Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Experimental Methods
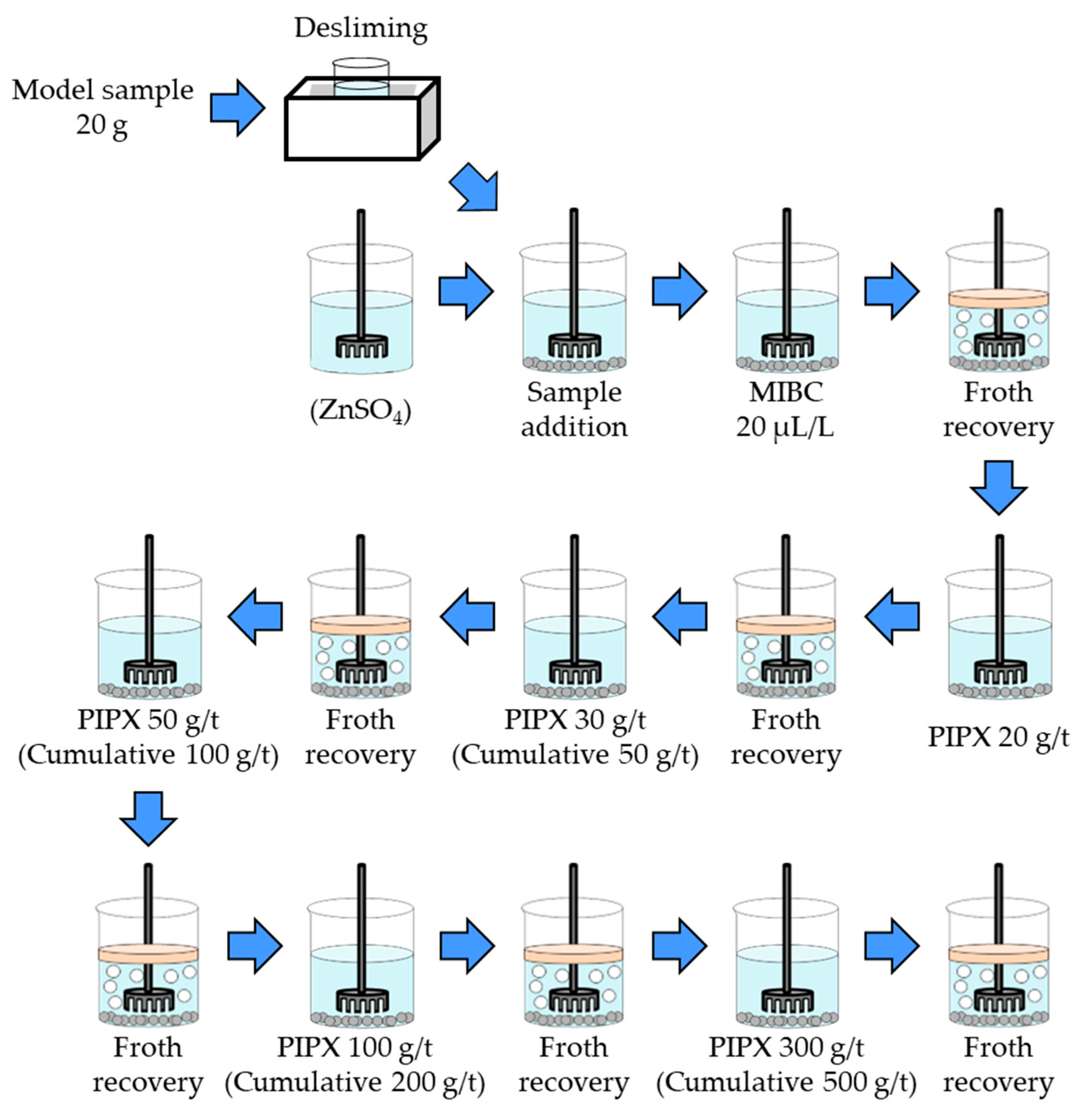
2.2.1. Flotation
2.2.2. Contact Angle Measurements
3. Results and Discussion
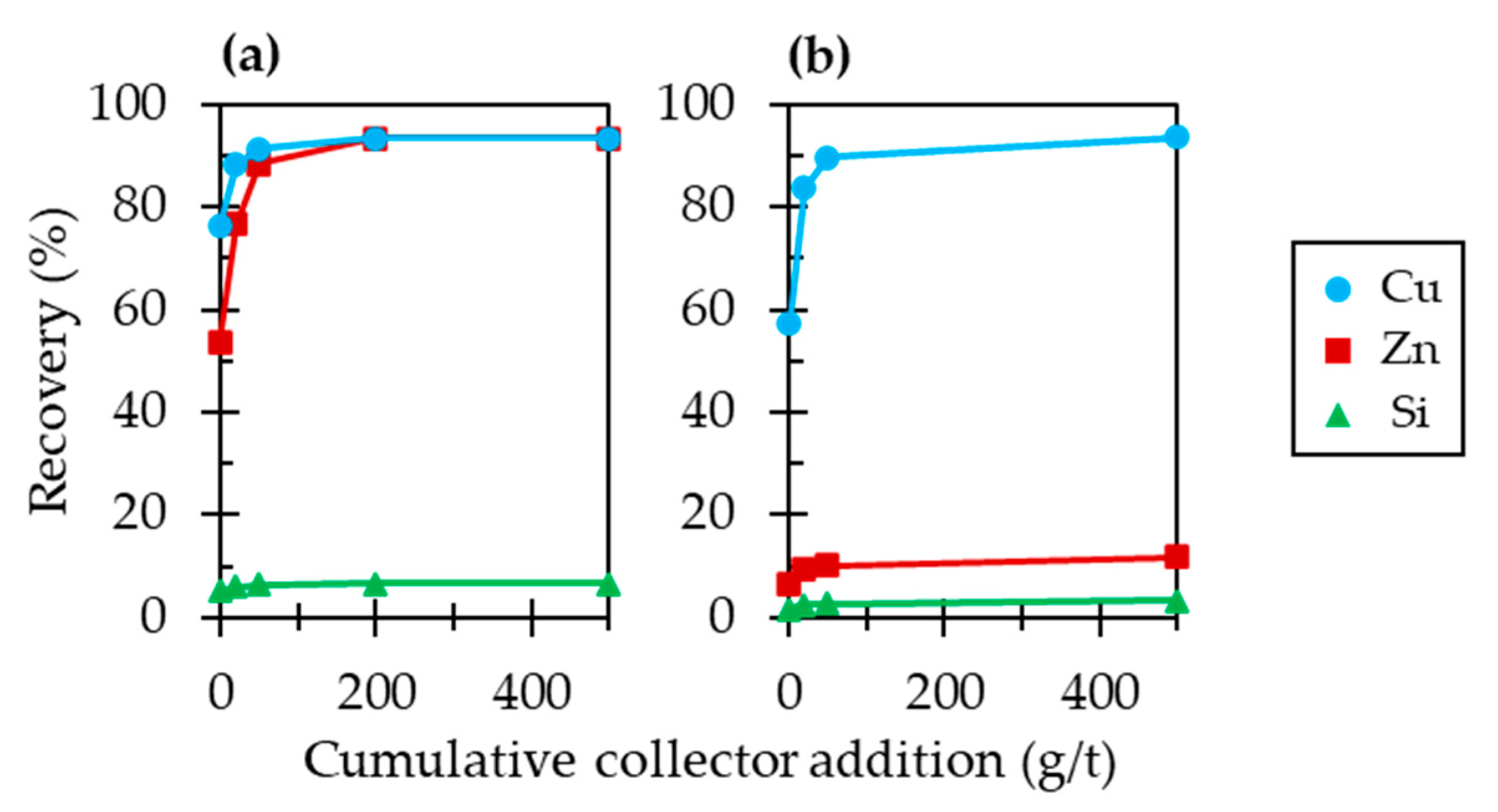
3.1. Single Mineral Flotation of Chalcopyrite or Sphalerite
3.2. Effects of Chalcopyrite on the Floatability of Sphalerite
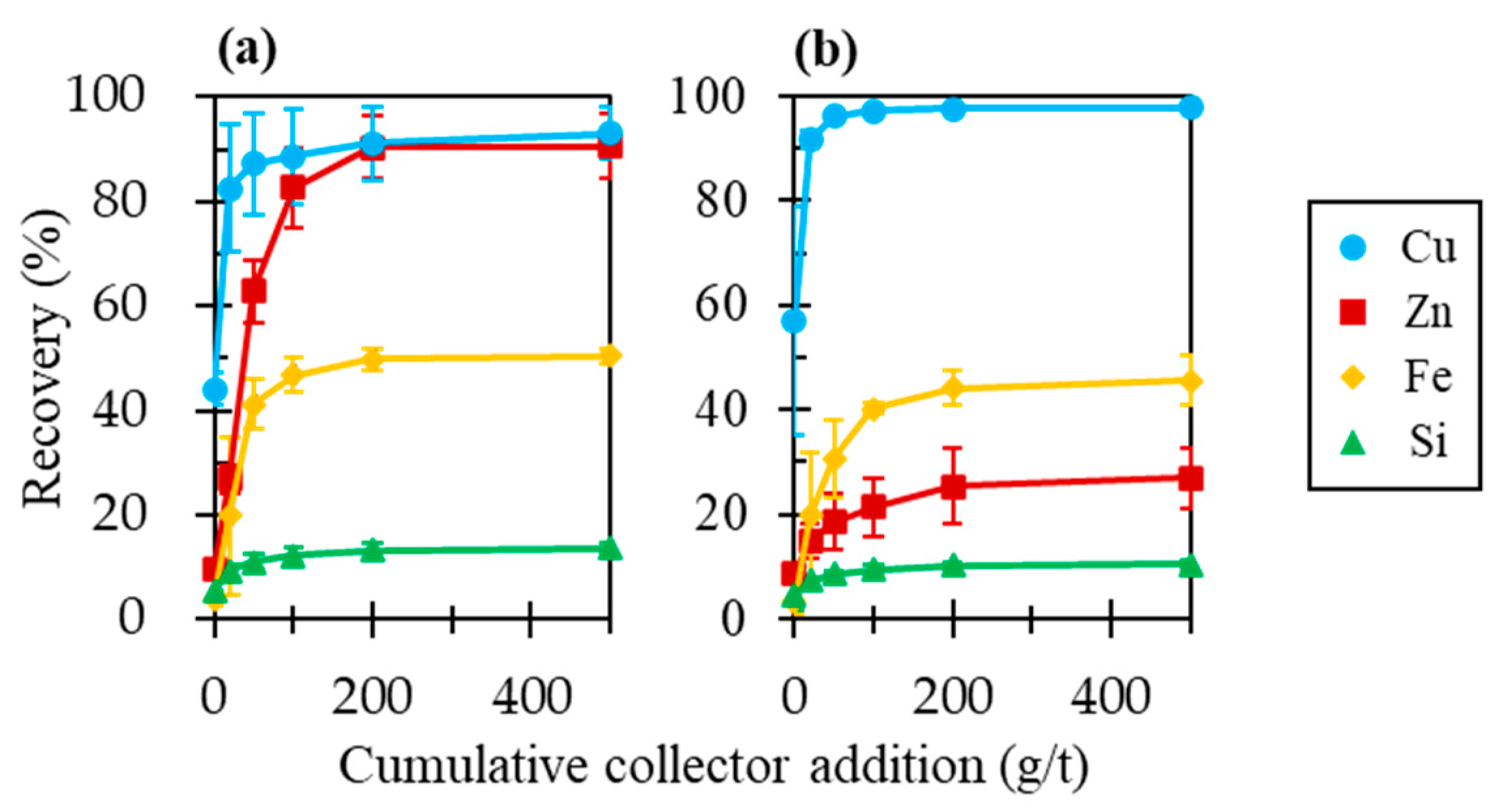
3.3. Effects of Pyrite on Sphalerite Floatability in the Presence of Chalcopyrite
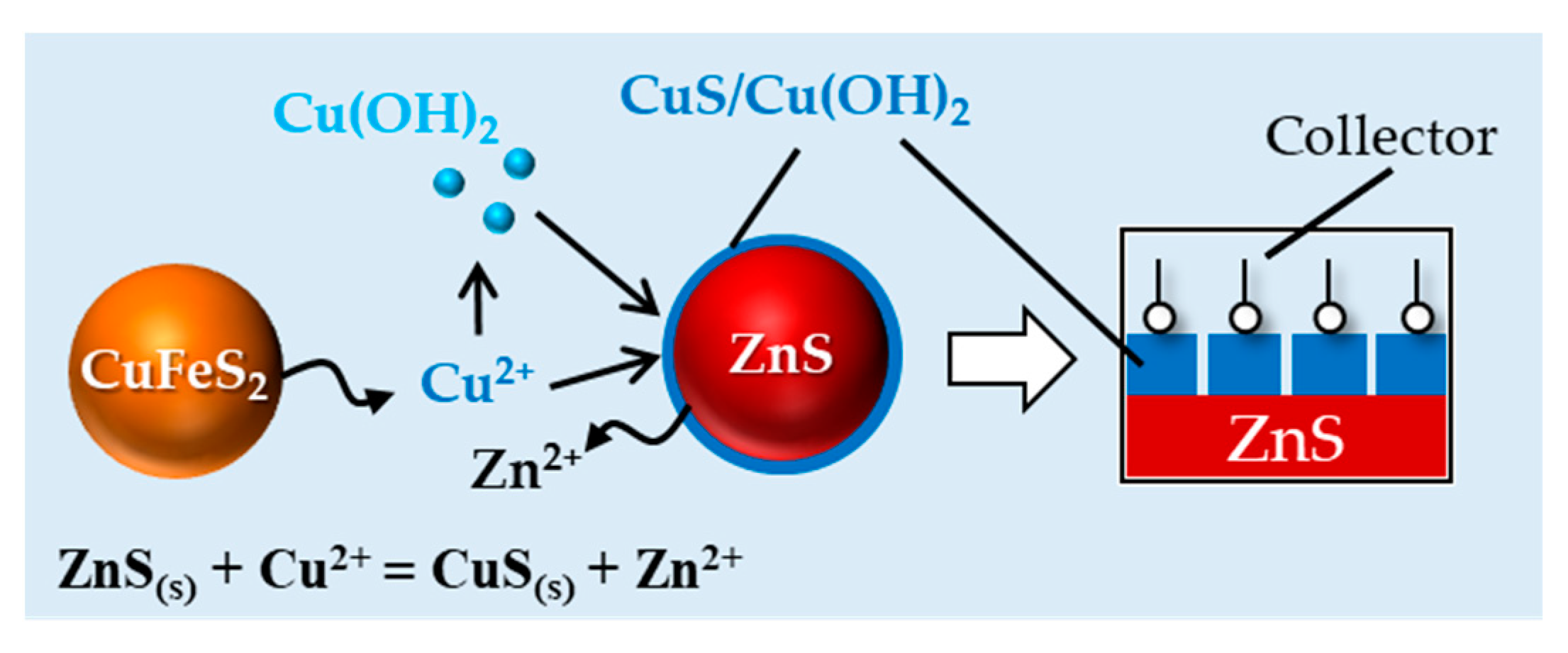
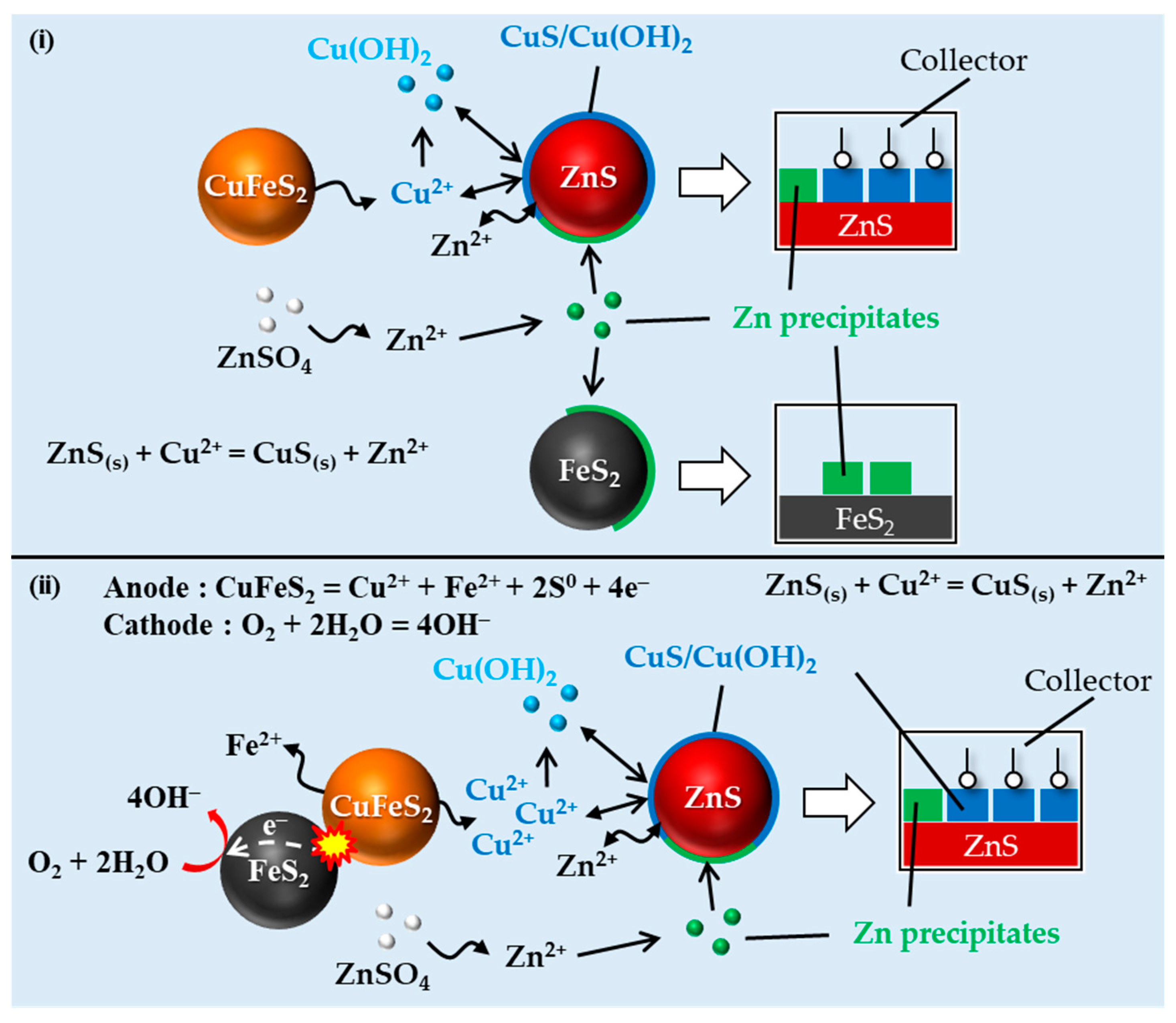
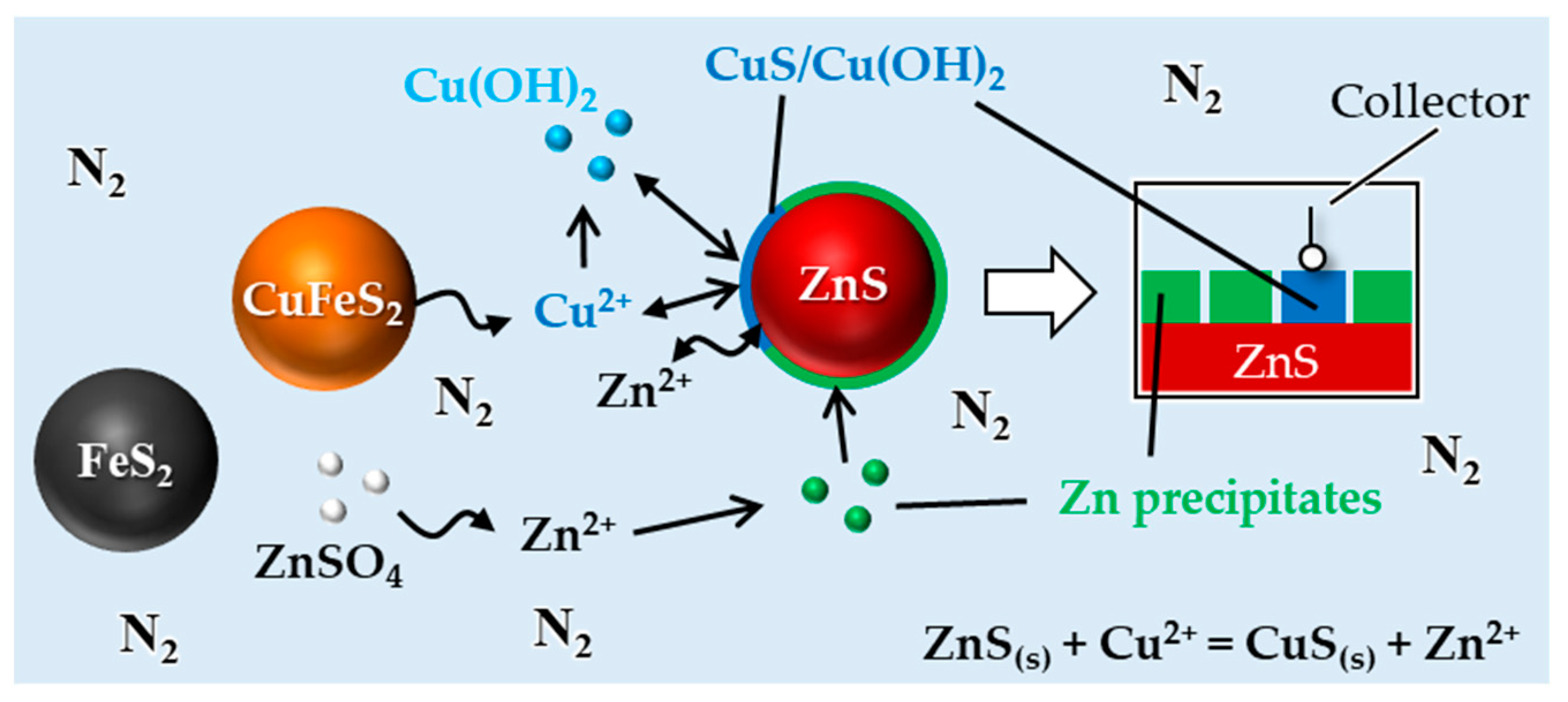
3.4. Mechanisms of Losing the Depressive Effect of Zinc Sulfate toward Sphalerite in the Presence of Both Pyrite and Chalcopyrite
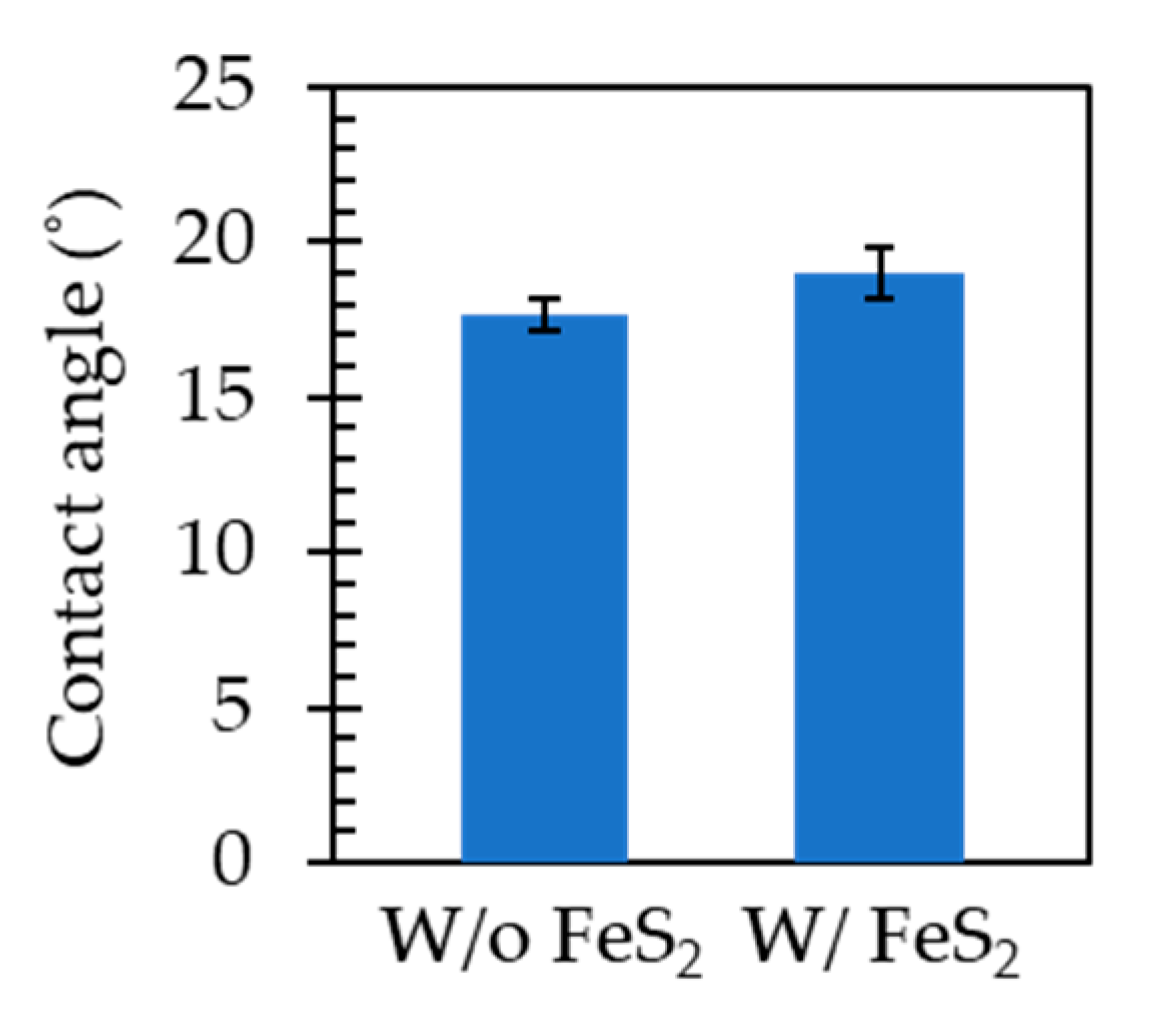
