Removal Mechanism of Mineral Impurities in Molybdenum Concentrate Treatment Process
Abstract
:1. Introduction
2. Experimental Method
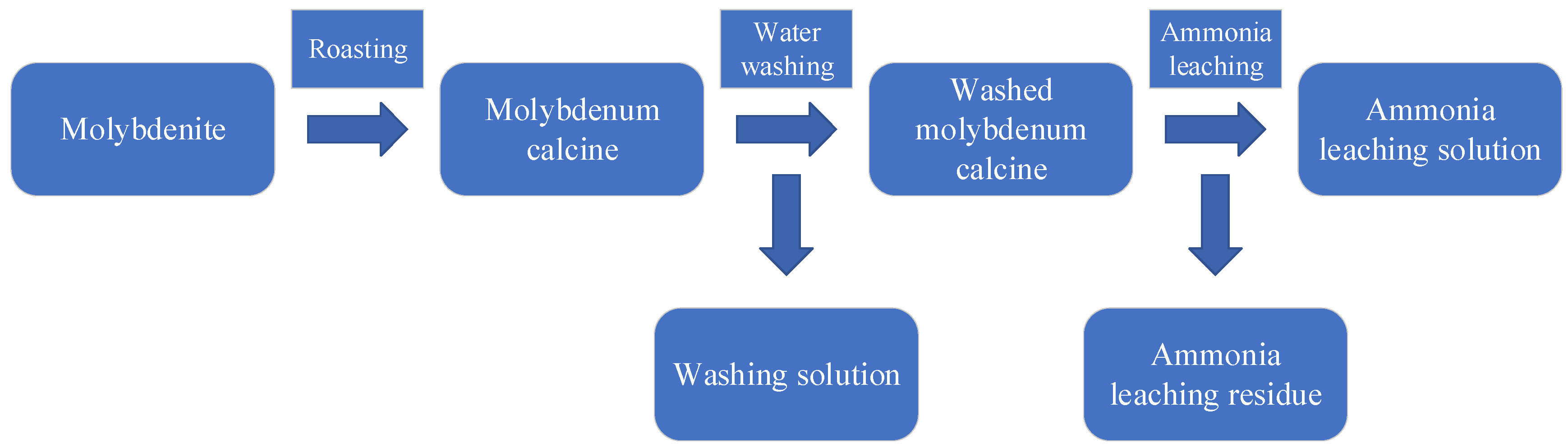
2.1. Experiment of Roasting-Water Washing-Ammonia Leaching
2.2. Chemical Composition Analysis of Different Products
2.3. Phase Analysis of Different Products
3. Results and Discussion
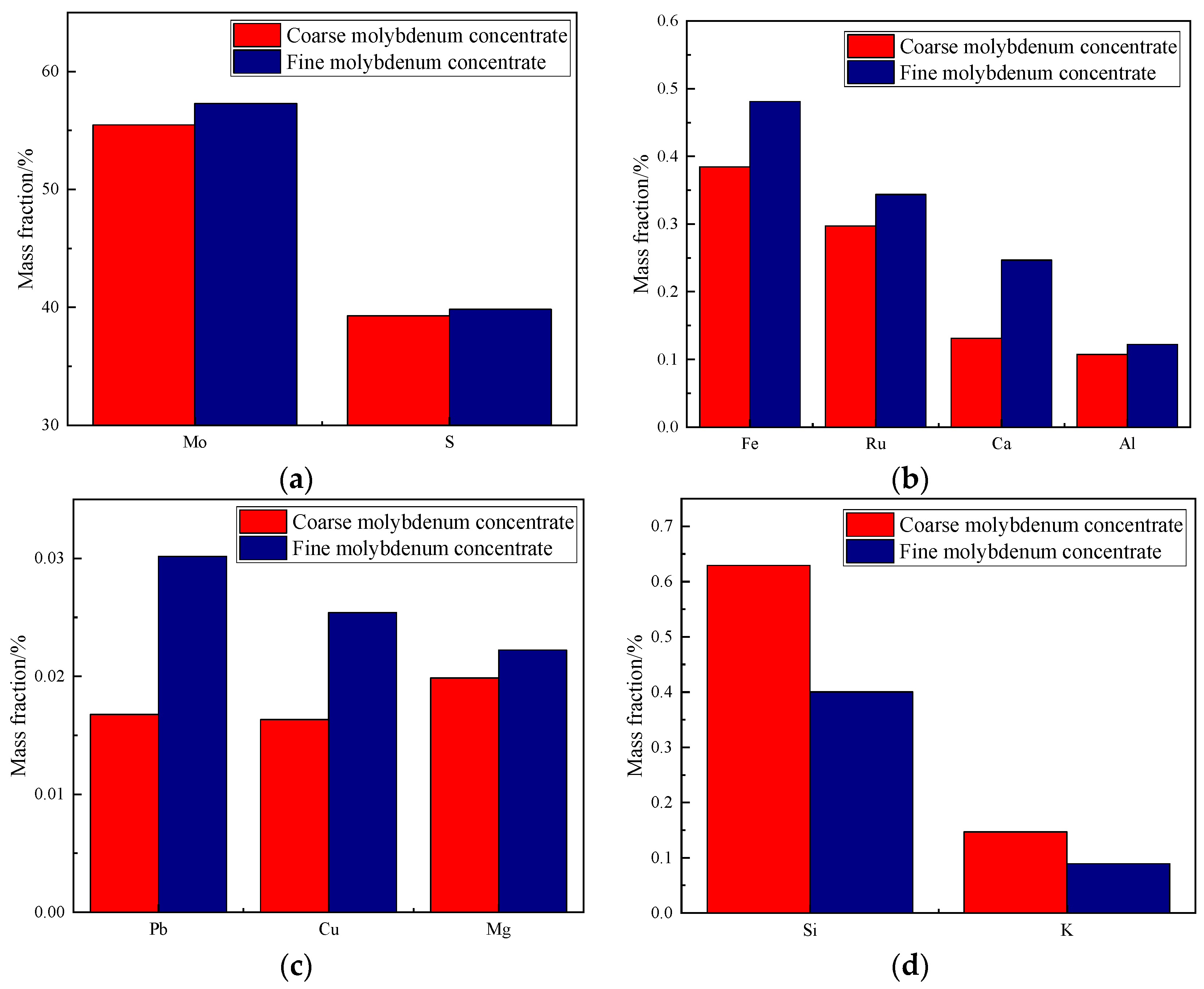
3.1. Main Elements in Molybdenum Concentrates with Different Particle Sizes
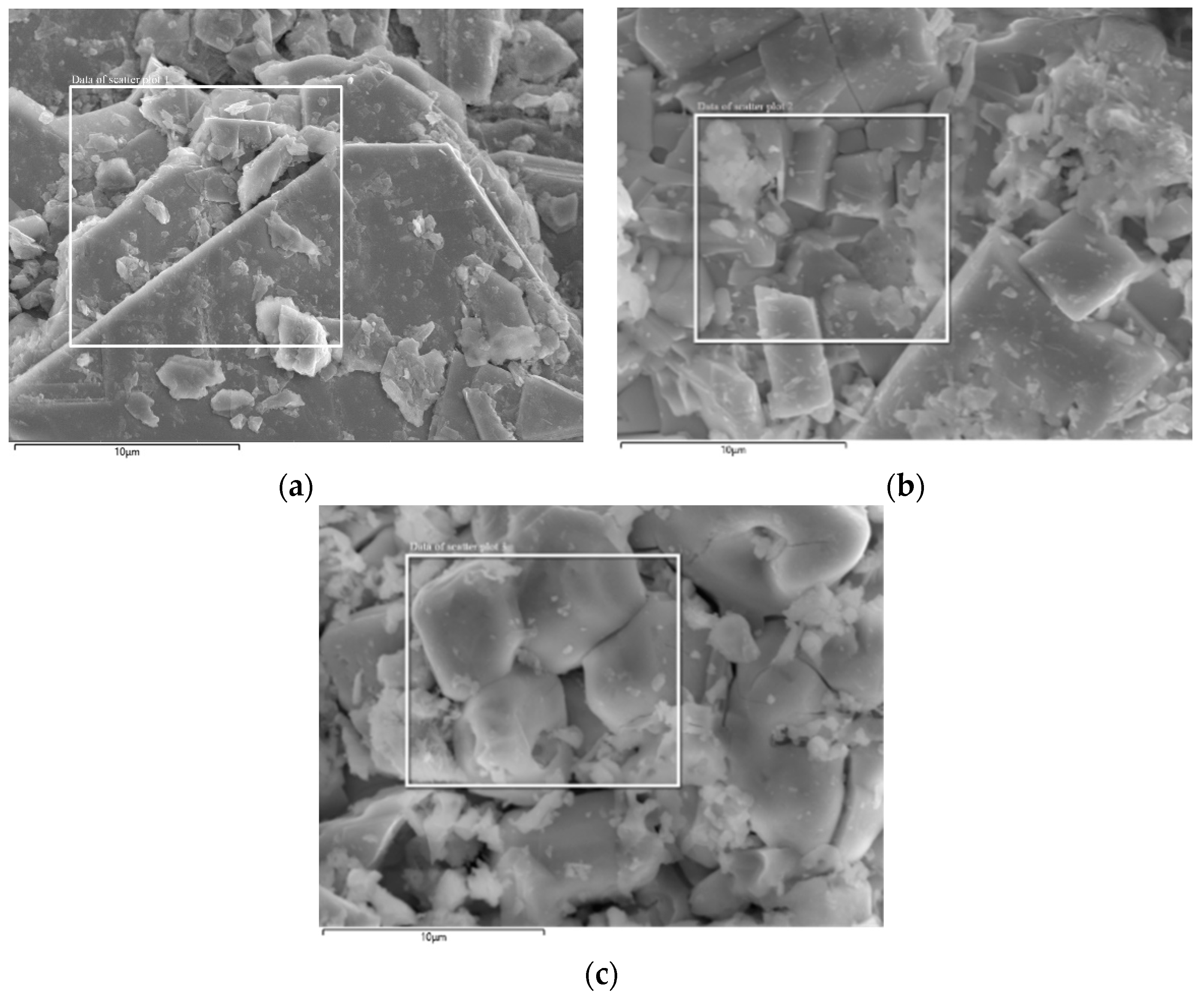
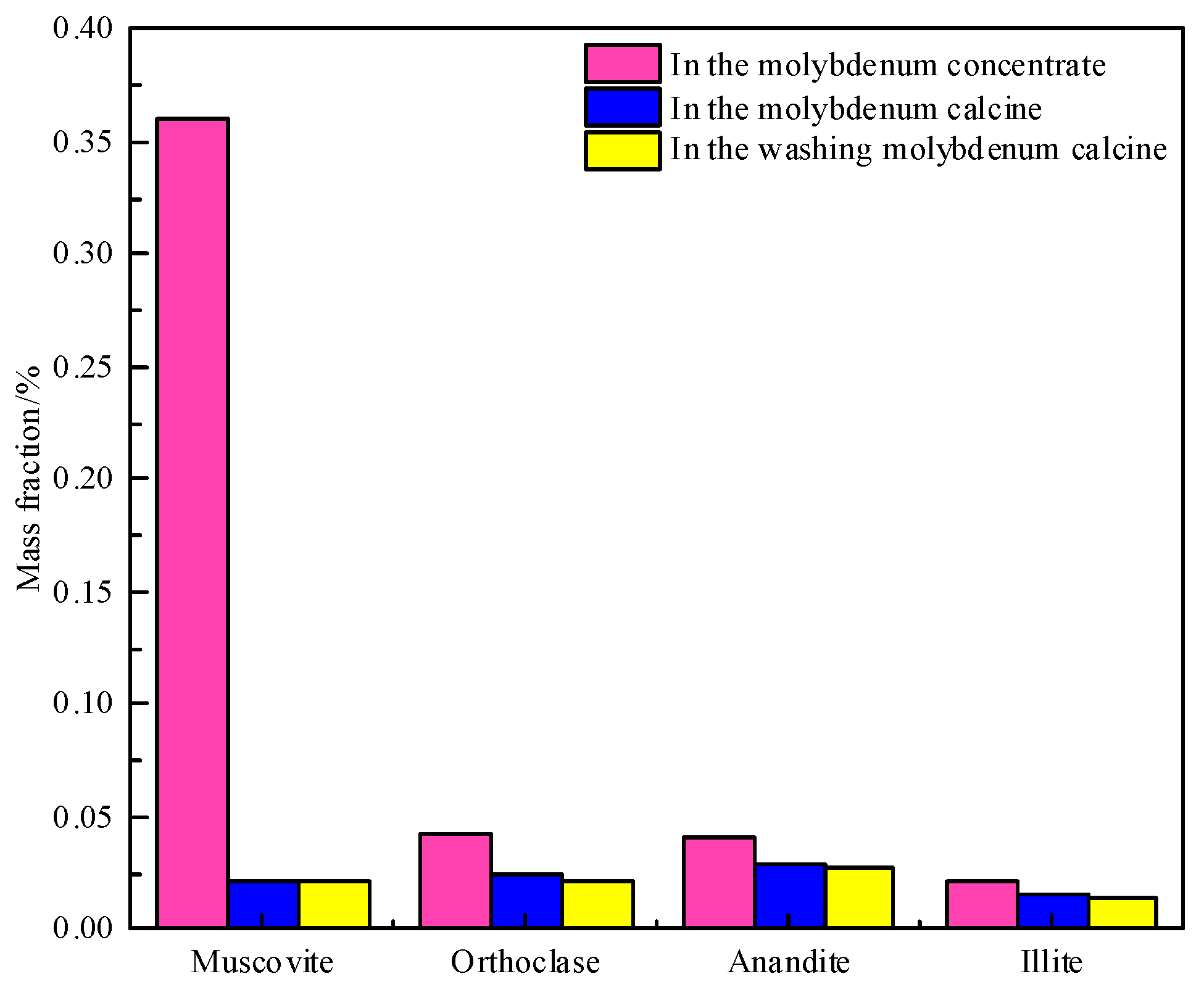
3.2. Speciation and Phase Analysis of Impurity Elements
3.3. Transformation Mechanism of Impurities during Roasting Process
3.4. Effect of Humidification on the Removal of Impurity Elements
3.5. Influence Mechanism of Impurities on Molybdenum Leaching
3.6. Effect of Particle Size on Leaching of Molybdenum and Potassium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, C.H.; Wang, H.H.; Zhang, L.Z.; Guo, M.C.; Feng, B. Flotation separation of molybdenite and talc by xanthan gum. Powder Technol. 2021, 388, 158–165. [Google Scholar] [CrossRef]
- Sheybani, K.; Paydar, M.H.; Shariat, M.H. Effect of mechanical activation on aluminothermic reduction of molybdenum trioxide. Int. J. Refract. Hard Met. 2019, 82, 245–254. [Google Scholar] [CrossRef]
- Shafiei Bafti, B.; Svojtka, M.; Abdolahi, M. Geochemistry of Rhenium and Other Trace Elements in Molybdenite; SarCheshmeh Porphyry Cu-Mo Deposit, Iran. Acta Geol. Sin. Engl. 2021, 95, 19. [Google Scholar] [CrossRef]
- Gan, M.; Fan, X.; Chen, X.; Wu, C. Reaction mechanisms of low-grade molybdenum concentrate during calcification roasting process. Trans. Nonferrous Met. Soc. China 2016, 26, 3015–3023. [Google Scholar] [CrossRef]
