Potential Toxicity of Natural Fibrous Zeolites: In Vitro Study Using Jurkat and HT22 Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Zeolite Samples
2.2. Scanning Electron Microscopy (SEM)
2.3. X-ray Powder Diffraction (XRPD)
2.4. Cell Culture and Treatments
2.5. Trypan Blue Test
2.6. Clonogenic Test
2.7. Statistical Analysis
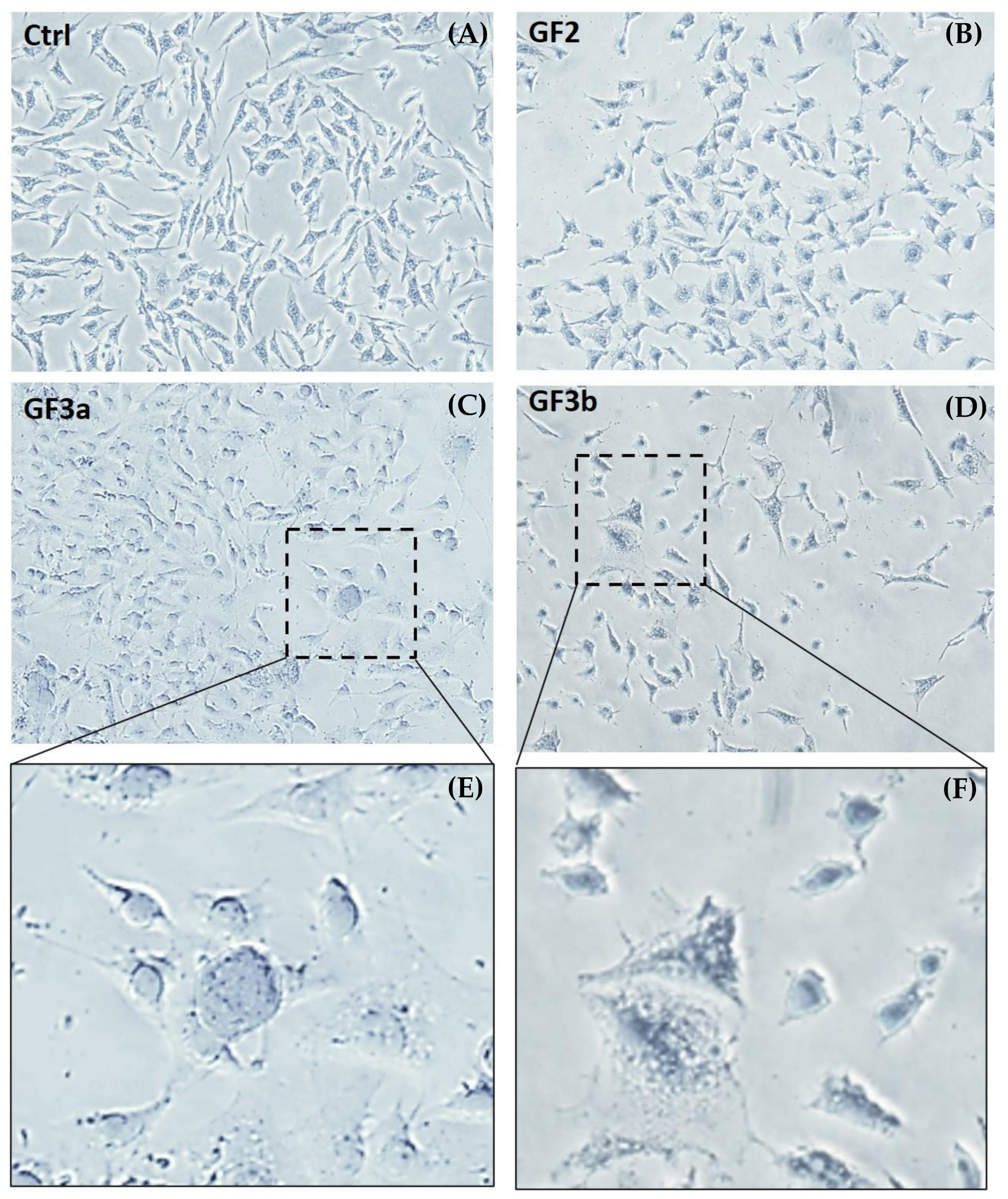
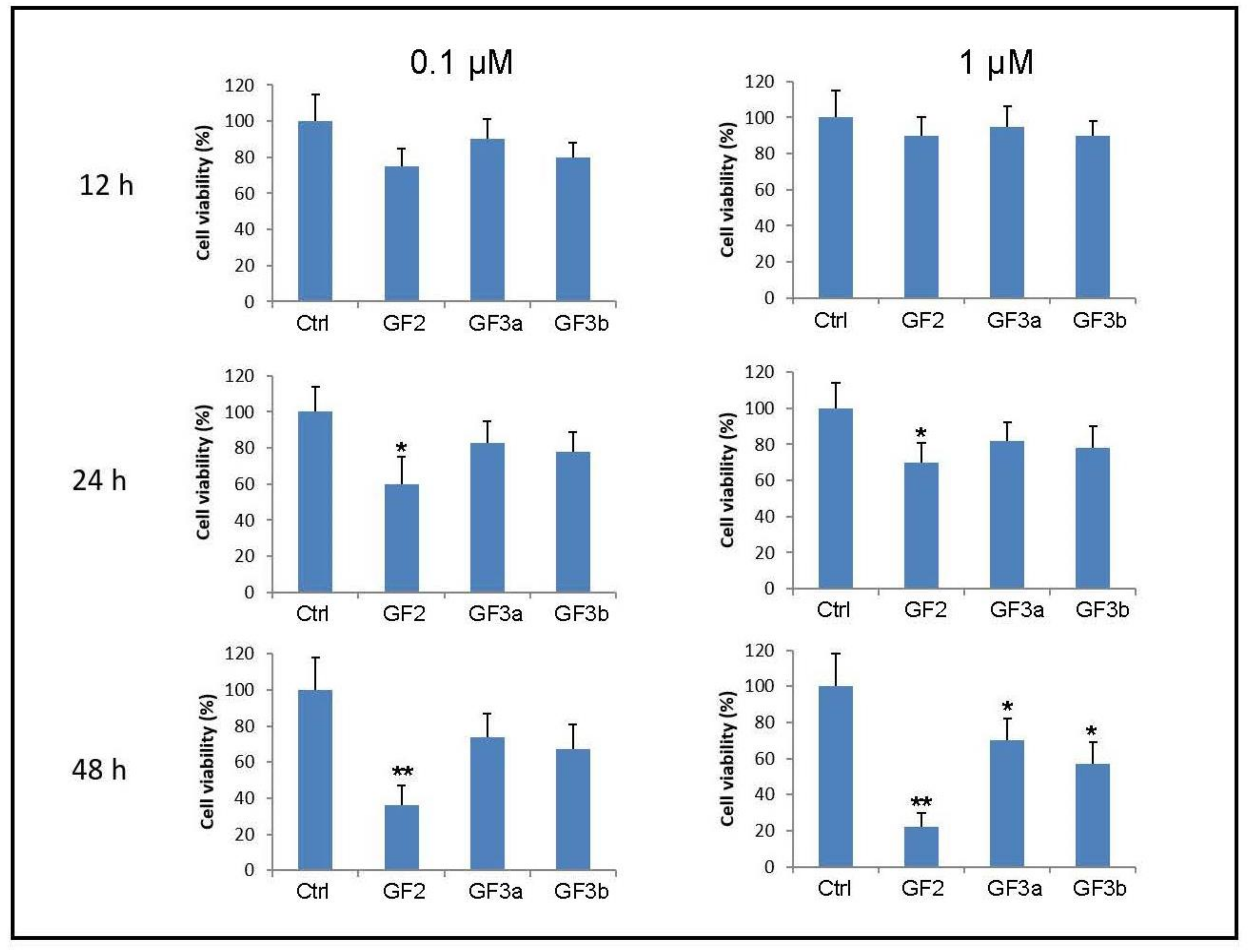
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sisko, A.; Boffetta, P. Occupational Cancers; Springer Nature: London, UK, 2020; p. 640. [Google Scholar]
- Berry, T.-A.; Belluso, E.; Vigliaturo, R.; Gieré, R.; Emmett, E.A.; Testa, J.R.; Steinhorn, G.; Wallis, S.L. Asbestos and Other Hazardous Fibrous Minerals: Potential Exposure Pathways and Associated Health Risks. Int. J. Environ. Res. Public Health 2022, 19, 4031. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). In IARC Monographs on the Evaluation Carcinogenic Risks to Humans; IARC Publication: Lyon, France, 2012; Volume 100C, pp. 219–309. [Google Scholar]
- Carbone, M.; Yang, H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- NIOSH (National Institute for Occupational Safety and Health). Asbestos Fibers and Other Elongate Mineral Particles: State of the Science and Roadmap for Research; Revised Edition; (NIOSH) Publication No. 2011-159 Current Intelligence Bulletin; Department of Health and Human Services DHHS: Cincinnati, OH, USA, 2011; Volume 62, p. 174. [Google Scholar]
- Cardile, V.; Renis, M.; Scifo, C.; Lombardo, L.; Gulino, R.; Mancari, B.; Panico, A. Behaviour of the new asbestos amphibole fluor-edenite in different lung cell systems. Int. J. Biochem. Cell. Biol. 2004, 36, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, G.B.; Ballirano, P.; Gianfagna, A.; Mazziotti-Tagliani, S.; Pacella, A. Structural and spectroscopic characterization of a suite of fibrous amphiboles with high environmental and health relevance from Biancavilla (Sicily, Italy). Am. Mineral. 2009, 94, 1333–1340. [Google Scholar] [CrossRef]
- Comba, P.; Gianfagna, A.; Paoletti, L. Pleural mesothelioma cases in Biancavilla are related to a new fluoro-edenite fibrous amphibole. Arch. Environ. Health 2003, 58, 229–232. [Google Scholar] [CrossRef]
- Erskine, B.G.; Bailey, M. Characterization of asbestiform glaucophane-winchite in the franciscan complex blueschist, northern diablo range, California. Toxicol. Appl. Pharmacol. 2018, 361, 3–13. [Google Scholar] [CrossRef]
