Abstract
Fluorite is a widespread mineral in porphyry and hydrothermal vein Mo-polymetallic deposits. Here, fluorite is utilised as a probe to trace the fluid source and reveal the fluid evolution process in the Chalukou giant Mo (Pb–Zn) deposit, Northeast China, which is characterised as early porphyry Mo and later vein-style Zn–Pb mineralisation. A detailed rare earth element (REE) and Sr–Nd isotope study of fluorite combined with Sr isotopes of sphalerite is conducted for the Chalukou deposit. The chondrite-normalised REE patterns of fluorites from molybdenite veins show light REE (LREE)-enriched patterns, with negative Eu anomalies (δEu = 0.60) and weakly negative Y anomalies (Y/Y* = 0.72). The fluorites associated with sphalerite veins exhibit rare earth element (REE)-flat patterns with negative Eu anomalies (δEu = 0.65 to 0.99) and positive Y anomalies (Y/Y* = 1.37 to 3.08). In addition, during the progression from Mo to Zn–Pb mineralisation, the total concentration of REEs decreases from 839 ppm to 53.7 ppm, and Y/Ho ratios increase from 22.1 to 92.5. These features may be explained by the different mobilities of REE complexes during fluid migration. The Eu anomalies are considered to be inherited from source fluids. All the initial 87Sr/86Sr ratios of fluorite and sphalerite are between those of ore-forming porphyries and wall rocks (rhyolite), with fluorite ratios ranging from 0.706942 to 0.707386 and sphalerite ratios varying from 0.705221 to 0.710417. The majority of εNd(t) values of fluorite varying from −6.4 to −3.6 are also located between the ratios exhibited by ore-forming porphyries and rhyolite, whereas three εNd(t) values of fluorites ranging from −0.26 to 0.36 are close to those of ore-forming porphyries. All the isotopic features indicate that the Sr-Nd isotope ratios of hydrothermal fluid are derived from porphyries and disturbed by fluid–rock reactions. Together with a two-stage Sr–Nd isotope mixing model, we suggest that different sources and fluid–rock interactions (syn-ore intrusions and strata) finally influence the Sr–Nd isotopes of the ore-forming fluids, which are recorded by the majority of fluorite and sphalerite.
1. Introduction
Fluorite (CaF2) has received considerable attention in petrogenetic studies of magmatic–hydrothermal deposits, including porphyry–skarn Cu–Mo–W deposits [1,2,3], granite Sn deposits [4,5], hydrothermal vein polymetallic deposits [6,7] and rare earth element (REE) deposits [8,9]. Fluorite has the potential to provide reliable ages for ore formation (Sm–Nd isotope dating) [10,11,12,13] and reveal the thermal history of ore deposits using low-temperature thermochronology [14,15,16,17]. Fluorite REE contents and fractionations are used to probe their associations with the fluids from which they crystallised, as well as with mineral dissolution–precipitation, surface sorption, or complexation during fluid migration [18,19,20,21,22], and to trace the source of the ore material [8,23,24,25,26,27,28,29,30]. Moreover, fluorite Sr–Nd isotope compositions can be used to identify the features of possible fluid sources, thereby comparing the initial 87Sr/86Sr and 143Nd/144Nd ratios of fluorite with those of potential Sr and Nd sources at the time of mineralisation [31,32,33,34,35,36,37,38,39]. Therefore, fluorite REE elements and Sr–Nd isotope compositions are important tools to derive information on the characteristics of hydrothermal fluids and their evolving processes.
The Chalukou giant porphyry Mo deposit, located in the Northern Great Xing’an Range, Northeast China (Mo resources of 2.46 Mt @0.087%; Zn and Pb metal resources of 0.143 Mt @0.61% and 13,000 tons @0.26%, [40]), is the largest Mo deposit in China and the third largest Mo deposit in the world. After detailed geological studies [41,42,43], the Chalukou Mo deposit is a Climax-type porphyry molybdenum deposit and contains a large amount of fluorite. A genetic model covering the magmatic source of ore-forming fluids and the importance of the involved meteoric water in the mineralising stage [44] or fluid immiscibility [45] in a single, short-lived (<650 kyr) hydrothermal event has been proposed [43]. However, less information about the water–rock interactions between exsolved fluids and causative rocks (or strata) has been proposed, hampering the understanding of the mechanism of fluid evolution and ore precipitation in the Chalukou porphyry Mo systems. Therefore, integrated fluorite REE and Sr–Nd isotope studies of the Chalukou porphyry Mo deposit can provide new insights into tracing the fluid source and water–rock interactions in fluorite-rich porphyry Mo deposits.
In this study, we focus on the fluorite REE and Sr–Nd isotope compositions from early porphyry Mo and later vein-style Zn–Pb mineralisation in the Chalukou deposit, combined with sphalerite Sr isotopes. Based on these results, we determine the origin and evolution of the ore-forming fluids in the porphyry Mo-vein-type Zn–Pb system. We propose a new perspective on the model of fluid mixing and water–rock interactions during fluid migration.
2. Geological Background
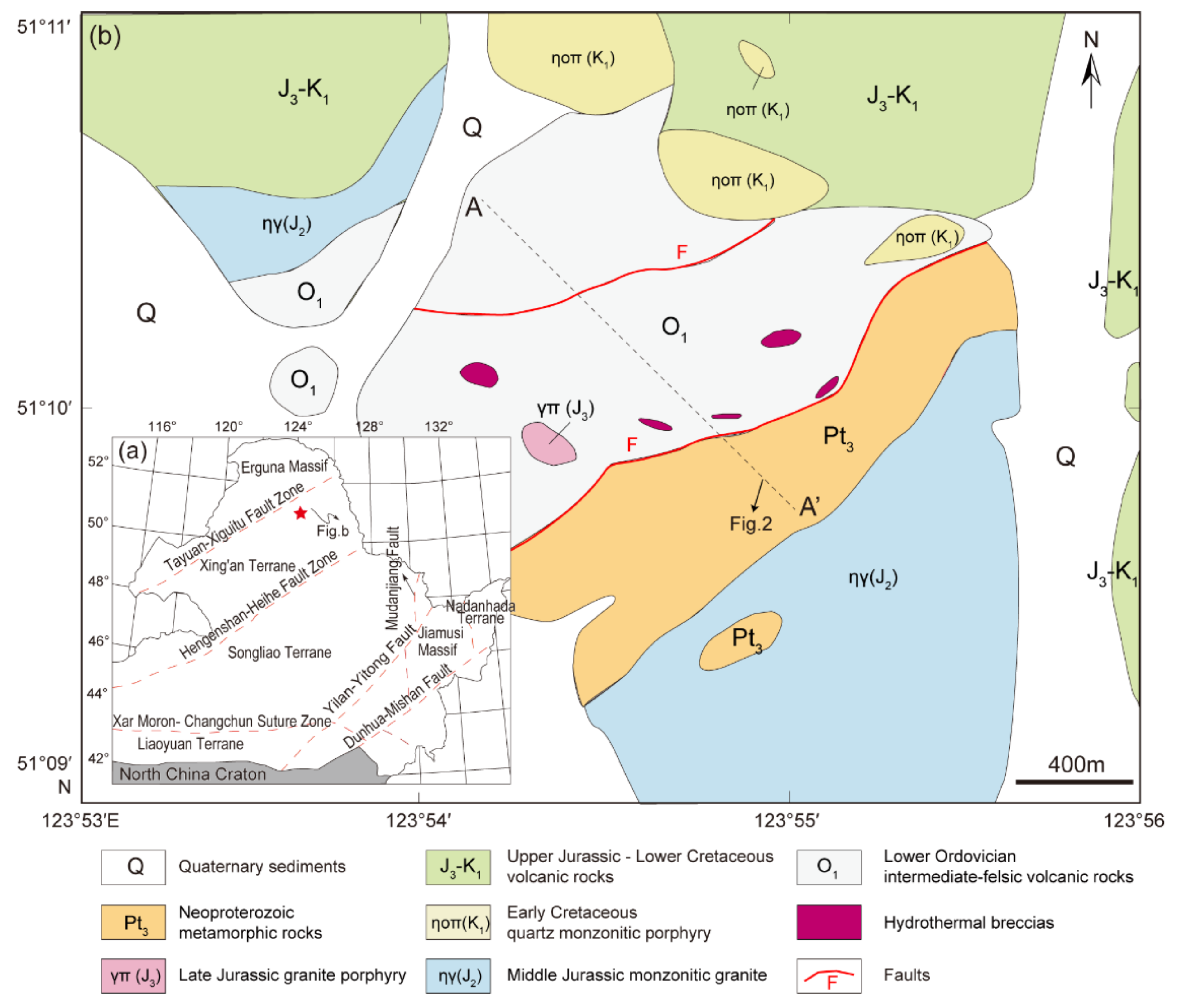
The Chalukou Mo–Zn–Pb deposit is located in the Great Xing’an Range district, Northeast China, in the eastern part of the Central Asian Orogenic Belt (CAOB) [46,47,48,49]. The tectonic evolution of NE China is characterised by the amalgamation of several microcontinental blocks (Figure 1a). In the early Palaeozoic, the Erguna Massif and Xing’an terrane collided along the Tayuan–Xiguitu fault [50,51,52], followed by the Palaeozoic collision of the Songnen terrane along the Hegenshan–Heihe fault [50,51,52,53]. The accretion of the Jiamusi Massif and Nadanhada terrane occurred in the eastern part of NE China during the Mesozoic, and this process was related to the subduction of the Pacific plate [52,54], whereas the subduction and collision of the Mongo–Okhotsk Ocean controlled the western part of NE China, including Manchuria and the Great Xing’an Range district [51,55]. During this evolution, early Palaeozoic island arc assemblages (andesite–dacite volcanic–sedimentary rocks) [56], widespread Mesozoic igneous rocks [52,57,58], and hydrothermal Pb–Zn–Ag vein deposits, epithermal Au–Ag–Cu deposits, and porphyry Cu ± Mo ± Au deposits developed in this NE China region [49,59,60,61].
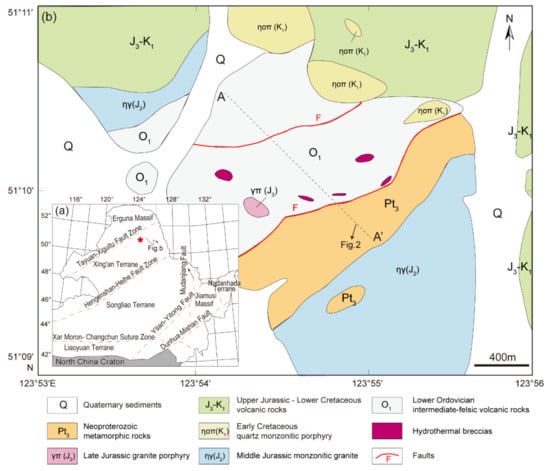
Figure 1.
(a) Tectonic subdivisions of Northeast (NE) China (modified from [52]); (b) geological map of the Chalukou Mo–Zn–Pb deposit modified from [40], with the A-A’ geological cross-section presented in Figure 2.
