Land Reclamation Using Typical Coal Gasification Slag in Xinjiang: A Full-Cycle Environmental Risk Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sampling and Testing Methods
2.2.1. Sample Collection
2.2.2. Selection of Typical Pollutants
2.2.3. Sample Determination
2.3. Environmental Risk Evaluation Methodology
2.3.1. Nemerow Composite Pollution Index Method
2.3.2. Potential Ecological Risk Index Method
2.3.3. Geo-Accumulation Index Method
2.3.4. Human Health Risk Assessment
- (1)
- Exposure calculation and parameter selection
- (2)
- Hazard quotient/carcinogenic risk calculation and parameter selection
2.4. Establishment of an Evaluation System
3. Results and Discussion
3.1. The Descriptive Statistics of Typical Pollutants
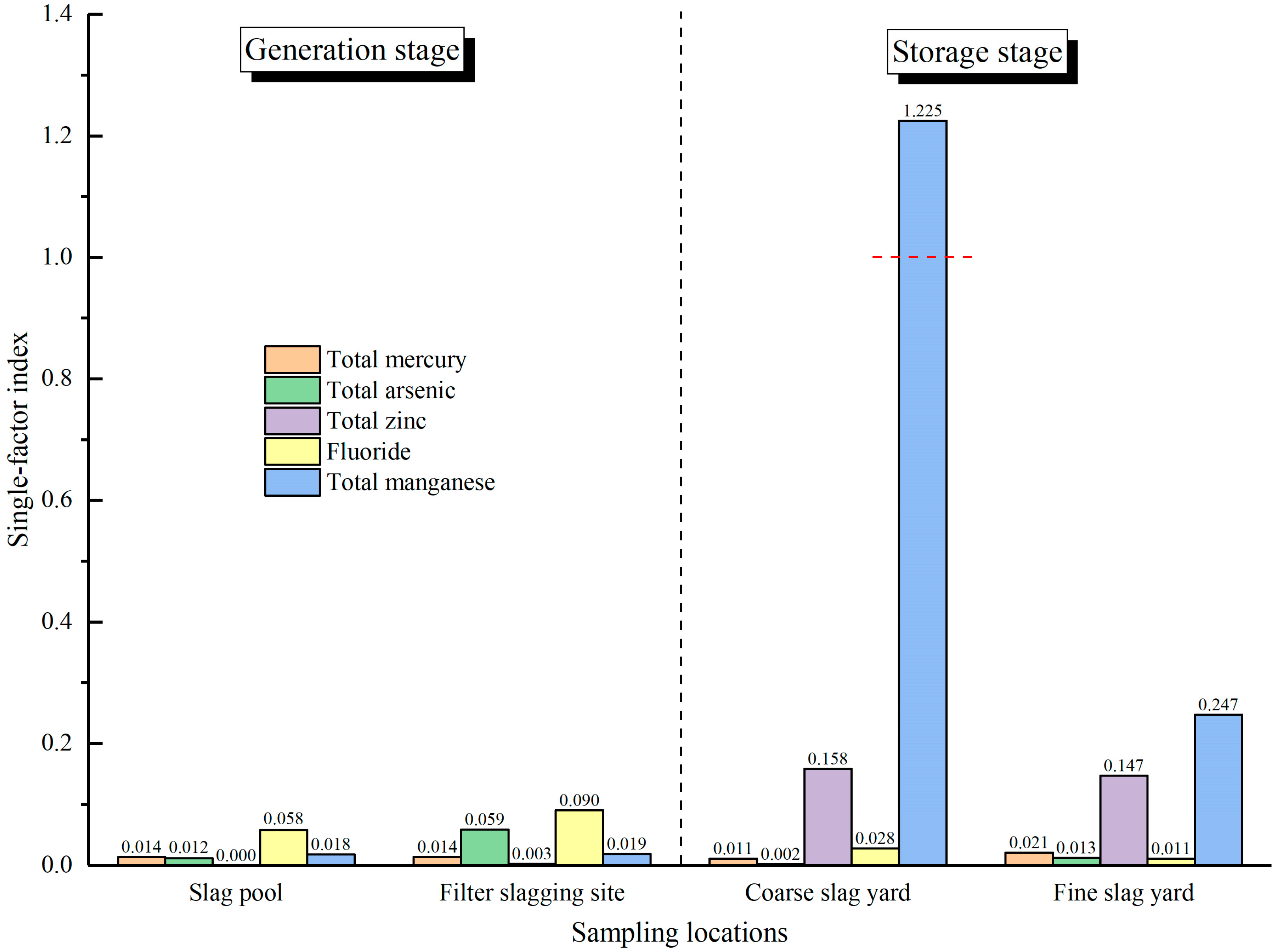
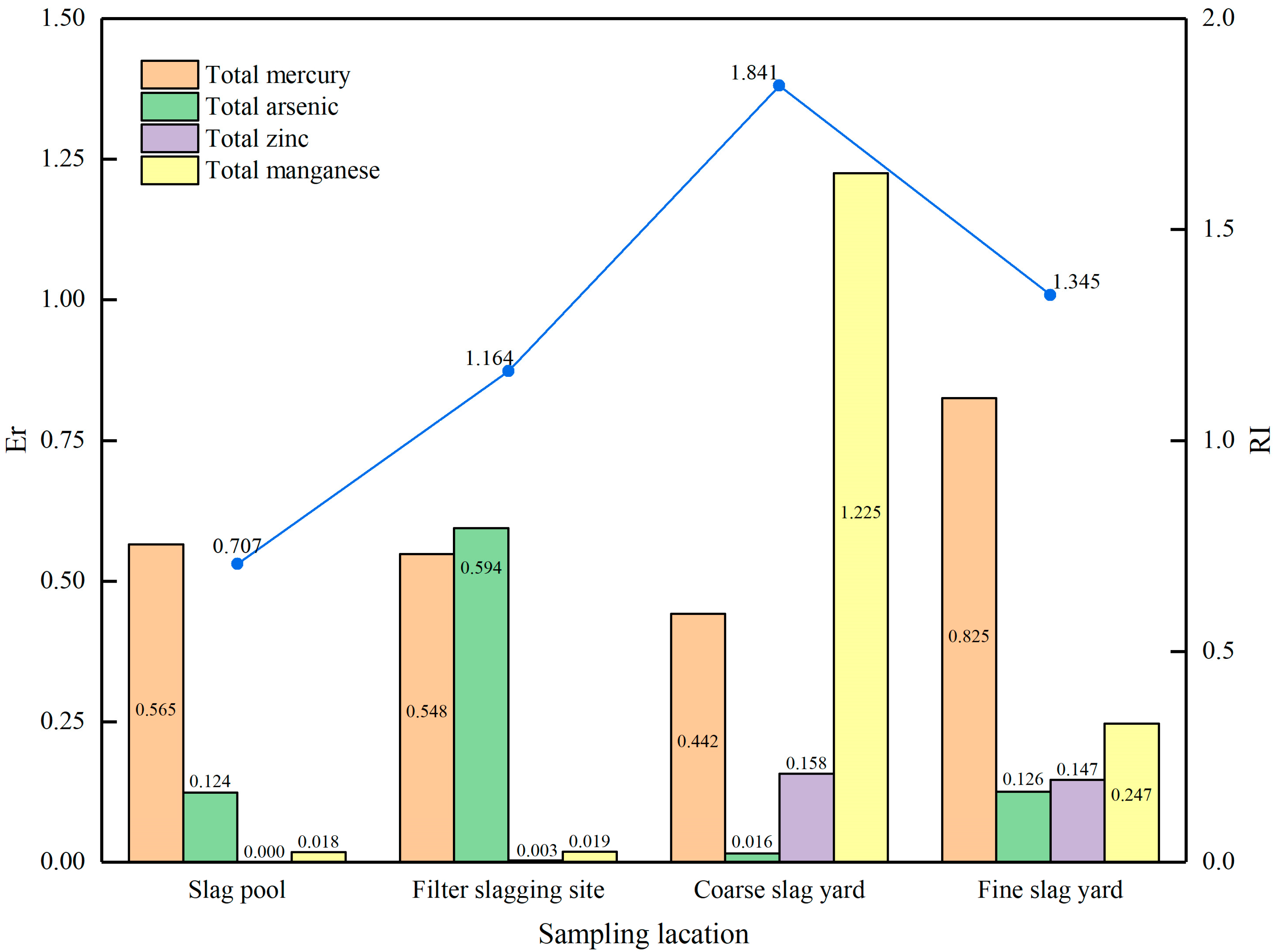
3.2. Environmental Risk Evaluation of Coal Gasification Slag Generation and Storage