- Tyagi, P.; Choudhary, S. Tuning the electronic and optical properties of molybdenite (MoS2) by adsorption of alkali metals and halogens. Opt. Mater. 2021, 118, 111248.1–111248.8. [Google Scholar] [CrossRef]
- Keilholz, S.; Kohlmann, H.; Uhlenhut, H.; Gabke, A.; García-Schollenbruch, M. In situ X-ray diffraction studies on the production process of molybdenum. Inorg. Chem. 2022, 61, 10126–10132. [Google Scholar] [CrossRef]
- Zhu, Q.; Xie, M.X.; Shang, X.T.; An, G.; Sun, J.; Wang, N.; Xi, S.; Bu, C.Y.; Zhang, J.P. Research Status and Progress of Welding Technologies for Molybdenum and Molybdenum Alloys. Metals 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Gui, D.; Hua, Y.N.; Xing, Z.X.; Zhao, S.P. Investigation of Potassium Contamination in SOI Wafer Using Dynamic SIMS. IEEE Trans. Device Mater. Reliab. 2007, 7, 369–372. [Google Scholar] [CrossRef]
- Hung, H.Y.; Lu, C.W.; Lee, C.Y.; Hsu, C.S.; Hsieh, Y.Z. Analysis of metal ion impurities in liquid crystals using high resolution inductively coupled plasma mass spectrometry. Anal. Methods 2012, 4, 3631–3637. [Google Scholar] [CrossRef]
- Shekhter, L.N.; Litz, J.E.; Shan, N.M.; Mchugh, L.F. Thermodynamic modelling of molybdenite roasting in a multiple-hearth furnace. JOM 2021, 73, 873–880. [Google Scholar] [CrossRef]
- Kan, S.; Benzeik, K.; Odabas, Ö.C.; Yvcel, O. Investigation of molybdenite Concentrate Roasting in Chamber and Rotary Furnaces. Min. Met. Explor. 2021, 38, 1597–1608. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, J.; Chang, Q.; Ma, D. Research progress of preparation technology of molybdenum powder. Trans. Mater. Heat Treat. 2020, 41, 14–24. (In Chinese) [Google Scholar]