3. Ore Geology
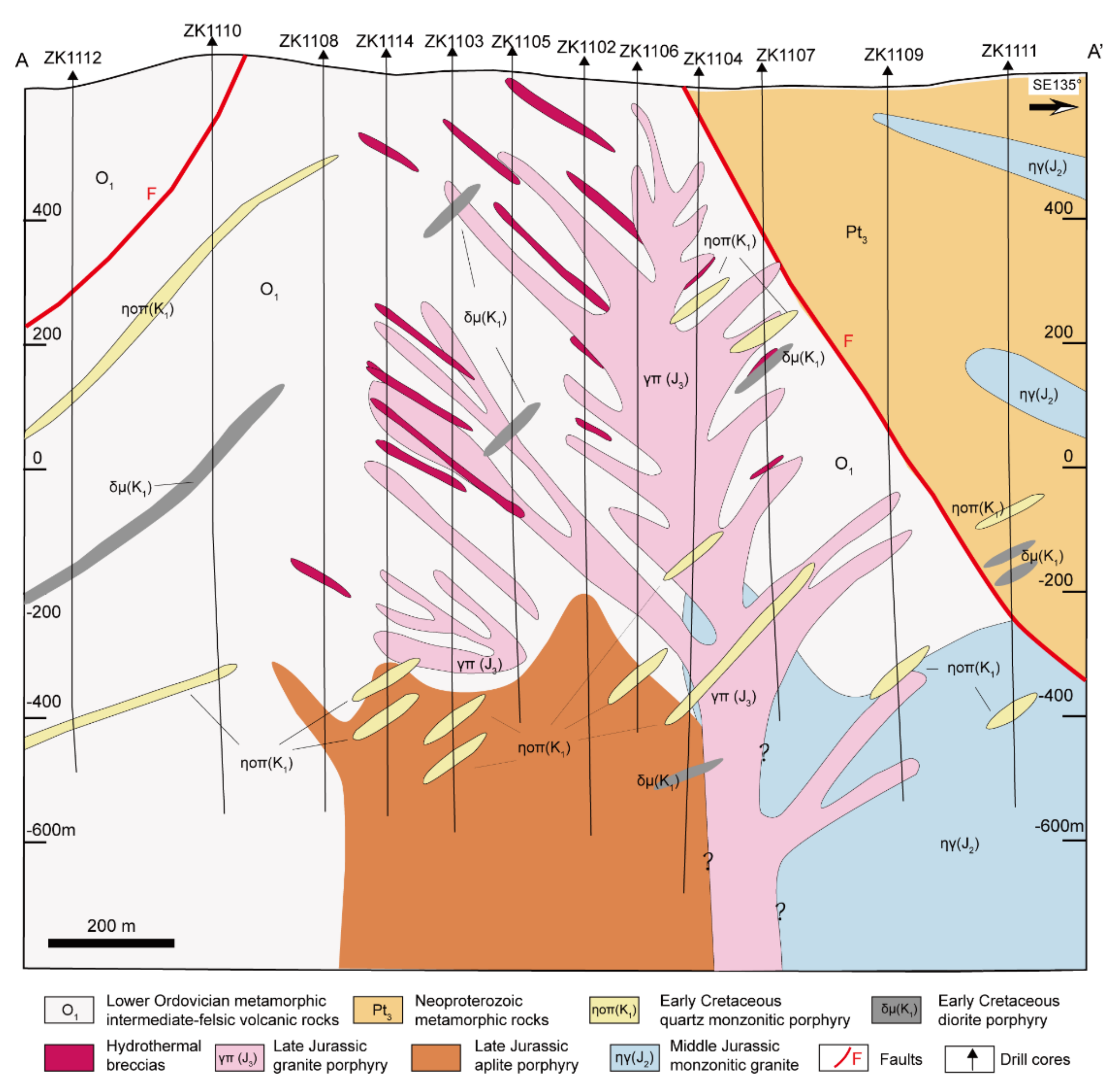
In terms of the strata and intrusions, the Chalukou deposit is located adjacent to ring-shaped concentric fractures and NE- and NW-striking faults around the western margin of the Jinsong caldera (Figure 1b). Strata that host the deposit form part of the Neoproterozoic–lower Cambrian Dawangzi Group (mainly quartz chlorite schist, quartz biotite schist, and marble); Lower Ordovician intermediate–felsic volcanic–sedimentary rocks, including dominant rhyolite (zircon U–Pb ages: 472–475 Ma, [62]), some dacite, andesite, and tuff that has been deformed slightly and undergone low-grade metamorphism; and the Upper Jurassic–Lower Cretaceous Baiyin’gaolao Group (zircon U–Pb ages in dacite: 145–146 Ma, [41]). The Upper Jurassic–Lower Cretaceous Baiyin’gaolao volcanic rocks overlie the Lower Ordovician strata observed in drill core logs [42]. The Chalukou intrusive sequence starts with the emplacement of late Jurassic monzogranite (zircon U–Pb ages: 147–149 Ma, [41,63]) into the Neoproterozoic–Lower Cambrian Dawangzi Group and Lower Ordovician strata (Figure 1b; Figure 2), and this batholith is intruded by a mass of stocks or dikes (zircon U–Pb ages: 147–149 Ma, [41,63]), including aplite porphyry stocks, granite porphyry dikes, and quartz porphyry dikes that are associated with the main pulse of Mo–Zn–Pb mineralisation. All the porphyries are observed only in the drill cores (Figure 3, [42]). The aplite porphyry stocks mainly intruded into Lower Ordovician volcanic–sedimentary strata and monzogranite in the south-eastern part of the district. The granite porphyry dikes also intruded into the Lower Ordovician strata. Additionally, quartz porphyry dikes were accompanied by hydrothermal breccia. The recent high-precision molybdenite ID-N-TIMS Re–Os ages show that the bulk of molybdenite deposition occurred between 147.67 ± 0.10/0.60/0.76 and 147.04 ± 0.12/0.72/0.86 Ma (analytical uncertainty /+tracer calibration /+decay constant uncertainties) [43], which is consistent with the emplacement of these causative intrusions. These strata and intrusions are finally cut by a series of post-ore porphyry dikes, including diorite porphyry dikes, feldspar porphyry dikes, and quartz monzonitic porphyry dikes that were emplaced at 142 Ma, 139 Ma and 128 Ma, respectively [41].
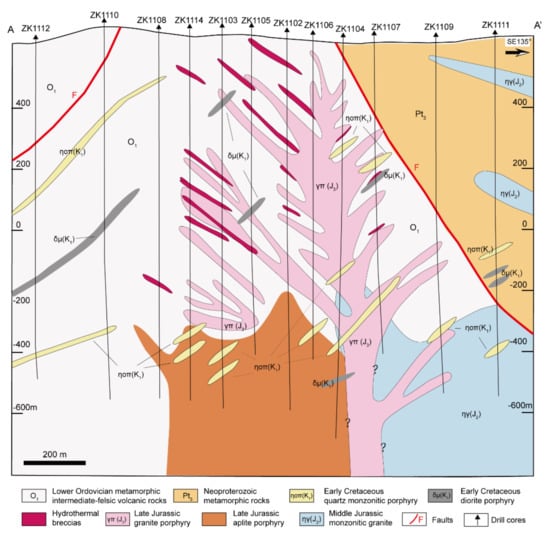
Figure 2.
Geological cross-section of prospecting line 11 of the Chalukou Mo–Zn–Pb deposit (line A-A’ marked in Figure 1b), showing strata, intrusions, and hydrothermal breccias. Quartz porphyry dikes exist at shallow depths close to the surface of the Chalukou deposit but do not occur in this geological cross-section.
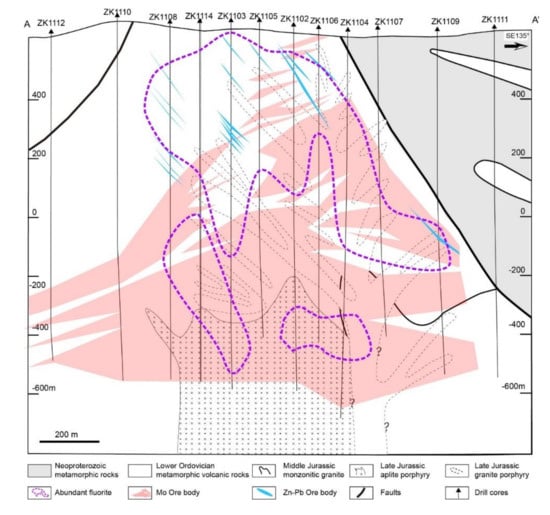
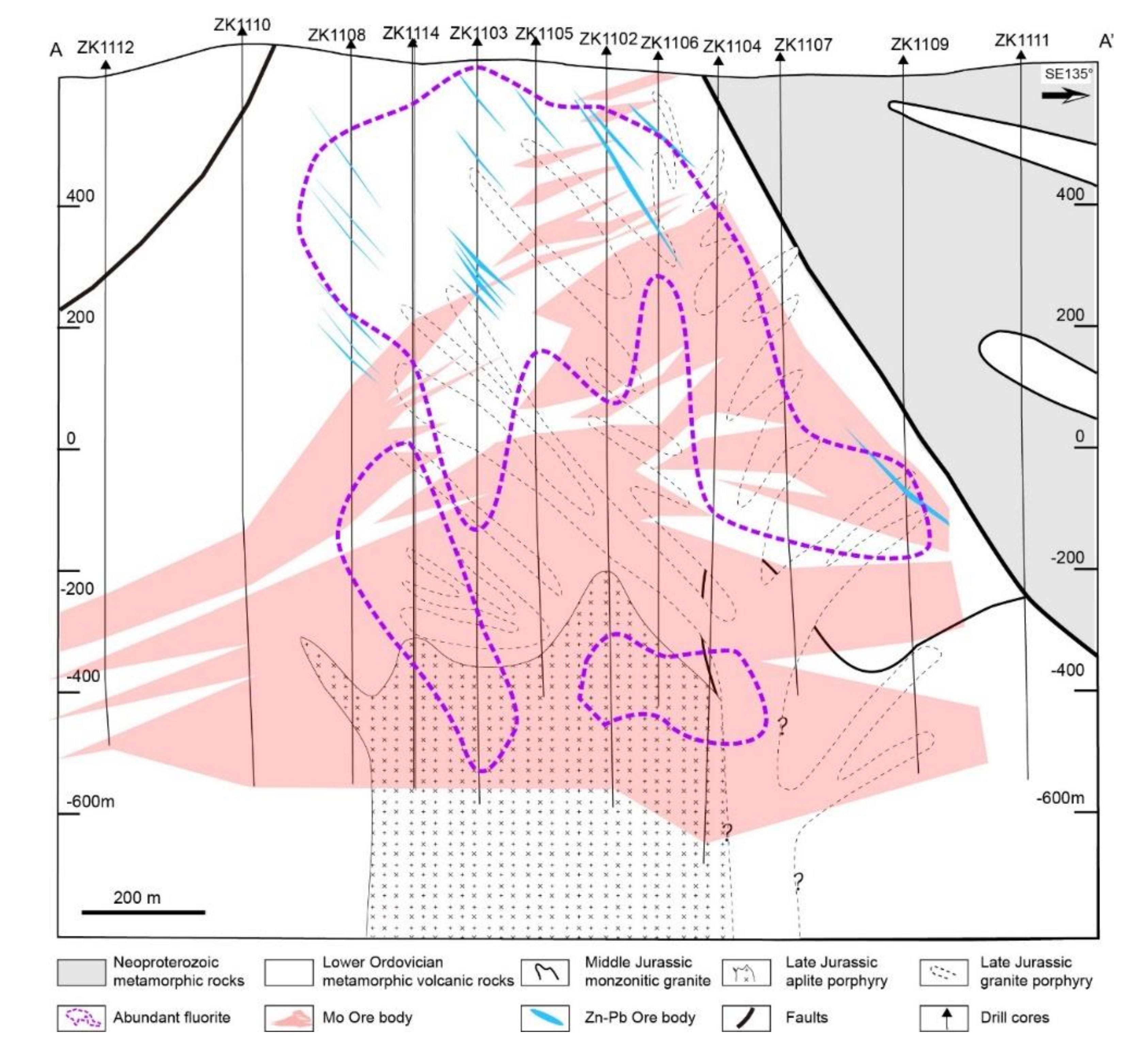
Figure 3.
Occurrence of Mo and Zn–Pb ore bodies modified from [40] and the distribution range of fluorite in the geological cross-section of the prospecting line.
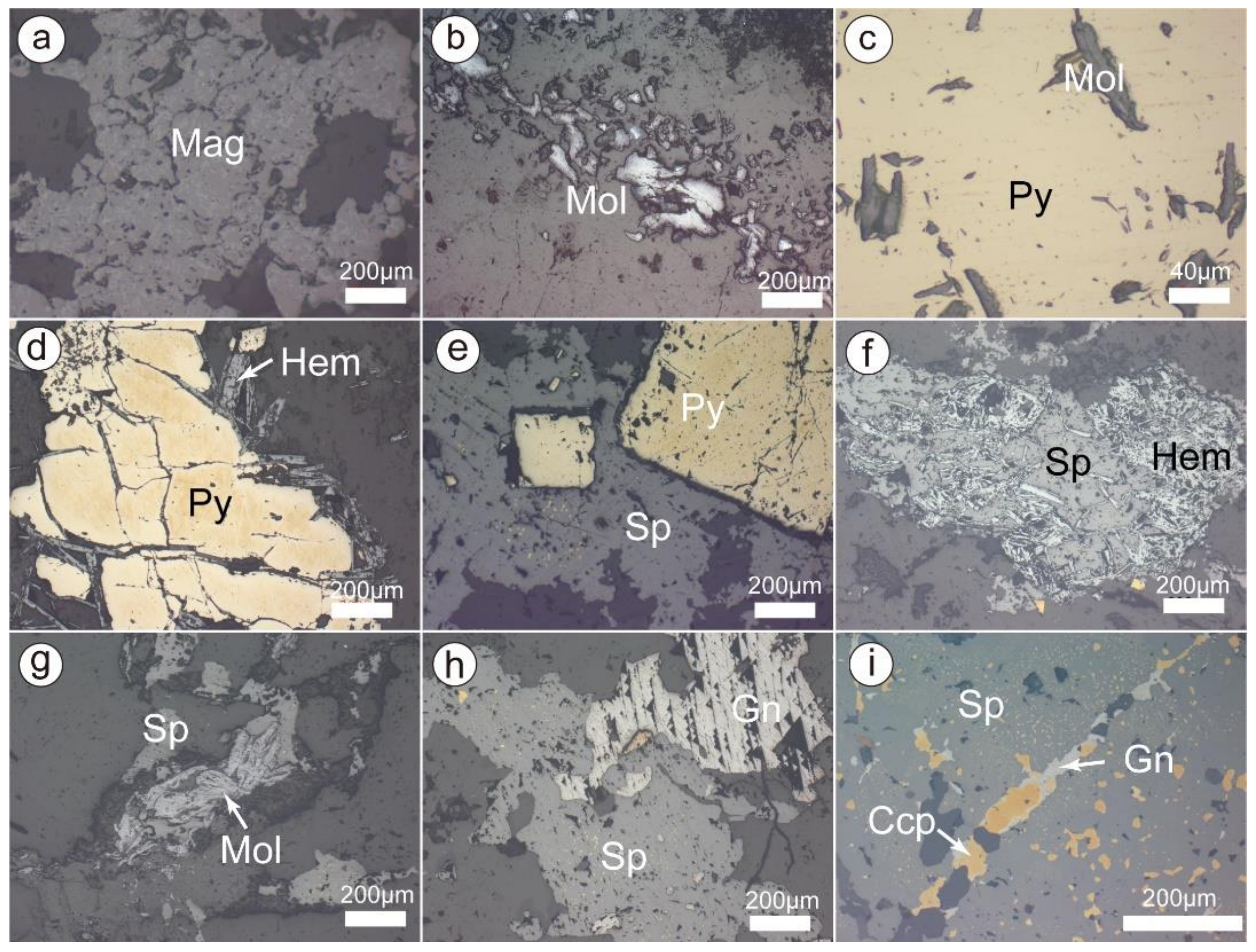
In the Mo–Pb–Zn mineralisation, two distinct mineralisation styles are recognised in the Chalukou deposit: porphyry Mo-style mineralisation (molybdenite Re–Os isotopic ages: 148–146 Ma, [41,43,63]) and Zn–Pb vein-style mineralisation [64]. Zn–Pb vein-style ore bodies are above and peripheral to the porphyry Mo-style ore bodies. Porphyry Mo mineralisation occurs as a dome-shaped ore body (Figure 3). The main Mo mineralisation is present from 500 to 1300 m below the surface, and some is present in the shallow area. The Mo ore body is hosted by rhyolite, monzogranite, and ore-forming porphyries (aplite porphyry, granite porphyry, and quartz porphyry). Molybdenite typically occurs in stockwork veins and veinlets and less commonly as molybdenite cement within hydrothermal breccias. Zn–Pb vein-style mineralisation is mainly hosted in near-surface rhyolite (Figure 3). Sphalerite and galena in the Zn–Pb ore bodies are present in veins or lodes, with crystals ranging from the mm to the cm scale. Generally, the ore minerals started on magnetite (Figure 4a) and then transitioned into vast molybdenite (Figure 4b), which comprised the Mo ore body. Later, pyrite became the dominant metallic mineral, and some hematite gradually precipitated (Figure 4c–e). Finally, the main sphalerite (Figure 4e–i), galena (Figure 4h,i), and minor chalcopyrite (Figure 4i) formed and produced the Zn–Pb ore body.