3.4.1. Effects of Pyrite on the Hydrophilicity of Sphalerite
3.4.2. Effects of the Galvanic Interaction between Chalcopyrite and Pyrite on the Floatability of Sphalerite
3.5. A Proposed Method to Efficiently Depress Sphalerite in Flotation of Cu Ores with a High Cu/Zn Ratio
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spatari, S.; Bertram, M.; Gordon, R.B.; Henderson, K.; Graedel, T.E. Twentieth Century Copper Stocks and Flows in North America: A Dynamic Analysis. Ecol. Econ. 2005, 54, 37–51. [Google Scholar] [CrossRef]
- Bilal, M.; Park, I.; Hornn, V.; Ito, M.; Hassan, F.; Jeon, S.; Hiroyoshi, N. The Challenges and Prospects of Recovering Fine Copper Sulfides from Tailings Using Different Flotation Techniques: A Review. Minerals 2022, 12, 586. [Google Scholar] [CrossRef]
- WBG Minerals for Climate Action: The Mineral Intensity of the Clean Energy Transition. Available online: https://pubdocs.worldbank.org/en/961711588875536384/Minerals-for-Climate-Action-The-Mineral-Intensity-of-the-Clean-Energy-Transition.pdf (accessed on 25 April 2022).
- Park, I.; Kanazawa, Y.; Sato, N.; Galtchandmani, P.; Jha, M.K.; Tabelin, C.B.; Jeon, S.; Ito, M.; Hiroyoshi, N. Beneficiation of Low-Grade Rare Earth Ore from Khalzan Buregtei Deposit (Mongolia) by Magnetic Separation. Minerals 2021, 11, 1432. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part I. Selective Coating Formation. Metals 2020, 10, 1667. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Akishino, R.; Bu, X.; Ul Hassan, F.; Park, I.; Jeon, S.; Aikawa, K.; Hiroyoshi, N. Heterogenous