- Pacella, A.; Ballirano, P. Chemical and structural characterization of fibrous richterite with high environmental and health relevance from Libby, Montana (USA). Period. Mineral. 2016, 85, 169–177. [Google Scholar]
- Rogers, A.J. Exposures estimates of the Wittenoom mining workforce and town residents–implications associated with risk estimation for persons exposed to asbestiform riebeckite. Toxicol. Appl. Pharmacol. 2018, 361, 168–170. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Harper, M.; Bailey, M.; Erskine, B.; Della Ventura, G.; Ardith, M.; Pasquali, L.; Tomaino, G.; Ray, R.; Mason, H.; et al. Characterization and assessment of the potential toxicity/pathogenicity of fibrous glaucophane. Environ. Res. 2019, 178, 108723. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Zoboli, A.; Filaferro, M.; Benassi, M.; Scarfì, S.; Mirata, S.; Avallone, R.; Vitale, G.; Bailey, M.; Harper, M.; et al. In vitro toxicity of fibrous glaucophane. Toxicology 2021, 454, 152743. [Google Scholar] [CrossRef]
- Petriglieri, J.R.; Laporte-Magoni, C.; Salvioli-Mariani, E.; Tomatis, M.; Gazzano, E.; Turci, F.; Cavallo, A.; Fubini, B. Identification and Preliminary Toxicological Assessment of a Non-Regulated Mineral Fiber: Fibrous Antigorite from New Caledonia. Environ. Eng. Geosci. 2020, 26, 89–97. [Google Scholar] [CrossRef]
- Turci, F.; Tomatis, M.; Compagnoni, R.; Fubini, B. Role of Associated Mineral Fibres in Chrysotile Asbestos Health Effects: The Case of Balangeroite. Ann. Occup. Hyg. 2009, 53, 491–497. [Google Scholar] [PubMed]
- Giordani, M.; Meli, M.A.; Roselli, C.; Betti, M.; Peruzzi, F.; Taussi, M.; Valentini, L.; Fagiolino, I.; Mattioli, M. Could soluble minerals be hazardous to human health? Evidence from fibrous epsomite. Environ. Res. 2022, 206, 112579. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Some nanomaterials and some fibres. In IARC Monographs on the Evaluation Carcinogenic Risks to Humans; IARC Publication: Lyon, France, 2017; Volume 111, pp. 215–240. [Google Scholar]
- Carbone, M.; Emri, S.; Dogan, A.U.; Steele, I.; Tuncer, M.; Pass, H.I.; Baris, Y.I. A mesothelioma epidemic in Cappadocia: Scientific developments and unexpected social outcomes. Nat. Rev. Cancer 2007, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zebedeo, C.N.; Davis, C.; Peña, C.; Ng, K.W.; Pfau, J.C. Erionite induces production of autoantibodies and IL-17 in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2014, 275, 257–264. [Google Scholar] [CrossRef]