- Zhang, M.P.; Liu, C.H.; Zhu, X.J.; Xiong, H.B.; Zhang, L.B.; Gao, J.Y.; Liu, M.H. Preparation of ammonium molybdate by oxidation roasting of molybdenum concentrate: A comparison of microwave roasting and conventional roasting. Chem. Eng. Process 2021, 167, 108550. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Z.F.; Yang, J.; Cao, L.M.; Zhang, W. Recovery of Mo and Ni from spent acrylonitrile catalysts using an oxidation leaching–chemical precipitation technique. Hydrometallurgy 2016, 164, 64–70. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, L.; Wang, G.H.; Qin, M.L.; Qu, X.H.; So, K.P. Influence of impurities on hot-rolled molybdenum for high temperature applications. Int. J. Refract. Met. Hard Mater. 2020, 92, 105294. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Z.; Zhao, Y.; Ding, W.; Zhang, Y.; Huang, W. Preparation of high purity molybdenum disulfide by one stage purification from molybdenum concentrate. Chem. Eng. 2018, 32, 11–13. (In Chinese) [Google Scholar]
- Gao, Z.; Xie, X.; Hui, S. New method for producing impurity of molybdenum disulfide. China Molybdenum Ind. 2019, 43, 42–45. (In Chinese) [Google Scholar]
- Aracena, A.; Sanino, A.; Jerez, O. Dissolution kinetics of molybdite in KOH media at different temperatures. Trans. Nonferrous Met. Soc. China 2018, 28, 177–185. [Google Scholar] [CrossRef]
- Yang, L.Q.; Li, X.B.; Qi, T.G.; Liu, G.H.; Peng, Z.H.; Zhou, Q.S. Direct synthesis of pure ammonium molybdates from ammonium tetramolybdate and ammonium bicarbonate. ACS Sustain. Chem. Eng. 2020, 8, 18237–18244. [Google Scholar] [CrossRef]
- Tang, L.; Li, X.; Liu, D. Soluble potassium change mechanism analysis in the process of roasting molybdenum concentrate mineral. China Molybdenum Ind. 2016, 40, 42–43+49. (In Chinese) [Google Scholar]
