Abstract
Melting phase relations in the eclogite-carbonate system were studied at 6 GPa and 900–1500 °C. Starting mixtures were prepared by blending natural bimineral eclogite group A (Ecl) with eutectic Na-Ca-Mg-Fe (N2) and K-Ca-Mg-Fe (K4) carbonate mixtures (systems Ecl-N2 and Ecl-K4). In the Ecl-N2 system, the subsolidus assemblage is represented by garnet, omphacite, eitelite, and a minor amount of Na2Ca4(CO3)5. In the Ecl-K4 system, the subsolidus assemblage includes garnet, clinopyroxene, K2Mg(CO3)2, and magnesite. The solidus of both systems is located at 950 °C and is controlled by the following melting reaction: Ca3Al2Si3O12 (Grt) + 2(Na or K)2Mg(CO3)2 (Eit) = Ca2MgSi3O12 (Grt) + [2(Na or K)2CO3∙CaCO3∙MgCO3] (L). The silica content (in wt%) in the melt increases with temperature from < 1 at 950 °C to 3–7 at 1300 °C, and 7–12 at 1500 °C. Thus, no gradual transition from carbonate to kimberlite-like (20–32 wt% SiO2) carbonate-silicate melt occurs even as temperature increases to mantle adiabat. This supports the hypothesis that the high silica content of kimberlite is the result of decarbonation at low pressure. As temperature increases from 950 to 1500 °C, the melt Ca# ranges from 58–60 to 42–46. The infiltration of such a melt into the peridotite mantle should lower its Ca# and causes refertilization from harzburgite to lherzolite and wehrlitization.
1. Introduction
Diamondiferous eclogite xenoliths derived from the base of the subcontinental lithospheric mantle (SCLM) often show traces of mantle metasomatism [1,2,3,4]. Three-dimensional, high-resolution X-ray computed tomography of eclogite xenoliths has revealed that diamonds grew in metasomatic alteration zones [5,6,7]. Numerous microinclusions of calcite and alkaline Cl-bearing carbonate melt found in diamonds from metasomatic veins in eclogite xenoliths [1,8,9] indicate that diamonds were formed during percolation of a carbonatitic melt through eclogite.
Mantle carbonatitic melts entrapped by diamonds from kimberlites and placers worldwide are rich in alkalis. Based on over a hundred analyses of carbonatitic inclusions containing <15 wt% SiO2 and ≤5 Cl wt% [10,11,12,13,14,15,16,17,18,19,20,21], the mantle carbonatitic melts contain (average/maximum) 6/19 wt% Na2O and 12/75 wt% K2O (see Supplementary Table S7 [22]). An affinity of high- and low-Mg carbonatitic melts to diamonds of peridotitic and eclogitic suits, respectively, was also revealed [23]. Moreover, inclusions intermediate between low-Mg carbonatitic and saline melts have been found in diamonds recovered from a xenolith of bimineralic eclogite Group B [8,13].
Alkali-rich carbonatitic inclusions have also been found in sheared garnet and spinel peridotite xenoliths derived from 110–230 km depths and magmatic minerals from kimberlites of Siberia, Canada, Greenland, and Africa [24,25,26,27,28,29,30,31,32,33,34,35]. Interestingly, alkaline carbonatite inclusions have been found in sheared peridotite xenoliths [30,32,33,34,35], while no such inclusions have been reported to occur in granular peridotite xenoliths. The latter was explained by the segregation of carbonatite melt in zones of intense deformation, by means of a dissolution–precipitation mechanism driven by mechanical stress [36].
Although the compositions of alkaline carbonate melts in equilibrium with peridotites under P-T conditions of the subcontinental lithospheric mantle and underlying asthenosphere were recently reported [37,38], data on the temperature stability range and compositions of carbonate melts equilibrium with eclogite in the mantle are rather contradictory. Estimates of the temperatures of carbonated eclogite solidus vary from ~1000 to 1350 °C at 5–6 GPa [39,40,41,42,43,44]. The experimental data on the composition of carbonate melts in equilibrium with eclogites are very limited and scattered. The reliability of a few available data points is questionable. A study of the melt compositions in experiments on the melting of the carbonated eclogites showed that these compositions do not undergo complete melting at the specified P-T conditions [45,46]. The results also showed that the stable melts have an alkaline carbonate composition similar to the eutectic melts in the Na2CO3-CaCO3-MgCO3 and K2CO3-CaCO3-MgCO3 systems at 6 GPa [47,48,49,50].
Given that alkaline carbonate melts are responsible for mantle metasomatism and diamond formation [8,51,52,53], it is interesting to know their compositions in equilibrium with eclogites at the base of SCLM and the underlying asthenosphere.
We have recently shown that such melts can be in equilibrium with eclogite at 1100 and 1200 °C at 6 GPa [54]. However, the temperature range, expected for the operation of metasomatizing carbonate melts in the mantle, is much wider. The temperature estimates for the formation of metasomatized and diamond-bearing eclogitic xenoliths and touching garnet-omphacite inclusions in diamonds fall in the range of 900–1500 °C [3,55]. The homogenization temperature of some carbonate-bearing microinclusions in cuboid diamonds reaches the temperature of the convective mantle [56], 1400–1500 °C [57].
Here, we present new experimental data on the solidus, melting reactions, and trends in the compositions of carbonate melts in the systems consisting of natural eclogite + Na-Ca-Mg-Fe and K-Ca-Mg-Fe carbonate mixtures over 900–1500 °C at 6 GPa.
2. Methods
2.1. Starting Materials
Starting materials were prepared by blending synthetic carbonate mixtures and powder of natural eclogite. The compositions of starting materials are given in Table 1 and S1–S3.

Table 1.
Composition (wt%) of starting materials.
Fresh xenolith of bimineral eclogite (UD-45-02) from the Udachnaya kimberlite pipe (Yakutia, Russia) [58], similar to that used in our previous study [54], was employed. The xenolith was chosen because it does not contain visible traces of secondary alterations and undoubtedly is of mantle origin. Compositions of clinopyroxene, Di78Jd12En4Fs6, and garnet, Prp65Alm21Grs14, correspond to Group A eclogite according to the classifications of Taylor and Neal [59] and Coleman, et al. [60], respectively (Table 1). Mass balance calculations indicate that the xenolith consists of 65 wt% garnet and 35 wt% clinopyroxene (Table S1). Although the eclogite does not contain phlogopite, the IR spectra of omphacite and garnet revealed the presence of a minor amount of water as hydroxyl structural defects. Considering the volume ratios of minerals, the bulk water content in the xenolith is estimated to be 576 ppm H2O [58]. Assuming a pressure of 5 GPa, the geothermometer of Ellis and Green [61] gives a temperature of 1193 °C [58]. It was also recently found that the xenolith UD-45-02 contains diamonds (V.S. Shatsky personal communication). Representative pieces of xenolith were ground with alcohol in a tungsten carbide mortar.
The starting carbonate mixtures, N2 and K4, were close to eutectics established at 6 GPa and 1050 °C in the Na2CO3-CaCO3-MgCO3 and K2CO3-CaCO3-MgCO3 systems, respectively [49,50]. The mixtures were blended from reagent grade Na2CO3, K2CO3, CaCO3, natural magnesite (<0.1% impurity) from Brumado (Bahia, Brazil), and siderite, Fe0.83Mn0.01Mg0.08Ca0.08CO3, from Farmsen Clay Pit, Schellerten, Hildesheim (Lower Saxony, Germany) (Table 1 and S2). The iron number, Fe# = 100∙Fe/(Fe + Mg), of carbonate mixtures, 22 mol%, was close to that of eclogite UD-45-02, 21 mol%.
2.2. High-Pressure Experiments
Since the starting mixtures contain hygroscopic compounds, K2CO3 and Na2CO3, special care was applied to prevent samples’ contamination with atmospheric water. The prepared assemblies with loaded samples were dried at 200 °C for ≥12 h under vacuum prior to experiment. To minimize the contamination of the dried cell with water during its loading into the press, indoor humidity was maintained at 15%–35%.
The design of the cell assembly is identical to that used by Shatskiy et al. [62]. The assembly includes an octahedral pressure medium made of ZrO2 ceramics [63], a graphite heater with 4.0/4.5 mm inner/outer diameter, and a W/Re (3%/25%) thermocouple, electrically insulated by Al2O3 tubes. The powdered samples were loaded in graphite capsules, electrically insulated from the heater by a thin (0.2 mm) MgO-SiO2 ceramic sleeve.
Eight tungsten carbide cubes (“Fujilloy N-05”) of 26 mm in size with 12-mm truncations were used as anvils to compress the octahedral cell assembly. Pyrophyllite gaskets, 4.0 mm in width and thickness, were fixed by rice glue at the edges of truncations to support anvil flanks. The experiments were run on a 1500-ton multianvil DIA-type press ‘Discoverer’.
The temperature gradients in the cell were examined using thermal modelling software [64]. The results revealed that the temperature gradient within individual samples varies from 7 to 14 °C/mm. The correctness of the modelling was verified experimentally [65] using the two-pyroxene thermometer [66].
Experiments were performed by 4-h compression to a load of 6.5 MN, corresponding to a sample pressure of 6 GPa, and heating to a target temperature at a rate of 25–50 °C/min. Then samples were annealed for 198 h at 900 °C, 168 h at 950 °C, 169 h at 1000 °C, 64 h at 1300 °C, 24 h at 1400 °C, and 5 h at 1500 °C. During annealing, the temperature was maintained within 2–3 °C of the desired value at a constant press load. The experiments were terminated by turning off the heater power, resulting in a temperature drop below 150 °C in a few seconds, followed by 5-h decompression.
2.3. Analytical Techniques
Immediately after experiments, the recovered graphite cassettes with samples were filled with epoxy under vacuum. Then capsules were sliced using a low-speed diamond saw to recover nearly axial, vertical cross-sections of samples. The obtained specimens were placed on a double-sided tape in a plexiglass holder with epoxy. The samples were then polished under oil using 400(37)-, 1000(13)-, and 1500(9)-mesh (μm) sandpapers. Finally, samples were polished using a 3 μm diamond paste. After polishing, the samples were cleaned using petroleum benzine and wipes and then stored in benzine before carbon coating.
Samples were studied using a MIRA 3 LMU scanning electron microscope (Tescan Orsay Holding, Brno-Kohoutovice, Czech Republic), coupled with an INCA energy-dispersive X-ray microanalysis system 450, equipped with liquid nitrogen-free Large area EDX X-Max-80 Silicon Drift Detector (Oxford Instruments Nanoanalysis Ltd., High Wycombe, UK) [67]. Energy-dispersive X-ray spectra (EDS) were collected by using an electron beam-rastering method, in which the stage was stationary while the electron beam moved over the surface area, with dimensions 5–30 μm (for silicate minerals) and 50–500 μm (for quenched melt) at 20 kV accelerating voltage and 1.5 nA beam current. The live counting time for X-ray spectra was 20 s. The silicon drift detector energy-dispersive X-ray spectrometry (SDD-EDS) enables accuracy and precision equivalent to that of wavelength-dispersive spectroscopy in the case of routine analysis of rock-forming silicate minerals [67,68] and even shows better performance in the case of alkali-rich carbonate samples, which are unstable (i.e., decompose and evaporate) under the strong stationary electron beam [69].
3. Results
The symbols used in the manuscript are given in the abbreviations section.