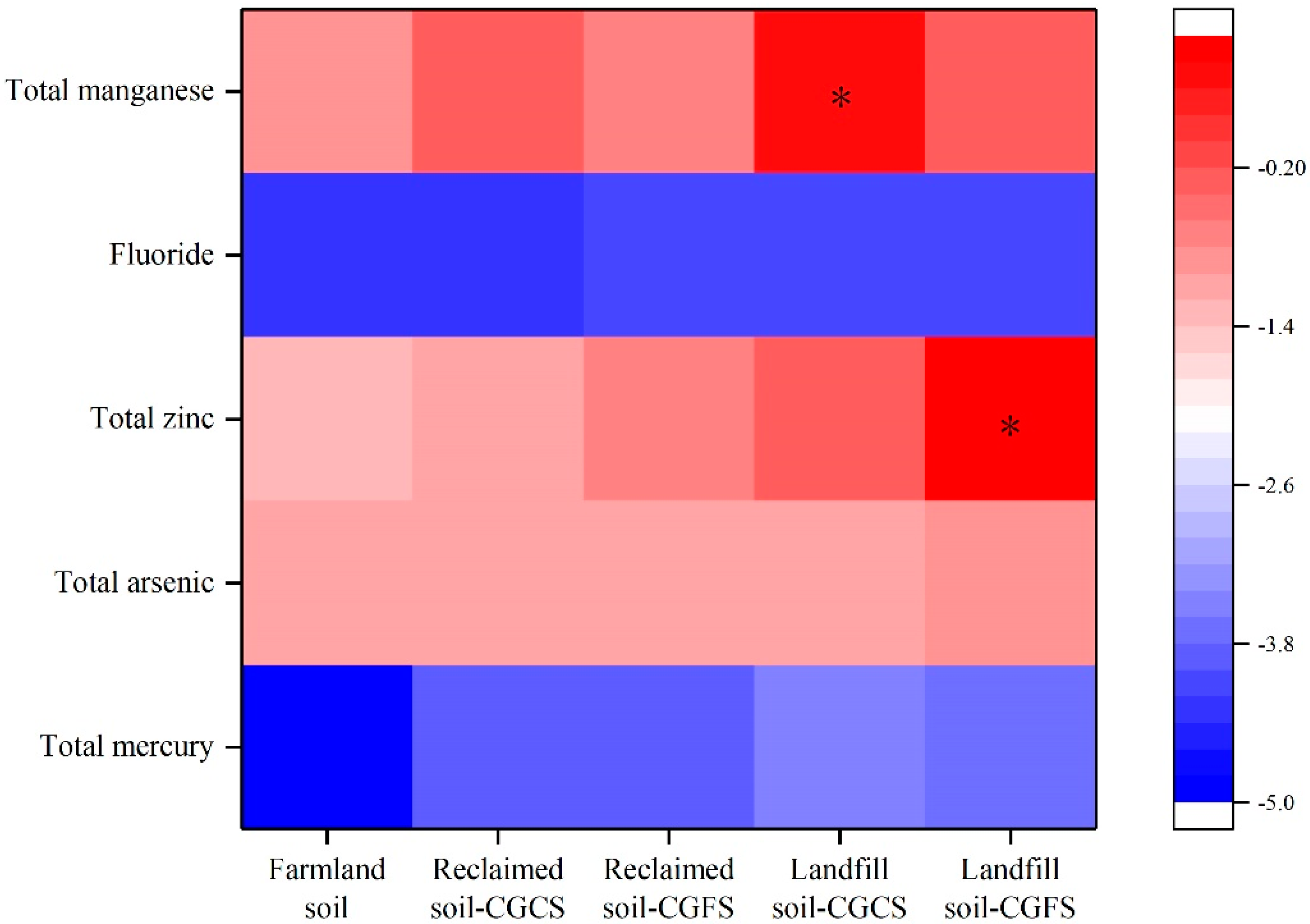
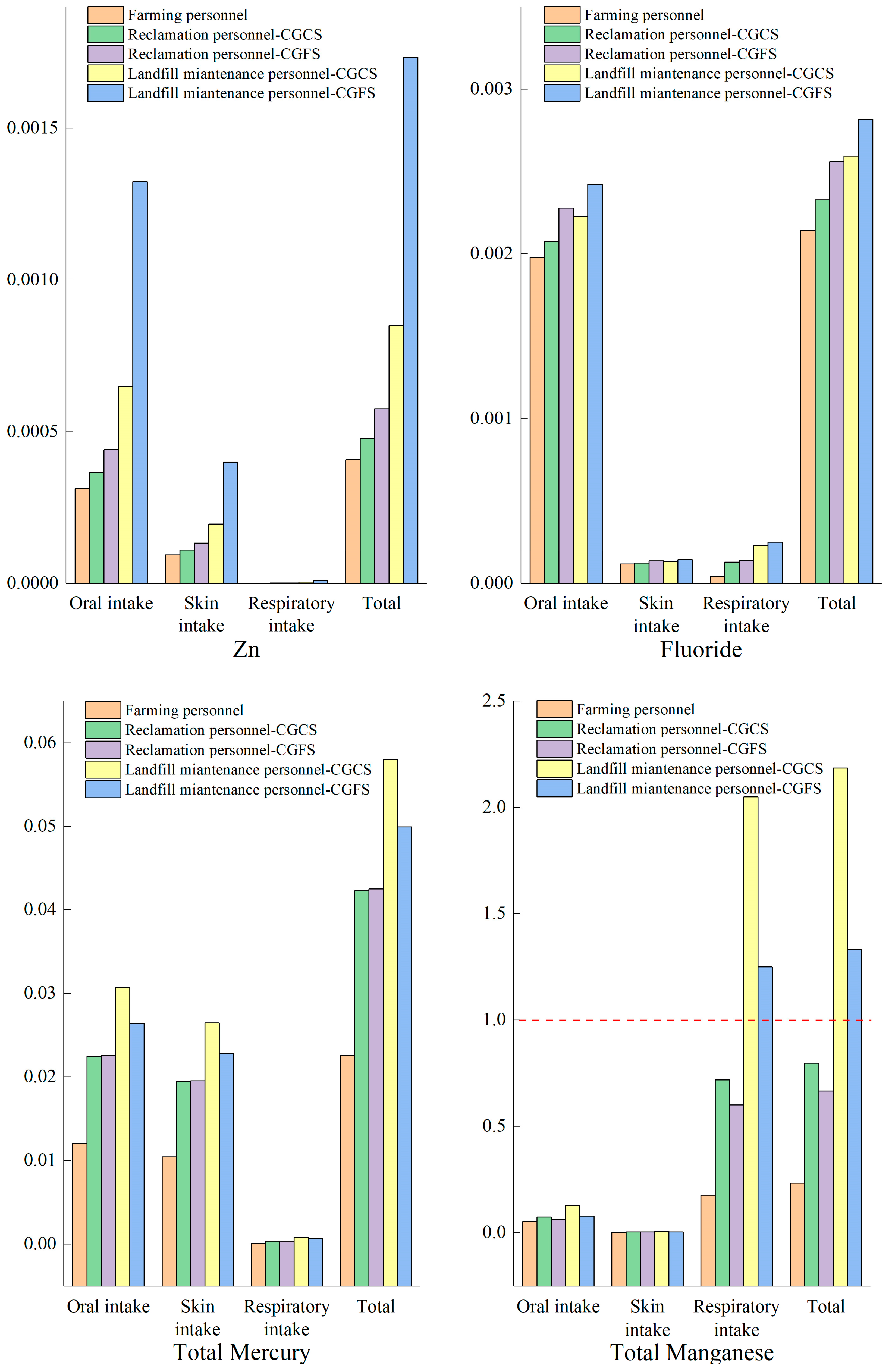
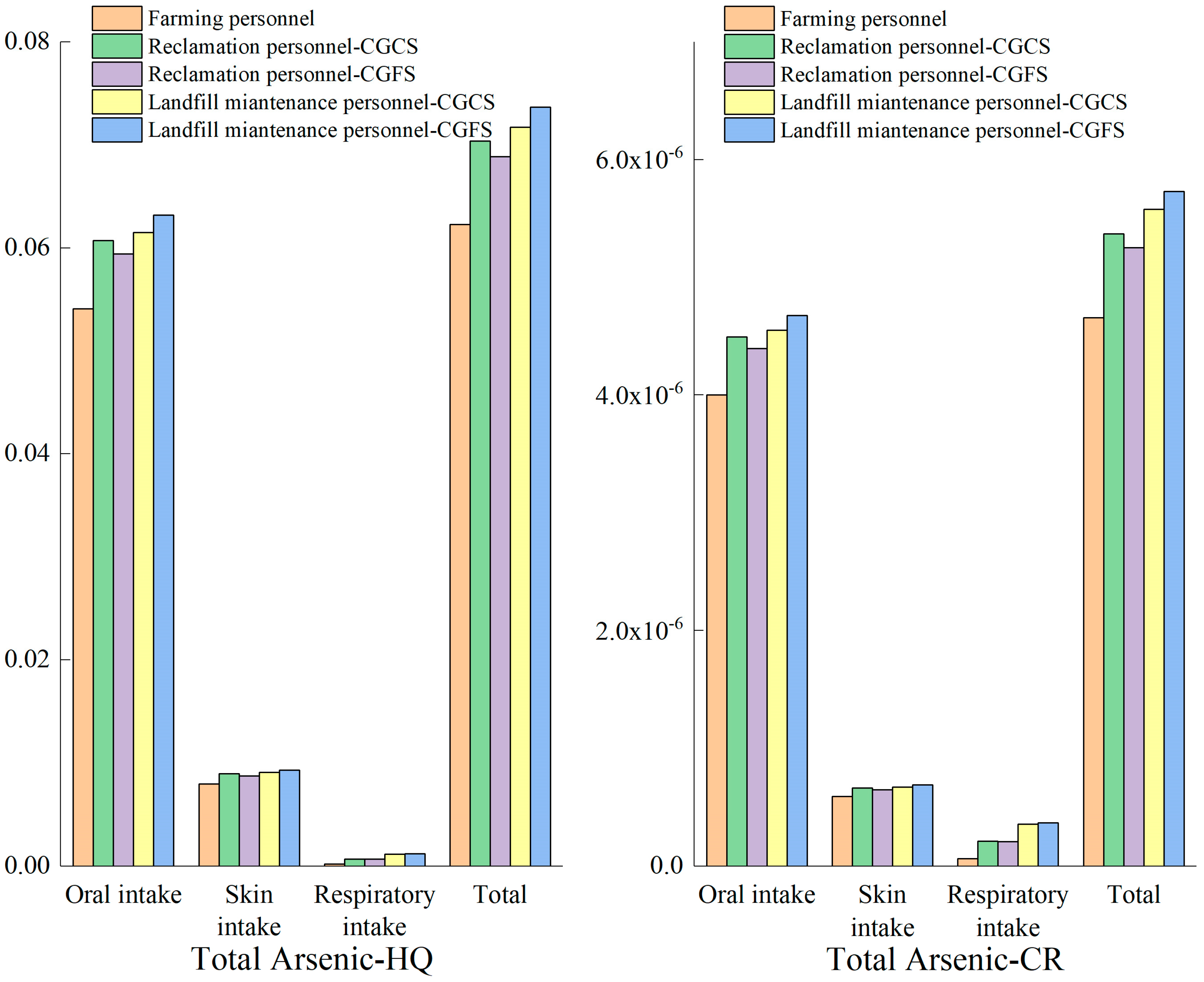
3.3. Environmental Risk Evaluation of Coal Gasification Slag Disposal
3.4. Prevention Measures for the Ecological Risk of Coal Gasification Slag
4. Conclusions
- (1)
- The Nemerow composite pollution index and comprehensive potential ecological risk index of the CGCS in the slag pool and the CGFS at the filter slagging site are both lower than the standard value of 1, indicating that there is no environmental risk from coal gasification slag at the generation stage.