- Yi, G.S.; Macha, E.; Dyke, J.V.; Macha, R.; Mckay, T.; Free, M.L. Recent progress on research of molybdenite flotation: A review. Adv. Colloid Interface Sci. 2021, 295, 102466. [Google Scholar] [CrossRef] [PubMed]
- Shalchian, H.; Khaki, J.V.; Babakhani, A.; Michelis, I.D.; Veglio, F.; Parizi, M.T. An enhanced dissolution rate of molybdenite and variable activation energy. Hydrometallurgy 2018, 175, 52–63. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Dang, J.; Chou, K.C. Oxidation roasting of molybdenite concentrate. Trans. Nonferrous Met. Soc. China 2015, 25, 4167–4174. [Google Scholar] [CrossRef]
- Tumen-Ulzii, N.; Batnasan, A.; Gunchin, B. Selective dissolution of copper and iron from molybdenite concentrate using acidic sodium nitrate solution. Miner. Eng. 2022, 185, 107715. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.H.; Wang, J.S.; Chou, K.C. Influences of different components on agglomeration behavior of MoS2 during oxidation roasting process in air. Metall. Mater. Trans. B 2016, 47, 2421–2432. [Google Scholar] [CrossRef]
- Popov, V.V.; Menushenkov, A.P.; Yastrebtsev, A.A.; Molokova, A.Y.; Pisarev, A.A.; Khramov, E.V.; Zubavichus, Y.V.; Shchetinin, I.V.; Ponkratov, K.V.; Tsarenko, N.A.; et al. The synthesis and studies of crystal/local structures and morphology of hydrated molybdenum oxides. J. Solid State Chem. 2021, 301, 122356. [Google Scholar] [CrossRef]
- Camalan, M.; Cavur, M. Development of a supervised classification method to construct 2D mineral maps on backscattered electron images. Turk. J. Electr. Eng. Comput. Sci. 2019, 28, 1030–1043. [Google Scholar] [CrossRef]