Carrier Flotation Technique for Recovering Finely Ground Chalcopyrite Particles Using Coarse Pyrite Particles as a Carrier. Miner. Eng. 2022, 180, 107518. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. A Review of Recent Advances in Depression Techniques for Flotation Separation of Cu–Mo Sulfides in Porphyry Copper Deposits. Metals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Park, I. Advances in Selective Flotation and Leaching Process in Metallurgy. Metals 2022, 12, 144. [Google Scholar] [CrossRef]
- Hornn, V.; Park, I.; Ito, M.; Shimada, H.; Suto, T.; Tabelin, C.B.; Jeon, S.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite Using Surfactant-Stabilized Oil Emulsions: Effects of Co-Existing Minerals and Ions. Miner. Eng. 2021, 171, 107076. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Park, I.; Phengsaart, T.; Jeon, S.; Villacorte-Tabelin, M.; Alonzo, D.; Yoo, K.; Ito, M.; Hiroyoshi, N. Copper and Critical Metals Production from Porphyry Ores and E-Wastes: A Review of Resource Availability, Processing/Recycling Challenges, Socio-Environmental Aspects, and Sustainability Issues. Resour. Conserv. Recycl. 2021, 170, 105610. [Google Scholar] [CrossRef]
- John, D.A.; Ayuso, R.A.; Barton, M.D.; Blakely, R.J.; Bodnar, R.J.; Dilles, J.H.; Gray, F.; Graybeal, F.T.; Mars, J.C.; McPhee, D.K.; et al. Porphyry Copper Deposit Model, Chapter B of Mineral Deposit Models for Resource Assessment; U.S. Geological Survey Scientific Investigations Report 2010-5070-B; U.S. Geological Survey: Menlo Park, CA, USA, 2010.