- Metintas, M.; Hillerdal, G.; Metintas, S.; Dumortier, P. Endemic malignant mesothelioma: Exposure to erionite is more important than genetic factors. Arch. Environ. Health 2010, 65, 86–93. [Google Scholar] [CrossRef]
- Mattioli, M.; Giordani, M.; Dogan, M.; Cangiotti, M.; Avella, G.; Giorgi, R.; Dogan, A.U.; Ottaviani, M.F. Morpho-chemical characterization and surface properties of carcinogenic zeolite fibers. J. Hazard. Mater. 2016, 306, 140–148. [Google Scholar] [CrossRef]
- Giordani, M.; Mattioli, M.; Dogan, M.; Dogan, A.U. Potential carcinogenic erionite from Lessini Mounts, NE Italy: Morphological, mineralogical and chemical characterization. J. Toxicol. Environ. Health A 2016, 79, 808–824. [Google Scholar] [CrossRef]
- Giordani, M.; Mattioli, M.; Ballirano, P.; Pacella, P.; Cenni, M.; Boscardin, M.; Valentini, L. Geological occurrence, mineralogical characterization and risk assessment of potentially carcinogenic erionite in Italy. J. Toxicol. Environ. Health B 2017, 20, 81–103. [Google Scholar] [CrossRef]
- Ballirano, P.; Andreozzi, G.B.; Dogan, M.; Dogan, A.U. Crystal structure and iron topochemistry of erionite-K from Rome, Oregon, USA. Am. Mineral. 2009, 94, 1262–1270. [Google Scholar] [CrossRef]
- Ballirano, P.; Pacella, A.; Bloise, A.; Giordani, M.; Mattioli, M. Thermal stability of woolly erionite-K and considerations about the heat induced behavior of the erionite group. Minerals 2018, 8, 28. [Google Scholar] [CrossRef]
- Mattioli, M.; Giordani, M.; Arcangeli, P.; Valentini, L.; Boscardin, M.; Pacella, A.; Ballirano, P. Prismatic to asbestiform offretite from Northern Italy: Occurrence, morphology and crystal-chemistry of a new potentially hazardous zeolite. Minerals 2018, 8, 69. [Google Scholar] [CrossRef]
- Giordani, M.; Cametti, G.; Di Lorenzo, F.; Churakov, S.V. Real-time observation of fibrous zeolites reactivity in contact with simulated lung fluids (SLFs) obtained by atomic force microscope (AFM). Minerals 2019, 9, 83. [Google Scholar] [CrossRef]
- Gualtieri, A.F.; Gandolfi, N.B.; Passaglia, E.; Pollastri, S.; Mattioli, M.; Giordani, M.; Ottaviani, M.F.; Cangiotti, M.; Bloise, A.; Barca, D.; et al. Is fibrous ferrierite a potential health hazard? Characterization and comparison with fibrous erionite. Am. Mineral. 2018, 103, 1044–1055. [Google Scholar] [CrossRef]