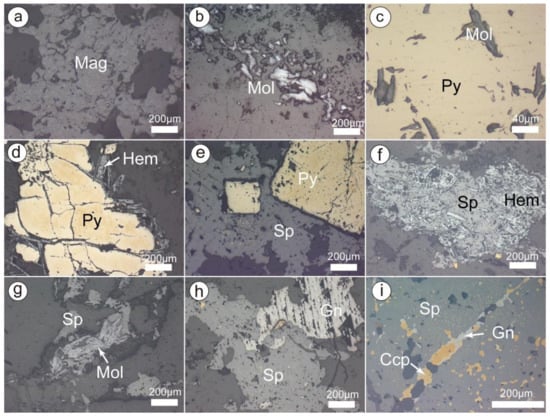
Figure 4.
Microphotographs of metallic minerals in the Chalukou deposit. (a) Magnetite; (b) molybdenite; (c) pyrite enclosed pre-existing molybdenite; (d) late hematite precipitated in the fractures of pyrite; (e) sphalerite enclosed pre-existing euhedral pyrite grains; (f) sphalerite enclosed pre-existing hematite; (g) sphalerite enclosed pre-existing molybdenite; (h) later galena overprinted partial sphalerite; (i) galena replaced sphalerite and then was overprinted by later chalcopyrite. Mineral abbreviations: Ccp-chalcopyrite, Gn-galena, Hem-haematite, Mag-magnetite, Mol-molybdenite, Sp-sphalerite.
In terms of hydrothermal alteration and veins, pervasive hydrothermal alteration (surface area: 3 km2) and various types of hydrothermal veins occur in the Chalukou deposit. The broad-scale alteration zoning pattern comprises potassic, silicic–phyllic, argillic, and propylitic alterations from the inner to the outside zone, with each alteration zone ranging from 300 m to 600 m in thickness. Mo mineralisation is mainly associated with potassic alteration and locally associated with phyllic alteration. Zn–Pb ore bodies are commonly associated with the argillic alteration. Five main types of hydrothermal veins are identified in the Chalukou deposit, as described in [42]. The earliest veins (V1) can be subdivided into barren quartz veins and magnetite − quartz ± hematite veins with K-feldspar alteration selvages. The later molybdenite veins (V2) can be divided into three subtypes, including quartz–molybdenite veins with K-feldspar alteration halos or minor muscovite ± pyrite alteration selvages, quartz–molybdenite ribbon veins, and molybdenite-only veins. The above vein types are cut by transitional pyrite-dominated veins with muscovite alteration (V3). They are, in turn, cut by hematite veins (minor) and sphalerite–galena-bearing veins with no alteration halos (V4). Finally, fluorite/chlorite/carbonate-dominated veins (V5) occur.
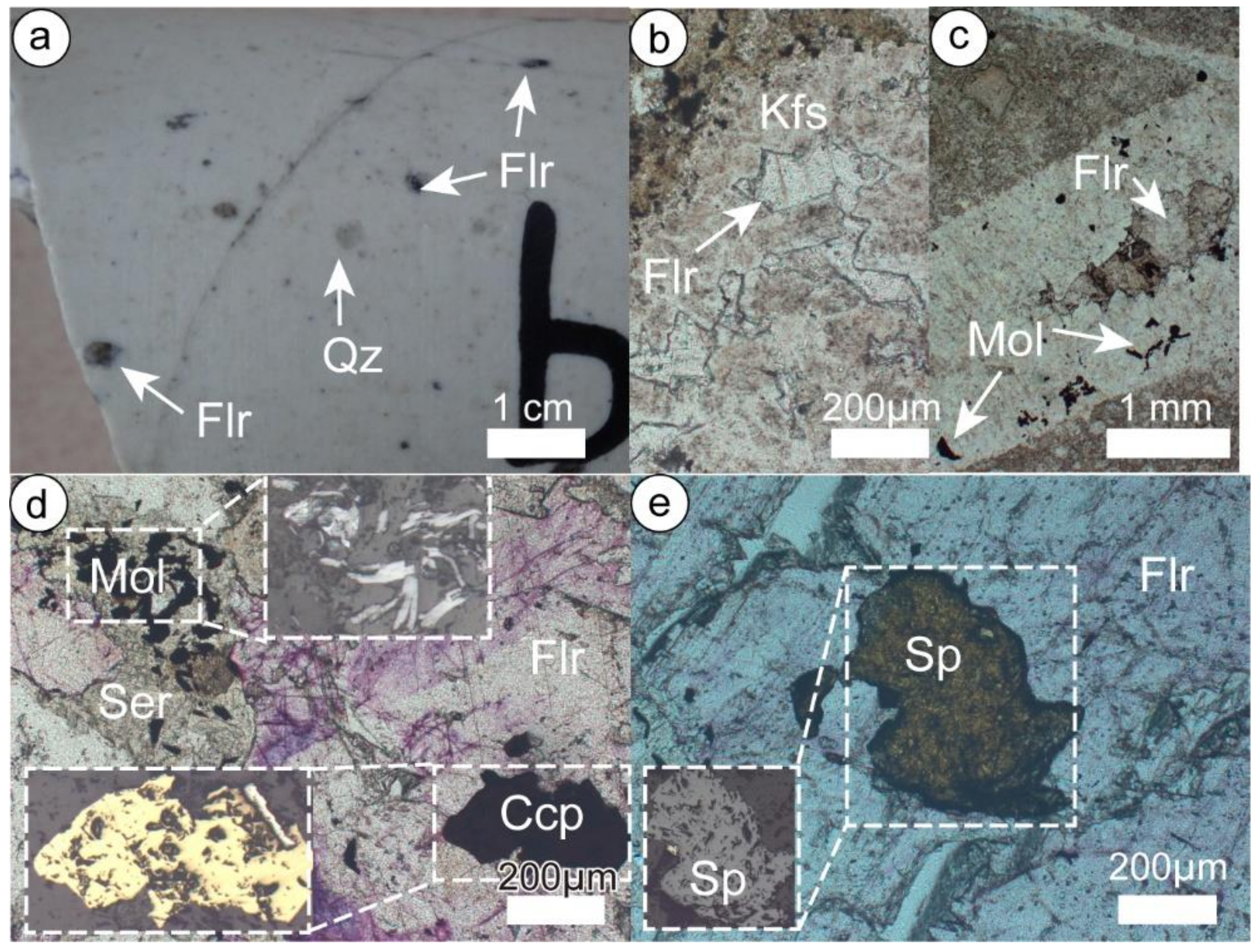
Fluorite is widely distributed and accompanied by both Chalukou Mo and Pb–Zn orebodies (Figure 3). Few fluorites are observed in the presence of altered rocks (Figure 5a), early K-feldspar-bearing veins (V1, Figure 5b), molybdenite-bearing veins (V2, Figure 5c), and quartz–pyrite veins. The majority of fluorite occurs in the sphalerite ± galena ± chalcopyrite ± pyrite veins (V4, Figure 5d,e) and fluorite ± carbonate veins (V5), indicating that the formation of fluorite is significantly associated with low-temperature Pb–Zn mineralisation and alteration.
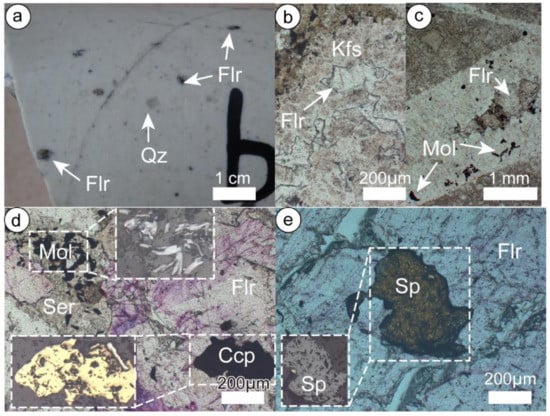
Figure 5.
Fluorite occurrence in the Chalukou deposit. (a) Fluorite disseminated in quartz porphyry; (b) fluorite in a quartz-K-feldspar vein (V1); (c) fluorite in a quartz–molybdenite vein (V2); (d) fluorite enclosing chalcopyrite, and molybdenite occurring with sericite; (e) fluorite enclosing sphalerite. Mineral abbreviations: Ccp-chalcopyrite, Flr-Fluorite, Kfs-K-feldspar, Mol-molybdenite, Qz-quartz, Ser-sericite, Sp-sphalerite.
4. Sampling and Analytical Methods
4.1. Sampling Strategy
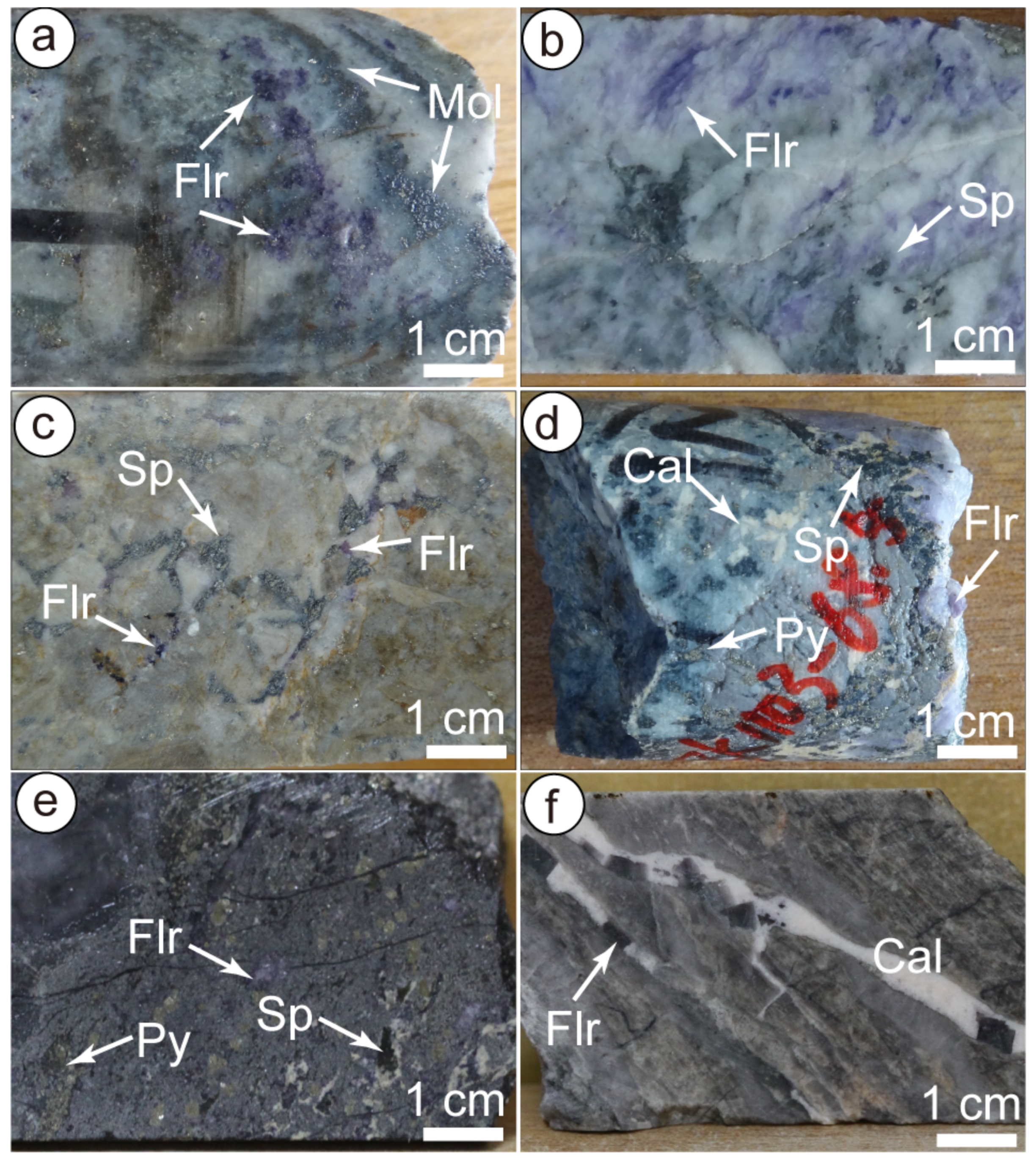
All the samples were collected from the drill holes and outcrops. Although fluorite occurred in some quartz–molybdenite veins, we were not able to isolate enough fluorite for analysis, as a sufficient amount of fluorite was rare in most veins. Hence, the REE concentrations of fluorite were analysed in nine samples, including one quartz–molybdenite–fluorite vein (V2, Figure 6a), seven sphalerite–galena–pyrite–fluorite veins (V4, Figure 6b–e), and one fluorite–carbonate vein (V5, Figure 6f). Most samples were also tested for Sr–Nd isotopes. Seven sphalerite samples from sphalerite–galena–pyrite veins were also examined for Rb–Sr isotopes. The detailed sample descriptions are listed in Table 1.