- Tongamp, W.; Takasaki, Y.; Shibayama, A. Arsenic Removal from Copper Ores and Concentrates through Alkaline Leaching in NaHS Media. Hydrometallurgy 2009, 98, 213–218. [Google Scholar] [CrossRef]
- Haga, K.; Tongamp, W.; Shibayama, A. Investigation of Flotation Parameters for Copper Recovery from Enargite and Chalcopyrite Mixed Ore. Mater. Trans. 2012, 53, 707–715. [Google Scholar] [CrossRef]
- Haga, K.; Altansukh, B.; Shibayama, A. Volatilization of Arsenic and Antimony from Tennantite/Tetrahedrite Ore by a Roasting Process. Mater. Trans. 2018, 59, 1396–1403. [Google Scholar] [CrossRef]
- Sinche-Gonzalez, M.; Fornasiero, D. Understanding the Effect of Sulphate in Mining-Process Water on Sulphide Flotation. Miner. Eng. 2021, 165, 106865. [Google Scholar] [CrossRef]
- Bıçak, Ö.; Ekmekçi, Z.; Can, M.; Öztürk, Y. The Effect of Water Chemistry on Froth Stability and Surface Chemistry of the Flotation of a Cu–Zn Sulfide Ore. Int. J. Miner. Process. 2012, 102–103, 32–37. [Google Scholar] [CrossRef]
- Natarajan, R.; Nirdosh, I.; Muthuswami, S.V. Flotation of a Copper-Zinc Ore Using p-Nonylcupferron as Collector. Dev. Chem. Eng. Miner. Processing 1997, 5, 183–193. [Google Scholar] [CrossRef]
- Soltani, F.; Koleini, S.M.J.; Abdollahy, M. Optimization of Cu–Zn Massive Sulphide Flotation by Selective Reagents. J. Inst. Eng. India Ser. D 2014, 95, 125–134. [Google Scholar] [CrossRef]
- Kant, C.; Rao, S.R.; Finch, J.A. Distribution of Surface Metal Ions among the Products of Chalcopyrite Flotation. Miner. Eng. 1994, 7, 905–916. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Kusano, A.; Jeon, S.; Park, I.; Hiroyoshi, N. Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals 2022, 12, 723. [Google Scholar] [CrossRef]
- Woodcock, J.T.; Sparrow, G.J.; Bruckard, W.J.; Johnson, N.W.; Dunne, R. Plant Practice: Sulfide Minerals and Precious Metals. In Froth Flotation a Century of Innovation; Fuerstenau, M., Jameson, G., Yoon, R.H., Eds.; SME: Littleton, CO, USA, 2007; pp. 781–843. ISBN 978-0-873-35252-9. [Google Scholar]
- Ito, M.; Orii, N.; Aikawa, K.; Jeon, S.; Park, I.; Hiroyoshi, N.; Magwaneng, R.; Kudo, K.; Sunada, K.; Takahashi, T. Effects of Several Depressants on the Floatability of Sphalerite in the Flotation of Copper-Zinc Sulfides. In Proceedings of the Mining and Materials Processing Institute of Japan (MMIJ) Fall Meeting 2020, online, 8–10 September 2020; Volume 7. [Google Scholar]
- Aikawa, K.; Ito, M.; Kusano, A.; Park, I.; Oki, T.; Takahashi, T.; Furuya, H.; Hiroyoshi, N. Flotation of Seafloor Massive Sulfide Ores: Combination of Surface Cleaning and Deactivation of Lead-Activated Sphalerite to Improve the Separation Efficiency of Chalcopyrite and Sphalerite. Metals 2021, 11, 253. [Google Scholar] [CrossRef]
- Bilal, M.; Ito, M.; Koike, K.; Hornn, V.; Ul Hassan, F.; Jeon, S.; Park, I.; Hiroyoshi, N. Effects of Coarse Chalcopyrite on Flotation Behavior of Fine Chalcopyrite. Miner. Eng. 2021, 163, 106776. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Yamazawa, R.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Kinetic Analysis for Agglomeration-Flotation of Finely Ground Chalcopyrite: Comparison of First Order Kinetic Model and Experimental Results. Mater. Trans. 2020, 61, 1940–1948. [Google Scholar] [CrossRef]