- Li, X.B.; Wu, T.; Zhou, Q.S.; Qi, T.G.; Peng, Z.H.; Liu, G.H. Kinetics of oxidation roasting of molybdenite with different particle sizes. Trans. Nonferrous Met. Soc. China. 2021, 31, 842–852. [Google Scholar] [CrossRef]
- Lv, L.; Li, C.; Zhang, G.H.; Hu, X.W.; Liang, B. Decomposition behavior of CaSO4 during potassium extraction from a potash feldspar-CaSO4 binary system by calcination. Chin. J. Chem. Eng. 2018, 26, 838–844. [Google Scholar] [CrossRef]
- Massonne, H.J.; Fockenberg, T. Melting of phengite-bearing eclogite at pressures of 4 and 9 GPa relevant to deep regions of a subduction zone. Earth Planet. Sci. Lett. 2022, 584, 117475. [Google Scholar] [CrossRef]
- Jena, S.K.; Mohanty, B.; Padhy, G.; Sahu, J.; Kandi, S.K. Potassium recovery from muscovite using NaCl-roasting followed by H2SO4-leaching. J. Cent. South Univ. 2022, 29, 1881–1894. [Google Scholar] [CrossRef]
- Yuan, J.Y.; Yang, J.; Ma, H.W.; Su, S.Q.; Chang, Q.Q.; Komarneni, S. Hydrothermal Synthesis of nano-kaolinite from K-feldspar. Ceram. Int. 2018, 44, 15611–15617. [Google Scholar] [CrossRef]
- Salimkhani, H.; Joodi, T.; Bordbar-Khiabani, A.; Dizaji, A.M.; Abdolalipour, B.; Azizi, A. Surface and structure characteristics of commercial K-Feldspar powders: Effects of temperature and leaching media. Chin. J. Chem. Eng. 2020, 28, 307–317. [Google Scholar] [CrossRef]
- Liu, C.J.; Ma, H.W. Hydrothermal decomposition process of K-feldspar in NaOH solution. Asia-Pac. J. Chem. Eng. 2020, 16, e2578. [Google Scholar] [CrossRef]
- Liu, J.N.; Zhai, Y.C.; Wu, Y.; Zhang, J.; Shen, X.Y. Kinetics of roasting potash feldspar in presence of sodium carbonate. J. Cent. South Univ. 2017, 24, 1544–1550. [Google Scholar] [CrossRef]