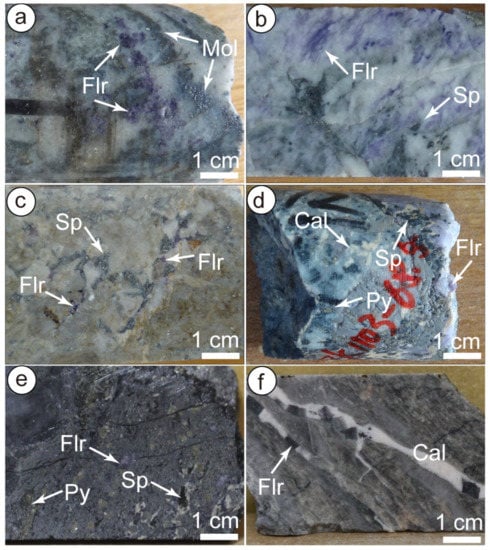
Figure 6.
Representative photographs of fluorite and sphalerite used for isotopic analysis. (a) Quartz-molybdenite-fluorite vein (V2); (b) Quartz-sphalerite-fluorite vein (V4); (c) Fluorite and sphalerite occur in the cement of hydrothermal breccia; (d) Quartz-sphalerite-pyrite-fluorite-calcite vein (V4); (e) Sphalerite-pyrite-quartz-fluorite vein (V4). (f) Calcite-fluorite vein. Mineral abbreviations: Cal-calcite, Flr-Fluorite, Mol-molybdenite, Py-pyrite, Sp-sphalerite.

Table 1.
Sample descriptions of the analysed fluorite and sphalerite at Chalukou in this study.
4.2. Analytical Methods
Fluorite and sphalerite were hand-picked and purified using heavy liquids, and then they were crushed to 200 mesh for REE analyses and to 80 to 100 mesh for Sr–Nd isotope analyses.
The REE analyses of fluorites were performed at the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS). Approximately 50 mg of sample powder was weighed and dissolved in a Teflon bomb using HF + HNO3. Then, the sealed bomb was placed in an electric oven and heated to 190 °C for 24 h. After cooling and removal, the solution was evaporated to dryness. Then, HNO3 was added to the residue again. These steps were repeated three times. The final residue was redissolved in HNO3 and diluted to 100 mL by the addition of distilled deionised water for inductively coupled plasma–mass spectrometry (ICP–MS) analysis. Reference materials GSR-1 and GSR-3 were also analysed to monitor the accuracy of the analytical procedures (GeoREM, http://georem.mpch-mainz.gwdg.de/ (accessed om 26 October 2022)).
The Rb–Sr and Sm–Nd isotope analyses were conducted at IGGCAS according to the procedures described by [65]. Approximately 100 mg of powder was weighed into 7 mL Teflon beakers, and then they were added to appropriate amounts of mixed 87Rb–84Sr and 149Sm–150Nd spikes. Then, HNO3 + HCl (with a ratio of 3:1) was used to dissolve fluorite and sphalerite. Rb, Sr, and REEs were separated using standard ion-exchange columns, and Sm and Nd were separated using Eichrom LN (LN-C-50B, 100–150 μm, 2 mL) chromatographic columns. The Rb–Sr and Sm–Nd isotopes were measured using an IsoProbe-T thermal ionisation mass spectrometer. The measured 87Sr/86Sr isotopes were mass fractionation-corrected using 86Sr/88Sr = 0.1194, and 143Nd/144Nd was corrected using 146Nd/144Nd = 0.7219. The measured values for the NBS987 and JNdi-1 standards were 87Sr/86Sr = 0.710250 ± 10 (2σ, n = 12) and 0.512135 ± 10 (2σ, n = 12), respectively. The Rb–Sr and Sm–Nd isotope compositions of the U.S. Geological Survey reference material BCR-2 were analysed to monitor the accuracy of the analytical procedures, and the analytical results agreed well with the reported reference values (GeoREM, http://georem.mpch-mainz.gwdg.de/ (accessed om 26 October 2022)). Total procedural blanks were <40 pg for Rb, <300 pg for Sr, <20 pg for Sm, and <70 pg for Nd.
5. Results
5.1. REE Concentrations of Fluorite
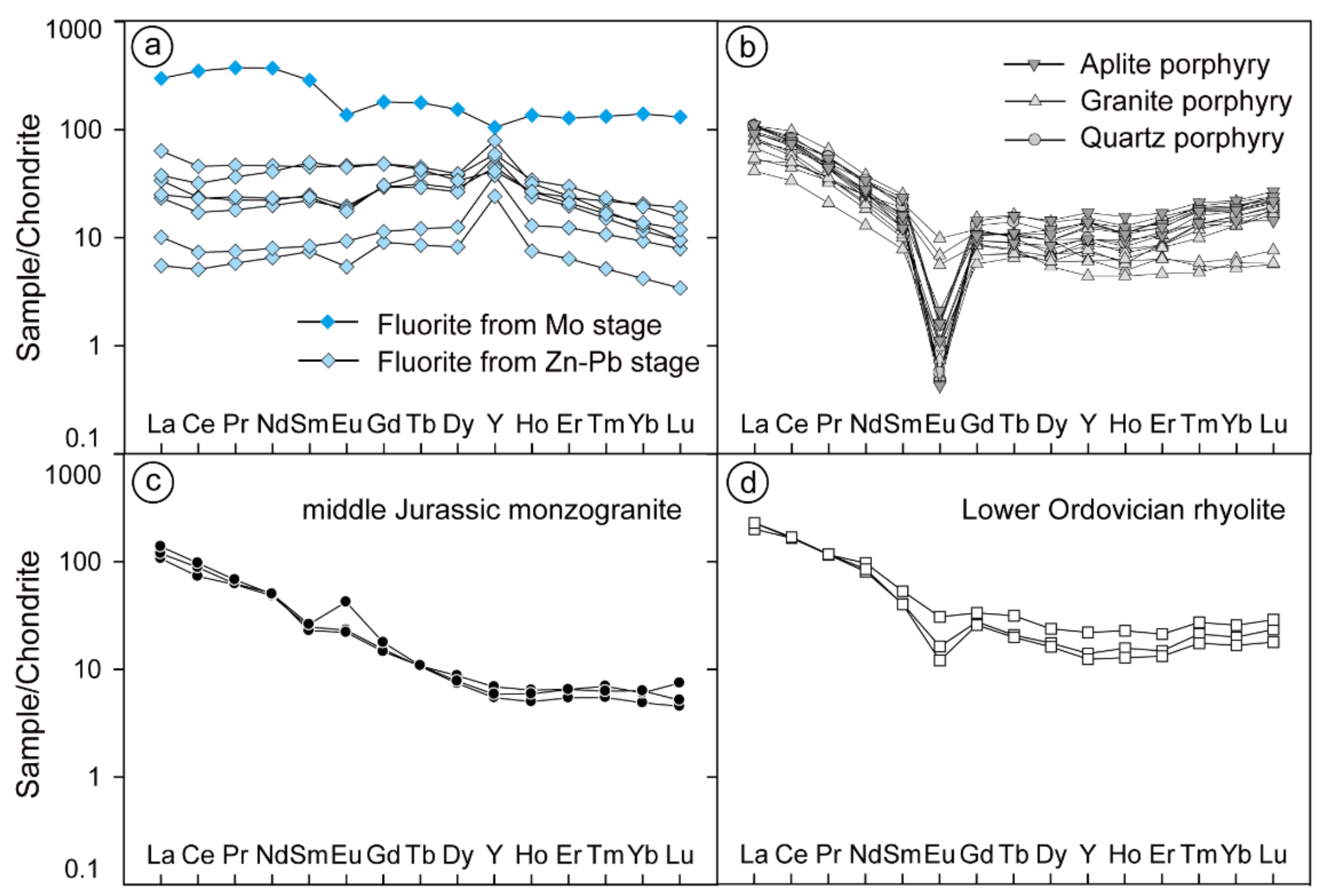
The REE compositions of fluorite are presented in Table 2. Fluorite separated from molybdenite-bearing veins (V2) shows a high total concentration of REEs (838.9 ppm) and light REE (LREE)-enriched distribution patterns with moderately negative Eu anomalies (δEu = 0.60) and weakly negative Y anomalies (Y/Y* = 0.72) (Figure 7a). In contrast, the fluorite in the sphalerite–pyrite veins and fluorite–carbonate veins have much lower REE total concentrations ranging from 53.71 to 230.8 ppm. The REE patterns exhibit REE-flat patterns with weakly negative Eu anomalies (δEu = 0.65 to 0.99), no or lightly negative Ce anomalies, and positive Y anomalies ranging from 1.37 to 3.08 (Figure 7a). Both patterns show no similarities with the Chalukou intrusions (Figure 7b,c) or strata (Figure 7d).

Table 2.
REE concentrations of fluorite in the Chalukou deposit (in ppm).
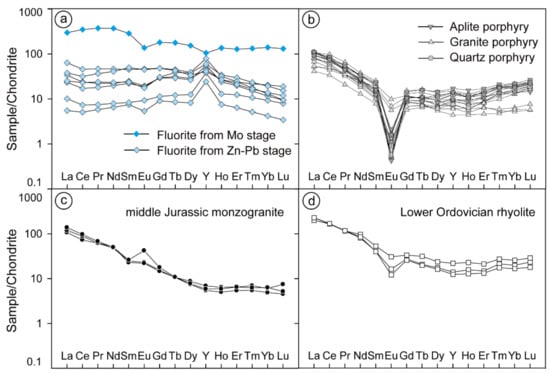
Figure 7.
Chondrite-normalised REE patterns of fluorite from different stages (a), as well as REE patterns of intrusions (b,c) and rhyolite (d) (data from [41]), and the REE composition of chondrites is from [66].
