Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry
Abstract
:1. Introduction
2. Materials and Methods
2.1. First Stage: Treatment of Waste Brine from RO Desalination
2.1.1. Waste Brine Samples and Reagents
2.1.2. Procedure for Waste Brine Treatment
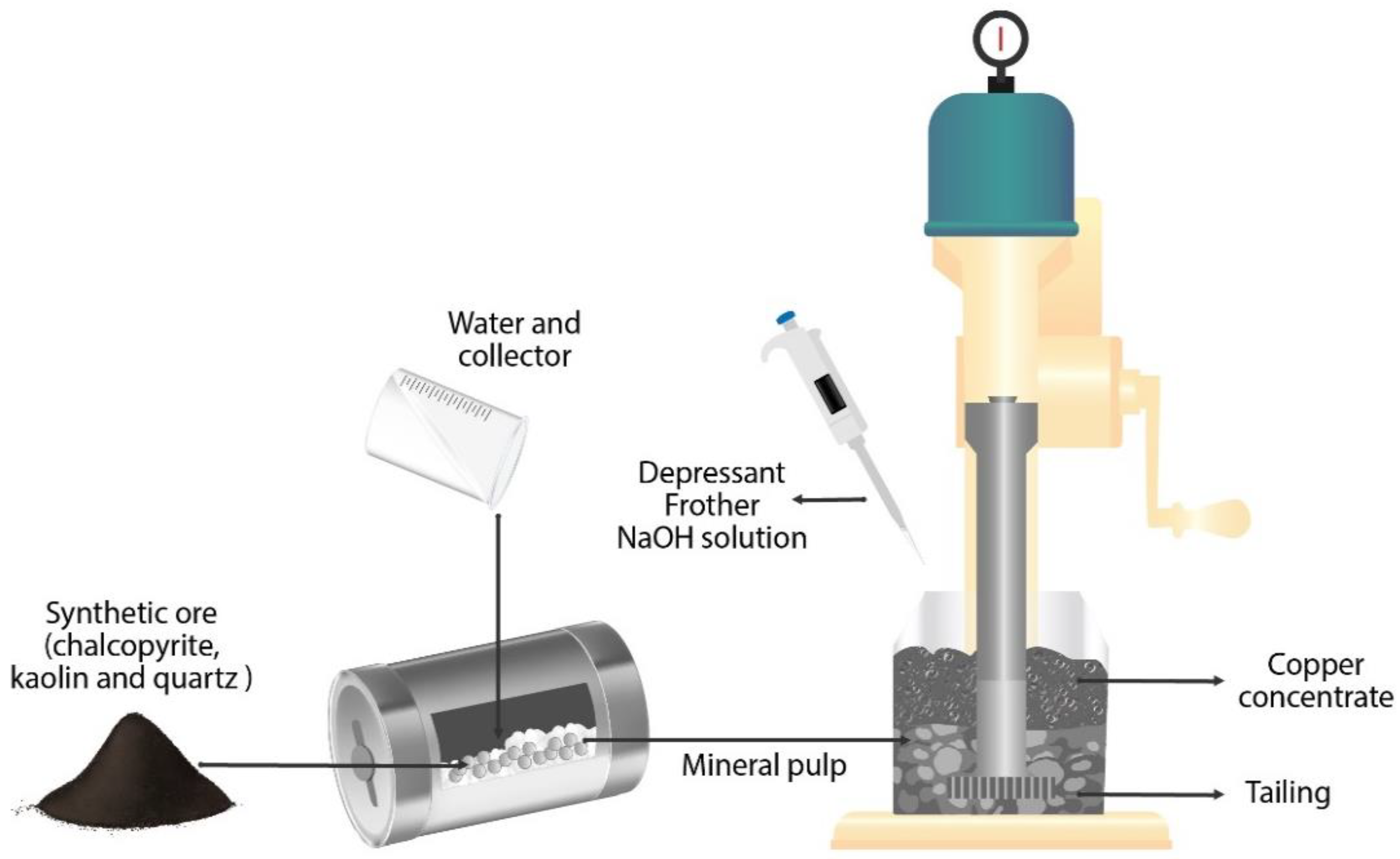
2.2. Second Stage: Froth Flotation Tests of Chalcopyrite with Clay Content
Copper Sulfide Ore Samples and Reagents
3. Results and Discussion
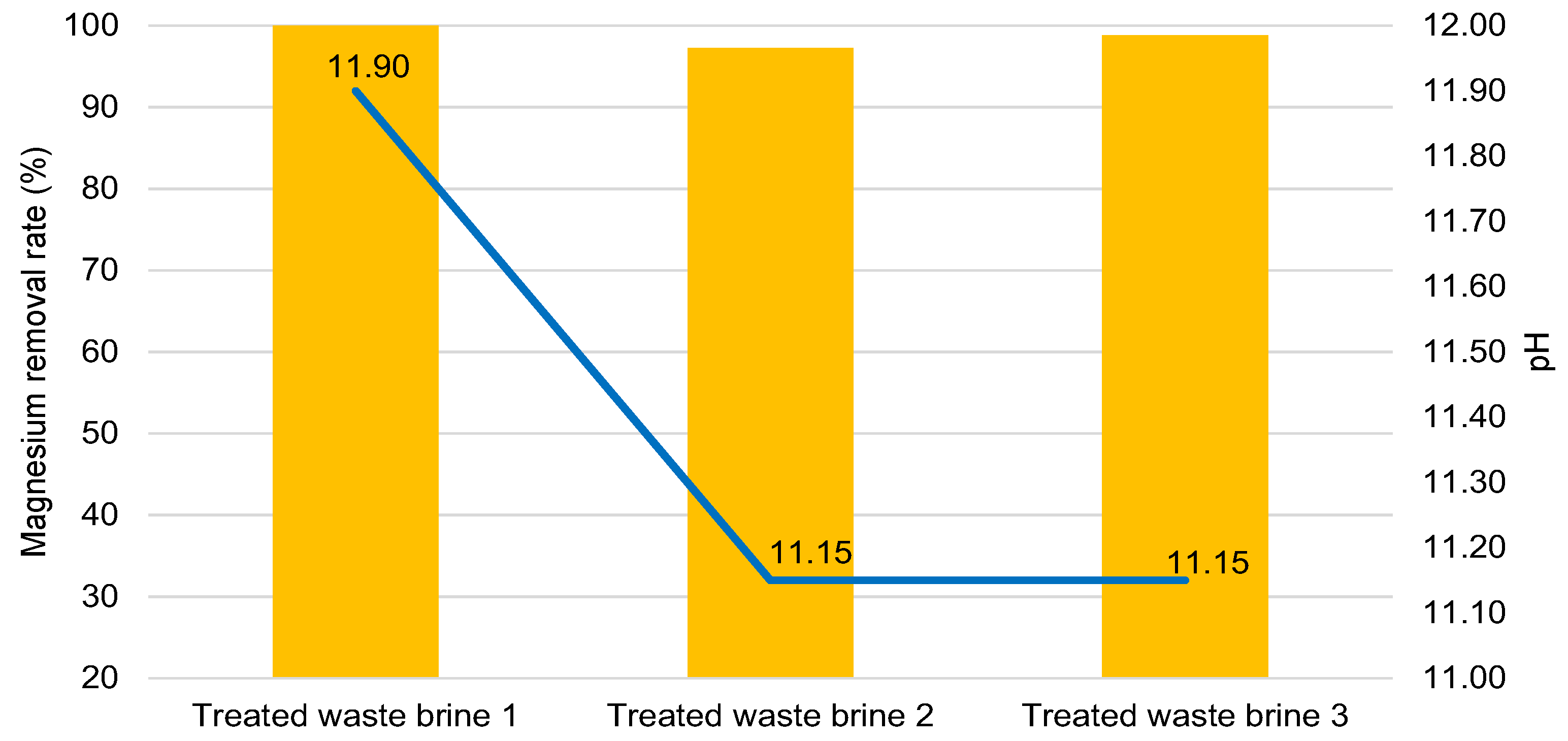
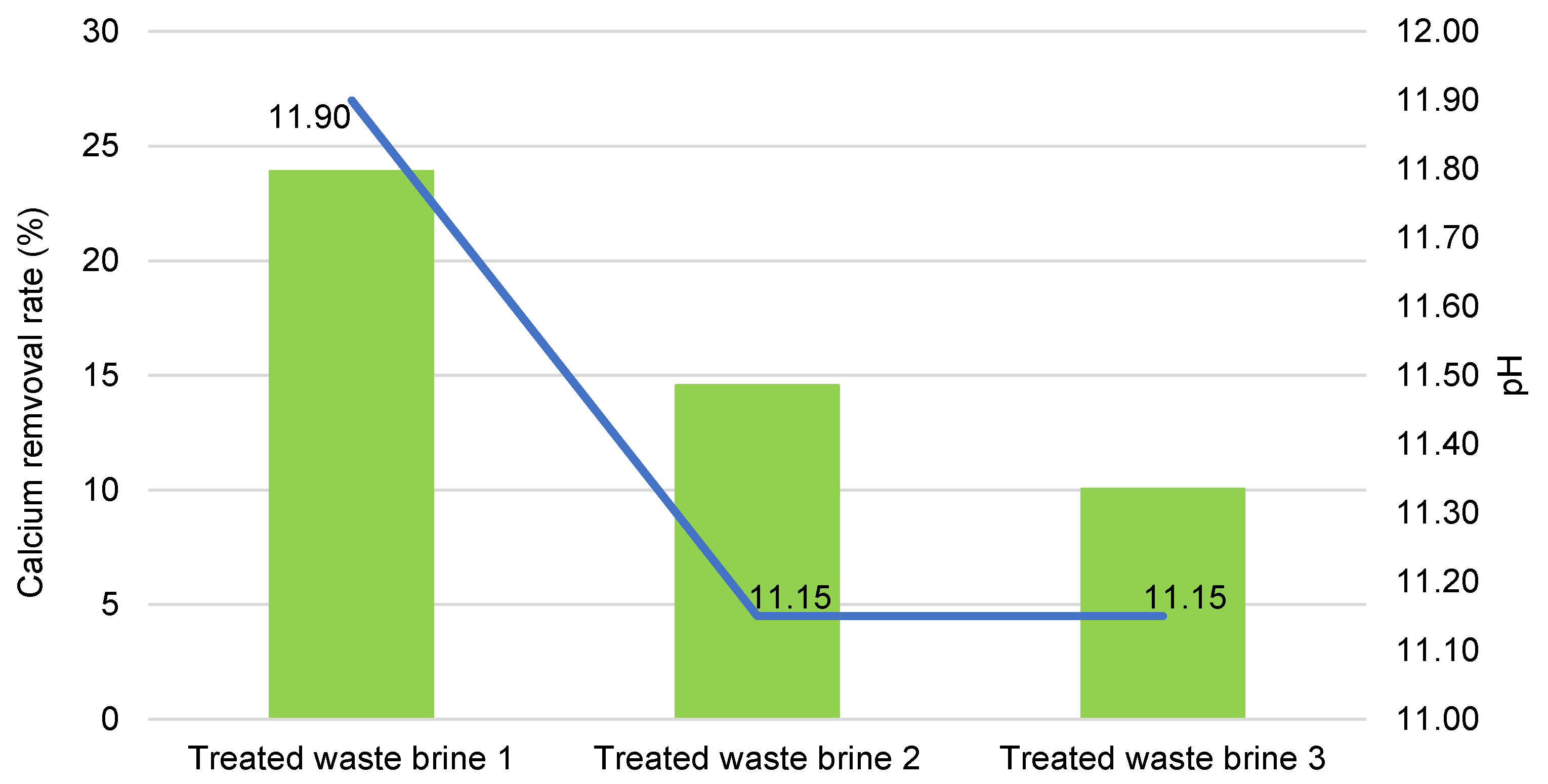
