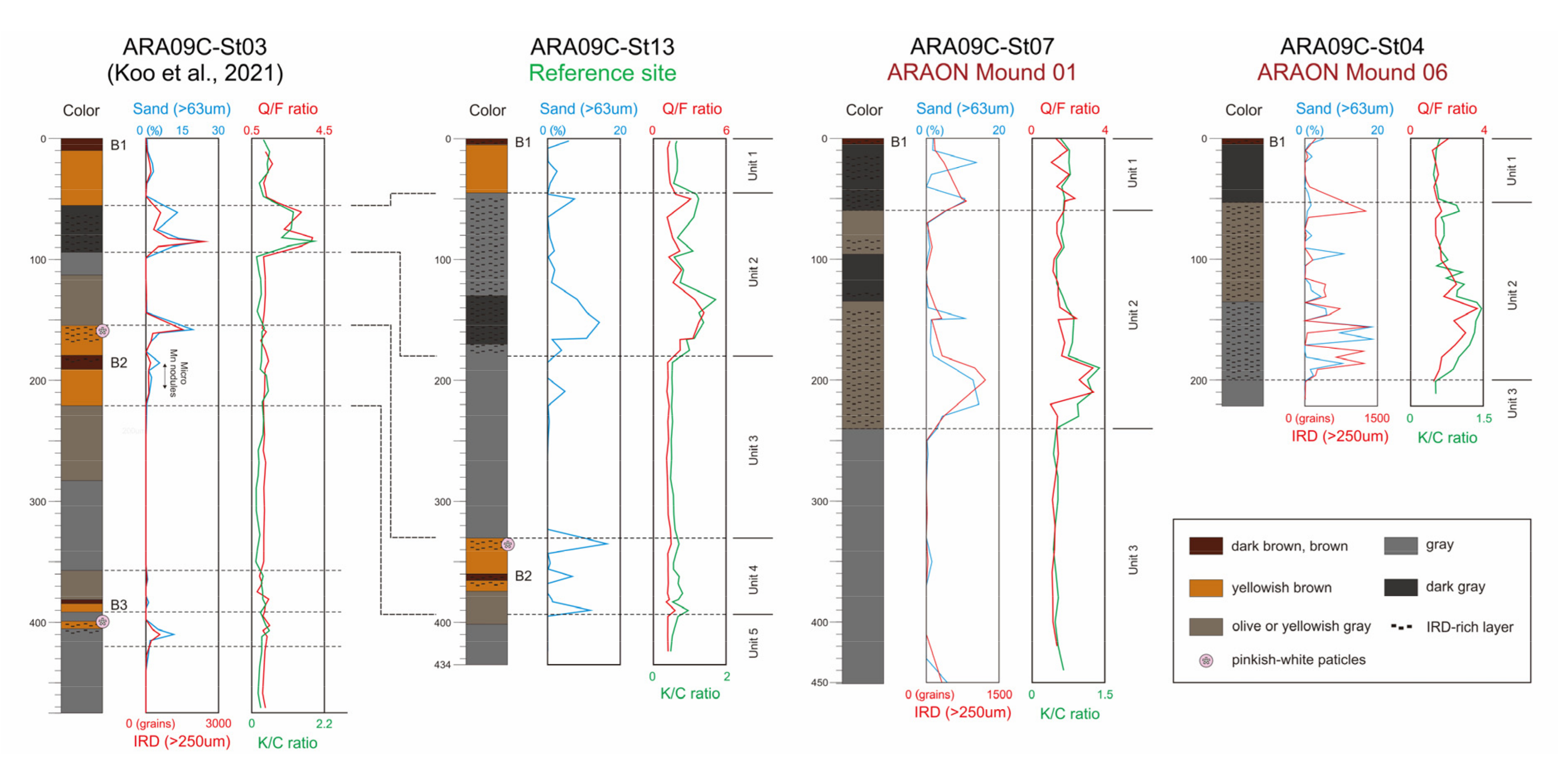
Sedimentary Units at the Background Site and ARAON Mounds
4. Discussion
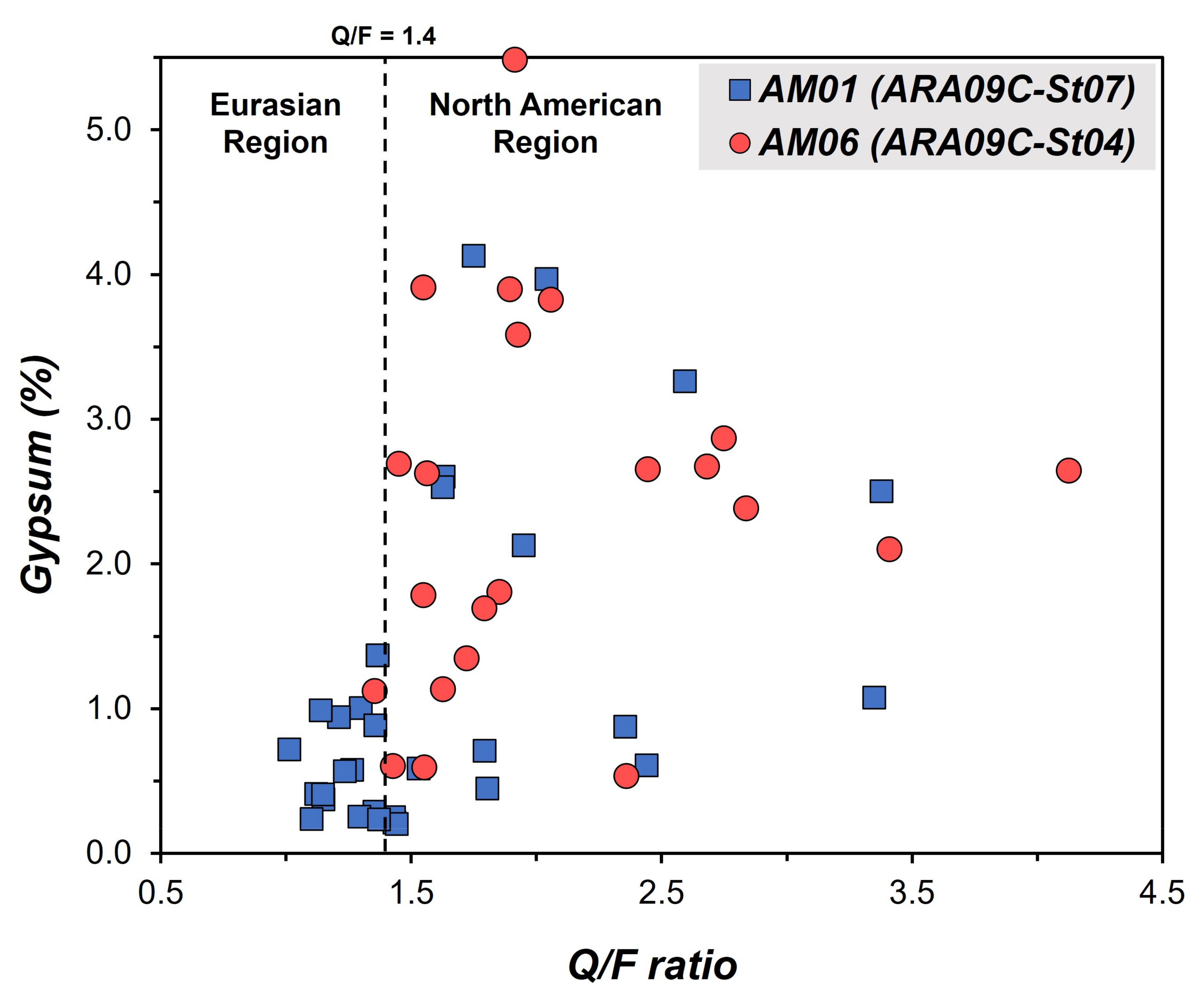
4.1. Sedimentary Units and Sediment Provenances at the Background Site and ARAON Mounds
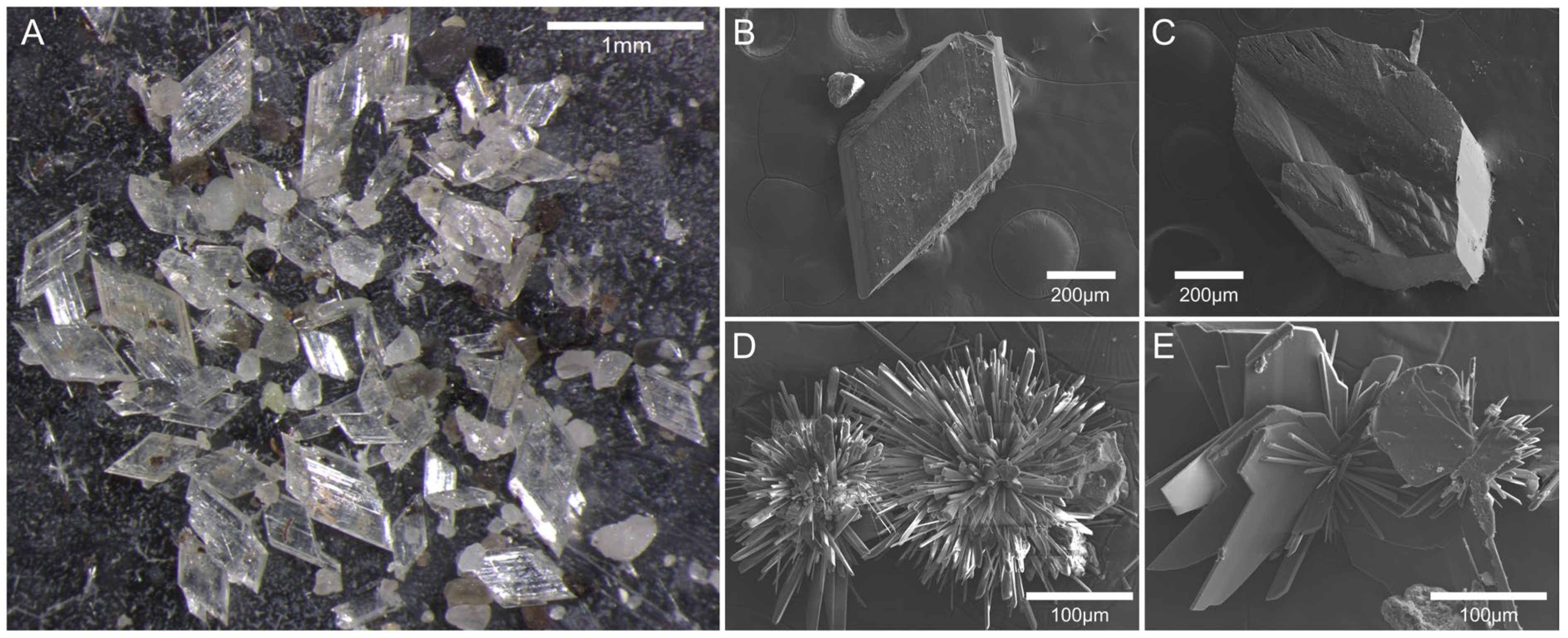
4.2. Methane Hydrate Evolution and Authigenic Gypsum
4.3. Possible Sources of Sulfate and Calcium for Gypsum Precipitation
5. Conclusions
- (1)
- Background core ARA09C-St13, located between the mound structures, consists of five sedimentary units that extend from the Chukchi Rise to the Chukchi Basin. Core sediments in the AMs are darker than the background core, although three sedimentary units in the same order are present. The main differences among sites are the absence of dolomite and the presence of gypsum in the AMs.
- (2)
- Gypsum was authigenically precipitated in situ based on its morphological characteristics. The vertical distribution of gypsum in sediment cores revealed that precipitation was more closely related to the absence of dolomite than the location of the SMT. Chemical weathering and gypsum overgrowth were observed on the surface of dolomite recovered from the AMs, demonstrating that dolomite dissolution is the primary source of Ca for gypsum precipitation.
- (3)
- Ion exclusion and dissolution of biological carbonates may also provide Ca for gypsum precipitation, but these processes might be secondary, as gypsum was found only in sedimentary units containing dolomite in this study.
- (4)
- The main source of sulfate was the disproportionation of sulfide and oxidation of H2S generated through POCSR and AOM, as no sulfides other than gypsum were present in the AM or background cores. For verification of this process, the isotopic analysis will be required.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collett, T. Natural Gas Hydrates: Vast Resources, Uncertain Future; US Geological Survey: Reston, VA, USA, 2001.