5.2. Sr–Nd Isotopes of Fluorite and Sphalerite
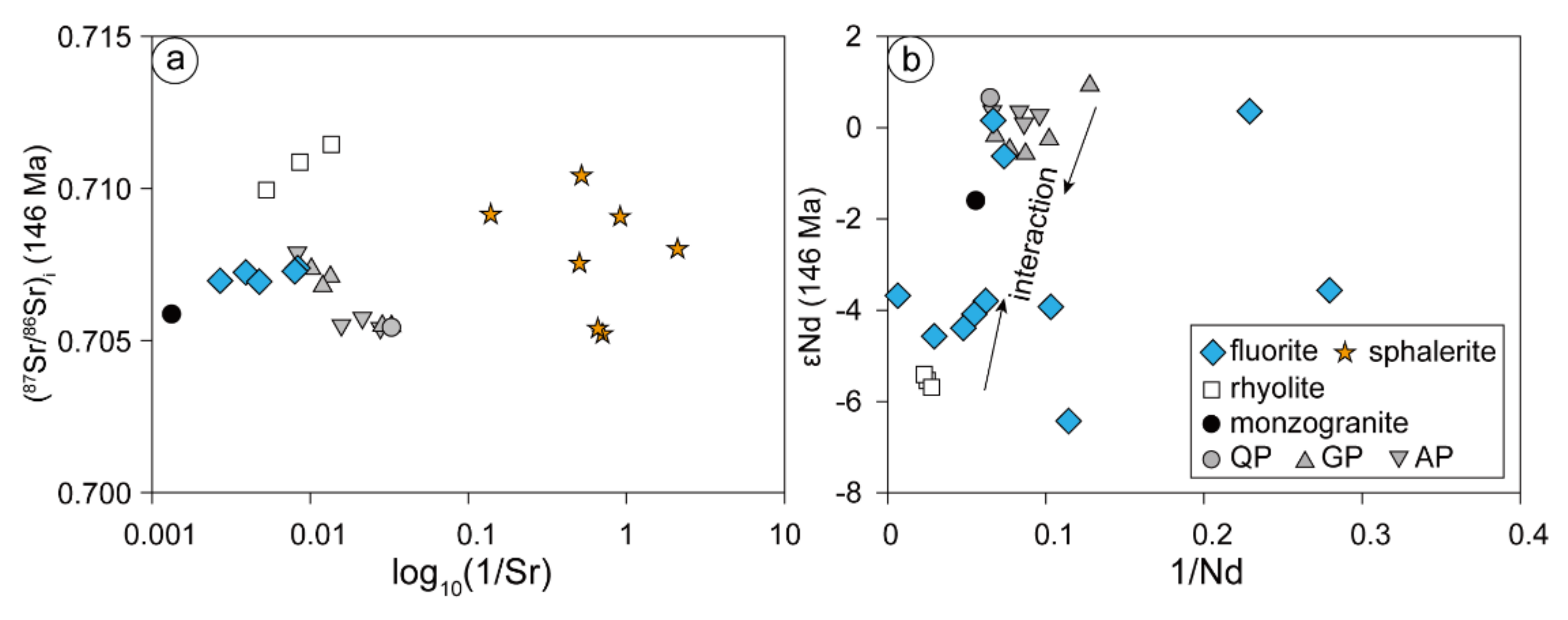
The Sr and Nd isotopic compositions of fluorite and sphalerite are presented in Table 3. Five fluorite samples from sphalerite-bearing veins and fluorite veins have relatively uniform Sr isotope compositions (87Sr/86Sr = 0.706942 to 0.707386). A total of 11 fluorite samples show two Nd isotope composition clusters. Three samples have high εNd(t) values between −0.62 and 0.36, and another eight samples have values that vary from −3.56 to −6.42. Seven sphalerite samples have a wide range of initial 87Sr/86Sr ratios from 0.705221 to 0.710417, and the lowest two values are very close to those of the ore-forming porphyries (87Sr/86Sri = 0.705413 to 0.707889, [41]).

Table 3.
Sr–Nd isotopic compositions of fluorite and Sr isotopes of sphalerite in the Chalukou deposit.
6. Discussion
6.1. Source of REEs and Fluids Revealed by Fluorite REE Patterns
The REE distribution of hydrothermal minerals is controlled by several factors and mechanisms, including fluid source features [37,67,68], REE adsorption on mineral surfaces [69], REE complexation during fluid migration [22,70], changes in the physicochemical conditions [71], and mineral precipitation–dissolution processes [20]. For early fluorite at Mo mineralising stage, petrographic observation in Figure 5c shows that the vein-type fluorite is distributed in the middle part of the vein, indicating that the early-stage fluorites are more likely the product of gradual crystallisation of the ore-forming fluid in a relatively close space (Figure 5c), that is, the formation of fluorite is the product of the residual fluid. The fluorite with Pb–Zn mineralisation (Figure 5e and Figure 6b–e) and late alteration (Figure 6f) was crystallised from late-evolved fluids. The discussion of how the REE distributions of the Chalukou fluorites form and the change of the fluid compositions includes the following.
6.1.1. REE Complexation and Absorption
We acknowledge that high REE concentrations and LREE enrichment characterise fluorites at the Mo mineralising stage, and fluorites associated with Pb–Zn mineralisation and low-temperature alteration have lower total REE concentrations, with flat REE patterns (Figure 7). This observation can be explained by REE fractionation occurring during fluid migration as the predominance of complexation or absorption, that is, the changes in fluid compositions.
High-temperature, low-pH fluids are facilitated by low concentrations of OH−, CO32− or halogen, and REE fractionation is controlled by equilibrium partitioning between fluid and crystallising mineral [22,72]. Under this circumstance, LREEs are abundant in solutions, and the fluorite that precipitates from the solutions shows LREE enrichment patterns. However, in alkaline fluids that have abundant uni- or bivalent CO32- and/or halogen ligands, REE patterns have ratios of (La/Lu)N < 1, as REE fractionation is predominated by complexation [19,20,21,70]. In the Chalukou deposit, fluorite, carbonate, gypsum, and anhydrite are widely developed. The bulk compositions of fluid inclusions analysed from vein quartz are rich in SO42− and halogens, such as Cl− and F− [42], which are crucial in REE complex migration [24,25,73,74,75,76,77]. We interpret this abundance of ligands in Chalukou fluids as having dominant complexation during REE immigration. The stability of the REE complex gradually weakens, accompanied by ionic radii and/or temperature decreases [18,19,37,78,79,80]. Therefore, this mechanism leads to the gradual change in the REE distribution from LREE-enriched to REE-flat patterns observed in the Chalukou deposit, finally leading to LREE depletion [71,81,82,83].
6.1.2. Y Anomalies and Y–Ho Fractionation
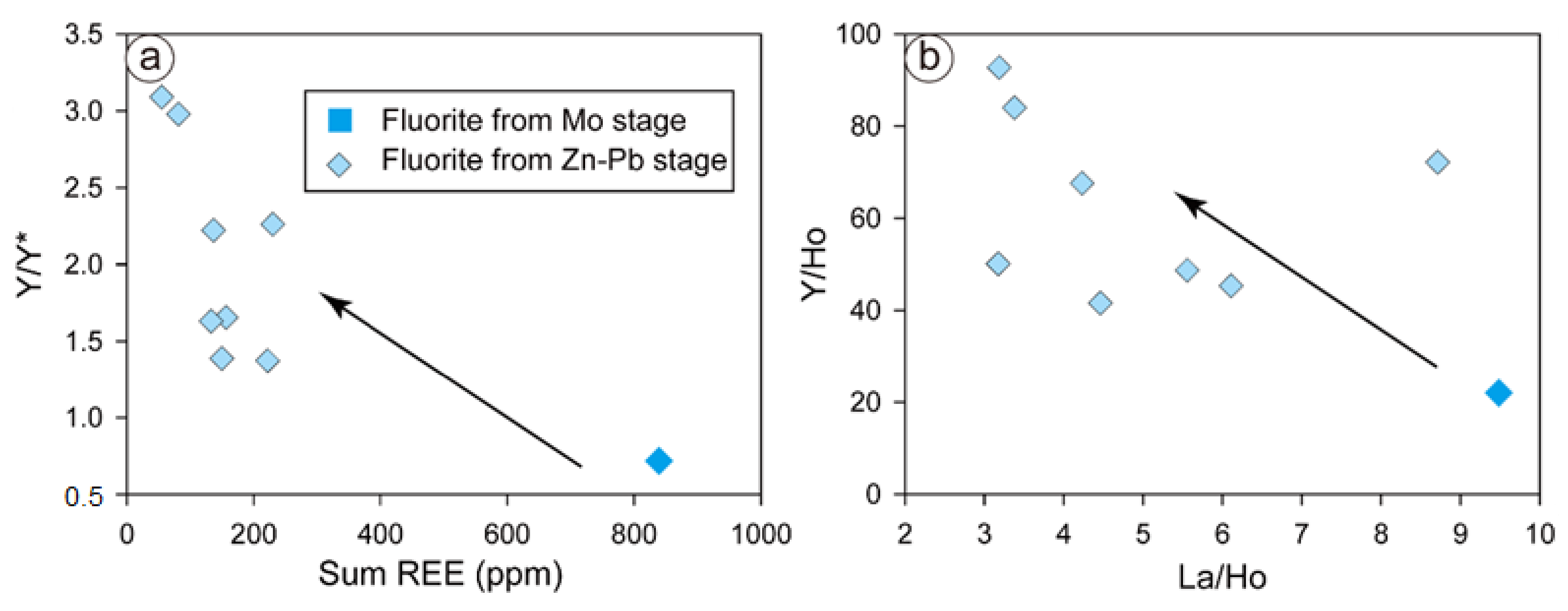
Positive Y anomalies and high Y/Ho ratios are always present in the REE-Y patterns of fluorite [3,9,26], and they are higher than the chondrite Y/Ho ratio of 28 [84]. This feature occurs because REE–fluoride is also an important complex to transport REEs despite the presence of REE–chlorine [24,25,75]. In fluorine-rich hydrothermal fluids, the Y–F complex is more stable than the Ho–F complex and any other REE–F2+ complexes [22,85,86]. Hence, positive Y anomalies and Y/Ho ratios would increase during fluid immigration and reaction with fluorine-rich aqueous fluid [22].
The massive precipitation of hydrothermal fluorite can indicate high–moderate fluorine activity in the Chalukou hydrothermal fluid, quartz vermicular resorption textures (such as in the Empire Mine, [87]), and high fluorine-content hydrothermal biotite (up to 4.5 wt.%) and muscovite (up to 2.7 wt.%) in the Chalukou deposit [42]. Under this circumstance, positive Y anomalies and high Y/Ho ratios are considered to be the result of the stability difference between the Y–F complex and other REE–F2+ complexes. Therefore, this high-fluorine fluid evolves from early-stage Mo to late-stage Zn–Pb mineralisation, resulting in changed Y anomalies (weakly negative to positive anomalies, Figure 8a) and increased Y/Ho ratios (Figure 8b). Therefore, we conclude that the Y–Ho fractionation in the Chalukou fluorite could also be caused by the high–moderate fluorine activity in the fluids.
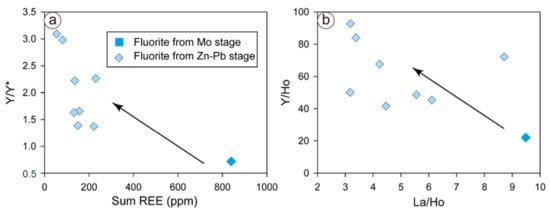
Figure 8.
REE–Y/Y* (a) and La/Ho–Y/Ho (b) plots of fluorite from different stages.
6.1.3. Eu Anomalies
We document that the weakly negative Eu anomalies in fluorite in the Chalukou deposit (Figure 7a) are facilitated by the temperature decrease and water–rock interactions, some changes in the fluid redox condition.
Commonly, Eu anomalies are controlled by temperature and redox conditions during fluorite precipitation [37]. At temperatures above 250 °C, Eu3+ changing to Eu2+ due to thermal–chemical reduction [19,20,70] restricts Eu2+ from entering the fluorite lattice and results in negative Eu anomalies [20,71]. At low temperatures below 200 °C, Eu3+ predominates in hydrothermal fluid, and the minerals crystallised from the fluids exhibit positive Eu anomalies [71]. Microthermometry of fluid inclusions in fluorite reveals that the majority of homogenisation temperatures are between 180 °C and 250 °C [42], implying that temperature may not be the crucial factor affecting Eu anomalies in the Chalukou deposit. The possibility lies in the inheritance of Eu anomalies from source fluids through water–rock interactions and leaching of rocks with Eu-rich minerals (i.e., early magmatic plagioclase, hydrothermal apatite, etc. [37]). This phenomenon could lead to negative Eu anomalies in the fluid phase [26,37,71,88,89] because Eu remains in the crystal lattice, while the incompatible rare earth elements and yttrium (REYs) are mobilised. No Eu anomalies of some samples can be attributed to the dissolution of Eu-rich minerals into the fluids during water–rock interactions (such as plagioclase in wall rocks, [26]). For change in the redox condition, the transition from magnetite to hematite at Chalukou could make the fluids more oxidised, also causing more Eu3+ to exist in fluids. Therefore, we should consider both water–rock interactions and the change of the redox condition in the fluid in Eu anomalies.
6.2. Origin of Ore-Forming Fluids and Fluid–Rock Interaction
The composition of hydrothermal fluid responsible for depositing fluorites is determined by the composition of the fluid source, the isotopic composition of the rocks along the fluid-flow path, and the extent of isotopic exchange between the rock and fluid [90]. Hence, the Sr–Nd isotopic features of fluorites could trace the origin and evolution of hydrothermal fluids in the ore deposits.
The relatively concentrated 87Sr/86Sr ratios of the Chalukou fluorites from 0.706942 to 0.707386 are between those of intrusions (0.70541–0.70789, [41]) and rhyolites (0.709945–0.711444, [91]) (Figure 9a). The range of 87Sr/86Sr ratios of sphalerite is wider than that of fluorite but is also located between them (Figure 9a). We infer that the formation of these minerals is affected by both intrusions and rhyolites, and different distributions of Sr isotopes from fluorite and sphalerite reveal different degrees of isotopic equilibrium during precipitation. Moreover, the initial 87Sr/86Sr ratios of two sphalerites (0.705221 and 0.705395, Figure 9a) are very close to the lowest ratio of ore-forming porphyries (0.70541, [41]), indicating that these minerals might retain the isotopic compositions of the source rocks (mainly ore-forming porphyries). This finding means that the ore-forming fluid was derived from ore-forming porphyries and influenced by wall rocks (monzogranite and rhyolites), which is also supported by hydrogen–oxygen isotopes of quartz and sulphur isotopes from sulphides [42,45].