- (2)
- At the slag yard, the Nemerow composite pollution index and the comprehensive potential ecological risk index of CGCS and CGFS are also below the standard value. However, the value of the single-factor index of manganese in CGCS exceeds the standard value. This suggests that there is no environmental risk from coal gasification slag during storage. Nevertheless, attention needs to be paid to the leaching risk of manganese in the CGCS.
- (3)
- When 15% of CGS is used for land reclamation, the geo-accumulation index, carcinogenicity risk, and hazard quotient of each typical pollutant do not exceed the standard. This indicates that the use of CGS for land reclamation poses no environmental risk. When all of the CGS is disposed of in a landfill, the geo-accumulation index of manganese in CGCS and zinc in CGFS surpasses the standard value. Additionally, the hazard quotient of manganese in CGS is greater than the standard value. These findings imply that landfill disposal of CGS will create environmental risks.
- (4)
- Based on the aforementioned research results, during the storage stage, CGS can be mitigated by spraying alkaline lime water on the coarse slag yard. By installing an anti-seepage membrane in the slag yard, curing and stabilizing the coal gasification slag, and incorporating adsorbents into the CGS, potential environmental hazards caused by the CGS in the landfill can be mitigated.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.D.; Jin, Z.W.; Jing, Y.H.; Fan, P.P.; Qi, Z.L.; Bao, W.R.; Wang, J.C.; Yan, X.H.; Lv, P.; Dong, L.P. Review of the characteristics and graded utilisation of coal gasification slag. Chin. J. Chem. Eng. 2021, 35, 92–106. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, H.; Wang, X.; Rahman, Z.U.; Shi, Z.; Bai, Y.; Wang, G.; Chen, Y.; Wang, J.; Liu, L. Enrichment of residual carbon from coal gasification fine slag by spiral separator. J. Environ. Manag. 2022, 315, 115149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, S.; Yilmaz, E. Influence of 3D-printed polymer structures on dynamic splitting and crack propagation behavior of cementitious tailings backfill. Constr. Build. Mater. 2022, 343, 128137. [Google Scholar] [CrossRef]
- Guo, Q.H.; Huang, Y.C.; Gong, Y.; Zhuang, X.D.; Richter, A.; Yu, G.S. Recovered Carbon from Coal Gasification Fine Slag as Electrocatalyst for Oxygen Reduction Reaction and Zinc-Air Battery. Energy Technol. 2021, 9, 2000890. [Google Scholar] [CrossRef]
- Wu, F.; Li, H.; Yang, K. Effects of Mechanical Activation on Physical and Chemical Characteristics of Coal-Gasification Slag. Coatings 2021, 11, 902. [Google Scholar] [CrossRef]
- Zhu, D.; Xue, B.; Jiang, Y.; Wei, C. Using chemical experiments and plant uptake to prove the feasibility and stability of coal gasification fine slag as silicon fertilizer. Environ. Sci. Pollut. Res. 2019, 26, 5925–5933. [Google Scholar] [CrossRef]
- Zhang, R.M.; Li, X.A.; Zhang, K.; Wang, P.F.; Xue, P.F.; Zhang, H.L. Research on the Application of Coal Gasification Slag in Soil Improvement. Processes 2022, 10, 2690. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Y.; Chen, L.; Jia, W.; Zhu, Y.; Li, Y.; Wang, H.; Yao, X.; Zhang, Y.; Wu, J. Multitudinous components recovery, heavy metals evolution and environmental impact of coal gasification slag: A review. Chemosphere 2023, 338, 139473. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Wan, S.; Zheng, R.; Tong, J.; Hou, H.; Wang, T. Synthesis and characterization of geopolymer composites based on gasification coal fly ash and steel slag. Constr. Build. Mater. 2019, 211, 646–658. [Google Scholar] [CrossRef]