3.1. Treated Waste Brine
3.2. Flotation of Chalcopyrite Using the Treated Waste Brine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gunson, A.J.; Klein, B.; Veiga, M.; Dunbar, S. Reducing Mine Water Requirements. J. Clean. Prod. 2012, 21, 71–82. [Google Scholar] [CrossRef]
- Northey, S.A.; Mudd, G.M.; Saarivuori, E.; Wessman-Jääskeläinen, H.; Haque, N. Water Footprinting and Mining: Where Are the Limitations and Opportunities? J. Clean. Prod. 2016, 135, 1098–1116. [Google Scholar] [CrossRef]
- Kunz, N.C. Towards a Broadened View of Water Security in Mining Regions. Water Secur. 2020, 11, 100079. [Google Scholar] [CrossRef]
- Northey, S.A.; Mudd, G.M.; Werner, T.T.; Jowitt, S.M.; Haque, N.; Yellishetty, M.; Weng, Z. The Exposure of Global Base Metal Resources to Water Criticality, Scarcity and Climate Change. Glob. Environ. Chang. 2017, 44, 109–124. [Google Scholar] [CrossRef]
- USGS; U.G.S. Minerals Commodity Summary—Copper. 2021. Available online: https://pubs.usgs.gov/periodicals/mcs2021/mcs2021-copper.pdf (accessed on 1 June 2021).