3.1. Textures of Recovered Samples
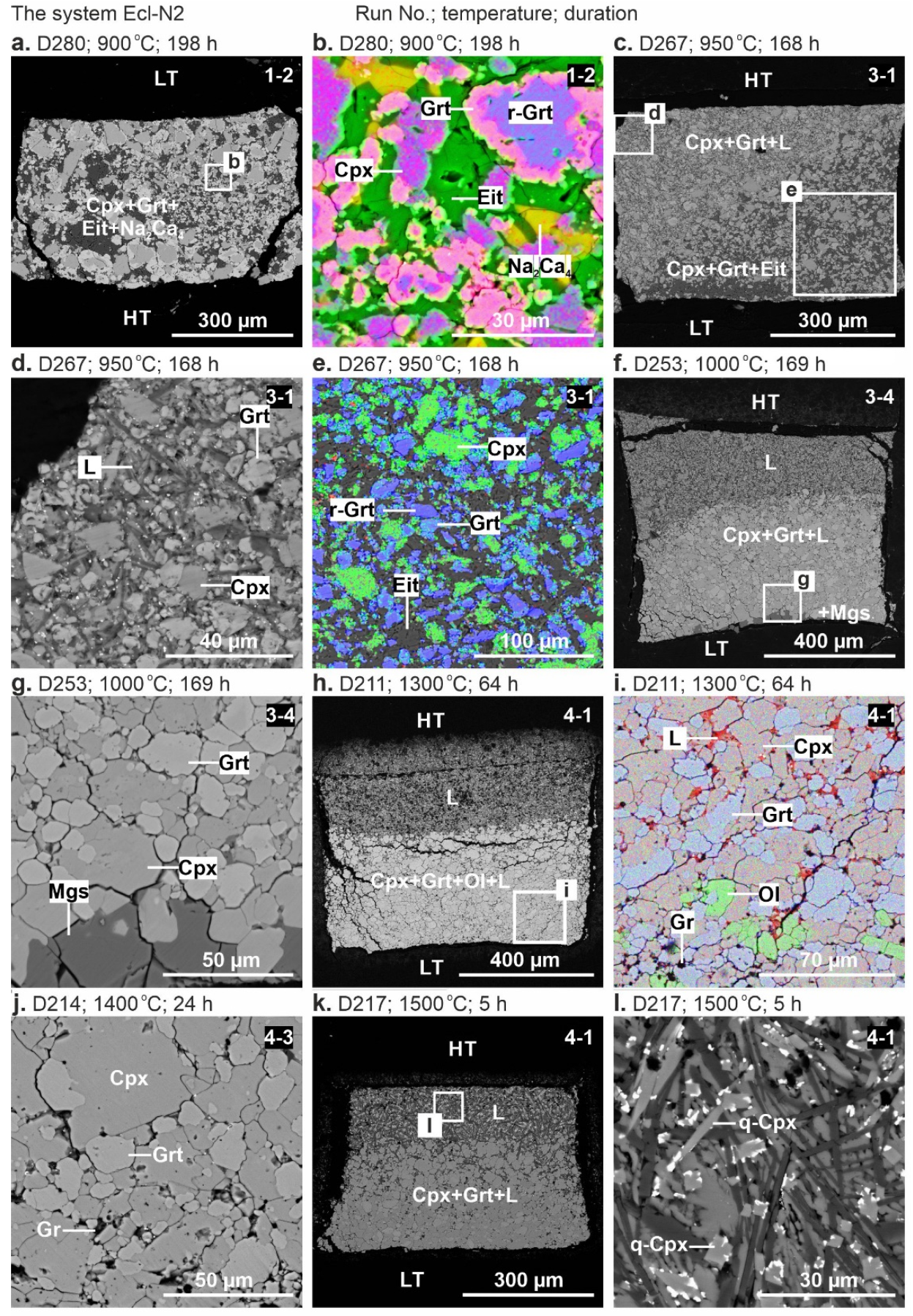
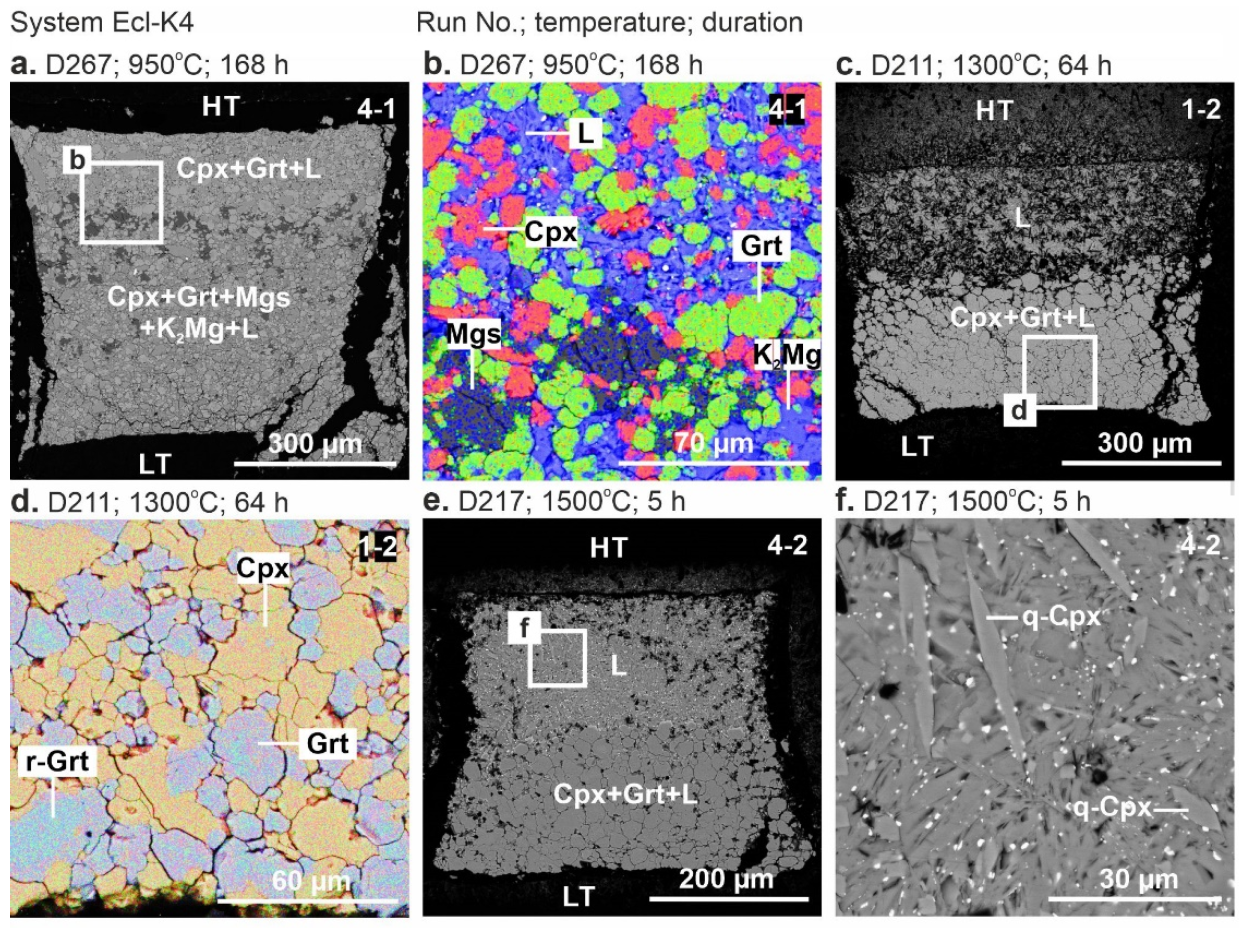
Representative backscattered electron (BSE) images of the samples are shown in Figure 1 and Figure 2. Below the solidus, at 900 °C, the crystalline phases are homogeneously distributed throughout the sample (Figure 1a,b). At 950 °C, the sample consists of the subsolidus assemblage in the low-temperature (LT) zone and quenched melt with suspended clinopyroxene and garnet crystals in the high-temperature (HT) zone (Figure 1c–e and Figure 2a,b). Above the solidus over 1000–1500 °C, the melt forms a separate pool in the HT sample side, while an aggregate of clinopyroxene and garnet crystals adjoins the LT side (Figure 1c–l and Figure 2c,e). The carbonate melt quenches to an aggregate of needle-shaped carbonate crystals up to 60 µm in length (Figure 1d). At 1500 °C, needle-shaped clinopyroxene crystals appear in addition to carbonate (Figure 1l and Figure 2f).
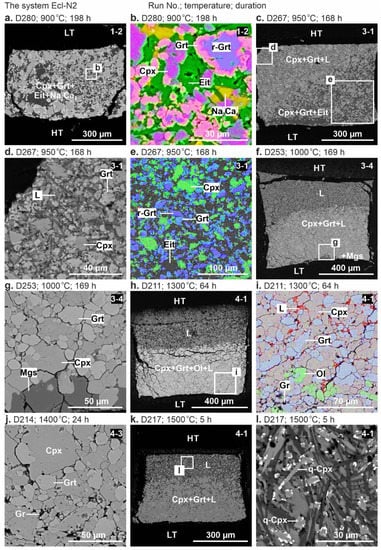
Figure 1.
BSE images of sample cross-sections from 6-GPa experiments in the system Ecl-N2 at 900 °C (a,b), 950 °C (c–e), 1000 °C (f,g), 1300 °C (h,i), 1400 °C (j), and 1500 °C (k,l). HT—high-temperature and LT—low-temperature sample sides. The gravity vector is directed downward. The color images (b,e,i) were taken in the element mapping mode. The numbers at the top-right corners of each subfigure are sample numbers. See the abbreviations section for mineral and phase symbols.
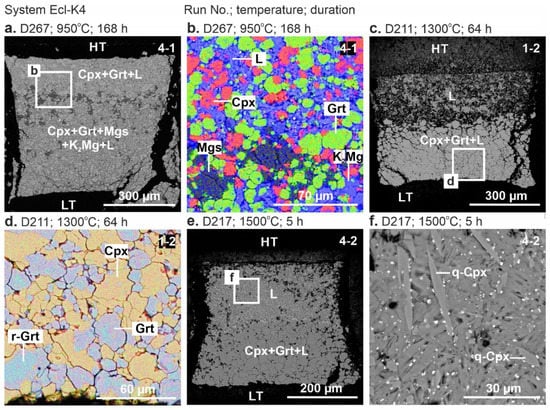
Figure 2.
BSE images of sample cross-sections from 6-GPa experiments in the systems Ecl-K4 at 950 °C (a,b), 1300 °C (c,d), 1500 °C (e,f). See the Figure 1 caption for other details. See the abbreviations section for mineral and phase symbols.
Clinopyroxene, garnet, and olivine form euhedral to subhedral grains 5–50 µm in size (Figure 1 and Figure 2). Relicts with the initial composition remain in the larger garnet crystals, while the smaller ones are free of relicts (Figure 1b). In the Ecl-N2 system over 1100–1300 °C, in addition to clinopyroxene and garnet, a minor amount of olivine appears as well-shaped euhedral crystals up to 15 μm in size (Figure 1i).
3.2. Phase Relations
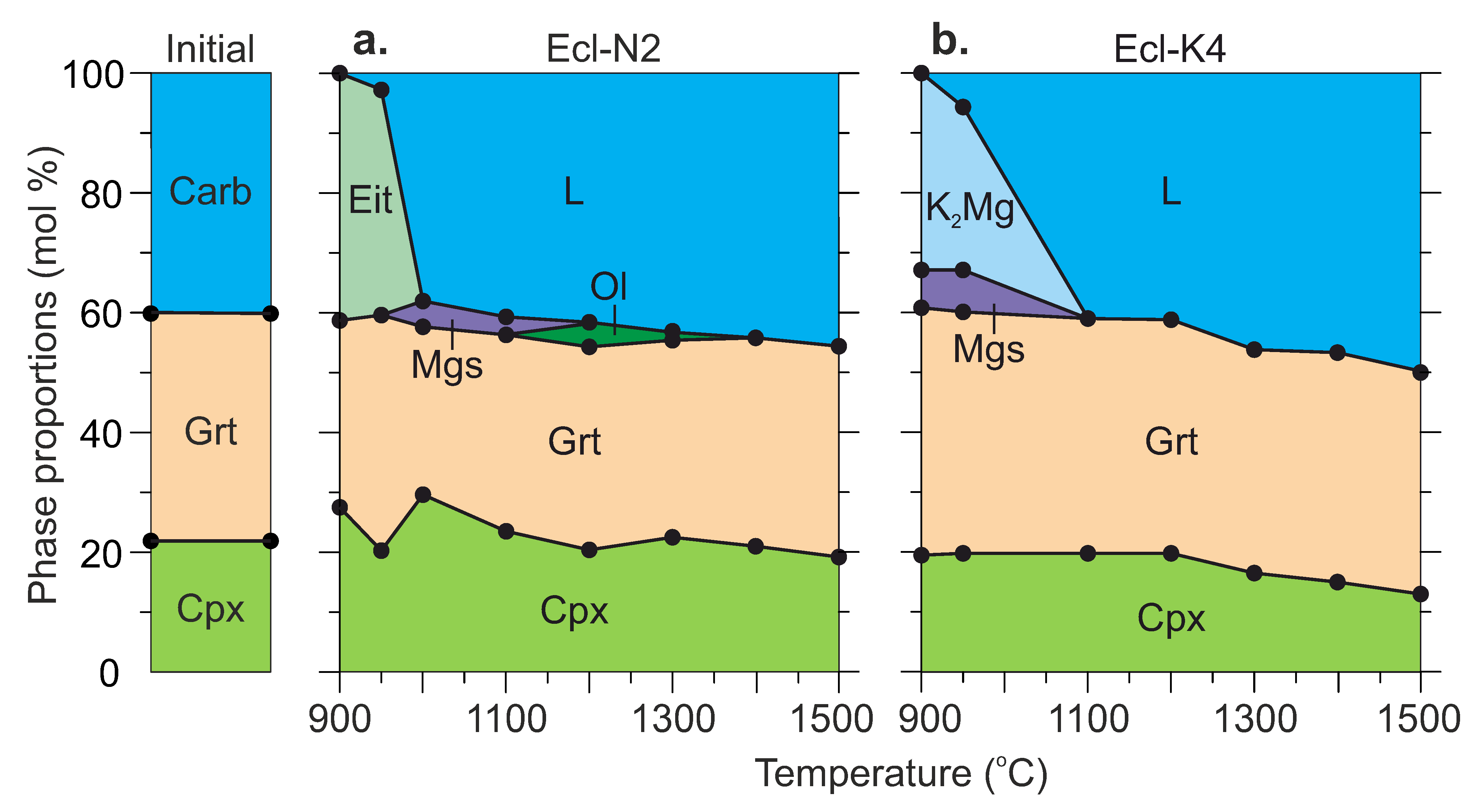
Successive changes in the phase assemblage with increasing temperature are shown in Figure 3. The run conditions and the modal abundance of phases are listed in Table 2 and Tables S1–S10 both in mol% and wt%. The mass balance calculations are given in Tables S5 and S6 in mol% and wt%, respectively.