- McGuire, A.D.; Anderson, L.G.; Christensen, T.R.; Dallimore, S.; Guo, L.; Hayes, D.J.; Heimann, M.; Lorenson, T.D.; Macdonald, R.W.; Roulet, N. Sensitivity of the carbon cycle in the Arctic to climate change. Ecol. Monogr. 2009, 79, 523–555. [Google Scholar] [CrossRef] [Green Version]
- Dlugokencky, E.J.; Bruhwiler, L.; White, J.; Emmons, L.; Novelli, P.C.; Montzka, S.A.; Masarie, K.A.; Lang, P.M.; Crotwell, A.; Miller, J.B. Observational constraints on recent increases in the atmospheric CH4 burden. Geophys. Res. Lett. 2009, 36, L18803. [Google Scholar] [CrossRef] [Green Version]
- Kerr, R.A. ‘Arctic Armageddon’ Needs More Science, Less Hype. Science 2010, 329, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Hachikubo, A.; Kida, M.; Minami, H.; Lee, D.-H.; Jin, Y.K.; Ryu, J.-S.; Lee, Y.M.; Hur, J.; Park, M.-H.; et al. Upwarding gas source and postgenetic processes in the shallow sediments from the ARAON Mounds, Chukchi Sea. J. Nat. Gas Sci. Eng. 2020, 76, 103223. [Google Scholar] [CrossRef]
- Andreassen, K.; Hart, P.; Grantz, A. Seismic studies of a bottom simulating reflection related to gas hydrate beneath the continental margin of the Beaufort Sea. J. Geophys. Res. Solid Earth 1995, 100, 12659–12673. [Google Scholar] [CrossRef]
- Petersen, C.J.; Bünz, S.; Hustoft, S.; Mienert, J.; Klaeschen, D. High-resolution P-Cable 3D seismic imaging of gas chimney structures in gas hydrated sediments of an Arctic sediment drift. Mar. Pet. Geol. 2010, 27, 1981–1994. [Google Scholar] [CrossRef]
- Shakhova, N.; Semiletov, I.; Salyuk, A.; Yusupov, V.; Kosmach, D.; Gustafsson, Ö. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf. Science 2010, 327, 1246–1250. [Google Scholar] [CrossRef]
- Kocherla, M. Authigenic Gypsum in Gas-Hydrate Associated Sediments from the East Coast of India (Bay of Bengal). Acta Geol. Sin.-Engl. Ed. 2013, 87, 749–760. [Google Scholar] [CrossRef]
- Pierre, C.; Blanc-Valleron, M.-M.; Demange, J.; Boudouma, O.; Foucher, J.-P.; Pape, T.; Himmler, T.; Fekete, N.; Spiess, V. Authigenic carbonates from active methane seeps offshore southwest Africa. Geo-Mar. Lett. 2012, 32, 501–513. [Google Scholar] [CrossRef]
- Pierre, C.; Bayon, G.; Blanc-Valleron, M.-M.; Mascle, J.; Dupré, S. Authigenic carbonates related to active seepage of methane-rich hot brines at the Cheops mud volcano, Menes caldera (Nile deep-sea fan, eastern Mediterranean Sea). Geo-Mar. Lett. 2014, 34, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Sassen, R.; Roberts, H.H.; Carney, R.; Milkov, A.V.; DeFreitas, D.A.; Lanoil, B.; Zhang, C. Free hydrocarbon gas, gas hydrate, and authigenic minerals in chemosynthetic communities of the northern Gulf of Mexico continental slope: Relation to microbial processes. Chem. Geol. 2004, 205, 195–217. [Google Scholar] [CrossRef]
- Wang, J. Authigenic gypsum found in gas hydrate-associated sediments from Hydrate Ridge, the eastern North Pacific. Sci. China Earth Sci. 2004, 47, 280. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Algeo, T.J.; Su, P.; Hu, G. Formation mechanism of authigenic gypsum in marine methane hydrate settings: Evidence from the northern South China Sea. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 210–220. [Google Scholar] [CrossRef]