- Park, I.; Hong, S.; Jeon, S.; Ito, M.; Hiroyoshi, N. Flotation Separation of Chalcopyrite and Molybdenite Assisted by Microencapsulation Using Ferrous and Phosphate Ions: Part II. Flotation. Metals 2021, 11, 439. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Froth flotation. In Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Elsevier: Oxford, UK, 2016; pp. 265–380. ISBN 9780080970530. [Google Scholar]
- Chandra, A.P.; Gerson, A.R. A Review of the Fundamental Studies of the Copper Activation Mechanisms for Selective Flotation of the Sulfide Minerals, Sphalerite and Pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef]
- Lascelles, D.; Finch, J.A. Quantifying Accidental Activation. Part I. Cu Ion Production. Miner. Eng. 2002, 15, 567–571. [Google Scholar] [CrossRef]
- Wei, Q.; Jiao, F.; Dong, L.; Liu, X.; Qin, W. Selective Depression of Copper-Activated Sphalerite by Polyaspartic Acid during Chalcopyrite Flotation. Trans. Nonferrous Met. Soc. China 2021, 31, 1784–1795. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Q. Reexamining the Functions of Zinc Sulfate as a Selective Depressant in Differential Sulfide Flotation—The Role of Coagulation. J. Colloid Interface Sci. 2006, 301, 523–531. [Google Scholar] [CrossRef]
- Pecina-Treviño, E.T.; Uribe-Salas, A.; Nava-Alonso, F. Effect of Dissolved Oxygen and Galvanic Contact on the Floatability of Galena and Pyrite with Aerophine 3418A. Miner. Eng. 2003, 16, 359–367. [Google Scholar] [CrossRef]
- Qin, W.; Wang, X.; Ma, L.; Jiao, F.; Liu, R.; Yang, C.; Gao, K. Electrochemical Characteristics and Collectorless Flotation Behavior of Galena: With and without the Presence of Pyrite. Miner. Eng. 2015, 74, 99–104. [Google Scholar] [CrossRef]
- Barker, G.J.; Gerson, A.R.; Menuge, J.F. The Impact of Iron Sulfide on Lead Recovery at the Giant Navan Zn–Pb Orebody, Ireland. Int. J. Miner. Process. 2014, 128, 16–24. [Google Scholar] [CrossRef]
- Qin, W.; Wang, X.; Ma, L.; Jiao, F.; Liu, R.; Gao, K. Effects of Galvanic Interaction between Galena and Pyrite on Their Flotation in the Presence of Butyl Xanthate. Trans. Nonferrous Met. Soc. China 2015, 25, 3111–3118. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.; Jiao, F.; Wu, J. The Influence of Galvanic Interaction on the Dissolution and Surface Composition of Galena and Pyrite in Flotation System. Miner. Eng. 2020, 156, 106525. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of Lead-Activated Sphalerite by Pyrite via Galvanic Interactions: Implications to the Selective Flotation of Complex Sulfide Ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Owusu, C.; Brito e Abreu, S.; Skinner, W.; Addai-Mensah, J.; Zanin, M. The Influence of Pyrite Content on the Flotation of Chalcopyrite/Pyrite Mixtures. Miner. Eng. 2014, 55, 87–95. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The Galvanic Interaction between Chalcopyrite and Pyrite in the Presence of Lignosulfonate-Based Biopolymers and Its Effects on Flotation Performance. Miner. Eng. 2018, 122, 91–98. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. Enhancing the Kinetics of Chalcopyrite Leaching in the GalvanoxTM Process. Hydrometallurgy 2011, 105, 251–258. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Kojima, M.; Hiroyoshi, N.; Ito, M. Galvanic Microencapsulation (GME) Using Zero-Valent Aluminum and Zero-Valent Iron to Suppress Pyrite Oxidation. Mater. Trans. 2019, 60, 277–286. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Makino, Y.; Chea, M.; Phengsaart, T.; Park, I.; Hiroyoshi, N.; Ito, M. Improvement of Flotation and Suppression of Pyrite Oxidation Using Phosphate-Enhanced Galvanic Microencapsulation (GME) in a Ball Mill with Steel Ball Media. Miner. Eng. 2019, 143, 105931. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Kuze, A.; Ito, M.; Hiroyoshi, N. A Kinetic Study on Enhanced Cementation of Gold Ions by Galvanic Interactions between Aluminum (Al) as an Electron Donor and Activated Carbon (AC) as an Electron Mediator in Ammonium Thiosulfate System. Minerals 2022, 12, 91. [Google Scholar] [CrossRef]