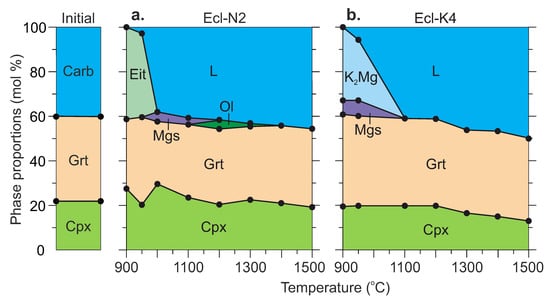
Figure 3.
Modal abundances of phases as a function of temperature in the systems Ecl-N2 (a) and Ecl-K4 (b) at 6 GPa. Modes (in mol%) were determined from the bulk compositions of starting mixtures and compositions of phases measured by EDS (Table S5). Modes in wt% are given in Table S6. See the abbreviations section for mineral and phase symbols. The data at 1100 and 1200 °C are after [54]. See the abbreviations section for mineral and phase symbols.

Table 2.
Modal abundance of phases (in wt%) in the experimental samples.
The system Ecl-N2. At 900 °C (run D280, 198 h), the sample is represented by the subsolidus assemblage consisting of clinopyroxene, garnet, eitelite, and a trace amount of Na2Ca4(CO3)5 (Figure 1a,b, Table 2). As the temperature increases to 950 °C (run D267, 168 h), melting begins, Na2Ca4(CO3)5 disappears, while eitelite is still present (Figure 1c–e, Table 2).
At 1000 °C (run D253, 169 h), eitelite disappears, while a minor amount of magnesite (3 wt%) is present (Figure 1f,g, Figure 3a and Table 2). At 1300 °C (run D211, 64 h), a minor amount of olivine (1 wt%) appears in addition to clinopyroxene and garnet similar to that observed at 1100 and 1200 °C in our earlier study [54] (Figure 1h,i, Figure 3a and Table 2). At 1400 °C (run D214, 24 h) and 1500 °C (run D217, 5 h), olivine disappears, the clinopyroxene fraction slightly decreases, and the melt fraction increases (Figure 3a).
The system Ecl-K4. At 900 °C (run D280, 198 h), the sample consists of clinopyroxene, garnet, K2Mg(CO3)2, and magnesite (Figure 3b, Table 2). At 950 °C (run D267, 168 h), the resulting mineral assemblage remains unchanged, while the melt fraction increases at the expense of K2Mg(CO3)2 (Figure 2c,d and Figure 3b). Over 1300–1500 °C (runs D211, D214, D217), the samples are represented by clinopyroxene, garnet, and quenched melt (Figure 2e–h and Figure 3b) as that observed by Shatskiy et al. [54] in the same system at 1100 and 1200 °C (Figure 3b, Table 2).
3.3. Composition of Phases
The chemical composition of phases is given in Table 3 in wt% and Tables S1–S10 both in mol% and wt%.

Table 3.
Phase compositions in wt%, normalized to 100%.
3.3.1. Clinopyroxene
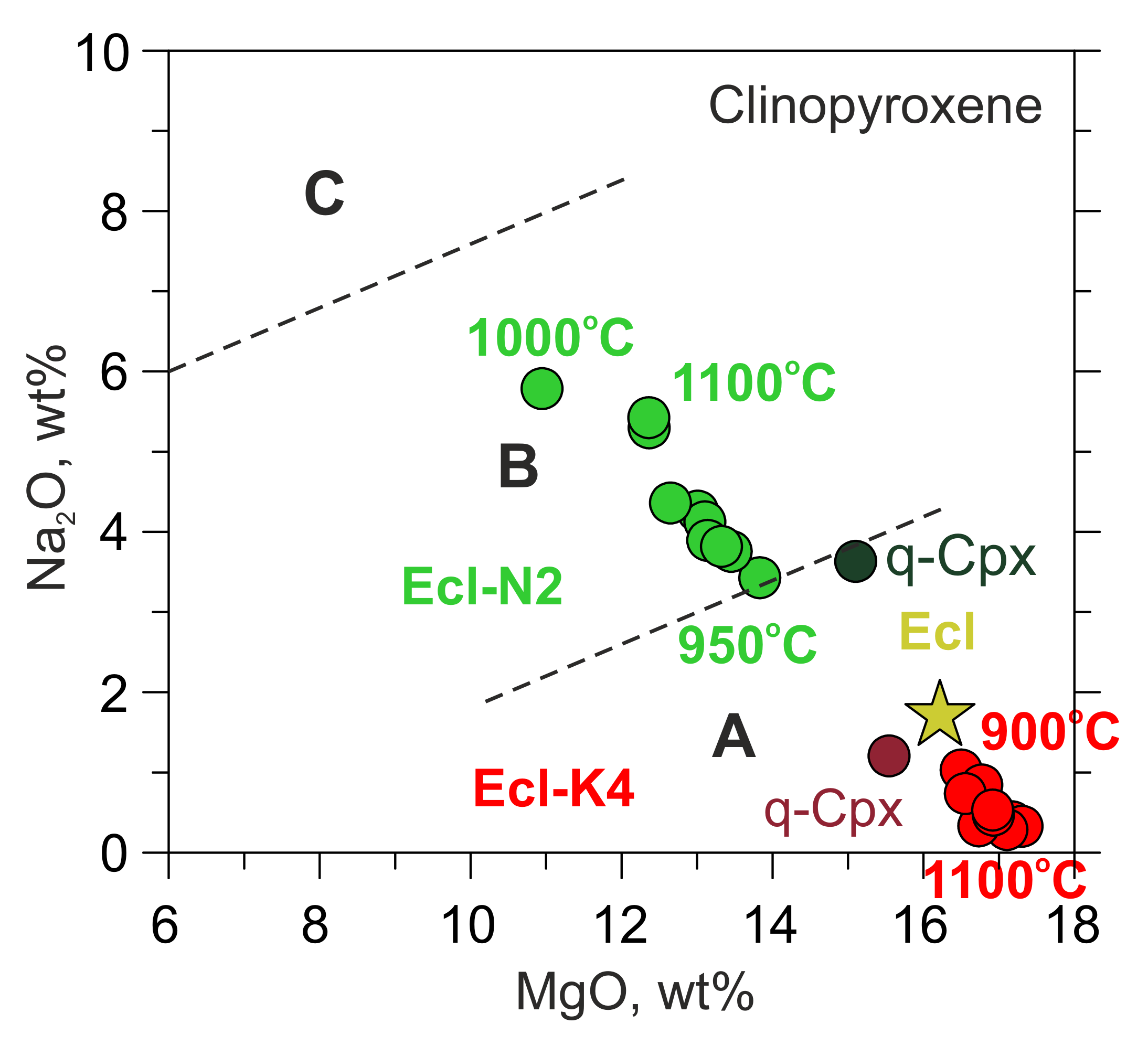
Equilibration with the Na-rich carbonate melt (N2) increases Na2O in clinopyroxene from the initial 1.7 to 5.8 wt% (Table 1, Table 3 and Table S8). In contrast, equilibration with the K-rich carbonate melt (K4) decreases Na2O from 1.7 to 0.3 wt% and increases K2O from the initial 0.03 to 0.6 wt% (Table 1, Table 3 and Table S8). Thus, after the experiments, the clinopyroxenes in Ecl-N2, belong to Group B eclogites, according to the classification of Taylor and Neal [59], whereas those in Ecl-K4 belong to Group A eclogites or garnet clinopyroxenes (Figure 4). Like the equilibrium clinopyroxene, the one in the melt quench products (q-Cpx) recovered from 1500 °C is almost free of K2O and contains 3.6 wt% Na2O in the Ecl-N2 system, while in the Ecl-K4 system, q-Cpx contains 1.2 wt% Na2O and 1.9 wt% K2O (Table 3 and Table S8). In both systems, q-Cpx is richer in FeO (6.5–7.7 wt%) and TiO2 (0.7–1.2 wt%) than the initial and equilibrium clinopyroxene (Table 1, Table 3 and Table S8).
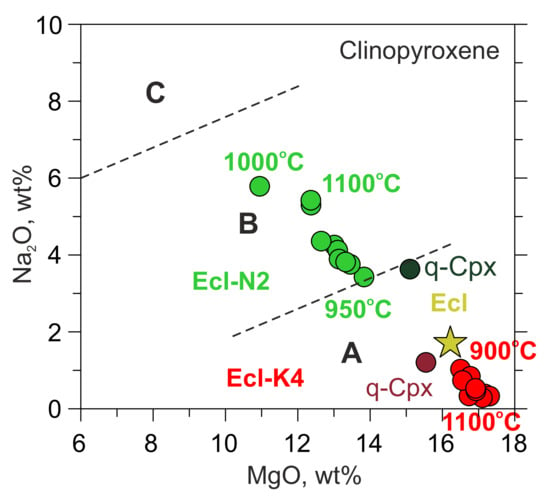
Figure 4.
Na2O vs. MgO in clinopyroxenes from 6-GPa experiments in the systems Ecl-N2 (green) and Ecl-K4 (red). q-Cpx clinopyroxene from the melt quench products. Clinopyroxene from the original eclogite is denoted by a yellow star. Group A, B, and C according to the classification of Taylor and Neal [59]. The data at 1100 and 1200 °C are after [54].
3.3.2. Garnet
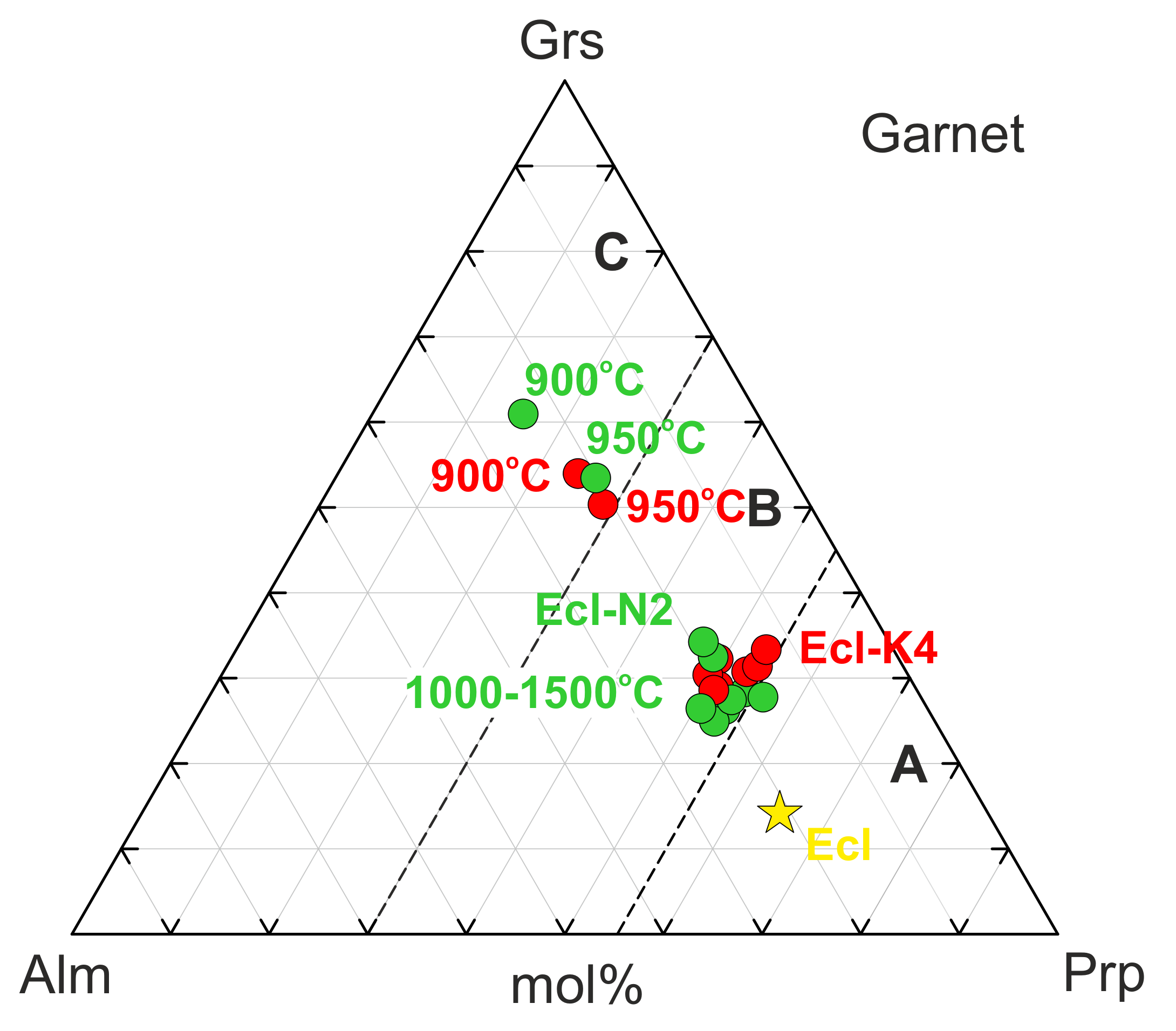
Garnet often contains relics of the original garnet in the core rimmed by the newly formed garnet (Figure 1b). The composition of the newly formed garnet is plotted on the classification scheme Prp-Grs-Alm [60] (Figure 5). Interaction with both N2 and K4 melts increases the grossular content in garnet. Over 900–950 °C, the garnet composition falls in the field of eclogites Group C, while over 1000–1500 °C—Group B (Figure 5, Table 3 and Table S9).