- Pierre, C. Origin of the authigenic gypsum and pyrite from active methane seeps of the southwest African Margin. Chem. Geol. 2017, 449, 158–164. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, H.; Guan, H.; Liu, L.; Wu, D.; Wu, N. Methane seepage intensities traced by sulfur isotopes of pyrite and gypsum in sediment from the Shenhu area, South China Sea. Acta Oceanol. Sin. 2018, 37, 20–27. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, J.; Phillips, S.C.; Liang, J.; Su, P.; Lin, Q.; Chen, C.; Liu, J. Non-evaporitic gypsum formed in marine sediments due to sulfate-methane transition zone fluctuations and mass transport deposits in the northern South China Sea. Mar. Chem. 2021, 233, 103988. [Google Scholar] [CrossRef]
- Chang, L.; Howe, R.; Zussman, J. Non-Silicates: Sulphates, Carbonates, Phosphates, Halides; Longman Group: London, UK, 1996; p. 383. [Google Scholar]
- Jin, Y.K.; Onboard Ship Scientific Party. ARA07C Cruise Report: 2016 Korea-Russia-Germany East Siberian Sea Research Program; Korea Polar Research Institute: Incheon, Korea, 2017; p. 103. [Google Scholar]
- Jin, Y.K.; Shipboard Scientific Party. ARA09C Cruise Report: 2018 Korea-Russia-Japan East Siberian/Chukchi Sea Research Program; Korea Polar Research Institute: Incheon, Korea, 2019; p. 205. [Google Scholar]
- Kim, Y.-G.; Kim, S.; Lee, D.-H.; Lee, Y.M.; Kim, H.J.; Kang, S.-G.; Jin, Y.K. Occurrence of active gas hydrate mounds in the southwestern slope of the Chukchi Plateau, Arctic Ocean. Episodes 2020, 43, 811–823. [Google Scholar] [CrossRef]
- Kang, S.-G.; Jang, U.; Kim, S.; Choi, Y.; Kim, Y.-G.; Hong, J.K.; Jin, Y.K. Exploration of the Gas Hydrates on the Southwestern Continental Slope of the Chukchi Plateau in the Arctic Ocean. J. Korean Soc. Miner. Energy Resour. Eng. 2021, 31, 418–432. [Google Scholar] [CrossRef]
- Koo, H.-J.; Jin, Y.-K.; Cho, H.-G. Change in Sediment Provenance on the Inner Slope of the Chukchi Rise and Their Paleoenvironmental Implications. Appl. Sci. 2021, 11, 6491. [Google Scholar] [CrossRef]
- Darby, D.A.; Bischof, J.F. A Holocene record of changing Arctic Ocean ice drift analogous to the effects of the Arctic Oscillation. Paleoceanography 2004, 19, 1027. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, D.; Yamamoto, M.; Irino, T.; Nam, S.-I.; Park, Y.-H.; Harada, N.; Nagashima, K.; Chikita, K.; Saitoh, S.-I. Distribution of detrital minerals and sediment color in western Arctic Ocean and northern Bering Sea sediments: Changes in the provenance of western Arctic Ocean sediments since the last glacial period. Polar Sci. 2016, 10, 519–531. [Google Scholar] [CrossRef]
- Biscaye, P.E. Mineralogy and sedimentation of recent deep-sea clay in the Atlantic Ocean and adjacent seas and oceans. Geol. Soc. Am. Bull. 1965, 76, 803–832. [Google Scholar] [CrossRef]
- Cho, H.G.; Kim, S.-O.; Kwak, K.Y.; Choi, H.; Khim, B.-K. Clay mineral distribution and provenance in the Heuksan mud belt, Yellow Sea. Geo-Mar. Lett. 2015, 35, 411–419. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters 1. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Darby, D.A.; Myers, W.B.; Jakobsson, M.; Rigor, I. Modern dirty sea ice characteristics and sources: The role of anchor ice. J. Geophys. Res. Ocean. 2011, 116, C09008. [Google Scholar] [CrossRef] [Green Version]
- Naidu, A.; Mowatt, T. Sources and dispersal patterns of clay minerals in surface sediments from the continental-shelf areas off Alaska. Geol. Soc. Am. Bull. 1983, 94, 841–854. [Google Scholar] [CrossRef]
- Wahsner, M.; Müller, C.; Stein, R.; Ivanov, G.; Levitan, M.; Shelekhova, E.; Tarasov, G. Clay-mineral distribution in surface sediments of the Eurasian Arctic Ocean and continental margin as indicator for source areas and transport pathways—A synthesis. Boreas 1999, 28, 215–233. [Google Scholar] [CrossRef]