- Ma, J.Y.; Zhang, Y.F.; Qin, Y.H.; Wu, Z.K.; Wang, T.L.; Wang, C.W. The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound. Ultrason. Sonochem. 2017, 35, 304–312. [Google Scholar] [CrossRef]
- Wang, L.; Li, M.; Que, B.; Xue, Z.; Zhang, G.; Lan, W. Oxidation roasting of molybdenite concentrate. Chin. J. Nonferr. Metal. 2021, 31, 1952–1964. (In Chinese) [Google Scholar] [CrossRef]
- Gomes, T.; Rosa, R.; Cargnin, M.; Quadri, M.B.; Peterson, M.; Oliveira, C.M.; RosaRabelo, N.; Angioletto, E. Pyrite roasting in modified fluidized bed: Experimental and modeling analysis. Chem. Eng. Sci. 2022, 261, 117977. [Google Scholar] [CrossRef]
- Aracena, A.; Jerez, O.; Ortiz, R.; Morales, J. Pyrite oxidation kinetics in an oxygen–nitrogen atmosphere at temperatures from 400 to 500 °C. Can. Metall. Q. 2016, 55, 195–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.L.; Xu, B.; Yang, Y.B.; Jiang, T. A Thermodynamic Analysis on the Roasting of Pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef]


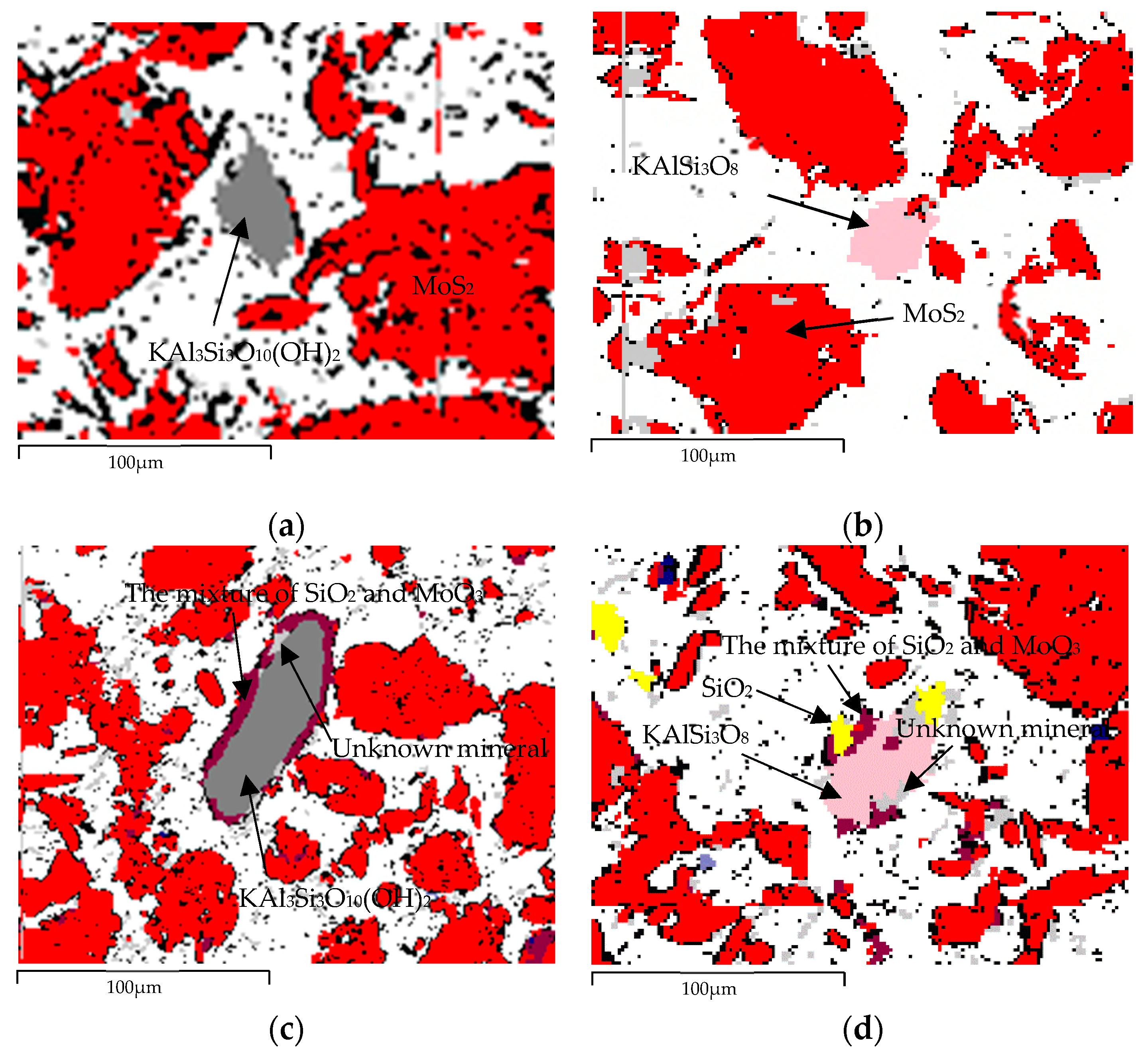
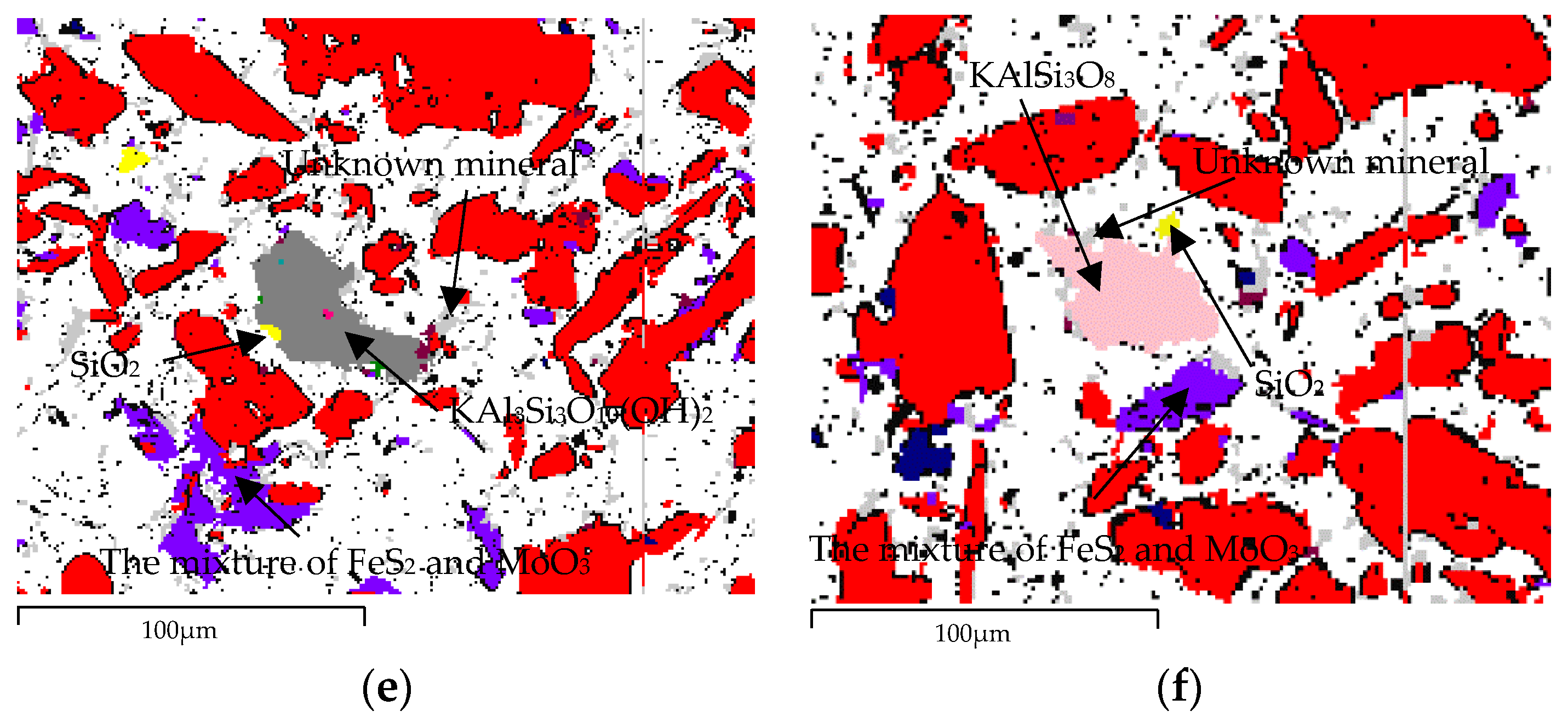

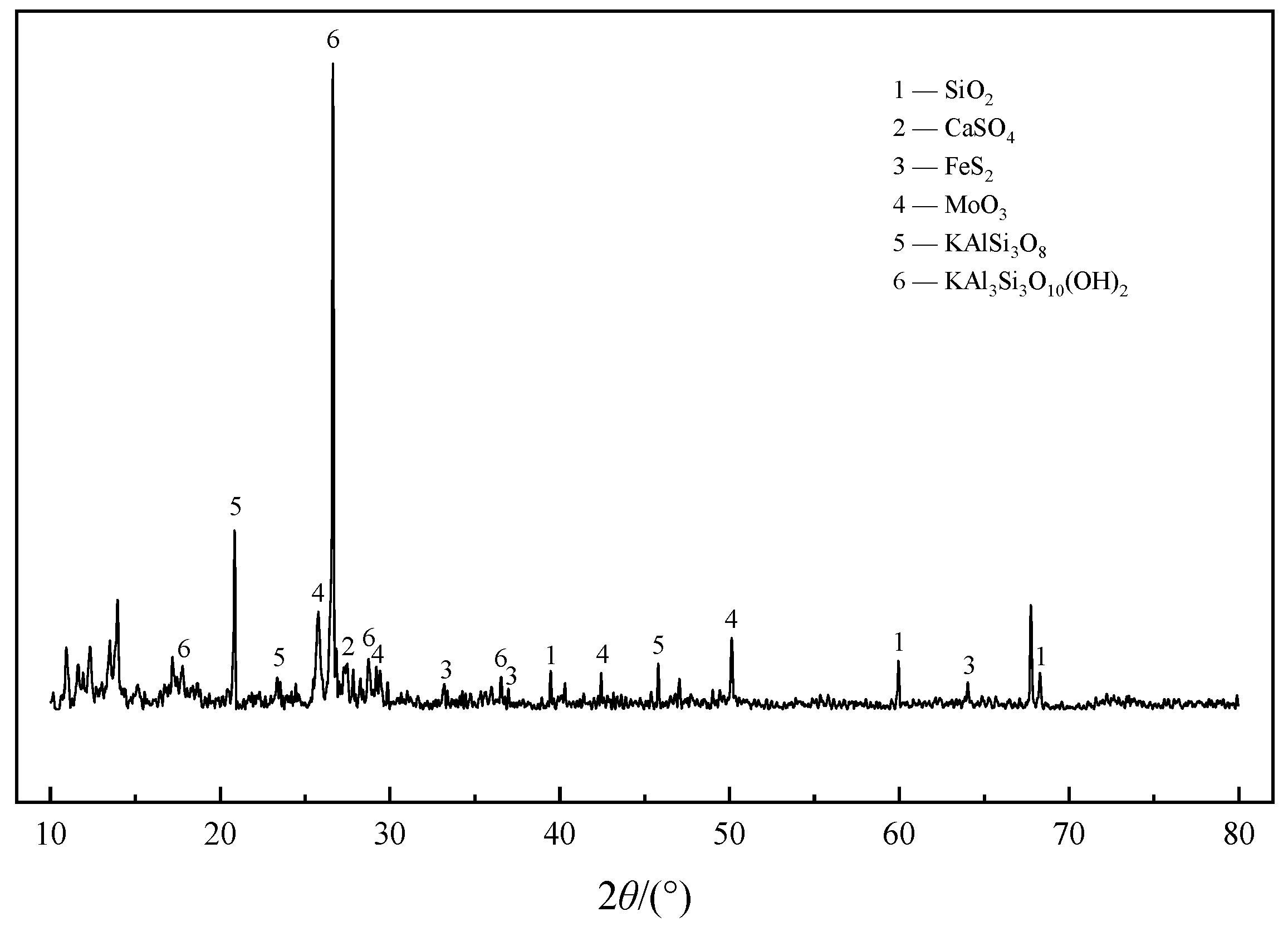
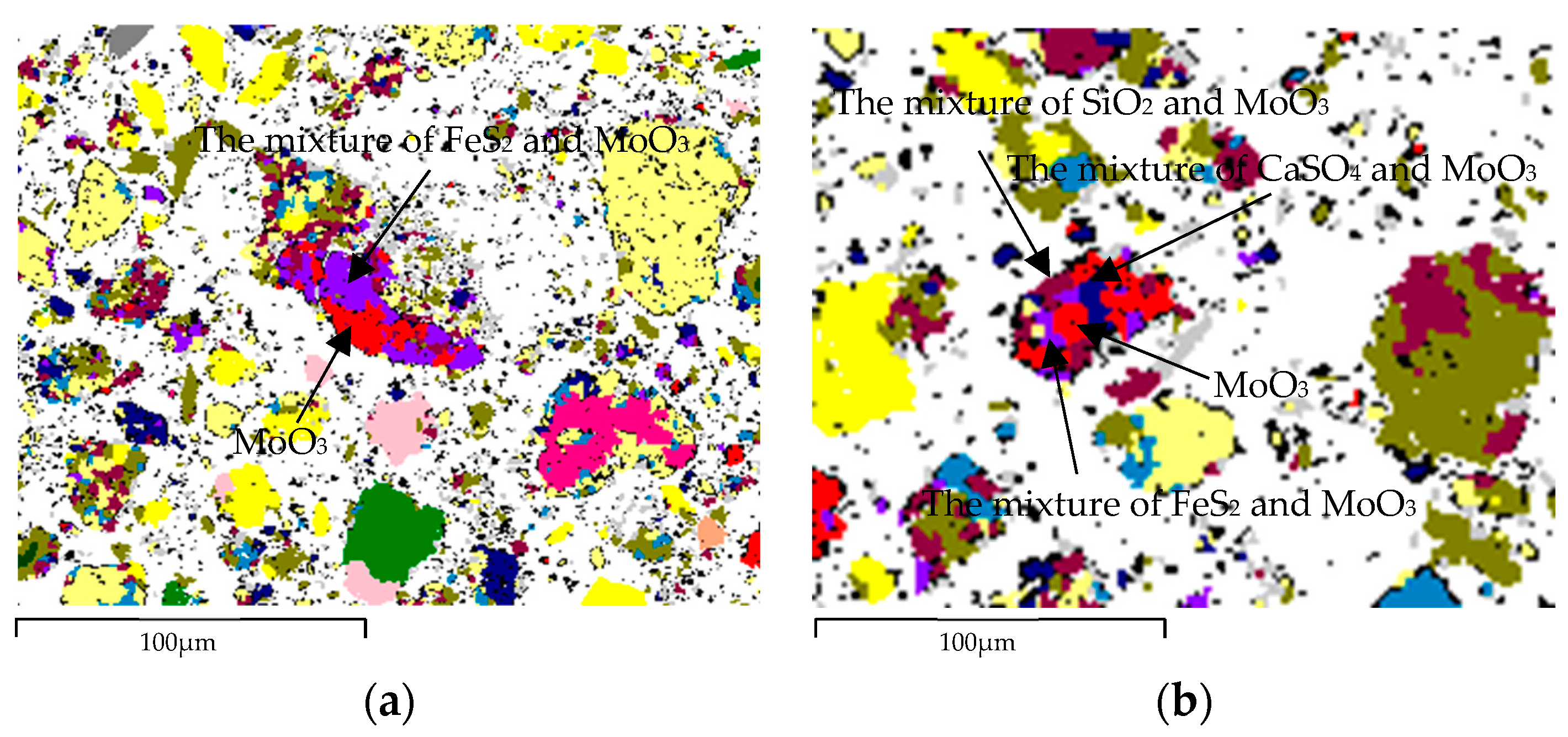
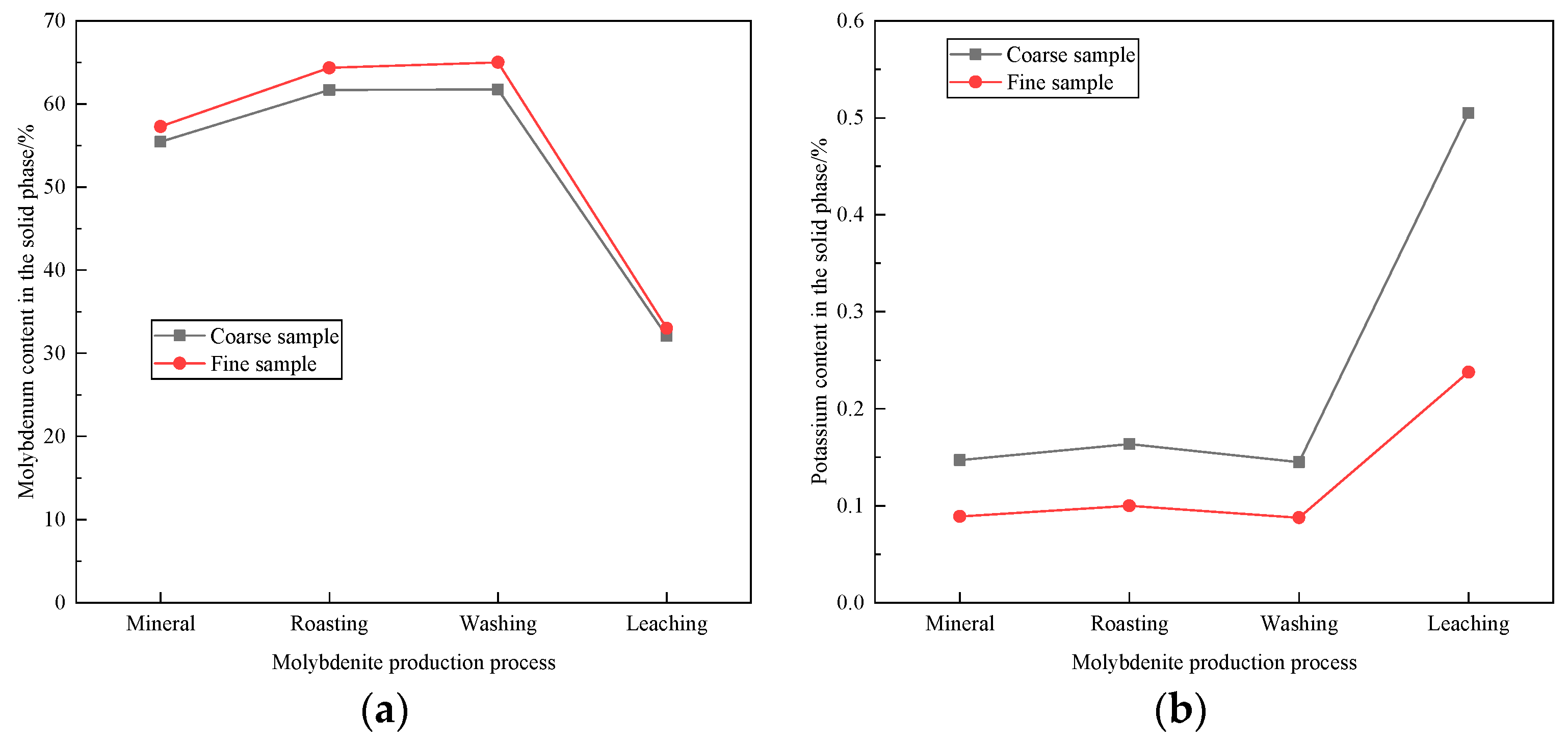
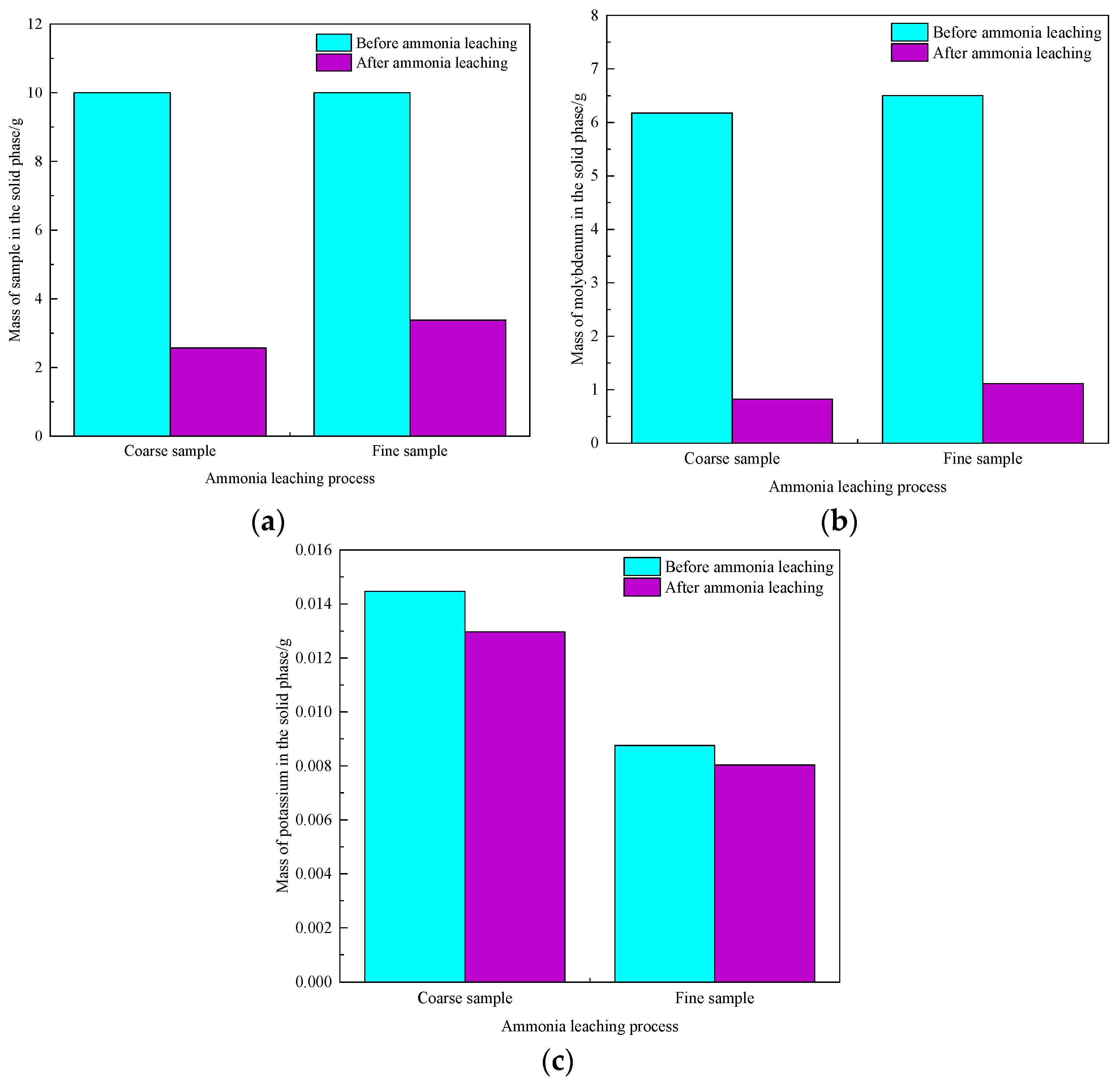
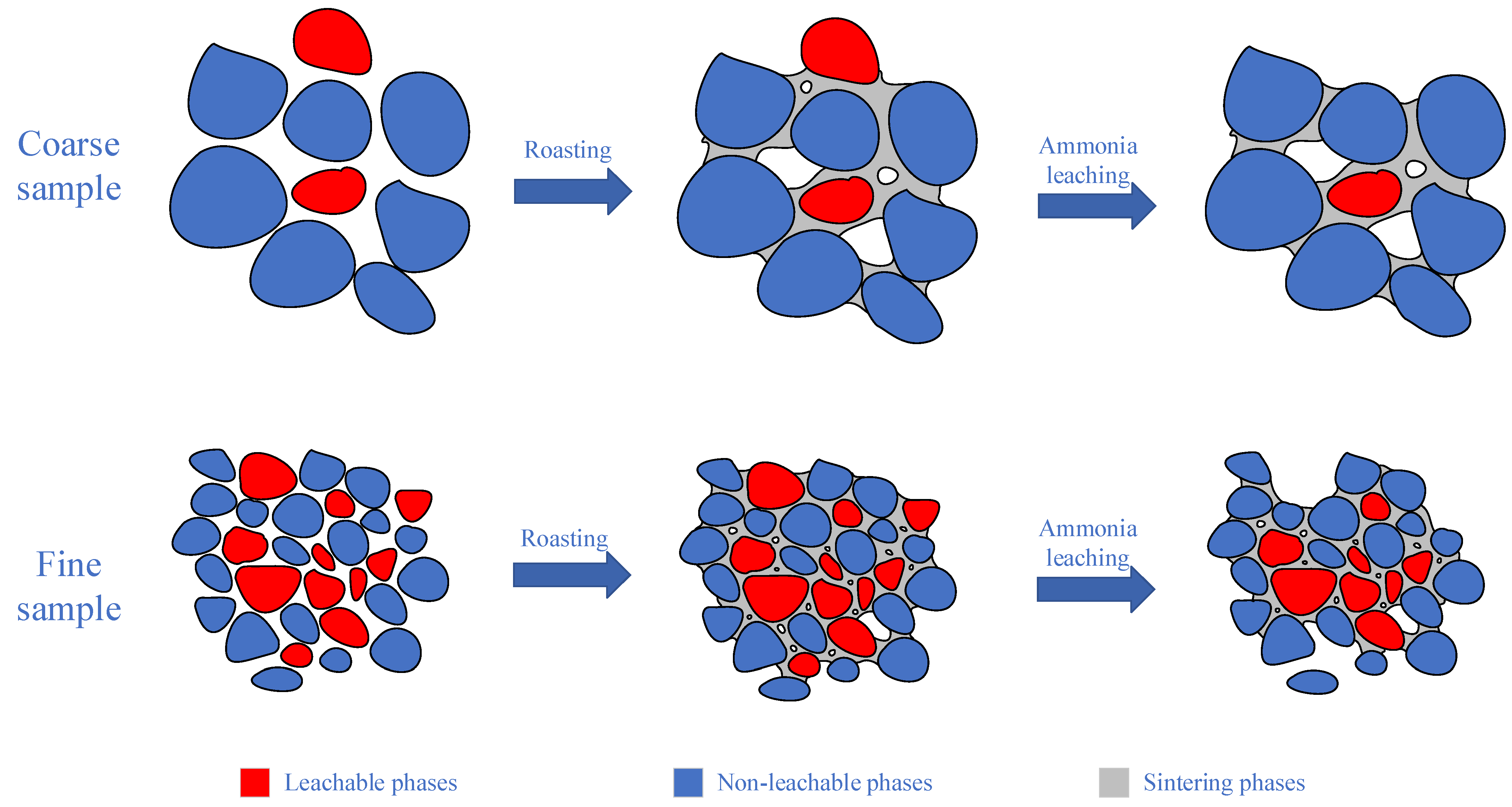
| Element | Molybdenum Concentrate | Molybdenum Calcine | Ammonia Leaching Residue |
|---|---|---|---|
| O | 1.49 | 40.54 | 41.49 |
| Al | 0.13 | 0.14 | 2.79 |
| S | 40.67 | 1.07 | 0.86 |
| K | 0.13 | 0.15 | 0.08 |
| Fe | 0.46 | 0.48 | 20.17 |
| Mo | 56.44 | 56.91 | 12.24 |
| Si | 0.68 | 0.71 | 22.37 |
| Name | Chemical Formula | Percentage of Weight/% | Percentage of Area/% | Area/μm2 | Number of Mineral Particles |