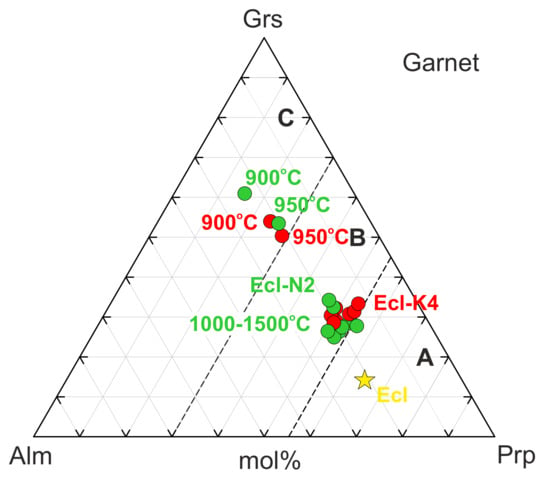
Figure 5.
Garnet compositions from 6-GPa experiments in the systems Ecl-N2 (green) and Ecl-K4 (red) expressed in terms of grossular–pyrope–almandine. Garnet from the original eclogite is denoted by a yellow star. Group A, B, and C according to the classification of Coleman et al. [60].
3.3.3. Olivine
3.3.4. Carbonates
At 900–950 °C, eitelite, (Na0.99K0.01)2(Mg0.91Fe0.05Ca0.04)(CO3)2, and (K0.98 Na0.02)2(Mg0.84Fe0.08Ca0.08)(CO3)2 crystallize in the Ecl-N2 and Ecl-K4 systems, respectively (Figure 3, Table 3 and Table S11). Magnesite with approximate composition, (Mg0.87Fe0.09Ca0.04)CO3, is a minor phase in both systems (Figure 3, Table 3 and Table S4).
3.3.5. Melt
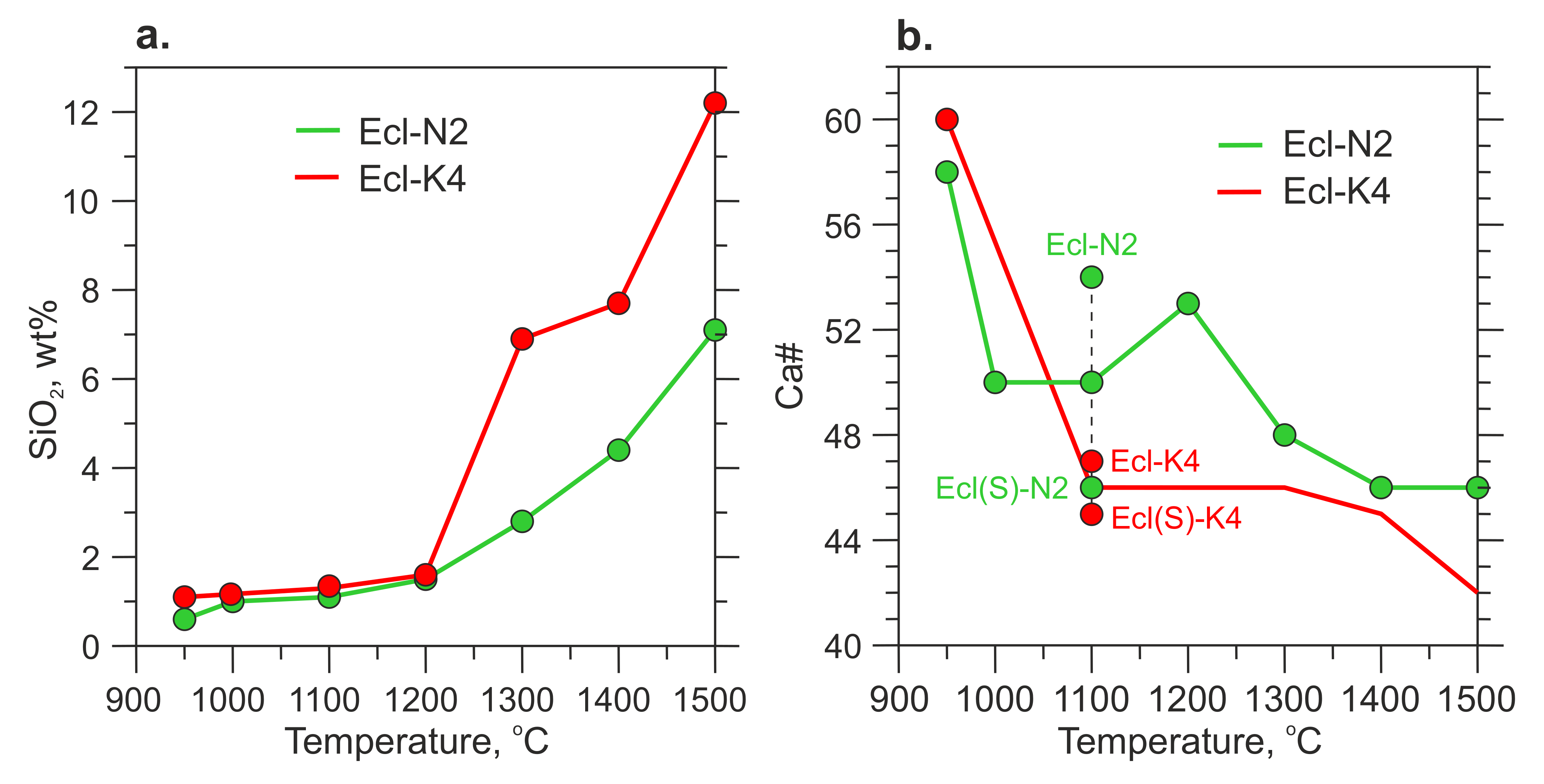
The melt has an alkali-rich carbonate composition (Table 3 and Table S7). At 900–950 °C, the melt has Ca# 58–60 and coexists with Mg-rich carbonates, eitelite in the Ecl-N2 system and K2Mg(CO3)2 + magnesite in the Ecl-K4 system (Figure 6b, Table 3). As temperature increases to 1100 °C, the melt consumes Mg-carbonates and its Ca# decreases to 47–54 (Figure 6b, Table 3 and Table S7). The silica content in the melt increases from <1 wt% over 900–950 °C to 3–7 wt% at 1300 °C, and 7–12 wt% at 1500 °C (Figure 6a, Table 3 and S7). The alumina content in the melt also increases with temperature but does not exceed 1.2 wt% (Table 3). Over 1300–1500 °C, the silica content in the K-rich melt is about two times higher than that in the Na-rich melt (Figure 6b, Table 3 and S7). An increase in the silica concentration in the melt is accompanied by a decrease in the clinopyroxene fraction. This is especially evident in the Ecl-K4 system (Figure 3, Table 2). Unlike clinopyroxene, the fraction of garnet remains unchanged. Thus, garnet is buffering the low Al2O3 in the melt.
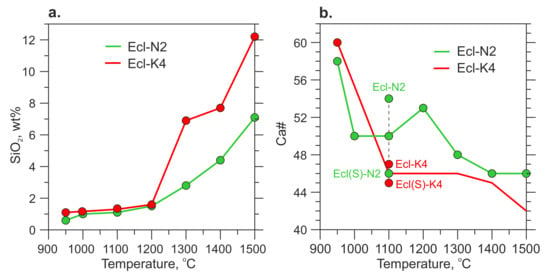
Figure 6.
Silica content (a) and Ca# (b) in the carbonate melt versus temperature. The data at 1100 and 1200 °C are after [54].
3.4. Approach to Equilibrium
To verify the approach to equilibrium, we calculated temperatures at 6 GPa using the geothermometers [61,70,71,72] based on the Mg-Fe2+ exchange between clinopyroxene and garnet. The calculations were performed using the PTEXL code developed by Thomas Koehler and Andrei Girnis (personal communication). In our calculation, we considered Fetotal = Fe2+ without the effect of Fe3+.
The runs in the Ecl-N2 system at 900–1000 °C exhibit a significant (by 150–300 °C) overestimation of the calculated temperatures (Table 4). This may be due either to an unsatisfactory approach to equilibrium only in experiments with the Na-rich carbonate melt at 900–1000 °C, or the inapplicability of the thermometers to experiments with the Na-rich carbonate melt. However, in all other experiments, calculated temperatures are within 100 °C of the nominal run temperatures (Table 4). Thus, in most of the experiments, equilibrium was approached sufficiently for the question being investigated.

Table 4.
Temperature estimates using Cpx-Grt geothermometers, p = 6 GPa.
3.5. Melt-–Solid Distribution Coefficients
The Fe-Mg distribution coefficients between silicate minerals and melt, S/LD(Fe-Mg) = S(Fe/Mg)/L(Fe/Mg) atomic ratio, where S—solid (Cpx, Grt, Ol) and L—liquid) are given in Table 5 and Table S5. For clinopyroxene S/LD(Fe-Mg) is 0.41–0.68 and 0.25–0.35 in the Na- and K-bearing systems, respectively. For garnet, S/LD(Fe-Mg) varies from 0.70 to 3.3 and for olivine S/LD(Fe-Mg) is 0.60. The S/LD(Fe-Mg) distribution coefficients from our experiments match the previously determined solid/melt phase equilibria under similar conditions in other experiments [42,43,54,76].

Table 5.
Partition coefficients between minerals and melt.
The partition coefficient of Na2O (wt%) between clinopyroxene and carbonate melt, Cpx/LD(Na2O) = Na2OCpx/Na2OL, established in the sodium system ranges from 0.142 to 0.237 (Table 5 and Table S5). The partition coefficient of K2O (wt%), Cpx/LD(K2O) = K2OCpx/K2OL, ranges from 0.01 to 0.02 (Table 5 and Table S5). These values are consistent with those established in the silicate system, CaMgSi2O6-NaAlSi2O6-KAlSi2O6 at 6–7 GPa and 1100–1300 °C, Cpx/LD(K2O) = 0.02–0.11 [77] and in the carbonate-silicate system (pyroxene-K2CO3) at 5–14 GPa and 1400–1700 °C, Cpx/LD(K2O) = 0.02–0.10 [78].
4. Discussion
4.1. Subsolidus Assemblage and Melting Reactions
The subsolidus assemblage consists of garnet, clinopyroxene, and carbonates. Although the starting carbonate mixtures are rich in CaCO3, with Ca# ~70, the subsolidus assemblages in the experiments consist of eitelite in Ecl-N2 and K2Mg(CO3)2 + magnesite in Ecl-K4 and do not contain Ca-bearing carbonates, except for the trace amount of Na2Ca4(CO3)5. Almost all calcium is redistributed from carbonate to garnet according to the following Ca-Mg exchange reaction:
3CaCO3 (in carbonates) + Mg3Al2Si3O12 (Grt) =
3MgCO3 (in carbonates) + Ca3Al2Si3O12 (Grt)
3MgCO3 (in carbonates) + Ca3Al2Si3O12 (Grt)
As a result of reaction (1), the garnet composition shifts from eclogite Group A to eclogite Group C (Figure 5). The pressure-induced partitioning of Ca from carbonates to garnet was reported earlier in several experimental studies on phase relations in carbonated eclogite [24,42,79].
Unlike the Ca-free subsolidus carbonates, the incipient carbonate melt has Ca# 58–60 (Figure 6b). Above the solidus, the garnet Ca# drops sharply from 49–60 to 25–30 (Figure 5, Table 3 and Table S9), while eitelite and K2Mg(CO3)2 disappear (Figure 3). Thus, at 6 GPa, the Ecl-N2 and Ecl-K4 solidi are situated at 950 °C and controlled by the following melting reaction:
Ca3Al2Si3O12 (Grt) + 2(Na or K)2Mg(CO3)2 (Eit) =
Ca2MgSi3O12 (Grt) + [2(Na or K)2CO3∙CaCO3∙MgCO3] (L).
Ca2MgSi3O12 (Grt) + [2(Na or K)2CO3∙CaCO3∙MgCO3] (L).
We also suppose that as pressure decreases below 6 GPa and Ca-bearing carbonates are stabilized, the Ecl-N2 and Ecl-K4 solidi will be controlled by the carbonate component. Melting of the Na- and K-carbonate (carbonatite) systems has been studied previously [45,46]. According to these data, the Na2Ca4(CO3)5 and then Na2Ca3(CO3)4 (below 4–5 GPa) compounds become stable and the solidus reactions can be approximated as follows:
above 5 GPa and
2Na2Mg(CO3)2 (Eit) + Na2Ca4(CO3)5 (Na2Ca4) =
[3Na2CO3∙2MgCO3∙4CaCO3] (L),
[3Na2CO3∙2MgCO3∙4CaCO3] (L),
2Na2Mg(CO3)2 (Eit) + Na2Ca3(CO3)5 (Na2Ca3) =
[3Na2CO3∙2MgCO3∙3CaCO3] (L),
[3Na2CO3∙2MgCO3∙3CaCO3] (L),
Below 4–5 GPa. In the K-carbonate system, dolomite stabilizes in addition to K2Mg(CO3)2 yielding the following melting reaction:
K2Mg(CO3)2 (K2Mg) + CaMg(CO3)2 (Dol) =
MgCO3 (Mgs) + [K2CO3∙MgCO3∙CaCO3] (L).
MgCO3 (Mgs) + [K2CO3∙MgCO3∙CaCO3] (L).
According to various estimates, at 3 GPa, the alkaline carbonate or carbonatite solidus is situated at about 750–850 °C [45,46,69,80].