- Myers, W.B. Circum-Arctic Mineralogy & Pan-Arctic Chronostratigraphy of Late Pleistocene Sediments: Developing a Comprehensive Age Model for the Western Arctic Ocean Using Unique Ice-Rafted Signals; Old Dominion University: Norfolk, UK, 2019. [Google Scholar]
- Viscosi-Shirley, C.; Mammone, K.; Pisias, N.; Dymond, J. Clay mineralogy and multi-element chemistry of surface sediments on the Siberian-Arctic shelf: Implications for sediment provenance and grain size sorting. Cont. Shelf Res. 2003, 23, 1175–1200. [Google Scholar] [CrossRef]
- Wang, R.; Xiao, W.; März, C.; Li, Q. Late Quaternary paleoenvironmental changes revealed by multi-proxy records from the Chukchi Abyssal Plain, western Arctic Ocean. Glob. Planet. Change 2013, 108, 100–118. [Google Scholar] [CrossRef]
- Fagel, N.; Not, C.; Gueibe, J.; Mattielli, N.; Bazhenova, E. Late Quaternary evolution of sediment provenances in the Central Arctic Ocean: Mineral assemblage, trace element composition and Nd and Pb isotope fingerprints of detrital fraction from the Northern Mendeleev Ridge. Quat. Sci. Rev. 2014, 92, 140–154. [Google Scholar] [CrossRef]
- Dong, L.; Liu, Y.; Shi, X.; Polyak, L.; Huang, Y.; Fang, X.; Liu, J.; Zou, J.; Wang, K.; Sun, F.; et al. Sedimentary record from the Canada Basin, Arctic Ocean: Implications for late to middle Pleistocene glacial history. Clim. Past 2017, 13, 511–531. [Google Scholar] [CrossRef] [Green Version]
- Stein, R. Arctic Ocean Sediments: Processes, Proxies, and Paleoenvironment; Elsevier: Amsterdam, Netherlands, 2008. [Google Scholar]
- März, C.; Stratmann, A.; Matthießen, J.; Meinhardt, A.-K.; Eckert, S.; Schnetger, B.; Vogt, C.; Stein, R.; Brumsack, H.-J. Manganese-rich brown layers in Arctic Ocean sediments: Composition, formation mechanisms, and diagenetic overprint. Geochim. Cosmochim. Acta 2011, 75, 7668–7687. [Google Scholar] [CrossRef]
- Schreck, M.; Nam, S.-I.; Polyak, L.; Vogt, C.; Kong, G.S.; Stein, R.; Matthiessen, J.; Niessen, F. Improved Pleistocene sediment stratigraphy and paleoenvironmental implications for the western Arctic Ocean off the East Siberian and Chukchi margins. arktos 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Zou, H. An X-ray diffraction approach: Bulk mineral assemblages as provenance indicator of sediments from the Arctic Ocean. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2016. [Google Scholar]
- Park, K.; Ohkushi, K.i.; Cho, H.G.; Khim, B.-K. Lithostratigraphy and paleoceanography in the Chukchi Rise of the western Arctic Ocean since the last glacial period. Polar Sci. 2017, 11, 42–53. [Google Scholar] [CrossRef]
- Burton, E.A. Controls on marine carbonate cement mineralogy: Review and reassessment. Chem. Geol. 1993, 105, 163–179. [Google Scholar] [CrossRef]
- Magalhães, V.H.; Pinheiro, L.M.; Ivanov, M.K.; Kozlova, E.; Blinova, V.; Kolganova, J.; Vasconcelos, C.; McKenzie, J.A.; Bernasconi, S.M.; Kopf, A.J. Formation processes of methane-derived authigenic carbonates from the Gulf of Cadiz. Sediment. Geol. 2012, 243, 155–168. [Google Scholar] [CrossRef]
- Serié, C.; Huuse, M.; Schødt, N.H. Gas hydrate pingoes: Deep seafloor evidence of focused fluid flow on continental margins. Geology 2012, 40, 207–210. [Google Scholar] [CrossRef]
- Hong, W.-L.; Torres, M.E.; Carroll, J.; Crémière, A.; Panieri, G.; Yao, H.; Serov, P. Seepage from an arctic shallow marine gas hydrate reservoir is insensitive to momentary ocean warming. Nat. Commun. 2017, 8, 15745. [Google Scholar] [CrossRef]