- Cisternas, L.A.; Gálvez, E.D. The Use of Seawater in Mining. Miner. Process. Extr. Metall. Rev. 2018, 39, 18–33. [Google Scholar] [CrossRef]
- Dirección General de Aguas (DGA) Atlas Del Agua. Ministerio de Obras Públicas. Available online: https://snia.mop.gob.cl/sad/Atlas2016parte1.pdf (accessed on 15 June 2021).
- Scheihing, K.; Tröger, U. Local Climate Change Induced by Groundwater Overexploitation in a High Andean Arid Watershed, Laguna Lagunillas Basin, Northern Chile. Hydrogeol. J. 2018, 26, 705–719. [Google Scholar] [CrossRef]
- Herrera-León, S.; Cruz, C.; Negrete, M.; Chacana, J.; Cisternas, L.A.; Kraslawski, A. Impact of Seawater Desalination and Wastewater Treatment on Water Stress Levels and Greenhouse Gas Emissions: The Case of Chile. Sci. Total Environ. 2022, 818, 151853. [Google Scholar] [CrossRef]
- Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E. Water Scarcity and the Impact of the Mining and Agricultural Sectors in Chile. Sustainability 2016, 8, 128. [Google Scholar] [CrossRef]
- Alvez, A.; Aitken, D.; Rivera, D.; Vergara, M.; McIntyre, N.; Concha, F. At the crossroads: Can desalination be a suitable public policy solution to address water scarcity in Chile’s mining zones? J. Environ. Manag. 2020, 258, 110039. [Google Scholar] [CrossRef]
- Cisternas, L.; Moreno, L. Seawater in Mining: Fundamentals and Applications; Ril Editores: Santiago, Chile, 2014; pp. 1–234. (In Spanish) [Google Scholar]
- Cisternas, L.; Gálvez, E.; Leyton, Y.; Valderrama, J. Seawater in Atacama: Opportunities and Advances for the Sustainable Use of Seawater in Mining; Ril Editores: Santiago, Chile, 2016; pp. 1–248. (In Spanish) [Google Scholar]
- Cruz, C.; Ramos, J.; Robles, P.; Leiva, W.H.; Jeldres, R.I.; Cisternas, L.A. Partial Seawater Desalination Treatment for Improving Chalcopyrite Floatability and Tailing Flocculation with Clay Content. Miner. Eng. 2020, 151, 1–7. [Google Scholar] [CrossRef]
- Castro, S. Physico-Chemical Factors in Flotation of Cu-Mo-Fe Ores with Seawater: A Critical Review. Physicochem. Probl. Miner. Process. 2018, 54, 1223–1236. [Google Scholar]
- Jeldres, R.I.; Forbes, L.; Cisternas, L.A. Effect of Seawater on Sulfide Ore Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2016, 37, 369–384. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y. The Effect of Saline Water on Mineral Flotation—A Critical Review. Miner. Eng. 2014, 66, 13–24. [Google Scholar] [CrossRef]
- Chilean Copper Commission (Cochilco). Consumo de Agua En La Minería Del Cobre Al 2019 (Water Consumption in Copper Mining by 2019). Available online: https://www.cochilco.cl/Listado%20Temtico/2020%2010%2030%20Consumo%20de%20agua%20en%20la%20mineria%20del%20cobre%20al%202019_version%20final.pdf (accessed on 25 June 2021).
- Herrera-Leon, S.; Cruz, C.; Kraslawski, A.; Cisternas, L.A. Others Current Situation and Major Challenges of Desalination in Chile. Desalin. Water Treat 2019, 171, 93–104. [Google Scholar] [CrossRef]
- Missimer, T.M.; Maliva, R.G. Environmental Issues in Seawater Reverse Osmosis Desalination: Intakes and Outfalls. Desalination 2018, 434, 198–215. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The State of Desalination and Brine Production: A Global Outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Sola, I.; Fernández-Torquemada, Y.; Forcada, A.; Valle, C.; del Pilar-Ruso, Y.; González-Correa, J.M.; Sánchez-Lizaso, J.L. Sustainable Desalination: Long-Term Monitoring of Brine Discharge in the Marine Environment. Mar. Pollut. Bull. 2020, 161, 111813. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torquemada, Y.; Carratalá, A.; Sánchez Lizaso, J.L. Others Impact of Brine on the Marine Environment and How It Can Be Reduced. Desalin. Water Treat. 2019, 167, 27–37. [Google Scholar] [CrossRef]