4.2. Comparison with the Various Solidi of the Carbonated Eclogites in Previous Experimental Studies
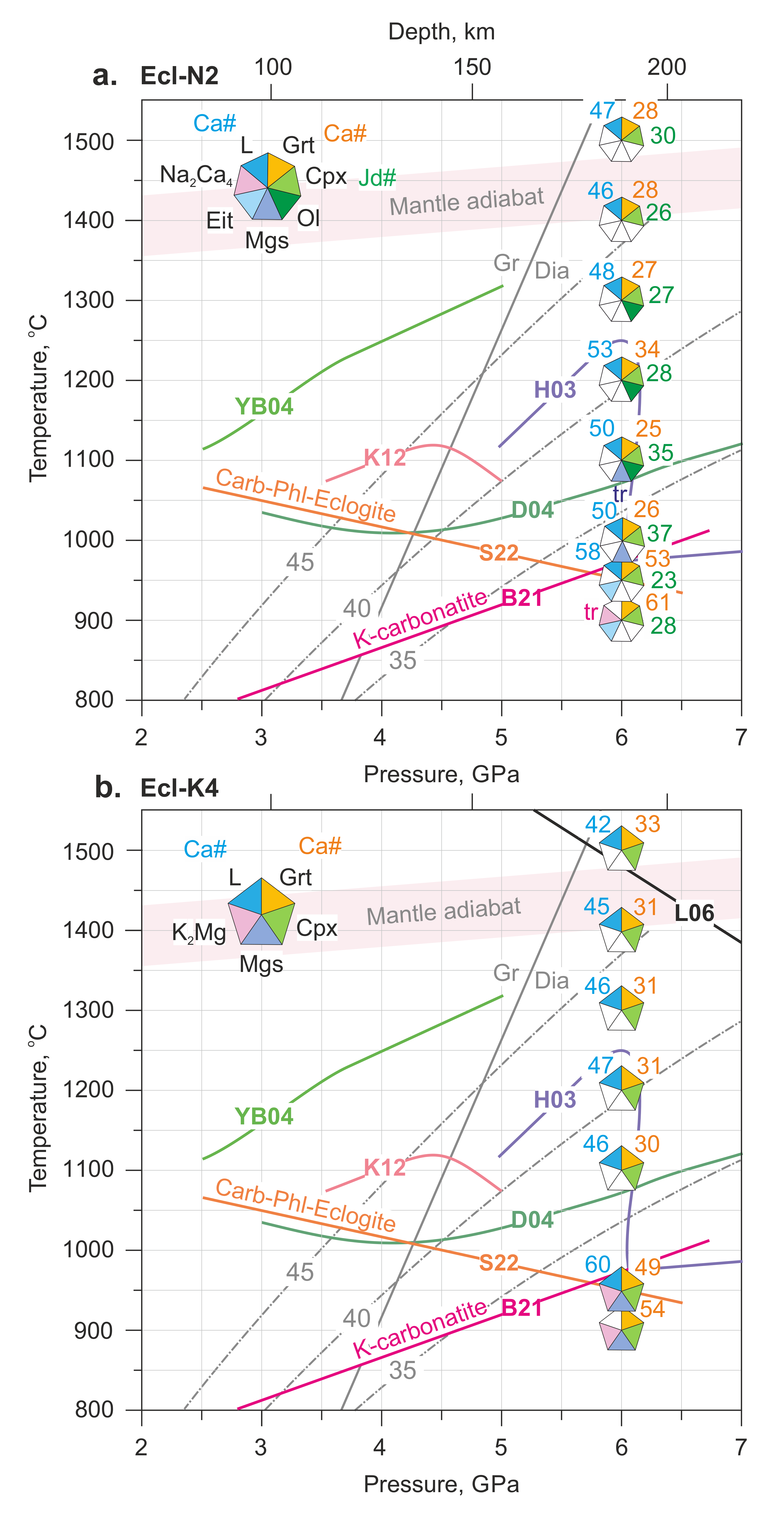
Phase relationships in carbonated eclogite under mantle P-T conditions have been studied in several works. The obtained solidi are shown in Figure 7. As can be seen, their temperatures differ significantly. Here we would like to discuss what this may be connected with.
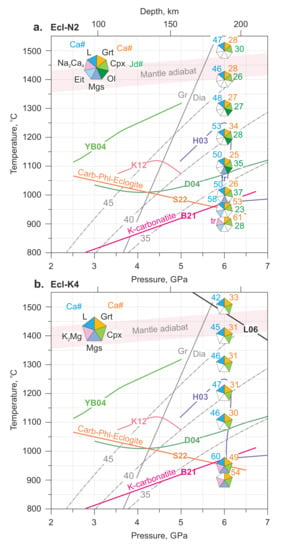
Figure 7.
P-T plot illustrating phase relations in the Ecl-N2 (a) and Ecl-K4 (b) systems at 6 GPa in comparison with the solidi of carbonated eclogite: H03—[39], D04—[40], YB04—[42], K12—[43]; carbonated phlogopite eclogite: S22—[82]; and carbonatite: B21—[46]. The solidus of the Di-CO2 system (L06) is after Luth [83], Gr-Dia—graphite-to-diamond phase transition [84]. The light-brown numbers, adjacent to corresponding segments in polygons, denote Ca# of garnet, Ca# of carbonate melt, and Jd# of clinopyroxene. The mantle adiabat is after [56]. The grey dash-dotted lines denote continental geotherms with a surface heat flux of 35, 40, and 45 mW/m2 [85]. The data at 1100 and 1200 °C are after [54]. See the abbreviations section for mineral and phase symbols.
Hammouda [39] studied the system OTBC consisting of 89.8 wt% basaltic glass, 10.1 wt% CaCO3, and 0.12 wt% H2O. He found that at 6 GPa and 1200 °C, the subsolidus assemblage is represented by garnet, Ca# 38, clinopyroxene, and magnesian calcite with Ca# 75. This is inconsistent with our data, according to which, at 6 GPa, garnet reacts with CaCO3 to form a more calcium garnet containing up to 60 mol% grossular. As temperature increases to 1250 °C, the first melt appears, while its composition resembles subsolidus calcite and has Ca# 80. Moreover, as temperature increases above the solidus, the garnet and clinopyroxene retain their composition almost unchanged. Given that Ca-Mg-Fe carbonates with Ca# 75–80 do not melt at such low temperatures under dry conditions [62,81], it appears that, in contrast to our experiments, the solidus of the OTBC system is controlled by the melting of magnesian calcite in the presence of water.
Dasgupta, et al. [40] reported the phase relations in the SLEC1 system, prepared by adding 5 wt% CO2 in the form of a mixture (mole ratio): 4(Na0.96K0.04)2CO3∙96(Ca0.32Mg0.44Fe0.36)CO2, to an eclogite from Salt Lake crater, Oahu, Hawaii. They found that partial melting yields carbonate melt, which appears near 1080 °C at 6.1 GPa (Figure 7). Above 5 GPa, the subsolidus assemblage in the SLEC1 system is represented by garnet, clinopyroxene, magnesite, and rutile. Similar to our study, calcium carbonates are absent in the subsolidus assemblage. However, the lack of data on the composition of subsolidus carbonate phases, the composition of garnets above and below the solidus at the same pressure, and the composition of the near-solidus melt prevent inferring the melting reaction controlling the SLEC1 solidus in the range of 5.1–7.0 GPa.
Yaxley and Brey [42] studied synthetic carbonated eclogite EC1 synthesized at 3.5 GPa and 1150 °C. The mineral composition of the starting material included garnet, clinopyroxene, and calcite-dolomite solid solution. It was found that as pressure increases from 3.5 to 5.5 GPa and temperature decreases from 1275 to 1200 °C, the carbonate Ca# decreases from 86 to 56, while the garnet Ca# increases from 22 to 27. Our experiments, where garnet with Ca# 49–60 coexists with magnesite at 6 GPa and 900–950 °C, are consistent with the established pattern. However, the solidus of the EC1 system is located 400–450 °C higher than that of the Ecl-N2 and Ecl-K4 systems. The difference is due to the presence of Na and K in the subsolidus carbonates in our experiment. In contrast to our study, in the experiments by Yaxley and Brey [42], K is absent, while sodium enters clinopyroxene. As it was shown early near 6 GPa, sodium is compatible in clinopyroxene and does not enter carbonates [86]. Therefore, its fluxing effect on the solidus of carbonated eclogite is not so significant and does not exceed 50 °C [87]. Thus, melting in the EC1 system at 5.5 GPa and 1340 °C is mainly controlled by the melting of calcium dolomite, whose composition is close to the CaCO3-MgCO3 eutectic. This is also in good agreement with the Ca# 62 of the solidus melt, which coincides with the CaCO3-MgCO3 eutectic established at 1400 °C [62].
Shatskiy, et al. [43] investigated the phase relations in a K-bearing altered mid-ocean ridge basalt (MORB) + 10% CaCO3. The composition of the system differs from previous works in higher contents of potassium and silica. As a result, melting in the system at 5 GPa occurs at only 1050 °C and is accompanied by the formation of a potassium aluminosilicate rather than a carbonate melt. As temperature increases to 1100 °C, the formation of two immiscible carbonate and silicate melts is observed. This behavior of the system resembles carbonated pelite (DG2) [22,88], where melting is mainly controlled by the assemblage of dolomite + K-feldspar/phengite [22,89].
Shatskiy, et al. [24] studied the phase relationships in carbonated phlogopite eclogite. They found that at 3–6 GPa, subsolidus assemblage consists of clinopyroxene, garnet, phlogopite, and Ca-Mg carbonate. As pressure increases from 3 to 6 GPa and temperature decreases from 1000 to 800 °C, the carbonate composition evolves from Mg-calcite to Ca-dolomite, dolomite, and then magnesite. At 6 GPa, melting consumes phlogopite and magnesite according to the following solidus reaction [24] accompanied by the Ca redistribution from garnet to carbonate:
Phl (KMg3AlSi3O10(OH)2) + Mgs (MgCO3) + Grs (Ca3Al2Si3O12) =
Prp (Mg3Al2Si3O12) + Cpx (CaMgSi2O6) + L (water-bearing carbonate melt)
Prp (Mg3Al2Si3O12) + Cpx (CaMgSi2O6) + L (water-bearing carbonate melt)
Thus, the addition of water at 6 GPa and bulk mole ratio H2O/K2O ≤ 2 yields redistribution of potassium from K2Mg(CO3)2 to phlogopite but does not affect solidus temperature at 6 GPa (Figure 7). Considering results under anhydrous conditions in K-carbonatite and Na-carbonatite systems [46], we expect a decrease in solidus temperatures of Ecl-N2 and Ecl-K4 as pressure decreases. Unlike that, the solidus of carbonated phlogopite eclogite has a negative Clapeyron slope, so that at shallower depths carbonated phlogopite eclogite becomes more refractory (Figure 7).
4.3. Composition of Carbonate Melt
In the range of 950–1200 °C, the silica content in the melt does not exceed 1.5 wt% (Figure 6). As temperature increases to 1300–1500 °C, the silica content in the melt increases sharply to 2–6 and 7–12 wt% in the Ecl-N2 and Ecl-K4 systems, respectively. A twofold higher concentration of silica in a potassium melt correlates with a twofold higher concentration of alumina (Table S7). At 6 GPa in the aluminosilicate system, Na and K are hosted by jadeite and K-feldspar, respectively. Jadeite is poorly soluble in the carbonate melt owing to the compatibility of Na with clinopyroxene [86]. The solubility of K-feldspar in carbonate melt should be higher since K is incompatible in the aluminosilicates in presence of carbonate [22,89]. This is consistent with the lower melting temperature of the Kfs + Dol system compared to Di + Jd + 2Mgs, which is 1050 °C and 1350 °C at 6 GPa, respectively [86,89]. Although potassium doubles the solubility of SiO2 and Al2O3 in the carbonate melt at 1300–1500 °C, their solubility is still low and an excess of the KAlSi3O8 component over its solubility in carbonate melt leads to the appearance of immiscible phonolitic melt [22,43,88,89,90].