- Mattioli, M.; Ballirano, P.; Pacella, A.; Cangiotti, M.; Di Lorenzo, F.; Valentini, L.; Meli, M.A.; Roselli, C.; Fagiolino, I.; Giordani, M. Fibrous ferrierite from Northern Italy: Mineralogical, chemical, and EPR characterization and assessment of potential toxicity. Minerals 2022, 12, 626. [Google Scholar] [CrossRef]
- Di Giuseppe, D. Characterization of Fibrous Mordenite: A First Step for the Evaluation of Its Potential Toxicity. Crystals 2020, 10, 769. [Google Scholar] [CrossRef]
- Giordani, M.; Ballirano, P.; Pacella, A.; Meli, M.A.; Roselli, C.; Di Lorenzo, F.; Fagiolino, I.; Mattioli, M. Fibrous mordenite from Northern Italy: Another potentially hazardous zeolite. Minerals 2022, 12, 627. [Google Scholar] [CrossRef]
- Giordani, M.; Mattioli, M.; Cangiotti, M.; Fattori, A.; Ottaviani, M.F.; Betti, M.; Ballirano, P.; Pacella, A.; Di Giuseppe, D.; Scognamiglio, V.; et al. Characterisation of potentially toxic natural fibrous zeolites by means of electron paramagnetic resonance spectroscopy and morphological-mineralogical studies. Chemosphere 2022, 291, 133067. [Google Scholar] [CrossRef]
- Cangiotti, M.; Battistelli, M.; Salucci, S.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. Electron paramagnetic resonance and transmission electron microscopy study of the interactions between asbestiform zeolite fibers and model membranes. J. Toxicol. Environ. Health A 2017, 80, 171–187. [Google Scholar] [CrossRef]
- Cangiotti, M.; Salucci, S.; Battistelli, M.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. EPR, TEM and cell viability study of asbestiform zeolite fibers in cell media. Colloids Surf. B Biointerfaces 2018, 161, 147–155. [Google Scholar] [CrossRef]
- Carbone, M.; Adusumilli, P.S.; Alexander, H.R., Jr.; Baas, P.; Bardelli, F.; Bononi, A.; Bueno, R.; Felley-Bosco, E.; Galateu-Salle, F.; Jablons, D.; et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA-Cancer J. Clin. 2019, 69, 402–429. [Google Scholar] [CrossRef] [PubMed]
- Mirata, S.; Almonti, V.; Di Giuseppe, D.; Fornasini, L.; Raneri, S.; Vernazza, S.; Bersani, D.; Gualtieri, A.F.; Bassi, A.M.; Scarfì, S. The Acute Toxicity of Mineral Fibres: A Systematic In Vitro Study Using Different THP-1 Macrophage Phenotypes. Int. J. Mol. Sci. 2022, 23, 2840. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, D.; Scarfì, S.; Alessandrini, A.; Bassi, A.M.; Mirata, S.; Almonti, V.; Ragazzini, G.; Mescola, A.; Filaferro, M.; Avallone, R.; et al. Acute cytotoxicity of mineral fibres observed by time-lapse video microscopy. Toxicology 2022, 466, 153081. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.F.; Layard, M.; Tegeris, A.; Miller, E.; May, M.; Morgan, E.; Smith, A. Relation of particles dimension to carcinogenicity in amphibole asbestoses and other fibrous minerals. J. Nat. Cancer Inst. 1981, 67, 965–975. [Google Scholar]
- Bertino, P.; Marconi, A.; Palumbo, L.; Bruni, B.M.; Barbone, D.; Germano, S.; Dogan, A.U.; Tassi, G.F.; Porta, C.; Mutti, L.; et al. Erionite and asbestos differently cause transformation of human mesothelial cells. Int. J. Cancer 2007, 121, 2766–2774. [Google Scholar] [CrossRef]
- De Assis, L.V.M.; Locatelli, J.; Isoldi, M.C. The role of key genes and pathways involved in the tumorigenesis of malignant mesothelioma. Biochim. Biophys. Acta 2014, 1845, 232–247. [Google Scholar] [CrossRef]
- Hillegass, J.M.; Miller, J.M.; MacPherson, M.B.; Westbom, C.M.; Sayan, M.; Thompson, J.K.; Macura, S.L.; Perkins, T.N.; Beuschel, S.L.; Alexeeva, V.; et al. Asbestos and erionite prime and activate the NLRP3 inflammasome that stimulates autocrine cytokine release in human mesothelial cells. Part. Fibre Toxicol. 2013, 10, 39. [Google Scholar] [CrossRef]
- Zanella, C.L.; Posada, J.; Tritton, T.R.; Mossman, B.T. Asbestos causes stimulation of the extracellular signal-regulated kinase 1 mitogen-activated protein kinase cascade after phosphorylation of the epidermal growth factor receptor. Cancer Res. 1996, 56, 5334–5338. [Google Scholar]
- Di Giuseppe, D.; Zoboli, A.; Nodari, L.; Pasquali, L.; Sala, O.; Ballirano, P.; Malferrari, D.; Raneri, S.; Hanuskova, M.; Gualtieri, A.F. Characterization and assessment of the potential toxicity/pathogenicity of Russian commercial chrysotile. Am. Mineral. 2021, 106, 1606–1621. [Google Scholar] [CrossRef]
- Staples, L.W.; Gard, J.A. The fibrous zeolite erionite: Its occurrence, unit cell, and structure. Mineral. Mag. 1959, 322, 261–281. [Google Scholar] [CrossRef]
- Pacella, A.; Cremisini, C.; Nardi, E.; Montereali, M.R.; Pettiti, I.; Giordani, M.; Mattioli, M.; Ballirano, P. Different Erionite Species Bind Iron into the Structure: A Potential Explanation for Fibrous Erionite Toxicity. Minerals 2018, 8, 36. [Google Scholar] [CrossRef]
- Gude, A.J.; Sheppard, R.A. Woolly erionite from the Reese River zeolite deposit, Lander County, Nevada, and its relationship to other erionites. Clays Clay Miner. 1981, 29, 378–384. [Google Scholar] [CrossRef]