- Grossowicz, M.; Ofir, E.; Shabtay, A.; Wood, J.; Biton, E.; Belkin, N.; Frid, O.; Sisma-Ventura, G.; Kress, N.; Berman-Frank, I.; et al. Modeling the Effects of Brine Outflow from Desalination Plants on Coastal Food-Webs of the Levantine Basin (Eastern Mediterranean Sea). Desalination 2020, 496, 114757. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental Impact and Impact Assessment of Seawater Desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Petersen, K.L.; Frank, H.; Paytan, A.; Bar-Zeev, E. Impacts of Seawater Desalination on Coastal Environments. In Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 437–463. [Google Scholar]
- Zarzo, D. Beneficial Uses and Valorization of Reverse Osmosis Brines. In Emerging Technologies for Sustainable Desalination Handbook; Elsevier: Amsterdam, The Netherlands, 2018; pp. 365–397. [Google Scholar]
- Ayoub, G.M.; Zayyat, R.M.; Al-Hindi, M. Precipitation Softening: A Pretreatment Process for Seawater Desalination. Environ. Sci. Pollut. Res. 2014, 21, 2876–2887. [Google Scholar] [CrossRef] [PubMed]
- Casas, S.; Aladjem, C.; Larrotcha, E.; Gibert, O.; Valderrama, C.; Cortina, J.L. Valorisation of Ca and Mg By-Products from Mining and Seawater Desalination Brines for Water Treatment Applications. J. Chem. Technol. Biotechnol. 2014, 89, 872–883. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Uribe, L.; Cisternas, L.A.; Gutierrez, L.; Leiva, W.H.; Valenzuela, J. The Effect of Clay Minerals on the Process of Flotation of Copper Ores-A Critical Review. Appl. Clay Sci. 2019, 170, 57–69. [Google Scholar] [CrossRef]
- Uribe, L.; Gutierrez, L.; Laskowski, J.S.; Castro, S. Role of Calcium and Magnesium Cations in the Interactions between Kaolinite and Chalcopyrite in Seawater. Physicochem. Probl. Miner. Process. 2017, 53, 737–749. [Google Scholar]
- Yepsen, R.; Gutierrez, L.; Laskowski, J. Flotation Behavior of Enargite in the Process of Flotation Using Seawater. Miner. Eng. 2019, 142, 105897. [Google Scholar] [CrossRef]
- Hu, P.; Li, Q.; Liang, L. A Review of Characterization Techniques of Heterocoagulation between Mineral Particles in Mineral Separation Process. Sep. Purif. Technol. 2021, 279, 119699. [Google Scholar] [CrossRef]


| Ions | Value (mg/L) | Ions | Value (mg/L) |
|---|---|---|---|
| Chloride | 24,231 | Potassium | 499 |
| Sodium | 14,174 | Bicarbonate | 133 |
| Sulphate | 3486 | Nitrate | 3.94 |
| Magnesium | 1693 | Fluoride | 0.98 |
| Calcium | 553 | Nitrite | <0.1 |
| Type of Brine | PH | Magnesium (mg/L) | Calcium (mg/L) |
|---|---|---|---|
| Waste brine | 8.00 | 1693 | 553 |
| Treated waste brine 1 | 11.90 | 0.15 | 420.80 |
| Treated waste brine 2 | 11.15 | 45.75 | 472.50 |
| Treated waste brine 3 | 11.15 | 19.80 | 497.50 |
| Water Quality | Cu (%) | Separation Efficiency (%) | Enrichment Ratio |
|---|---|---|---|
| Waste brine | 80.80 | 19.00 | 1.3 |
| Seawater | 92.40 | 21.40 | 1.3 |
| Treated waste brine | 94.70 | 48.70 | 2.1 |
| Tap water | 97.70 | 45.80 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, C.; Herrera-León, S.; Calisaya-Azpilcueta, D.; Salazar, R.; Cisternas, L.A.; Kraslawski, A. Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry. Minerals 2022, 12, 1162. https://doi.org/10.3390/min12091162
Cruz C, Herrera-León S, Calisaya-Azpilcueta D, Salazar R, Cisternas LA, Kraslawski A. Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry. Minerals. 2022; 12(9):1162. https://doi.org/10.3390/min12091162
Chicago/Turabian StyleCruz, Constanza, Sebastián Herrera-León, Daniel Calisaya-Azpilcueta, Ruth Salazar, Luis A. Cisternas, and Andrzej Kraslawski. 2022. "Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry" Minerals 12, no. 9: 1162. https://doi.org/10.3390/min12091162
APA StyleCruz, C., Herrera-León, S., Calisaya-Azpilcueta, D., Salazar, R., Cisternas, L. A., & Kraslawski, A. (2022). Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry. Minerals, 12(9), 1162. https://doi.org/10.3390/min12091162