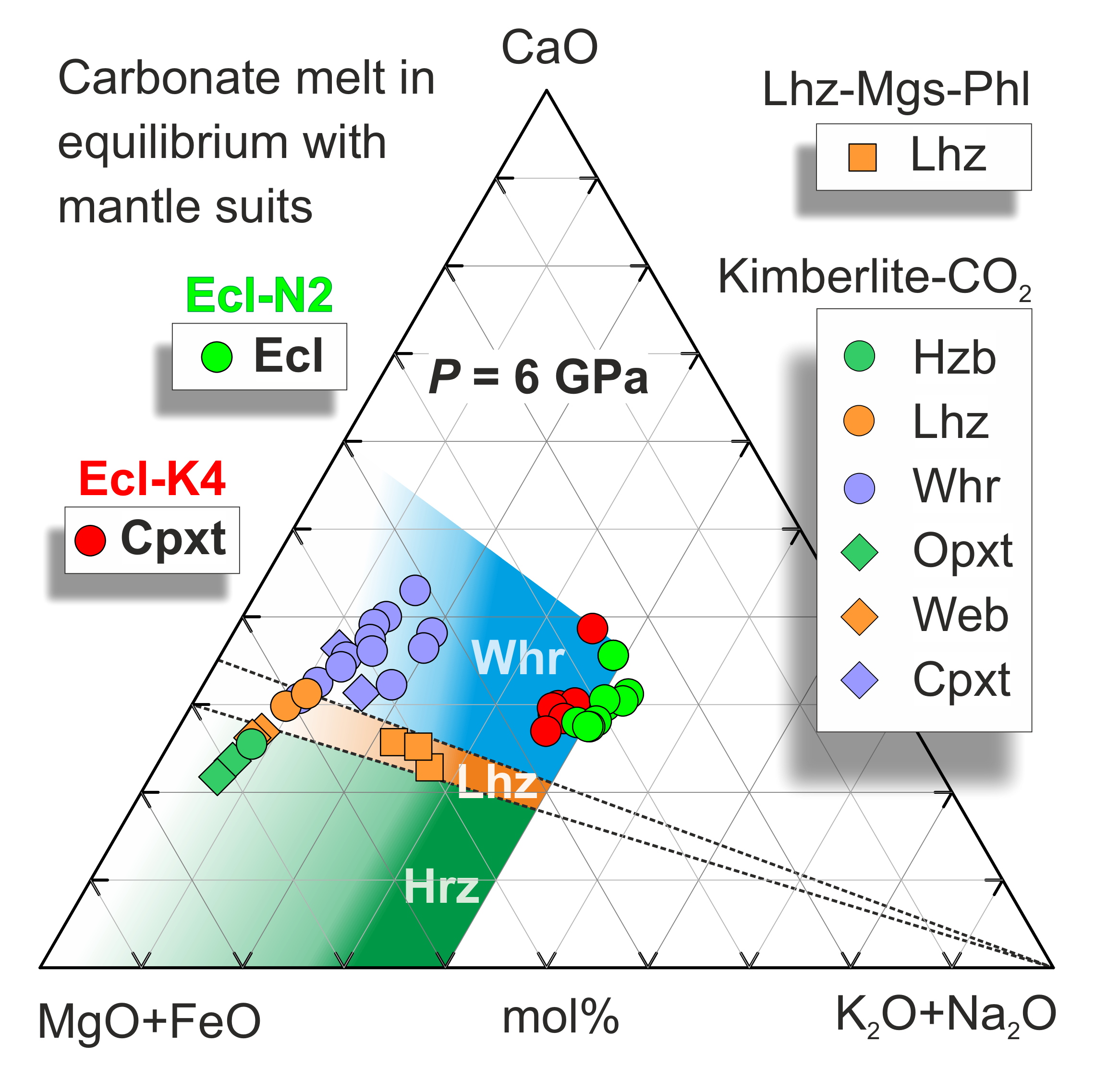
Ca# of carbonate melt coexisting with eclogite and garnet clinopyroxenite varies from 42 to 60 (Figure 6), similar to that established in the carbonated pelite system (Ca# 52–59) [22] and in equilibrium with wehrlite (Ca# 40–60) [37,38], but higher than Ca# of K-rich carbonate melt in equilibrium with garnet lherzolite (~30–34) and harzburgite (˂30) [37,38,91] (Figure 8).
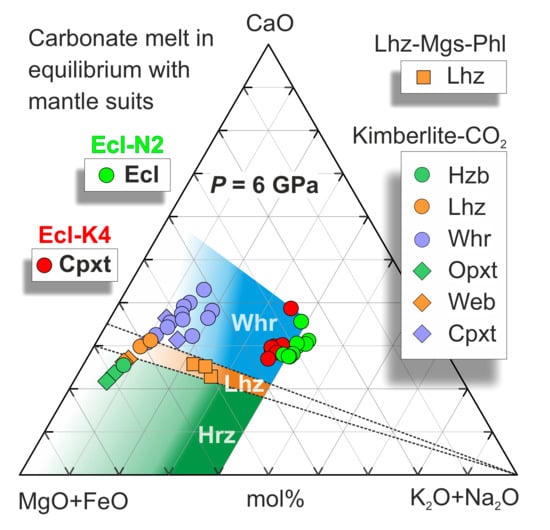
Figure 8.
The pseudo-ternary projection of carbonate melt in equilibrium with bimineral eclogite in the system Ecl-N2 and clinopyroxenite in the system Ecl-K4 at 6 GPa (this study). The colored areas correspond to the compositions of the carbonate melt in equilibrium with harzburgite (green), lherzolite (brown), and wehrlite (blue) [37,38]. The compositions of the carbonate melt obtained under hydrous conditions in the system carbonated phlogopite lherzolite (Lhz-Mgs-Phl) in equilibrium with lherzolite (Lhz) are after [82]. The compositions of carbonate melt obtained in the system kimberlite-CO2 in equilibrium with ultramafic assemblies including Hzb, Lhz, Whr, orthopyroxenite (Opxt), websterite (Web), and Cpxt are after [97].
Carbonate melts are very mobile owing to their excellent wetting properties, low viscosity, and density [92,93,94,95,96]. Therefore, carbonate melts, derived by partial melting of carbonated oceanic crust, can readily impregnate the overlying peridotitic mantle. Re-equilibration of eclogite-derived carbonate melt with peridotite should lower its Ca# and cause refertilization from harzburgite to lherzolite and wehrlitization [37].
5. Implications
5.1. Carbonatite Metasomatism
The present results show that the interaction of the Na-Ca-Mg-Fe carbonate melt with eclogite at 950–1500 °C is accompanied by an increase in the jadeite component in omphacite (Figure 4) and the grossular component in garnet (Figure 5) according to the reaction:
Prp (Mg3Al2Si3O12) + Di (CaMgSi2O6) + L (carbonate melt) =
Jd (NaAlSi2O6) + Grs (Ca3Al2Si3O12) + Ol (Mg2SiO4) + L (carbonate melt)
Jd (NaAlSi2O6) + Grs (Ca3Al2Si3O12) + Ol (Mg2SiO4) + L (carbonate melt)
As a result, eclogite Group A evolves to eclogite Group B (Figure 4 and Figure 5). In addition, reaction (4) produces olivine (Figure 3). The lack of nickel and low Mg# (86) distinguish this olivine from peridotitic olivine (Table 3 and Table S10). Similar olivine was found in a coesite-bearing diamondiferous eclogite xenolith along with secondary metasomatic mineralization including phlogopite, K-feldspar, orthopyroxene, and secondary clinopyroxene [98,99].
The interaction of the K-Ca-Mg-Fe carbonate melt with the eclogite lowers the sodium in the clinopyroxene and shifts the composition of the eclogite towards garnet clinopyroxenite (Figure 4). The above tendency may explain the formation of Na- and Al-depleted clinopyroxene with ‘spongy’ texture replacing primary omphacite in diamondiferous eclogites [1,100,101]. High potassium concentrations, up to 0.6 wt% K2O, in this clinopyroxene [1] indicate its deep, >3 GPa, origin [102]. Moreover, this indicates that the metasomatic melt was rich in potassium [78,103]. A direct finding of the K- and Cl-rich carbonatite melt as microinclusions in diamonds in the alteration veins in the eclogite xenoliths containing Na-poor ‘spongy’ textured clinopyroxene [8] supports our experimental observations.
5.2. Hosts for Potassium in Carbonated Eclogite
Present experiments on the K-rich carbonated eclogite system suggest that under water-poor conditions at 6 GPa, potassium enters K2Mg(CO3)2. This is supported by the findings of K2Mg(CO3)2 microinclusions in kimberlitic diamonds [20]. In contrast, under hydrous conditions, potassium is mainly hosted by phlogopite, as shown experimentally in the systems KMAS–H2O–CO2 [104], KCMAS–H2O–CO2 [105], and carbonated phlogopite eclogite [82]. This is also confirmed by the findings of syngenetic phlogopite inclusions in lithospheric diamonds [55,106,107,108] and diamondiferous eclogite xenoliths [1,2]. Thus, in the presence of volatiles (CO2 and/or H2O), potassium is hosted either by K2Mg(CO3)2 containing 40 wt% K2O, or phlogopite containing 11 wt% K2O, or carbonate melt containing up to 30 wt% K2O.
5.3. The Link between Kimberlites and Mantle Carbonatites
Although the temperatures in our experiments cover the entire range of geotherms of the lithospheric and the asthenospheric mantle at a depth of 200 km [56,85], no gradual transition from carbonate to kimberlite-like carbonate-silicate melt was observed. The highest silica content (7–12 wt% SiO2) in the obtained melt is comparable with that in the alkali-poor (9–15 wt% SiO2) and K-rich (12–16 wt% SiO2) carbonate melts in equilibrium with natural peridotite at 6 GPa and 1500 °C [37,91,109]. This is consistent with the idea that during ascent through the lithospheric mantle, the kimberlite magma was a combination of alkaline carbonate melt and solid silicate matter (xenoliths and xenocrysts) [25,28,29]. Decarbonation [110] and the subsequent loss of CO2 during the explosive emplacement of the kimberlites [111] led to a significant loss of the carbonate component and solidification of kimberlite magma in the form of silicate rock. Postmagmatic leaching of the alkaline carbonates that withstood decarbonation reinforces this trend [28,29]. The essentially carbonate composition of the liquid component of kimberlite magma is confirmed by the alkaline carbonate composition of inclusions in kimberlite magmatic minerals and xenoliths [24,25,27,33,34,35], as well as experiments on kimberlite melting [97,112,113].
Sokol et al. [114] have experimentally shown that a successive increase in the water content from 2.5 to 11.6 wt% in the kimberlite magma system over 6.3–7.5 GPa and 1400–1500 °C promotes the fusion of the silicate constituent yielding the transformation of the melt from essentially carbonate (5–12 wt% SiO2 at 2.5 wt% H2O) to carbonate-silicate (17–19 wt% SiO2 at 6–6.5 wt% H2O) and finally complete melting of kimberlite at 11–12 wt% H2O. Comparing these data with the alkaline carbonatitic composition of the melt inclusions in the igneous minerals of the kimberlites worldwide [24,25,27], we conclude that the water content in kimberlite magma did not exceed a few percent.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/min13010082/s1, Table S1: Composition of natural eclogite used as a component of starting mixtures; Table S2: Compositions of carbonate component of starting mixtures; Table S3: Compositions of starting mixtures made of carbonates and natural eclogite; Table S4: Summary of run conditions, composition of phases (in mol% and wt%) and mole/weight fraction of phases (MFP/WFP) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S5: Calculation of mole fraction of phases (MFP) from experiments on the interaction eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S6: Calculation of weight fraction of phases (WFP) from experiments on the interaction eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S7: Compositions of carbonatitic melts (L) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S8: Compositions of clinopyroxenes (Cpx) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S9: Compositions of garnets (Grt) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S10: Compositions of olivine (Grt) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S7: Compositions of carbonate from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S8: Compositions of clinopyroxenes (Cpx) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S9: Compositions of garnets (Grt) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S10: Compositions of olivine (Grt) from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C; Table S11: Compositions of carbonate from experiments on the interaction of eclogite with carbonatitic melts at 6.0 GPa and 900–1500 °C.
Author Contributions
Conceptualization, A.S.; methodology, A.S. and K.D.L.; validation, K.D.L.; formal analysis, A.B., A.V.A., and A.S.; investigation, A.B.; resources, K.D.L.; data curation, A.B. and A.S.; writing—original draft preparation, A.B. and A.S.; writing—review and editing, A.S.; visualization, A.B. and A.S.; supervision, A.S.; project administration, A.S. and A.B.; funding acquisition, A.V.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, project No 21-77-10057.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to three anonymous referees for constructive reviews; T.F.D. Nielsen and S. Keshav for the review of an early version of our manuscript which helped significantly improve the manuscript; V.S. Shatsky and A.L. Ragozin for providing natural eclogite and discussion; N.S. Karmanov, A.T. Titov, and M.V. Khlestov for assistance in the analytical works.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Alm—almandine, Arg — aragonite, Cal—calcite, Cpx—clinopyroxene, Cpxt—clinopyroxenite, Di—diopside, Dol—dolomite, Ecl—eclogite, Eit—eitelite Na2Mg(CO3)2, Gr—graphite, Grs —grossular, Grt—garnet, Hd—hedendergite, Hrz—harzburgite, Jd—jadeite, K2Mg–K2Mg(CO3)2, L—carbonate melt, Lhz—lherzolite, Mgs—magnesite, Na2Ca4 –Na2Ca4(CO3)5, Ol—olivine, Opxt—orthopyroxenite, Prp—pyrope, q-Cpx—quench clinopyroxene, Ca# = 100∙Ca/(Ca + Mg), Web—websterite, Whr—wehrlite.