- Paull, C.K.; Dallimore, S.; Caress, D.; Gwiazda, R.; Melling, H.; Riedel, M.; Jin, Y.; Hong, J.; Kim, Y.G.; Graves, D. Active mud volcanoes on the continental slope of the Canadian Beaufort Sea. Geochem. Geophys. Geosystems 2015, 16, 3160–3181. [Google Scholar] [CrossRef]
- Borowski, W.S.; Paull, C.K.; Ussler, W., III. Marine pore-water sulfate profiles indicate in situ methane flux from underlying gas hydrate. Geology 1996, 24, 655–658. [Google Scholar] [CrossRef]
- Briskin, M.; Schreiber, B.C. Authigenic gypsum in marine sediments. Mar. Geol. 1978, 28, 37–49. [Google Scholar] [CrossRef]
- Haffert, L.; Haeckel, M.; Liebetrau, V.; Berndt, C.; Hensen, C.; Nuzzo, M.; Reitz, A.; Scholz, F.; Schönfeld, J.; Perez-Garcia, C. Fluid evolution and authigenic mineral paragenesis related to salt diapirism–The Mercator mud volcano in the Gulf of Cadiz. Geochim. Cosmochim. Acta 2013, 106, 261–286. [Google Scholar] [CrossRef]
- Harouaka, K.; Eisenhauer, A.; Fantle, M.S. Experimental investigation of Ca isotopic fractionation during abiotic gypsum precipitation. Geochim. Cosmochim. Acta 2014, 129, 157–176. [Google Scholar] [CrossRef]
- Balci, N.; Shanks, W.C., III; Mayer, B.; Mandernack, K.W. Oxygen and sulfur isotope systematics of sulfate produced by bacterial and abiotic oxidation of pyrite. Geochim. Cosmochim. Acta 2007, 71, 3796–3811. [Google Scholar] [CrossRef]
- Schippers, A.; Jørgensen, B.B. Oxidation of pyrite and iron sulfide by manganese dioxide in marine sediments. Geochim. Cosmochim. Acta 2001, 65, 915–922. [Google Scholar] [CrossRef]
- Canfield, D.E.; Thamdrup, B. The production of 34S-depleted sulfide during bacterial disproportionation of elemental sulfur. Science 1994, 266, 1973–1975. [Google Scholar] [CrossRef]
- Blanchet, C.L.; Kasten, S.; Vidal, L.; Poulton, S.W.; Ganeshram, R.; Thouveny, N. Influence of diagenesis on the stable isotopic composition of biogenic carbonates from the Gulf of Tehuantepec oxygen minimum zone. Geochem. Geophys. Geosystems 2012, 13, Q04003. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Kasten, S. Sulfur cycling and methane oxidation. In Marine geochemistry; Springer: Berlin/Heidelberg, Germany, 2006; pp. 271–309. [Google Scholar]
- Pirlet, H.; Wehrmann, L.M.; Brunner, B.; Frank, N.; Dewanckele, J.A.N.; Van Rooij, D.; Foubert, A.; Swennen, R.; Naudts, L.; Boone, M.; et al. Diagenetic formation of gypsum and dolomite in a cold-water coral mound in the Porcupine Seabight, off Ireland. Sedimentology 2010, 57, 786–805. [Google Scholar] [CrossRef]
- Riedinger, N.; Brunner, B.; Formolo, M.J.; Solomon, E.; Kasten, S.; Strasser, M.; Ferdelman, T.G. Oxidative sulfur cycling in the deep biosphere of the Nankai Trough, Japan. Geology 2010, 38, 851–854. [Google Scholar] [CrossRef]
- Schnitker, D.; Mayer, L.; Norton, S. Loss of calcareous microfossils from sediments through gypsum formation. Mar. Geol. 1980, 36, M35–M44. [Google Scholar] [CrossRef]
- Ussler, W.; Paull, C. Effects of ion exclusion and isotopic fractionation on pore water geochemistry during gas hydrate formation and decomposition. Geo-Mar. Lett. 1995, 15, 37–44. [Google Scholar] [CrossRef]



| Site | Latitude (°N) | Longitude (°W) | Water Depth (m) | Core Length (cm) | Depth of SMT (m) [5] | Remark |
|---|---|---|---|---|---|---|
| ARA09C-St07 | 75.7116 | 169.7931 | 699 | 434 | ~3.3 | Summit of AM01 |
| ARA09C-St04 | 75.6797 | 169.7388 | 605 | 221 | ~1.2 | Summit of AM06 |