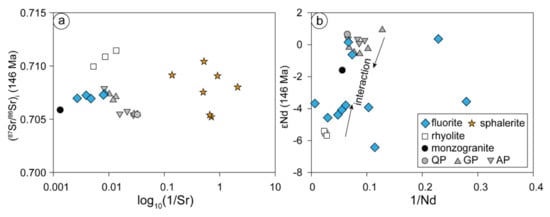
Figure 9.
The (87Sr/86Sr)i-1/Sr diagram of fluorite and sphalerite (a), as well as the 1/Nd-εNd (146 Ma) diagram of fluorite (b) in the Chalukou deposit (the Sr–Nd data of rhyolite and intrusions are from [91]). Abbreviations: QP-quartz porphyry, GP-granite porphyry, AP-aplite porphyry.
As compositions and temperature (180–240 °C) of the hydrothermal fluids in the fluorite-bearing veins were relatively constant at Chalukou [42], a much higher degree of fluid–rock reactions is required to equilibrate Nd to rock values compared to Sr [90]. Therefore, hydrothermal fluid, having reacted with wall rocks slightly or without sufficient time to reach equilibrium, preserves the Nd isotopic signature of the source [92]. Although εNd(t) values of most fluorite samples (εNd(t)= −3.56 to −3.42, Figure 9b) between intrusions and rhyolite reveal isotopic re-equations with two endmembers, three relatively uniform εNd values of fluorite show the magmatic source information (Figure 9b). Three fluorites possessing higher εNd(t) values (from −0.6 to 0.36) could represent a direct effect of Nd ratios in the source (Figure 9b, ore-forming porphyries, εNd(t): −0.57 to 0.93, [41]) or only partial re-equilibration of the mineralising fluid with host rocks. This finding is similar to the results of some other deposits [9,35,38,39,93] in that the Nd isotope compositions show the source of the mineralising fluid.
Commonly, ore-forming fluid can gradually be equilibrated with the host rock via dissolution–precipitation and exchange reactions along a flow path [36,37,90,94,95]. Considering most of the analysed fluorite was sampled from the hydrothermal veins hosted in the rhyolitic rocks (Table 1), and the former studies showing that different isotope systems could become equilibrated over different distances [90,95], we recognised that the exsolved fluids could easily become rock-buffered with respect to the Sr isotopes in the early stage of the fracture generation (opening of hydrothermal veins, Figure 9a), and turned to be later partially rock-buffered with respect to Nd during the precipitation of the fluorite (Figure 9b). We use a modified version of the equations to calculate the water/rock ratio (W/R) for modelling fluid/rock interaction using radiogenic isotopes [93]:
where C is the Nd concentrations and (i) and (f) represent the initial and final rock εNd values (= 0.93 and ) = −0.18, [41]). The calculated results show that water/rock ratios are varying between 0.08 and 3.57. This suggests different degrees of water–rock interaction and isotopic exchange happened between the fluid and rocks at Chalukou. This hydrothermal fluid altered the aplite porphyry and rhyolitic strata, causing higher concentrations of Pb and Zn in both rocks (average values: 72.8 ppm Zn and 20.3 ppm Pb in strata, 82.1 ppm Zn and 46.3 ppm Pb in aplite porphyry, [91]), and led the shallow vein-type Pb-Zn mineralisation. However, because some Nd isotopes cannot be equilibrated with the host rocks, we still need to consider the possibility of the fluid isotopic mixing from different sources in the following sections.
6.3. Mixing Model of Fluid Sr–Nd Isotopes
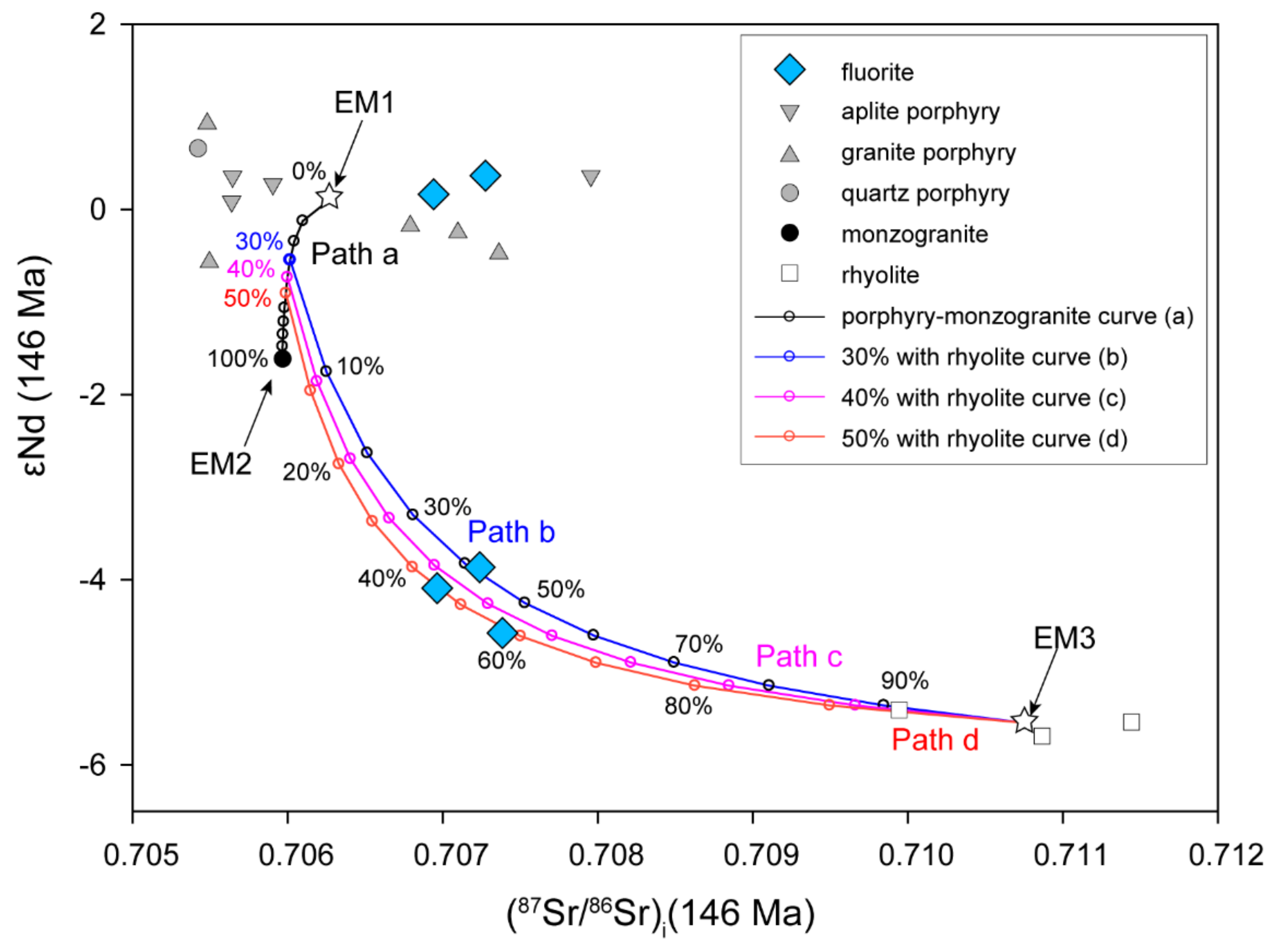
Considering the source mixing process of fluids recorded by Sr-Nd isotopes, the isotopic compositions of hydrothermal fluorite can show the mixed signature in Figure 10. We first selected possible candidates for a binary system (the isotope compositions of end-members in the calculation are listed in Table 4). Since the ore-forming materials have been proven to be derived from ore-forming porphyries (including aplite porphyry, granite porphyry, and quartz porphyry) [42,45], we select these low 87Sr/86Sr ratios and high εNd (t) values of porphyries as endmember one (averaged initial Sr ratio and εNd value noted as endmember 1, EM1, Figure 10). The second endmember (noted as EM2, Figure 10) should be characterised by higher 87Sr/86Sr ratios and lower εNd (t) values, which are most likely rhyolite or any fluid equilibrating with it (noted as EM3, Figure 10). However, a simple two-component mixing model between porphyries and rhyolite can hardly generate the observed Sr–Nd isotopes of the fluorites (Figure 10). Because monzogranite is also the ore host rock and is strongly altered, we infer that monzogranite also played a significant role in Sr-Nd mixing processes after fluid exsolution. Therefore, it is reasonable to deduce that hydrothermal fluids derived from porphyries first contacted monzogranites and formed intense potassic alterations (Figure 10, path a). Then, the fluid continued to migrate outwards and upwards and interacted with the rhyolite with various alterations. In this case, the two-stage mixing model curves can reasonably fit the observed Sr–Nd isotopes of fluorite (Figure 10). In our model, an intermediate fluid was generated by the 30–50% isotopic exchange between the initial fluids and monzogranite along pathway A at the first stage. Later, in the second stage, this mixed fluid made a 40–60% isotopic exchange with the rhyolite along pathways b, c, or d. Thus, the observed Sr-Nd isotopes in fluorite can be modelled as the mixing processes from different sources, and both the water–rock interaction and fluid mixing processes from different sources make the contribution to the features of the Chalukou fluorite Sr-Nd isotopes.
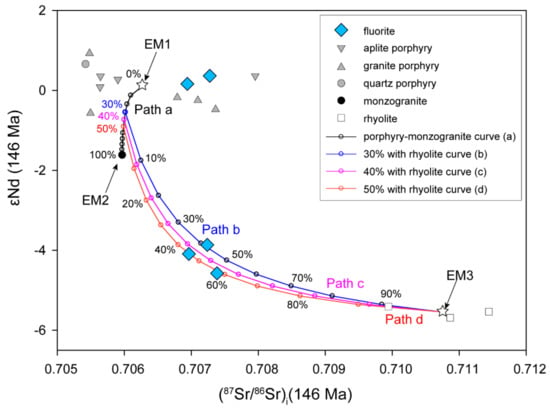
Figure 10.
The (87Sr/86Sr)i–εNd (t) diagram of the Chalukou fluorite, showing mixed fluids from multiple sources.

Table 4.
The isotope compositions of endmember used in the binary Sr-Nd mixing calculation (Figure 10).
7. Conclusions
REE and Sr–Nd isotopes of fluorite, as well as the Sr isotopes of sphalerite in the Chalukou deposit, record the features of fluid sources and water–rock interactions in the Mo–Zn–Pb deposit. The chondrite-normalised REE patterns of fluorite from early molybdenite-bearing veins show LREE enrichment, negative Eu anomalies, and weakly negative Y anomalies, whereas the REE patterns of fluorite associated with sphalerite show flat REE curves, negative Eu anomalies, and positive Y anomalies. From the Mo mineralising stage to the Zn–Pb stage, the REE concentrations of fluorites decrease, and Y/Ho ratios increase. All of these characteristics can be explained by the different mobilities of REE complexes during fluid migration. Two initial 87Sr/86Sr values of sphalerite and three εNd (t) values of fluorite are close to those of source rock porphyries (aplite porphyry, granite porphyry, and quartz porphyry), implying that they inherited the isotopic features of the magmatic source. Most fluorite Sr–Nd values are between those of intrusions and rhyolites, indicating that ore-forming fluids were derived from ore-forming porphyries and influenced by wall rocks. Together with a two-stage Sr–Nd isotope mixing model, we suggest that different sources and fluid–rock interactions (syn-ore intrusions and strata) finally influence the Sr–Nd isotopes of the ore-forming fluids, which are recorded by the majority of fluorite and sphalerite.
Author Contributions
Conceptualisation, L.J. and K.Q.; field investigation, L.J., Z.L., G.S. and G.L.; methodology, J.Z., L.J. and Z.C.; data curation, L.J. and J.Z.; writing—original draft preparation, L.J.; writing—review and editing, J.Z. and K.Q.; Supervision, G.L. and K.Q.; funding acquisition, K.Q., J.Z. and L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation Project (No. 41390444 and 41872086), the Society of Economic Geologists Hugh McKinstry Fund to Luying Jin, and the Youth Innovation Promotion Association CAS (2019070) to Junxing Zhao.
Data Availability Statement
All the data are presented in the paper.