References
- Shatsky, V.; Ragozin, A.; Zedgenizov, D.; Mityukhin, S. Evidence for multistage evolution in a xenolith of diamond-bearing eclogite from the Udachnaya kimberlite pipe. Lithos 2008, 105, 289–300. [Google Scholar] [CrossRef]
- Smart, K.A.; Heaman, L.M.; Chacko, T.; Simonetti, A.; Kopylova, M.; Mah, D.; Daniels, D. The origin of high-MgO diamond eclogites from the Jericho Kimberlite, Canada. Earth Planet. Sci. Lett. 2009, 284, 527–537. [Google Scholar] [CrossRef]
- Agashev, A.M.; Pokhilenko, L.N.; Pokhilenko, N.P.; Shchukina, E.V. Geochemistry of eclogite xenoliths from the Udachnaya Kimberlite Pipe: Section of ancient oceanic crust sampled. Lithos 2018, 314–315, 187–200. [Google Scholar] [CrossRef]
- Aulbach, S.; Viljoen, K.S.; Gerdes, A. Diamondiferous and barren eclogites and pyroxenites from the western Kaapvaal craton record subduction processes and mantle metasomatism, respectively. Lithos 2020, 368, 105588. [Google Scholar] [CrossRef]
- Taylor, L.A.; Keller, R.A.; Snyder, G.A.; Wang, W.; Carlson, W.D.; Hauri, E.H.; Mccandless, T.; Kim, K.-R.; Sobolev, N.V.; Bezborodov, S.M. Diamonds and their mineral inclusions, and what they tell us: A detailed “pull-apart” of a diamondiferous eclogite. Int. Geol. Rev. 2000, 42, 959–983. [Google Scholar] [CrossRef]
- Anand, M.; Taylor, L.A.; Misra, K.C.; Carlson, W.D.; Sobolev, N.V. Nature of diamonds in Yakutian eclogites: Views from eclogite tomography and mineral inclusions in diamonds. Lithos 2004, 77, 333–348. [Google Scholar] [CrossRef]
- Taylor, L.A.; Anand, M. Diamonds: Time capsules from the Siberian Mantle. Geochemistry 2004, 64, 1–74. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Griffin, W.L. Diamond formation during metasomatism of mantle eclogite by chloride-carbonate melt. Contrib. Mineral. Petrol. 2018, 173, 84. [Google Scholar] [CrossRef]
- Zedgenizov, D.; Ragozin, A.; Shatsky, V. Chloride-carbonate fluid in diamonds from the eclogite xenolith. In Doklady Earth Sciences; Springer Nature BV: Berlin/Heidelberg, Germany, 2007; pp. 961–964. [Google Scholar]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Et Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Mantle fluid evolution—A tale of one diamond. Lithos 2004, 77, 243–253. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Et Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Rege, S.; Griffin, W.L.; Kagi, H.; Shatsky, V.S. Composition of trapped fluids in cuboid fibrous diamonds from the Udachnaya kimberlite: LAM-ICPMS analysis. Chem. Geol. 2007, 240, 151–162. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Logvinova, A.M.; Schrauder, M.; Spetius, Z.V.; Weiss, Y.; Hauri, E.H.; Kaminsky, F.V.; Sobolev, N.V.; Navon, O. High-Mg carbonatitic microinclusions in some Yakutian diamonds—A new type of diamond-forming fluid. Lithos 2009, 112, 648–659. [Google Scholar] [CrossRef]
- Weiss, Y.; Kessel, R.; Griffin, W.L.; Kiflawi, I.; Klein-BenDavid, O.; Bell, D.R.; Harris, J.W.; Navon, O. A new model for the evolution of diamond-forming fluids: Evidence from microinclusion-bearing diamonds from Kankan, Guinea. Lithos 2009, 112, 660–674. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L.; Kagi, H. Mg and Fe-rich carbonate-silicate high-density fluids in cuboid diamonds from the Internationalnaya kimberlite pipe (Yakutia). Lithos 2009, 112, 638–647. [Google Scholar] [CrossRef]
- Skuzovatov, S.Y.; Zedgenizov, D.A.; Shatsky, V.S.; Ragozin, A.L.; Kuper, K.E. Composition of cloudy microinclusions in octahedral diamonds from the Internatsional'naya kimberlite pipe (Yakutia). Russ. Geol. Geophys. 2011, 52, 85–96. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L. Fibrous diamonds from the placers of the northeastern Siberian Platform: Carbonate and silicate crystallization media. Russ. Geol. Geophys. 2011, 52, 1298–1309. [Google Scholar] [CrossRef]
- Weiss, Y.; Kiflawi, I.; Davies, N.; Navon, O. High-density fluids and the growth of monocrystalline diamonds. Geochim. Et Cosmochim. Acta 2014, 141, 145–159. [Google Scholar] [CrossRef]
- Jablon, B.M.; Navon, O. Most diamonds were created equal. Earth Planet. Sci. Lett. 2016, 443, 41–47. [Google Scholar] [CrossRef]
- Skuzovatov, S.; Zedgenizov, D.; Howell, D.; Griffin, W.L. Various growth environments of cloudy diamonds from the Malobotuobia kimberlite field (Siberian craton). Lithos 2016, 265, 96–107. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Origin of K-rich diamond-forming immiscible melts and CO2 fluid via partial melting of carbonated pelites at a depth of 180–200 km. Gondwana Res. 2019, 75, 154–171. [Google Scholar] [CrossRef]
- Gubanov, N.; Zedgenizov, D.; Sharygin, I.; Ragozin, A. Origin and evolution of high-Mg carbonatitic and low-Mg carbonatitic to silicic high-density fluids in coated diamonds from Udachnaya kimberlite pipe. Minerals 2019, 9, 734. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V. Origin of alkaline carbonates in kimberlites of the Siberian craton: Evidence from melt inclusions in mantle olivine of the Udachnaya-East pipe. Chem. Geol. 2017, 455, 357–375. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Weiss, Y.; Navon, O.; Nielsen, T.F.D.; Mernagh, T.P. How unique is the Udachnaya-East kimberlite? Comparison with kimberlites from the Slave Craton (Canada) and SW Greenland. Lithos 2009, 112, 334–346. [Google Scholar] [CrossRef]
- Abersteiner, A.; Kamenetsky, V.S.; Goemann, K.; Giuliani, A.; Howarth, G.H.; Castillo-Oliver, M.; Thompson, J.; Kamenetsky, M.; Cherry, A. Composition and emplacement of the Benfontein kimberlite sill complex (Kimberley, South Africa): Textural, petrographic and melt inclusion constraints. Lithos 2019, 324, 297–314. [Google Scholar] [CrossRef]
- Abersteiner, A.; Giuliani, A.; Kamenetsky, V.S.; Phillips, D. Petrographic and melt-inclusion constraints on the petrogenesis of a magmaclast from the Venetia kimberlite cluster, South Africa. Chem. Geol. 2017, 455, 331–341. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Golovin, A.V.; Maas, R.; Giuliani, A.; Kamenetsky, M.B.; Weiss, Y. Towards a new model for kimberlite petrogenesis: Evidence from unaltered kimberlites and mantle minerals. Earth-Sci. Rev. 2014, 139, 145–167. [Google Scholar] [CrossRef]
- Kamenetsky, M.B.; Sobolev, A.V.; Kamenetsky, V.S.; Maas, R.; Danyushevsky, L.V.; Thomas, R.; Pokhilenko, N.P.; Sobolev, N.V. Kimberlite melts rich in alkali chlorides and carbonates: A potent metasomatic agent in the mantle. Geology 2004, 32, 845–848. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Korsakov, A.V.; Pokhilenko, N.P. Eitelite in sheared peridotite xenoliths from Udachnaya-East kimberlite pipe (Russia)—A new locality and host rock type. Eur. J. Mineral. 2013, 25, 825–834. [Google Scholar] [CrossRef]
- Golovin, A.; Sharygin, I.; Kamenetsky, V.; Korsakov, A.; Yaxley, G. Alkali-carbonate melts from the base of cratonic lithospheric mantle: Links to kimberlites. Chem. Geol. 2018, 483, 261–274. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V.; Kamenetsky, V.S.; Abersteiner, A. Can primitive kimberlite melts be alkali-carbonate liquids: Composition of the melt snapshots preserved in deepest mantle xenoliths. J. Raman Spectrosc. 2020, 51, 1849–1867. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Dymshits, A.M.; Kalugina, A.D.; Solovev, K.A.; Malkovets, V.G.; Pokhilenko, N.P. Relics of deep alkali–carbonate melt in the mantle xenolith from the Komsomolskaya–Magnitnaya kimberlite pipe (Upper Muna field, Yakutia). Dokl. Earth Sci. 2021, 500, 842–847. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Tarasov, A.A.; Dymshits, A.M.; Kovaleva, E. Confocal Raman spectroscopic study of melt inclusions in olivine of mantle xenoliths from the Bultfontein kimberlite pipe (Kimberley cluster, South Africa): Evidence for alkali-rich carbonate melt in the mantle beneath Kaapvaal Craton. J. Raman Spectrosc. 2022, 53, 508–524. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Borzdov, Y.M.; Katsura, T.; Yamazaki, D.; Ohtani, E. Silicate diffusion in alkali-carbonatite and hydrous melts at 16.5 and 24 GPa: Implication for the melt transport by dissolution-precipitation in the transition zone and uppermost lower mantle. Phys. Earth Planet. Inter. 2013, 225, 1–11. [Google Scholar] [CrossRef]
- Amundsen, H.E.F. Evidence for liquid immiscibility in the upper mantle. Nature 1987, 327, 692–695. [Google Scholar] [CrossRef]
- Sumiya, H.; Yusa, H.; Inoue, T.; Ofuji, H.; Irifune, T. Conditions and mechanism of formation of nano-polycrystalline diamonds on direct transformation from graphite and non-graphitic carbon at high pressure and temperature. High Press. Res. 2006, 26, 63–69. [Google Scholar] [CrossRef]
- Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 2003, 214, 357–368. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Withers, A.C. Deep global cycling of carbon constrained by the solidus of anhydrous, carbonated eclogite under upper mantle conditions. Earth Planet. Sci. Lett. 2004, 227, 73–85. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; Dellas, N. The effect of bulk composition on the solidus of carbonated eclogite from partial melting experiments at 3 GPa. Contrib. Mineral. Petrol. 2005, 149, 288–305. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Kiseeva, E.S.; Yaxley, G.M.; Hermann, J.; Litasov, K.D.; Rosenthal, A.; Kamenetsky, V.S. An experimental study of carbonated eclogite at 3.5–5.5 GPa—Implications for silicate and carbonate metasomatism in the cratonic mantle. J. Petrol. 2012, 53, 727–759. [Google Scholar] [CrossRef]
- Shirasaka, M.; Takahashi, E. A genesis of carbonatitic melt within subducting oceanic crust: High pressure experiments in the system MORB-CaCO3. In Proceedings of the 8th International Kimberlite Conference Long Abstract, Victoria, BC, Canada, 22–27 June 2003; pp. 1–5. [Google Scholar]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E.; Yaxley, G.M. The solidus of alkaline carbonatite in the deep mantle. Geology 2013, 41, 79–82. [Google Scholar] [CrossRef]
- Bekhtenova, A.; Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D. Phase relations in carbonate component of carbonatized eclogite and peridotite along subduction and continental geotherms. Gondwana Res. 2021, 94, 186–200. [Google Scholar] [CrossRef]
- Bustarret, E.; Gheeraert, E.; Watanabe, K. Optical and electronic properties of heavily boron-doped homo-epitaxial diamond. Phys. Status Solidi 2003, 199, 9–18. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Egonin, I.A.; Mironov, A.M.; Palyanov, Y.N.; Ohtani, E. The system Na2CO3–CaCO3–MgCO3 at 6 GPa and 900–1250 °C and its relation to the partial melting of carbonated mantle. High Press. Res. 2016, 36, 23–41. [Google Scholar] [CrossRef]
- Pyle, J.M.; Haggerty, S.E. Silicate-carbonate liquid immiscibility in upper-mantle eclogites: Implications for natrosilicic and carbonatitic conjugate melts. Geochim. Et Cosmochim. Acta 1994, 58, 2997–3011. [Google Scholar] [CrossRef]
- Girnis, A.; Bulatov, V.; Brey, G.; Gerdes, A.; Höfer, H. Trace element partitioning between mantle minerals and silico-carbonate melts at 6–12GPa and applications to mantle metasomatism and kimberlite genesis. Lithos 2013, 160, 183–200. [Google Scholar] [CrossRef]
- Pal'yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation from mantle carbonate fluids. Nature 1999, 400, 417–418. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.; Rossman, G.; Wasserburg, G. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Weiss, Y.; Czas, J.; Navon, O. Fluid inclusions in fibrous diamonds. Rev. Mineral. Geochem. 2022, 88, 475–532. [Google Scholar] [CrossRef]
- Shatskiy, A.; Bekhtenova, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D. Carbonate melt interaction with natural eclogite at 6 GPa and 1100–1200 °C: Implications for metasomatic melt composition in subcontinental lithospheric mantle. Chem. Geol. 2020, 558, 119915. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.J.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the geology of mantle carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef]
- Katsura, T. A revised adiabatic temperature profile for the mantle. J. Geophys.Res. Solid Earth 2022, 127, e2021JB023562. [Google Scholar] [CrossRef]
- Ragozin, A.L.; Palyanov, Y.N.; Zedgenizov, D.A.; Kalinin, A.A.; Shatsky, V.S. The homogenization of carbonate-containing microinclusions in diamond at P-T parameters of the upper mantle. Doklady Akademii Nauk in press. 2016. [Google Scholar]