- Jeon, S.; Bright, S.; Park, I.; Tabelin, C.B.; Ito, M.; Hiroyoshi, N. A Simple and Efficient Recovery Technique for Gold Ions from Ammonium Thiosulfate Medium by Galvanic Interactions of Zero-Valent Aluminum and Activated Carbon: A Parametric and Mechanistic Study of Cementation. Hydrometallurgy 2022, 208, 105815. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Igarashi, T.; Villacorte-Tabelin, M.; Park, I.; Opiso, E.M.; Ito, M.; Hiroyoshi, N. Arsenic, Selenium, Boron, Lead, Cadmium, Copper, and Zinc in Naturally Contaminated Rocks: A Review of Their Sources, Modes of Enrichment, Mechanisms of Release, and Mitigation Strategies. Sci. Total Environ. 2018, 645, 1522–1553. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Tabelin, C.B.; Jeon, S.; Li, X.; Seno, K.; Ito, M.; Hiroyoshi, N. A Review of Recent Strategies for Acid Mine Drainage Prevention and Mine Tailings Recycling. Chemosphere 2019, 219, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Ekmekçi, Z.; Demirel, H. Effects of Galvanic Interaction on Collectorless Flotation Behaviour of Chalcopyrite and Pyrite. Int. J. Miner. Process. 1997, 52, 31–48. [Google Scholar] [CrossRef]
- Lee, R.L.J.; Chen, X.; Peng, Y. Flotation Performance of Chalcopyrite in the Presence of an Elevated Pyrite Proportion. Miner. Eng. 2022, 177, 107387. [Google Scholar] [CrossRef]
- Allison, S.A.; Goold, L.A.; Nicol, M.J.; Granville, A. A Determination of the Products of Reaction between Various Sulphide Minerals and Aqueous Xanthate. Metall. Trans. 1972, 3, 2613–2618. [Google Scholar] [CrossRef]
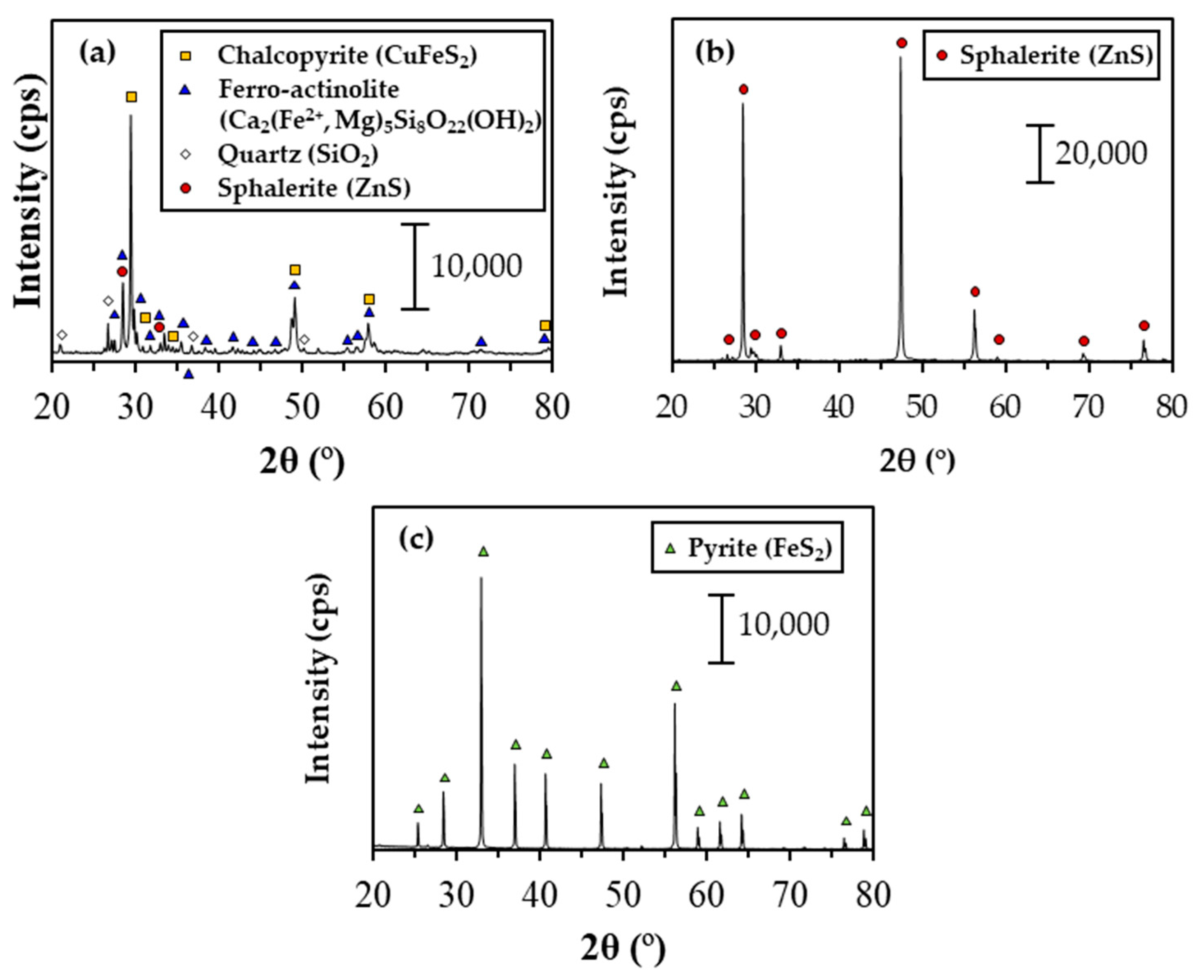


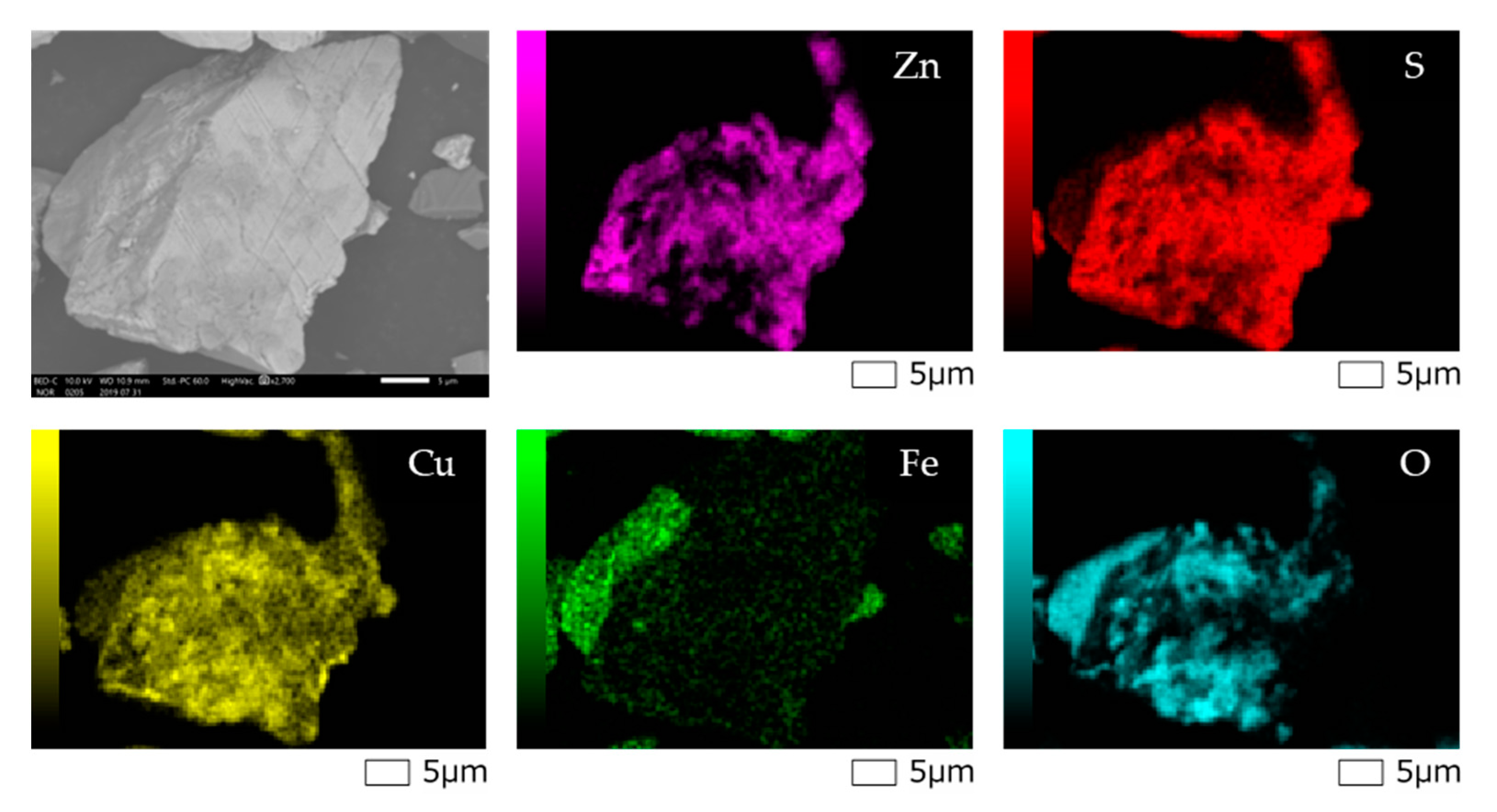

| Sample | Mass Fraction (%) | ||||
|---|---|---|---|---|---|
| Cu | Zn | Fe | S | Si | |
| Chalcopyrite | 28.3 | 0.5 | 32.8 | 17.7 | 11.0 |
| Sphalerite | – | 54.0 | 6.4 | 32.3 | 3.3 |
| Pyrite | – | – | 42.3 | 52.5 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aikawa, K.; Ito, M.; Orii, N.; Jeon, S.; Park, I.; Haga, K.; Kamiya, T.; Takahashi, T.; Sunada, K.; Sakakibara, T.; et al. Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression. Minerals 2022, 12, 1103. https://doi.org/10.3390/min12091103
Aikawa K, Ito M, Orii N, Jeon S, Park I, Haga K, Kamiya T, Takahashi T, Sunada K, Sakakibara T, et al. Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression. Minerals. 2022; 12(9):1103. https://doi.org/10.3390/min12091103
Chicago/Turabian StyleAikawa, Kosei, Mayumi Ito, Nodoka Orii, Sanghee Jeon, Ilhwan Park, Kazutoshi Haga, Taro Kamiya, Tatsuru Takahashi, Kazuya Sunada, Taisuke Sakakibara, and et al. 2022. "Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression" Minerals 12, no. 9: 1103. https://doi.org/10.3390/min12091103
APA StyleAikawa, K., Ito, M., Orii, N., Jeon, S., Park, I., Haga, K., Kamiya, T., Takahashi, T., Sunada, K., Sakakibara, T., Ono, T., Magwaneng, R. S., & Hiroyoshi, N. (2022). Flotation of Copper Ores with High Cu/Zn Ratio: Effects of Pyrite on Cu/Zn Separation and an Efficient Method to Enhance Sphalerite Depression. Minerals, 12(9), 1103. https://doi.org/10.3390/min12091103