Acknowledgments
The authors are greatly indebted to Yan Yan, Xia-Nan Zhang, and Dong-Mei Tang for assistance with Sr-Nd analyses, Wen-Jun Li, Bing-Yu Gao, Chun-long Wang, and Xin-Di Jin for guidance on REE analyses. We thank Qi-Feng Zhou and Ming-Jian Cao for their constructive suggestions. The authors would like to acknowledge the editors-in-chief and three anonymous reviewers for their constructive comments, which helped to significantly improve the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- A McPhie, J.; Kamenetsky, V.; Allen, S.; Ehrig, K.; Agangi, A.; Bath, A. The fluorine link between a supergiant ore deposit and a silicic large igneous province. Geology 2011, 39, 1002–1006. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Liu, C.Z.; Wang, J.G.; Su, B.; Gao, Y.J.; Wu, F.Y.; Sein, K.; Yang, Y.H.; Mao, Q. Scheelite and coexisting F-rich zoned garnet, vesuvianite, fluorite, and apatite in calc-silicate rocks from the Mogok metamorphic belt, Myanmar: Implications for metasomatism in marble and the role of halogens in W mobilisation and mineralisation. J. Asian Eart Sci. 2016, 117, 82–106. [Google Scholar] [CrossRef]
- Deng, X.H.; Chen, Y.J.; Yao, J.M.; Bagas, L.; Tang, H.S. Fluorite REE-Y (REY) geochemistry of the ca. 850 Ma Tumen molybdenite–fluorite deposit, eastern Qinling, China: Constraints on ore genesis. Ore Geol. Rev. 2014, 63, 532–543. [Google Scholar] [CrossRef]
- Aksyuk, A.M. Estimation of fluorine concentrations in fluids of mineralised skarn systems. Econ. Geol. 2000, 95, 1339–1347. [Google Scholar] [CrossRef]
- Xie, L.; Wang, R.C.; Groat, L.A.; Zhu, J.C.; Huang, F.F.; Cempírek, J. A combined EMPA and LA-ICP-MS study of Li-bearing mica and Sn–Ti oxide minerals from the Qiguling topaz rhyolite (Qitianling District, China): The role of fluorine in origin of tin mineralisation. Ore Geol. Rev. 2015, 65, 779–792. [Google Scholar] [CrossRef]
- Gonza’lez-Partidaa, E.; Carrillo-Cha´veza, A.; Grimmerb, J.O.W.; Pirononb, J.; Muttererc, J.; Levressea, G. Fluorite deposits at Encantada–Buenavista, Mexico: Products of Mississippi Valley type processes. Ore Geol. Rev. 2003, 23, 107–124. [Google Scholar] [CrossRef]
- Pelch, M.A.; Appold, M.S.; Emsbo, P.; Bodnar, R.J. Constraints from Fluid Inclusion Compositions on the Origin of Mississippi Valley-Type Mineralization in the Illinois-Kentucky District. Econ. Geol. 2015, 110, 787–808. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Samson, I.M.; Olivo, G.R. The genesis of hydrothermal fluorite–REE deposits in the Gallinas Mountains, New Mexico. Econ. Geol. 2000, 95, 327–342. [Google Scholar] [CrossRef]
- Anenburg, M.; Mavrogenes, J.A.; Frigo, C.; Wall, F. Rare earth element mobility in and around carbonatites controlled by sodium, potassium, and silica. Sci. Adv. 2020, 6, eabb6570. [Google Scholar] [CrossRef]
- Halliday, A.N.; Shepherd, T.J.; Dickin, A.P.; Chesley, J.T. Sm–Nd evidence for the age and origin of a Mississippi Valley type ore deposits. Nature 1990, 344, 54–56. [Google Scholar] [CrossRef]
- Chesley, J.T.; Halliday, A.N.; Scrivener, C. Samarium–Neodymium direct dating of fluorite mineralisation. Science 1991, 252, 949–951. [Google Scholar] [CrossRef]
- Chesley, J.T.; Halliday, A.N.; Kyser, T.K.; Spry, P.G. Direct dating of Mississippi Valley-type mineralisation: Use of Sm–Nd in fluorite. Econ. Geol. 1994, 89, 1192–1199. [Google Scholar] [CrossRef]
- Pei, Q.M.; Li, C.H.; Zhang, S.T.; Zou, H.; Liang, Y.; Wang, L.; Li, S.L.; Cao, H.W. Vein-type fluorite mineralisation of the Linxi district in the Great Xing’an Range, Northeast China: Insights from geochronology, mineral geochemistry, fluid inclusion and stable isotope systematics. Ore Geol. Rev. 2022, 142, 104708. [Google Scholar] [CrossRef]
- Evans, N.J.; Wilson, N.; Cline, J.; McInnes, B.I.A.; Byrne, J. Fluorite (U-Th)/He thermochronology: Constraints on the low temperature history of Yucca Mountain, Nevada. Appl. Geochem. 2005, 20, 1099–1105. [Google Scholar] [CrossRef]
- Pi, T.; Solé, J.; Taran, Y. (U-Th)/He dating of fluorite: Application to the La Azul fluorspar deposit in the Taxco mining district, Mexico. Min. Depos. 2005, 39, 976–982. [Google Scholar] [CrossRef]
- Siebel, W.; Hann, H.P.; Danišík, M.; Shang, C.K.; Berthold, C.; Rohrmüller, J.; Wemmer, K.; Evans, N.J. Age constraints on faulting and fault reactivation: A multi-chronological approach. Int. J. Earth Sci. Geol. Rundsch. 2010, 99, 1187–1197. [Google Scholar] [CrossRef]
- Wolff, R.F.; Dunkl, I.; Kempe, U.; Eynatten, H.V. The Age of the Latest Thermal Overprint of Tin and Polymetallic Deposits in the Erzgebirge, Germany: Constraints from Fluorite (U-Th-Sm)/He Thermochronology. Econ. Geol. 2015, 110, 2025–2040. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium: 1. Review of available low-temperature data for inorganic complexes and the inorganic REE speciation of natural waters. Chem. Geol. 1990, 82, 159–186. [Google Scholar] [CrossRef]
- Wood, S.A. The aqueous geochemistry of the rare-earth elements and yttrium: 2. Theoretical predictions of speciation in hydrothermal solutions to 350 °C at saturation water vapor pressure. Chem. Geol. 1990, 88, 99–125. [Google Scholar] [CrossRef]
- Bau, M. Rare-earth element mobility during hydrothermal and metamorphic fluid–rock interaction and the significance of the oxidation state of europium. Chem. Geol. 1991, 93, 219–230. [Google Scholar] [CrossRef]
- Möller, P. REE fractionation in hydrothermal fluorite and calcite. In Source, Transport and Deposition of Metals; Pagel, M., Leroy, J.L., Eds.; Balkema: Rotterdam, The Netherlands, 1991; pp. 91–94. [Google Scholar]
- Bau, M.; Dulski, P. Comparative study of yttrium and rare-earth element behaviours in fluorine-rich hydrothermal fluids. Contrib Mineral Petrol. 1995, 119, 213–223. [Google Scholar] [CrossRef]
- Strong, D.F.; Fryer, B.J.; Kerrich, R. Genesis of the St. Lawrence fluospar deposits as indicated by fluid inclusions, rare earth elements, and isotopic data. Econ. Geol. 1984, 79, 1142–1158. [Google Scholar] [CrossRef]
- Salvi, S.; Fontan, F.; Monchoux, P.; Williams-Jones, A.E.; Moine, B. Hydrothermal mobilisation of high field strength elements in alkaline igneous systems: Evidence from the Tamazeght complex (Morocco). Econ. Geol. 2000, 95, 559–576. [Google Scholar]
- Tagirov, B.; Schott, J.; Harrichourry, J.C.; Salvi, S. Experimental study of aluminum speciation in fluoride-rich supercritical fluids. Geochim. Cosmochim. Acta. 2002, 66, 2013–2024. [Google Scholar] [CrossRef]
- Schӧenberger, J.; Kӧler, J.; Markl, G. REE systematics of fluorides, calcite and siderite in peralkaline plutonic rocks from the Gardar Province, South Greenland. Chem. Geol. 2008, 247, 16–35. [Google Scholar] [CrossRef]
- Agangi, A.; Kamenetsky, V.S.; McPhie, J. The role of fluorine in the concentration and transport of lithophile trace elements in felsic magmas: Insights from the Gawler Range Volcanics, South Australia. Chem. Geol. 2010, 273, 314–325. [Google Scholar] [CrossRef]
- Sánchez, V.; Cardellach, E.; Corbella, M.; Vindel, E.; Martín-Crespo, T.; Boyce, A.J. Variability in fluid sources in the fluorite deposits from Asturias (N Spain): Further evidences from REE, radiogenic (Sr, Sm, Nd) and stable (S, C, O) isotope data. Ore Geol. Rev. 2010, 37, 87–100. [Google Scholar] [CrossRef]
- Cangelosi, D.; Broom-Fendley, S.; Banks, D.; Morgan, D.; Yardley, B. Light rare earth element redistribution during hydrothermal alteration at the Okorusu carbonatite complex, Namibia. Mineral. Mag. 2020, 84, 49–64. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Y.; Zhang, L.S. The role of sulfate-, alkali-, and halogen-rich fluids in mobilization and mineralization of rare earth elements: Insights from bulk fluid compositions in the Mianning–Dechang carbonatite-related REE belt, southwestern China. Lithos 2021, 386–387, 106008. [Google Scholar] [CrossRef]
- Ruiz, J.; Kesler, S.E.; Jones, L.M. Strontium isotope geochemistry of fluorite mineralisation associated with fluorine-rich igneous rocks from the Sierra Madre Occidental, Mexico: Possible exploration significance. Econ. Geol. 1985, 80, 33–42. [Google Scholar] [CrossRef]
- Ruiz, J.; Richardson, C.K.; Patchett, P.J. Strontium isotope geochemistry of fluorite, calcite, and barite of the Cave-in-Rock Fluorite District, Illinois. Econ. Geol. 1988, 83, 203–210. [Google Scholar] [CrossRef]
- Ronchi, L.H.; Touray, J.C.; Michard, A.; Dardenne, M.A. The Ribeira Fluorite District, southern Brazil. Geological and geochemical (REE, Sm–Nd isotopes) characteristics. Min. Depos. 1993, 28, 240–252. [Google Scholar] [CrossRef]
- Galindo, C.; Tornos, F.; Darbyshire, D.P.F.; Casquet, C. The age and origin of the barite–fluorite (Pb–Zn) veins of the Sierra del Guadarrama (Spanish Central System, Spain): A radiogenic (Nd, Sr) and stable isotope study. Chem. Geol. 1994, 112, 351–364. [Google Scholar] [CrossRef][Green Version]
- Simonetti, A.; Bell, K. Nd, Pb, and Sr isotopes systematics of fluorite at the Amba Dongar carbonatite complex, India: Evidence for hydrothermal and crustal fluid mixing. Econ. Geol. 1995, 90, 2018–2027. [Google Scholar] [CrossRef]
- Partey, F.; Lev, S.; Casey, R.; Widom, E.; Lueth, V.W.; Rakovan, J. Source of fluorine and petrogenesis of the Rio Grande Rift type barite-fluorite-galena deposits. Econ. Geol. 2004, 104, 505–520. [Google Scholar] [CrossRef]
- Sallet, R.; Moritz, R.; Fontignie, D. The use of vein fluorite as probe for paleofluid REE and Sr–Nd isotope geochemistry: The Santa Catarina Fluorite District, Southern Brazil. Chem. Geol. 2005, 223, 227–248. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Ling, H.F.; Jiang, S.Y.; Shen, W.Z.; Fan, H.H.; Ni, P. Trace element and Sr-Nd isotope geochemistry of fluorite from the Xiangshan uranium deposit, Southeast China. Econ. Geol. 2006, 101, 1613–1622. [Google Scholar] [CrossRef]
- Castorina, F.; Masi, U.; Padalino, G.; Palomba, M. Trace-element and Sr–Nd isotopic evidence for the origin of the Sardinian fluorite mineralisation (Italy). Appl. Geochem. 2008, 23, 2906–2921. [Google Scholar] [CrossRef]
- 706 Geological Party of Heilongjiang Geological Exploration Bureau for Nonferrous Metal. Geological Exploration Report of the Chalukou Molybdenum Deposit; 706 Geological Party of Heilongjiang Geological Exploration Bureau for Nonferrous Metal: Qiqihaer, China, 2012; pp. 1–219, (In Chinese). unpublished. [Google Scholar]
- Li, Z.Z.; Qin, K.Z.; Li, G.M.; Ishihara, S.; Jin, L.Y.; Song, G.X.; Meng, Z.J. Formation of the giant Chalukou porphyry Mo deposit in northern Great Xing’an Range, NE China: Partial melting of the juvenile lower crust in intra-plate extensional environment. Lithos 2014, 202, 138–156. [Google Scholar] [CrossRef]
- Jin, L.Y. Metallogenesis of Chalukou Porphyry Mo and Vein type Zn-Pb Mineralisation System in Northern Great Xing’an Range. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2016, unpublished. [Google Scholar]
- Zhao, Q.; Zhai, D.; Mathur, R.; Liu, J.; Selby, D.; Williams-Jones, A.E. The giant Chalukou porphyry Mo deposit, northeast China: The product of a short-lived, high flux mineralising event. Econ. Geol. 2021, 116, 1209–1225. [Google Scholar] [CrossRef]
- Li, Z.Z.; Qin, K.Z.; Li, G.M.; Jin, L.Y.; Song, G.X.; Han, R. Incursion of meteoric water triggers molybdenite precipitation in porphyry Mo deposits: A case study of the Chalukou giant Mo deposit. Ore Geol. Rev. 2019, 109, 144–162. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.W.; Wu, G.; Wang, F.; Luo, D.F.; Hu, Y.Q.; Li, T.G. Fluid inclusions and H–O–S–Pb isotope systematics of the Chalukou giant porphyry Mo deposit, Heilongjiang Province, China. Ore Geol. Rev. 2014, 59, 83–96. [Google Scholar] [CrossRef]