- Ragozin, A.L.; Karimova, A.A.; Litasov, K.D.; Zedgenizov, D.A.; Shatsky, V.S. The water content in mantle xenoliths from Udachnaya pipe (Yakutia). Russ. Geol. Geophys. 2014, 55, 428–442. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with oceanic crustal and mantle signatures from the Bellsbank kimberlite, South Africa, Part I: Mineralogy, petrography, and whole rock chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- Coleman, R.G.; Lee, D.E.; Beatty, L.B.; Brannock, W.W. Eclogites and eclogites: Their differences and similarities. Geol. Soc. Am. Bull. 1965, 76, 483–508. [Google Scholar] [CrossRef]
- Ellis, D.J.; Green, D.H. An experimental study of the effect of Ca upon garnet-clinopyroxene Fe-Mg exchange equilibria. Contrib. Mineral. Petrol. 1979, 71, 13–22. [Google Scholar] [CrossRef]
- Sidorov, V.; Ekimov, E.; Stishov, S.; Bauer, E.; Thompson, J. Superconducting and normal-state properties of heavily hole-doped diamond. Phys. Rev. B 2005, 71, 060502. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Terasaki, H.; Katsura, T.; Ohtani, E. Performance of semi-sintered ceramics as pressure-transmitting media up to 30 GPa. High Press. Res. 2010, 30, 443–450. [Google Scholar] [CrossRef]
- Hernlund, J.; Leinenweber, K.; Locke, D.; Tyburczy, J.A. A numerical model for steady-state temperature distributions in solid-medium high-pressure cell assemblies. Am. Mineral. 2006, 91, 295–305. [Google Scholar] [CrossRef]
- Shatskiy, A.; Sharygin, I.S.; Gavryushkin, P.N.; Litasov, K.D.; Borzdov, Y.M.; Shcherbakova, A.V.; Higo, Y.; Funakoshi, K.-I.; Palyanov, Y.N.; Ohtani, E. The system K2CO3-MgCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2013, 98, 1593–1603. [Google Scholar] [CrossRef]
- Brey, G.P.; Kohler, T. Geothermobarometry in four-phase lherzolites II. New thermobarometers, and practical assessment of existing thermobarometers. J. Petrol. 1990, 31, 1353–1378. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.S.; Usova, L.V. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Newbury, D.E.; Ritchie, N.W.M. Performing elemental microanalysis with high accuracy and high precision by scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS). J. Mater. Sci. 2015, 50, 493–518. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Behtenova, A.; Litasov, K.D. The system K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for carbonatite melt compositions in the subcontinental lithospheric mantle. Minerals 2019, 9, 296. [Google Scholar] [CrossRef]
- Powell, R. Regression diagnostics and robust regression in geothermometer/geobarometer calibration: The garnet-clinopyroxene geothermometer revisited. J. Metamorph. Geol. 1985, 3, 231–243. [Google Scholar] [CrossRef]
- Krogh Ravna, E. The garnet–clinopyroxene Fe2+–Mg geothermometer: An updated calibration. J. Metamorph. Geol. 2000, 18, 211–219. [Google Scholar] [CrossRef]
- Krogh, E.J. The garnet-clinopyroxene Fe-Mg geothermometer—A reinterpretation of existing experimental data. Contrib. Mineral. Petrol. 1988, 99, 44–48. [Google Scholar] [CrossRef]
- Korsakov, A.V.; Dieing, T.; Golovin, A.V.; Toporski, J. Raman imaging of fluid inclusions in garnet from UHPM rocks (Kokchetav massif, Northern Kazakhstan). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 80, 88–95. [Google Scholar] [CrossRef]
- Dawson, J.B. Contrasting types of upper-mantle metasomatism. In Developments in Petrology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 11, pp. 289–294. [Google Scholar]
- McNeil, A.M.; Edgar, A.D. Sodium-rich metasomatism in the upper mantle: Implications of experiments on the pyrolite-Na2O-rich fluid system at 950 °C, 20 kbar. Geochim. Et Cosmochim. Acta 1987, 51, 2285–2294. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. A modified iterative sandwich method for determination of near-solidus partial melt compositions. II. Application to determination of near-solidus melt compositions of carbonated peridotite. Contrib. Mineral. Petrol. 2007, 154, 647–661. [Google Scholar] [CrossRef]
- Safonov, O.G.; Litvin, Y.A.; Perchuk, L.L. Synthesis of omphacites and isomorphic features of clinopyroxenes in the system CaMgSi2O6-NaAlSi2O6-KAlSi2O6. Petrology 2004, 12, 70–81. [Google Scholar]
- Harlow, G.E. K in clinopyroxene at high pressure and temperature: An experimental study. Am. Mineral. 1997, 82, 259–269. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H. Experimental demonstration of refractory carbonate-bearing eclogite and siliceous melt in the subduction regime. Earth Planet. Sci. Lett. 1994, 128, 313–325. [Google Scholar] [CrossRef]
- Safonov, O.G.; Perchuk, L.L.; Yapaskurt, V.O.; Litvin, Y.A. Immiscibility of carbonate-silicate and chloride-carbonate melts in the kimberlite-CaCO3–Na2CO3–KCl system at 4.8 GPa. Dokl. Earth Sci. 2009, 424, 388–392. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Kupriyanov, I.N.; Ohtani, E.; Palyanov, Y.N. Phase relations in the system FeCO3-CaCO3 at 6 GPa and 900–1700 °C and its relation to the system CaCO3-FeCO3-MgCO3. Am. Mineral. 2014, 99, 773–785. [Google Scholar] [CrossRef]
- Harker, R.I.; Tuttle, O.F. Studies in the system CaO-MgO-CO2; Part 1, The thermal dissociation of calcite, dolomite and magnesite. Am. J. Sci. 1955, 253, 209–224. [Google Scholar] [CrossRef]
- Luth, R.W. Experimental study of the CaMgSi2O6-CO2 system at 3–8 GPa. Contrib. Mineral. Petrol. 2006, 151, 141–157. [Google Scholar] [CrossRef]
- Day, H.W. A revised diamond-graphite transition curve. Am. Mineral. 2012, 97, 52–62. [Google Scholar] [CrossRef]
- Hasterok, D.; Chapman, D.S. Heat production and geotherms for the continental lithosphere. Earth Planet. Sci. Lett. 2011, 307, 59–70. [Google Scholar] [CrossRef]
- Irifune, T.; Isobe, F.; Shinmei, T. A novel large-volume Kawai-type apparatus and its application to the synthesis of sintered bodies of nano-polycrystalline diamond. Phys. Earth Planet. Inter. 2014, 228, 255–261. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Bekhtenova, A.; Vinogradova, Y.G.; Stepanov, K.M.; Litasov, K.D. Pyroxene-carbonate reactions in the CaMgSi2O6 ± NaAlSi2O6 + MgCO3 ± Na2CO3 ± K2CO3 system at 3–6 GPa: Implications for partial melting of carbonated peridotite. Contrib. Mineral. Petrol. 2021, 176, 34. [Google Scholar] [CrossRef]
- Thomsen, T.B.; Schmidt, M.W. Melting of carbonated pelites at 2.5–5.0 GPa, silicate–carbonatite liquid immiscibility, and potassium–carbon metasomatism of the mantle. Earth Planet. Sci. Lett. 2008, 267, 17–31. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Liquid immiscibility and phase relations in the join KAlSi3O8–CaMg(CO3)2±NaAlSi2O6±Na2CO3 at 6 GPa: Implications for diamond-forming melts. Chem. Geol. 2020, 550, 119701. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Effect of water on carbonate-silicate liquid immiscibility in the system KAlSi3O8–CaMgSi2O6–NaAlSi2O6–CaMg(CO3)2 at 6 GPa: Implications for diamond-forming melts. Am. Mineral. 2021, 106, 165–173. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V. Melting of K-rich carbonated peridotite at 6–10 GPa and the stability of K-phases in the upper mantle. Chem. Geol. 2011, 281, 333–342. [Google Scholar] [CrossRef]
- Minarik, W.G.; Watson, E.B. Interconnectivity of carbonate melt at low melt fraction. Earth Planet. Sci. Lett. 1995, 133, 423–437. [Google Scholar] [CrossRef]
- Dobson, D.P.; Jones, A.P.; Rabe, R.; Sekine, T.; Kurita, K.; Taniguchi, T.; Kondo, T.; Kato, T.; Shimomura, O.; Urakawa, S. In-situ measurement of viscosity and density of carbonate melts at high pressure. Earth Planet. Sci. Lett. 1996, 143, 207–215. [Google Scholar] [CrossRef]
- Hammouda, T.; Laporte, D. Ultrafast mantle impregnation by carbonatite melts. Geology 2000, 28, 283–285. [Google Scholar] [CrossRef]
- Kono, Y.; Kenney-Benson, C.; Hummer, D.; Ohfuji, H.; Park, C.; Shen, G.; Wang, Y.; Kavner, A.; Manning, C.E. Ultralow viscosity of carbonate melts at high pressures. Nat. Commun. 2014, 5, 5091. [Google Scholar] [CrossRef]
- Stagno, V.; Stopponi, V.; Kono, Y.; Manning, C.E.; Irifune, T. Experimental determination of the viscosity of Na2CO3 melt between 1.7 and 4.6 GPa at 1200–1700°C: Implications for the rheology of carbonatite magmas in the Earth's upper mantle. Chem. Geol. 2018, 501, 19–25. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Ohtani, E. Composition of primary kimberlite melt in a garnet lherzolite mantle source: Constraints from melting phase relations in anhydrous Udachnaya-East kimberlite with variable CO2 content at 6.5 GPa. Gondwana Res. 2017, 45, 208–227. [Google Scholar] [CrossRef]
- Mikhailenko, D.S.; Rezvukhin, D.I.; Korsakov, A.V.; Sobolev, N.V. Olivine in a coesite-bearing eclogite from the Udachnaya kimberlite pipe. Dokl. Earth Sci. 2019, 489, 1358–1362. [Google Scholar] [CrossRef]
- Mikhailenko, D.; Golovin, A.; Korsakov, A.; Aulbach, S.; Gerdes, A.; Ragozin, A. Metasomatic evolution of coesite-bearing diamondiferous eclogite from the Udachnaya kimberlite. Minerals 2020, 10, 383. [Google Scholar] [CrossRef]
- Misra, K.C.; Anand, M.; Taylor, L.A.; Sobolev, N.V. Multi-stage metasomatism of diamondiferous eclogite xenoliths from the Udachnaya kimberlite pipe, Yakutia, Siberia. Contrib. Mineral. Petrol. 2004, 146, 696–714. [Google Scholar] [CrossRef]
- Spetsius, Z.V.; Taylor, L.A. Partial melting in mantle eclogite xenoliths: Connections with diamond paragenesis. Int. Geol. Rev. 2002, 44, 973–987. [Google Scholar] [CrossRef]
- Sobolev, N.V. Deep Seated Inclusions in Kimberlites and the Problem of the Composition of the Upper Mantle; AGU: Washington, DC, USA, 1977; p. 304. [Google Scholar]
- Perchuk, L.L.; Safonov, O.G.; Yapaskurt, V.O.; Barton, J.M., Jr. Crystal-melt equilibria involving potassium-bearing clinopyroxene as indicator of mantle-derived ultrahigh-potassic liquids: An analytical review. Lithos 2002, 60, 89–111. [Google Scholar] [CrossRef]
- Enggist, A.; Chu, L.L.; Luth, R.W. Phase relations of phlogopite with magnesite from 4 to 8 GPa. Contrib. Mineral. Petrol. 2012, 163, 467–481. [Google Scholar] [CrossRef]
- Enggist, A.; Luth, R.W. Phase relations of phlogopite and pyroxene with magnesite from 4 to 8 GPa: KCMAS–H2O and KCMAS–H2O–CO2. Contrib. Mineral. Petrol. 2016, 171, 88. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Kaminsky, F.V.; Griffin, W.L.; Yefimova, E.S.; Win, T.T.; Ryan, C.G.; Botkunov, A.I. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Logvinova, A.M.; Efimova, E.S. Syngenetic phlogopite inclusions in kimberlite-hosted diamonds: Implications for role of volatiles in diamond formation. Russ. Geol. Geophys. 2009, 50, 1234–1248. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Zedgenizov, D.A.; Ragozin, A.L.; Kalinina, V.V. Diamondiferous subcontinental lithospheric mantle of the northeastern Siberian Craton: Evidence from mineral inclusions in alluvial diamonds. Gondwana Res. 2015, 28, 106–120. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V.; Lahaye, Y. Experimental melting of carbonated peridotite at 6–10 GPa. J. Petrol. 2008, 49, 797–821. [Google Scholar] [CrossRef]
- Wyllie, P.J.; Huang, W.L. Inflence of mantle CO2 ingeneration of carbonatites and kimberlites. Nature 1975, 257, 297–299. [Google Scholar] [CrossRef]
- Moussallam, Y.; Morizet, Y.; Massuyeau, M.; Laumonier, M.; Gaillard, F. CO2 solubility in kimberlite melts. Chem. Geol. 2014, 418, 198–205. [Google Scholar] [CrossRef]
- Sharygin, I.; Litasov, K.; Shatskiy, A.; Golovin, A.; Ohtani, E.; Pokhilenko, N. Melting phase relations of the Udachnaya-East group-I kimberlite at 3.0–6.5 GPa: Experimental evidence for alkali-carbonatite composition of primary kimberlite melts and implications for mantle plumes. Gondwana Res. 2015, 28, 1391–1414. [Google Scholar] [CrossRef]
- Litasov, K.D.; Sharygin, I.S.; Shatskiy, A.F.; Ohtani, E.; Pokhilenko, N.P. Experimental constraints on the role of chloride in the origin and evolution of kimberlitic magma. Dokl. Earth Sci. 2010, 435, 1641–1646. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kupriyanov, I.N.; Palyanov, Y.N.; Kruk, A.N.; Sobolev, N.V. Melting experiments on the Udachnaya kimberlite at 6.3–7.5 GPa: Implications for the role of H2O in magma generation and formation of hydrous olivine. Geochim. Et Cosmochim. Acta 2013, 101, 133–155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).