- Gonda, I.; Abd El Khalik, A.F. On the calculation of aerodynamic diameters of fibers. Aerosol. Sci. Technol. 1985, 4, 233–238. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley & Sons Inc.: New York, NY, USA, 1974. [Google Scholar]
- Gottardi, G.; Galli, E. Natural Zeolites; Minerals and Rocks Series; Springer: Berlin, Heidelberg, 1985; Volume 18, p. 409. [Google Scholar]
- Passaglia, E.; Sheppard, R.A. The Crystal Chemistry of Zeolites. Rev. Mineral. Geochem. 2011, 45, 69–116. [Google Scholar] [CrossRef]
- Lehman, S.E.; Larsen, S.C. Zeolite and mesoporous silica nanomaterials: Greener syntheses, environmental applications and biological toxicity. Environ. Sci. Nano 2014, 1, 200–213. [Google Scholar] [CrossRef]
- Gualtieri, A.F. Bridging the gap between toxicity and carcinogenicity of mineral fibres by connecting the fibre crystal-chemical and physical parameters to the key characteristics of cancer. Curr. Res. Toxicol. 2021, 26, 42–52. [Google Scholar] [CrossRef]
- Yu, S.; Choi, H.H.; Park, G.; Kim, I.W.; Kim, T.J. Fibrogenic effects of crocidolite, amosite, and chrysotile asbestos fibers on lung fibroblasts. Toxicol. Environ. Chem. 2019, 101, 148–164. [Google Scholar] [CrossRef]
- Yu, S.; Choi, H.H.; Kim, I.W.; Kim, T.J. Conditioned medium from asbestos-exposed fibroblasts affects proliferation and invasion of lung cancer cell lines. PLoS ONE 2019, 14, e0222160. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans. In Overall Evaluations of Carcinogenicity: And Updating IARC Monographs Vol. 1 to 42; IARC: Lyon, France, 1987. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betti, M.; Nasoni, M.G.; Luchetti, F.; Giordani, M.; Mattioli, M. Potential Toxicity of Natural Fibrous Zeolites: In Vitro Study Using Jurkat and HT22 Cell Lines. Minerals 2022, 12, 988. https://doi.org/10.3390/min12080988
Betti M, Nasoni MG, Luchetti F, Giordani M, Mattioli M. Potential Toxicity of Natural Fibrous Zeolites: In Vitro Study Using Jurkat and HT22 Cell Lines. Minerals. 2022; 12(8):988. https://doi.org/10.3390/min12080988
Chicago/Turabian StyleBetti, Michele, Maria Gemma Nasoni, Francesca Luchetti, Matteo Giordani, and Michele Mattioli. 2022. "Potential Toxicity of Natural Fibrous Zeolites: In Vitro Study Using Jurkat and HT22 Cell Lines" Minerals 12, no. 8: 988. https://doi.org/10.3390/min12080988