- Sengör, A.M.C.; Natal’in, B.A.; Burtman, V.S. Evolution of the Altaid tectonic collage and Palaeozoic crustal growth in Eurasia. Nature 1993, 364, 299–307. [Google Scholar] [CrossRef]
- Jahn, B.M.; Wu, F.Y.; Chen, B. Massive granitoid generation in Central Asia: Nd isotope evidence and implication for continental growth in the Phanerozoic. Episodes 2000, 23, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.J.; Windley, B.F.; Hao, J.; Zhai, M.G. Accretion leading to collision and the Permian Solonker suture, Inner Mongolia, China: Termination of the central Asian orogenic belt. Tectonics 2003, 22, 1–20. [Google Scholar] [CrossRef]
- Qin, K.Z.; Zhai, M.G.; Li, G.M.; Zhao, J.X.; Zhai, M.G.; Zeng, Q.D.; Gao, J.; Xiao, W.J.; Li, J.L.; Sun, S. Links of Collage orogenesis of multiblocks and crust evolution to characteristic metallogeneses in China. Acta Petrol. Sin. 2017, 33, 305–325, (In Chinese with English Abstract). [Google Scholar]
- Li, J.Y. Permian geodynamic setting of Northeast China and adjacent regions: Closure of the Paleo-Asian Ocean and subduction of the Paleo-Pacific Plate. J. Asian Eart Sci. 2006, 26, 207–224. [Google Scholar] [CrossRef]
- Ge, W.C.; Sui, Z.M.; Wu, F.Y.; Zhang, J.H.; Xu, X.C.; Cheng, R.Y. Zircon U-Pb ages, Hf isotopic characteristics and their implications of the Early Paleozoic granites in the northeastern Da Hinggan Mts., northeastern China. Acta Petrol. Sin. 2007, 23, 423–440, (In Chinese with English Abstract). [Google Scholar]
- Wu, F.Y.; Sun, D.Y.; Ge, W.C.; Zhang, Y.B.; Grant, M.L.; Wilde, S.A.; Jahn, B.M. Geochronology of the Phanerozoic granitoids in northeastern China. J. Asian Eart Sci. 2011, 41, 1–30. [Google Scholar] [CrossRef]
- Zhou, J.B.; Wilde, S.A. The crustal accretion history and tectonic evolution of the NE China segment of the Central Asian Orogenic Belt. Gondwana Res. 2013, 23, 1365–1377. [Google Scholar] [CrossRef]
- Zhou, J.B.; Wilde, S.A.; Zhang, X.; Zhao, G.C.; Zheng, C.Q.; Wang, Y.J.; Zhang, X.H. The onset of Pacific margin accretion in NE China: Evidence from the Heilongjiang high-pressure metamorphic belt. Tectonophysics 2009, 478, 230–246. [Google Scholar] [CrossRef]
- Kravchinsky, V.A.; Cogné, J.P.; Harbert, W.P.; Kuzmin, M.I. Evolution of the Mongol-Okhotsk Ocean as constrained by new palaeomagnetic data from the Mongol-Okhotsk suture zone, Siberia. Geophys. J. Int. 2002, 148, 34–57. [Google Scholar] [CrossRef]
- Shi, G.H.; Liu, D.Y.; Zhang, F.Q.; Jian, P.; Miao, L.C.; Shi, Y.R.; Tao, H. SHRIMP U-Pb zircon geochronology and its implications on the Xilin Gol Complex, Inner Mongolia, China. Geol. Bull. China 2003, 48, 2742–2748. [Google Scholar]
- Wang, F.; Zhou, X.H.; Zhang, L.C.; Ying, J.F.; Zhang, Y.T.; Wu, F.Y.; Zhu, R.X. Late Mesozoic volcanism in the Great Xing’an Range (NE China): Timing and implications for the dynamic setting of NE Asia. Earth Planet. Sci. Lett. 2006, 251, 179–198. [Google Scholar] [CrossRef]
- Zhang, J.H.; Gao, S.; Ge, W.C.; Wu, F.Y.; Yang, J.H.; Wilde, S.A.; Li, M. Geochronology of the Mesozoic volcanic rocks in the Great Xing’an Range, northeastern China: Implications for subduction-induced delamination. Chem. Geol. 2010, 276, 144–165. [Google Scholar] [CrossRef]
- Zeng, Q.D.; Liu, J.P.; Yu, C.M.; Ye, J.; Liu, H.T. Metal deposits in the Da Hinggan Mountains, NE China: Styles, characteristics, and exploration potential. Int. Geol. Rev. 2011, 53, 846–878. [Google Scholar] [CrossRef]
- Liu, J.; Wu, G.; Li, Y.; Zhu, M.T.; Zhong, W. Re-Os sulfide (chalcopyrite, pyrite and molybdenite) systematics and fluid inclusion study of the Duobaoshan porphyry Cu (Mo) deposit, Heilongjiang Province, China. J. Asian Earth Sci. 2012, 49, 300–312. [Google Scholar] [CrossRef]
- Deng, K.; Li, N.; Yang, Y.F.; Zhang, C.; Yu, Y.B.; Zhang, D.C. Fluid inclusion constraints on the origin of the Zhengguang gold deposit, Heihe City, Heilongjiang Province. Acta Petrol. Sin. 2013, 29, 231–240, (In Chinese with English Abstract). [Google Scholar]
- Li, Z.Z.; Qin, K.Z.; Li, G.M.; Jin, L.Y.; Song, G.X. Neoproterozoic and Early Paleozoic magmatic records from the Chalukou ore district, northern Great Xing’an Range, NE China: Implications for tectonic evolution and Mesozoic Mo mineralisation. J. Asian Eart Sci. 2018, 165, 96–113. [Google Scholar] [CrossRef]
- Liu, J.; Mao, J.W.; Wu, G.; Wang, F.; Luo, D.F.; Hu, Y.Q. Zircon U–Pb and molybdenite Re–Os dating of the Chalukou porphyry Mo deposit in the northern Great Xing’an Range, China and its geological significance. J. Asian Earth Sci. 2014, 79, 696–709. [Google Scholar] [CrossRef]
- Jin, L.Y.; Qin, K.Z.; Li, G.M.; Zhao, J.X.; Li, Z.Z. Characteristics, controlling factors and exploration implications of porphyry molybdenum-hydrothermal vein-style lead-zinc-silver metallogenic systems. Acta Petrol. Sin. 2020, 36, 3813–3829, (In Chinese with English Abstract). [Google Scholar]
- Chu, Z.Y.; Wu, F.Y.; Walker, R.J.; Rudnick, R.L.; Pitcher, L.; Puchtel, I.S.; Yang, Y.H.; Wilde, S.A. Temporal evolution of the lithospheric mantle beneath the eastern North China craton. J. Petrol. 2009, 50, 1857–1898. [Google Scholar] [CrossRef]
- Sun, S.S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313–345. [Google Scholar] [CrossRef]
- Fleischer, M.; Altschuler, Z. The relationship of the rare-earth composition of minerals to geological environment. Geochim. Cosmochim. Acta. 1969, 33, 725–732. [Google Scholar] [CrossRef]
- Sallet, R.; Moritz, R.; Fontignie, D. Fluorite 87Sr/86Sr and REE constraints on fluid–melt relations, crystallisation time span and bulk DSr of evolved high-silica granites. Tabuleiro granites, Santa Catarina, Brazil. Chem. Geol. 2000, 164, 81–92. [Google Scholar] [CrossRef]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta. 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P. Rare earth element fractionation in metamorphogenic hydrothermal calcite, magnesite and siderite. Miner. Petrol. 1992, 45, 231–246. [Google Scholar] [CrossRef]
- Schwinn, G.; Markl, G. REE systematics in hydrothermal fluorite. Chem. Geol. 2005, 216, 225–248. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Gammons, C.H.; Wood, S.A.; Williams-Jones, A.E. The aqueous geochemistry of the rare earth elements and yttrium: VI. Stability of neo-dynium chloride complexes from 250 °C to 300 °C. Geochim. Cosmochim. Acta. 1996, 60, 4615–4630. [Google Scholar] [CrossRef]
- Gammons, C.H.; Wood, S.A.; Li, Y. Complexation of the rare earth elements with aqueous chloride at 200 °C and 300 °C and saturated water vapor pressure. Geol. Soc. Lond. Spec. Publ. 2002, 7, 191–207. [Google Scholar]
- Pan, Y.; Fleet, M.E. Rare earth element mobility during prograde granulite facies metamorphism: Significance of fluorine. Contrib Mineral Petrol. 1996, 123, 251–262. [Google Scholar] [CrossRef]
- Smith, M.; Henderson, P.; Campbell, L. Fractionation of the REE during hydrothermal processes: Constraints from the Bayan Obo Fe-REE-Nb deposit, Inner Mongolia, China. Geochim. Cosmochim. Acta. 2000, 64, 3141–3160. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A. Hydrothermal transport and deposition of the rare earth elements by fluorine-bearing aqueous liquids. Min. Depos. 2014, 49, 987–997. [Google Scholar] [CrossRef]
- Haas, J.R.; Shock, E.L.; Sassani, D.C. Rare earth elements in hydrothermal systems: Estimates of standard partial molal thermodynamic properties of aqueous complexes of the rare earth elements at high pressures and temperatures. Geochim. Cosmochim. Acta. 1995, 59, 4329–4350. [Google Scholar] [CrossRef]
- Luo, Y.R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta. 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Williams-Jones, A.; Wagner, T. An experimental study of the solubility and speciation of the Rare Earth Elements (III) in fluoride-and chloride-bearing aqueous solutions at temperatures up to 300 °C. Geochim. Cosmochim. Acta. 2009, 73, 7087–7109. [Google Scholar] [CrossRef]
- Lüders, V.; Möller, P.; Dulski, P. REE fractionation in carbonates and fluorite. In Formation of Hydrothermal Vein Deposits; Möller, P., Lüders, V., Eds.; Borntraeger: Berlin, Germany, 1993; pp. 133–150. [Google Scholar]
- Xu, C.; Huang, Z.L.; Qi, L.; Xiao, H.Y.; Li, W.B.; Liu, C.Q. Source and evolution of ore- forming fluids of Maoniuping rare-earth deposit, evidence from REE geochemistry of fluorites. Geol. Prosp. 2001, 37, 24–28, (In Chinese with English Abstract). [Google Scholar]
- Trinkler, M.; Monecke, T.; Thomas, R. Constraints on the genesis of yellow fluorite in hydrothermal barite–fluorite veins of the Erzgebirge, Eastern Germany: Evidence from optical absorption spectroscopy, rare-earth-element data, and fluid-inclusion investigations. Can. Miner. 2005, 43, 883–898. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Veklser, I.V.; Dorfman, A.M.; Kamenetsky, M.; Dulski, P.; Dingwell, D. Partitioning of lanthanides and Y between immiscible silicate and fluoride melts, fluorite and cryolite and the origin of the lanthanide tetrad effect in igneous systems. Geochim. Cosmochim. Acta. 2005, 69, 2847–2860. [Google Scholar]
- Chang, Z.S.; Meinert, L.D. The magmatic-hydrothermal transition—Evidence from quartz phenocryst textures and endoskarn abundance in Cu-Zn skarns at the Empire Mine, Idaho, USA. Chem. Geol. 2004, 210, 149–171. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophys. Res.Solid Earth. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Kraemer, D.; Kopf, S.; Bau, M. Oxidative mobilisation of cerium and uranium and enhanced release of “immobile” high field strength elements from igneous rocks in the presence of the biogenic siderophore desferrioxamine B. Geochim. Cosmochim. Acta. 2015, 165, 263–279. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Hickey, K.A.; Cline, J.S.; Dipple, G.M.; Kilburn, M.R.; Vaughan, J.R.; Longo, A.A. Uncloaking invisible gold: USE of nanosims to evaluate gold, trace elements, and sulfur isotopes in pyrite from Carlin-type gold deposits. Econ. Geol. 2009, 104, 897–904. [Google Scholar] [CrossRef]
- Li, Z.Z. Fluorine-Rich and Highly Oxidised Magmatic-Hydrothermal Evolution and Metallogenesis of Chalukou Giant Porphyry Mo Deposit in Northern Great Xing’an Range. Ph.D. Thesis, University of Chinese Academy of Sciences, Beijing, China, 2014, unpublished. [Google Scholar]
- Bau, M.; Romer, R.L.; Lüders, V.; Dulski, P. Tracing element sources of hydrothermal mineral deposits: REE and Y distribution and Sr-Nd-Pb isotopes in fluorite from MVT deposits in the Pennine Orefield, England. Min. Depos. 2003, 38, 992–1008. [Google Scholar] [CrossRef]
- Voicu, G.; Bardoux, M.; Stevenson, R.; Jebrak, M. Nd and Sr isotope study of hydrothermal scheelite and host rocks at Omai, Guiana Shield: Implications for ore fluid source and flow path during the formation of orogenic gold deposits. Min. Depos. 2000, 35, 302–314. [Google Scholar] [CrossRef]
- Walshaw, R.D.; Menuge, J.F.; Tyrrell, S. Metal sources of the Navan carbonate-hosted base metal deposit, Ireland: Nd and Sr isotope evidence for deep hydrothermal convection. Min. Depos. 2006, 41, 803–819. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Bennett, V.C.; Cox, S.F.; Norman, M.D.; Gagan, M.K. Sm–Nd, Sr, C and O isotope systematics in hydrothermal calcite–fluorite veins: Implications for fluid–rock reaction and geochronology. Chem. Geol. 2009, 268, 58–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).