|---|---|---|---|---|---|
| Iron | Fe | 0.02 | 0.01 | 479.27 | 1 |
| Rutile | TiO2 | 0.01 | 0.01 | 260.85 | 3 |
| Calcite | CaCO3 | 0.01 | 0.01 | 419.36 | 9 |
| Chlorite | Fe2+3Mg1.5AlFe3+0.5Si3AlO12(OH)6 | 0.02 | 0.04 | 1092.09 | 16 |
| Hematite | Fe2O3 | 0.04 | 0.03 | 999.73 | 18 |
| Kaolinite | Al2Si2O5(OH)4 | 0.04 | 0.06 | 1699.92 | 16 |
| Illite | K0.6(H3O)0.4Al1.3Mg0.3Fe2+0.1Si3.5O10(OH) | 0.02 | 0.04 | 1102.08 | 18 |
| Fluorite | CaF2 | 0.08 | 0.13 | 3774.28 | 19 |
| Orthoclase | KAlSi3O8 | 0.04 | 0.07 | 2045.65 | 20 |
| Chalcopyrite | CuFeS2 | 0.04 | 0.04 | 1253.1 | 20 |
| Anandite | Ba0.75K0.25Fe2+2.25Mg0.75Si3Al0.7Fe3+0.3O10S1.5(OH)0.5 | 0.04 | 0.04 | 1190.69 | 26 |
| Minium | Pb2PbO4 | 0.11 | 0.07 | 2020.69 | 58 |
| Muscovite | KAl3Si3O10(OH)1.8F0.2 | 0.36 | 0.63 | 18,799 | 102 |
| Quartz | SiO2 | 0.27 | 0.51 | 15,371.7 | 93 |
| Anhydrite | CaSO4 | 0.15 | 0.25 | 7366.33 | 144 |
| Pyrite | FeS2 | 1.23 | 0.21 | 36,359.88 | 297 |
| Molybdenite | MoS2 | 95.57 | 85.61 | 2,554,936.8 | 25,206 |
| Unknown mineral | 1.95 | 4.81 | 143,533.6 | 15,992 |
| Ion Name | Washed Sample | Concentration/(mmol·L−1) |
|---|---|---|
| K+ | Molybdenite concentrate | 0.0317 |
| K+ | Molybdenum calcine | 0.2859 |
| Al3+ | Molybdenum calcine | 0.7297 |
| SO42- | Molybdenum calcine | 1.1049 |
| Element | Al | K | S | Si | Fe | Mo | Ca |
|---|---|---|---|---|---|---|---|
| Humidified | 0.09 | 0.12 | 1.06 | 1.09 | 0.98 | 56.4 | 0.23 |
| Non-humidified | 0.14 | 0.15 | 1.08 | 1.12 | 1.04 | 56.7 | 0.25 |
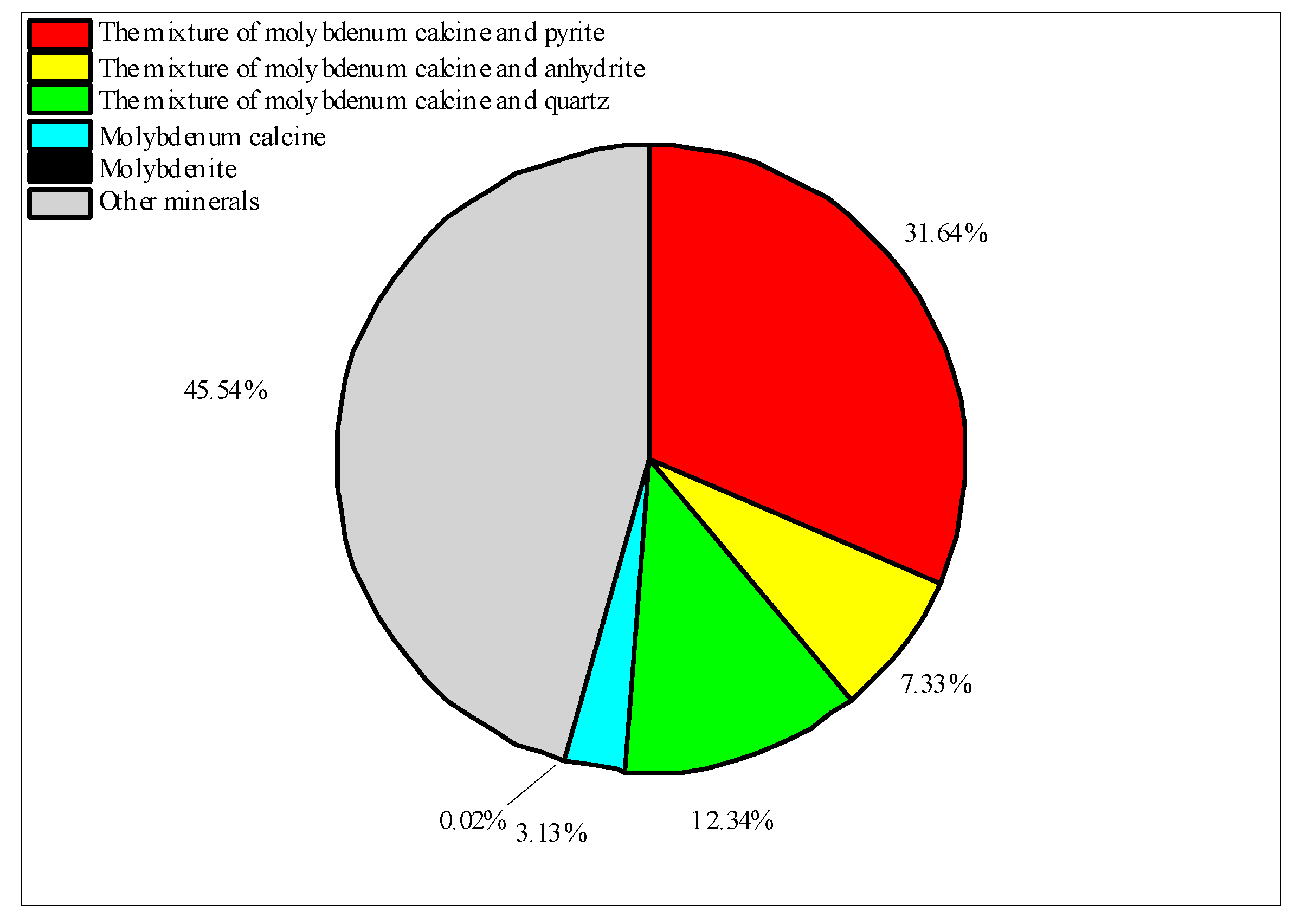
| Name | Chemical Formula | Percentage of Weight/% (Humidified) | Percentage of Weight/% (Non-Humidified) |
|---|---|---|---|
| Molybdenum Oxide | MoO3 | 86.43 | 86.80 |