| ARA09C-St13 | 75.6731 | 169.7391 | 615 | 434 | - | Background site (between AM06 and AM07) |
| Samples | n | Qz | Afs | Pl | Dol | Mica | Gp | Clay Minerals | Q/F | |
|---|---|---|---|---|---|---|---|---|---|---|
| ARA09C-St07 | Unit 1 | 8 | 28.5 | 5.1 | 12.1 | 0.6 | 13.3 | 2.4 | 35.7 | 1.7 |
| Unit 2 | 18 | 27.0 | 4.9 | 11.3 | 0.8 | 12.4 | 1.8 | 39.6 | 1.8 | |
| Unit 3 | 9 | 21.1 | 3.4 | 13.7 | 0.5 | 12.9 | 0.4 | 45.4 | 1.2 | |
| ARA09C-St04 | Unit 1 | 11 | 23.8 | 3.5 | 13.0 | 0.2 | 14.1 | 1.1 | 42.0 | 1.5 |
| Unit 2 | 24 | 31.0 | 5.0 | 9.8 | 1.3 | 13.4 | 2.4 | 35.2 | 2.2 | |
| Unit 3 | 2 | 23.5 | 4.3 | 16.6 | 0.2 | 11.0 | 0.2 | 42.8 | 1.1 | |
| ARA09C-St13 | Unit 1 | 5 | 21.0 | 3.8 | 12.6 | 1.0 | 13.0 | - | 45.4 | 1.3 |
| Unit 2 | 14 | 31.9 | 3.9 | 9.9 | 2.5 | 12.5 | - | 36.8 | 2.4 | |
| Unit 3 | 11 | 23.2 | 4.3 | 14.9 | 0.5 | 11.5 | - | 43.2 | 1.2 | |
| Unit 4 | 10 | 22.7 | 4.5 | 13.3 | 1.6 | 11.7 | - | 44.1 | 1.3 | |
| Unit 5 | 3 | 21.8 | 4.2 | 13.9 | 0.3 | 13.3 | - | 43.2 | 1.2 | |
| Laptev Sea | 1 | 23.6 | 16.3 | 21.1 | 0.1 | 7.3 | - | 28.8 | 0.6 | |
| East Siberian Sea | 5 | 27.1 | 9.2 | 17.3 | 0.1 | 14.9 | - | 31.1 | 1.0 | |
| Chukchi Sea | 4 | 28.5 | 5.5 | 15.1 | 1.1 | 17.3 | - | 31.1 | 1.4 | |
| Canada Archipelago | 7 | 21.8 | 3.7 | 6.4 | 16.9 | 14.2 | - | 31.4 | 2.2 | |
| Beaufort Sea | 3 | 39.3 | 3.7 | 5.1 | 8.0 | 10.2 | - | 25.4 | 4.5 | |
| Samples | n | Illite | Chlorite | Kaolinite | Smectite | K/C | Sand | |
|---|---|---|---|---|---|---|---|---|
| ARA09C-St07 | Unit 1 | 8 | 63.1 | 20.1 | 13.8 | 3.1 | 0.7 | 4.2 |
| Unit 2 | 18 | 62.1 | 19.9 | 15.4 | 2.7 | 0.8 | 2.5 | |
| Unit 3 | 10 | 67.0 | 20.2 | 10.2 | 2.6 | 0.5 | 0.6 | |
| ARA09C-St04 | Unit 1 | 7 | 64.6 | 21.4 | 12.5 | 1.5 | 0.6 | 1.1 |
| Unit 2 | 20 | 61.1 | 18.6 | 17.0 | 3.3 | 0.9 | 3.3 | |
| Unit 3 | 1 | 65.8 | 20.1 | 10.4 | 3.7 | 0.5 | 0.1 | |
| ARA09C-St13 | Unit 1 | 5 | 68.4 | 17.3 | 10.8 | 3.4 | 0.6 | 1.9 |
| Unit 2 | 14 | 59.1 | 18.8 | 19.8 | 2.3 | 1.1 | 4.4 | |
| Unit 3 | 11 | 67.6 | 19.2 | 10.1 | 3.2 | 0.5 | 0.5 | |
| Unit 4 | 10 | 69.3 | 16.6 | 11.1 | 3.0 | 0.7 | 3.8 | |
| Unit 5 | 3 | 68.2 | 18.4 | 10.1 | 3.3 | 0.6 | 0.0 | |
| Laptev Sea | 243 | 40.4 | 22.3 | 13.0 | 24.3 | 0.58 | - | |
| East Siberian Sea | 104 | 66.3 | 19.9 | 8.3 | 5.9 | 0.42 | - | |
| Chukchi Sea | 25 | 58.1 | 23.7 | 9.4 | 8.7 | 0.40 | - | |
| Canada Archipelago | 17 | 57.8 | 10.0 | 14.4 | 17.7 | 1.44 | - | |
| Beaufort Sea | 59 | 63.1 | 16.7 | 12.0 | 8.1 | 0.72 | - | |
| Gypsum Forms | Rhombic | Fiber Rosette | |
|---|---|---|---|
| Morphology | n | 30 | 226 |
| Major axis (μm) | 912 (36–2596) | 138 (32–360) | |
| Minor axis (μm) | 477 (20–1428) | 10 (1–46) | |
| Major axis/minor axis | 1.9 (1.5–2.5) | 17.2 (3.4–202.0) | |
| Chemistry | n | 109 | 209 |
| CaO (wt.%) | 39.8 (35.4–45.8) | 40.9 (32.0–49.7) | |
| SO3 (wt.%) | 59.8 (54.2–61.6) | 59.0 (50.3–61.9) | |
| Ca/S | 0.95 (0.89–1.20) | 0.99 (0.76–1.41) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koo, H.J.; Jang, J.K.; Lee, D.H.; Cho, H.G. Authigenic Gypsum Precipitation in the ARAON Mounds, East Siberian Sea. Minerals 2022, 12, 983. https://doi.org/10.3390/min12080983
Koo HJ, Jang JK, Lee DH, Cho HG. Authigenic Gypsum Precipitation in the ARAON Mounds, East Siberian Sea. Minerals. 2022; 12(8):983. https://doi.org/10.3390/min12080983
Chicago/Turabian StyleKoo, Hyo Jin, Jeong Kyu Jang, Dong Hun Lee, and Hyen Goo Cho. 2022. "Authigenic Gypsum Precipitation in the ARAON Mounds, East Siberian Sea" Minerals 12, no. 8: 983. https://doi.org/10.3390/min12080983