| Orthoclase | KAlSi3O8 | 0.03 | 0.17 |
| Rutile | TiO2 | 0.01 | 0.01 |
| Corundum | Al2O3 | 0.38 | 0.52 |
| Hematite | Fe2O3 | 0.29 | 0.48 |
| Ferrotschermakite | Ca2MgFe2Al3FeSi6O22(OH)2 | 0.01 | 0.07 |
| Illite | K0.6(H3O)0.4Al1.3Mg0.3Fe2+0.1Si3.5O10(OH) | 0.01 | 0.02 |
| Fluorite | CaF2 | 0.07 | 0.09 |
| Ferrimolybdite | Fe3+2(MoO4)3·n(H2O) | 0.75 | 1.11 |
| Anandite | Ba0.75K0.25Fe2+2.25Mg0.75Si3Al0.7Fe3+0.3O10S1.5(OH)0.5 | 0.44 | 0.46 |
| Muscovite | KAl3Si3O10(OH)1.8F0.2 | 0.05 | 0.10 |
| Quartz | SiO2 | 1.12 | 1.67 |
| Molybdenite | MoS2 | 0.03 | 0.02 |
| Mixture of molybdenum oxide and pyrite | MoO3, FeS2 | 1.81 | 1.61 |
| Mixture of molybdenum oxide and anhydrite | MoO3, CaSO4 | 0.95 | 0.83 |
| Mixture of molybdenum oxide and quartz | MoO3, SiO2 | 1.66 | 1.53 |
| Mixture of quartz and pyrite | SiO2, FeS2 | 0.37 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, R.; Yang, S.; Feng, W.; Wang, M. Removal Mechanism of Mineral Impurities in Molybdenum Concentrate Treatment Process. Minerals 2023, 13, 35. https://doi.org/10.3390/min13010035
Liu Q, Liu R, Yang S, Feng W, Wang M. Removal Mechanism of Mineral Impurities in Molybdenum Concentrate Treatment Process. Minerals. 2023; 13(1):35. https://doi.org/10.3390/min13010035
Chicago/Turabian StyleLiu, Qihang, Ruilin Liu, Shuangping Yang, Weiguo Feng, and Miao Wang. 2023. "Removal Mechanism of Mineral Impurities in Molybdenum Concentrate Treatment Process" Minerals 13, no. 1: 35. https://doi.org/10.3390/min13010035
APA StyleLiu, Q., Liu, R., Yang, S., Feng, W., & Wang, M. (2023). Removal Mechanism of Mineral Impurities in Molybdenum Concentrate Treatment Process. Minerals, 13(1), 35. https://doi.org/10.3390/min13010035