- Guo, Y.; Li, H.; Qiu, G.; Li, Y.; Niu, Y.; Xu, J.; Jia, W.; Zhang, Y.; Wu, J.; Guo, F. Processing of coal gasification fine slag by different physical separation methods: Fate of typical heavy metals and comparison analysis on products. Sep. Purif. Technol. 2023, 306, 122675. [Google Scholar] [CrossRef]
- Xiang, Y.; Xiang, Y.; Wang, L.; Li, X. Effects of sewage sludge modified by coal gasification slag and electron beam irradiation on the growth of Alhagi sparsifolia Shap. and transfer of heavy metals. Environ. Sci. Pollut. Res. 2018, 25, 11636–11645. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Zhao, A.; Hu, Z.; Tan, K.; Zhang, J. Preparation and application of porous materials from coal gasification slag for wastewater treatment: A review. Chemosphere 2022, 287, 132227. [Google Scholar] [CrossRef]
- Xiao, H.P.; Li, Y.; Wang, M.W.; Guo, Z.W.; Yan, D.H.; Liu, Z. Distributions of and environmental risks posed by Cr and Zn when co-treating solid waste in different kilns. Waste Manag. 2023, 165, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.X.; Zhao, X.; Xu, J.; Qiu, G.F.; Jia, W.K.; Wu, J.J.; Guo, F.H. Multifaceted evaluation of distribution, occurrence, and leaching features of typical heavy metals in different-sized coal gasification fine slag from Ningdong region, China: A case study. Sci. Total Environ. 2022, 831, 154726. [Google Scholar] [CrossRef]
- Qu, J.S.; Zhang, J.B.; Li, H.Q.; Li, S.P.; Shi, D.; Chang, R.Q.; Wu, W.F.; Zhu, G.Y.; Yang, C.; Wang, C.Y. Occurrence, leaching behavior, and detoxification of heavy metal Cr in coal gasification slag. Chin. J. Chem. Eng. 2023, 58, 11–19. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, Y.; Li, R.; Guo, X.; Hurley, J.P.; Finkelman, R.B. Measurements of the leachability of potentially hazardous trace elements from solid coal gasification wastes in China. Sci. Total Environ. 2021, 759, 143463. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, H. Accumulation of Heavy Metals in Roadside Soil in Urban Area and the Related Impacting Factors. Int. J. Environ. Res. Public Health 2018, 15, 1064. [Google Scholar] [CrossRef]
- Technical Specifications on Sampling and Sample Preparation from Industry Solid Waste. HJ/T 20-1998. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/gthw/qtxgbz/199807/W020190128557848529134.pdf (accessed on 25 September 2023). (In Chinese)
- Shen, F.; Mao, L.; Sun, R.; Du, J.; Tan, Z.; Ding, M. Contamination Evaluation and Source Identification of Heavy Metals in the Sediments from the Lishui River Watershed, Southern China. Int. J. Environ. Res. Public Health 2019, 16, 336. [Google Scholar] [CrossRef]
- Integrated Wastewater Discharge Standard. GB 8978-1996. Available online: https://english.mee.gov.cn/Resources/standards/water_environment/Discharge_standard/200710/W020061027521858212955.pdf (accessed on 25 September 2023). (In Chinese)
- Zhang, S.; Wang, T.; Wang, H.; Kang, Q.; Zhou, Q.; Chen, B. Spatial Pattern, Sources Identification, and Risk Assessment of Heavy Metals in a Typical Soda Soil from Bayannur, Northwestern China. Int. J. Environ. Res. Public Health 2022, 19, 13880. [Google Scholar] [CrossRef]
- Ahamad, M.I.; Song, J.X.; Sun, H.T.; Wang, X.X.; Mehmood, M.S.; Sajid, M.; Su, P.; Khan, A.J. Contamination Level, Ecological Risk, and Source Identification of Heavy Metals in the Hyporheic Zone of the Weihe River, China. Int. J. Environ. Res. Public Health 2020, 17, 1070. [Google Scholar] [CrossRef]
- Jia, S.; Changchun, X.; Yingxue, L.; Yuanyuan, Y. Analysis of environmental risk and spatial distribution characteristics of heavy metal in soils of Zhundong coal area. Jiangsu Agric. Sci. 2019, 47, 241–247. [Google Scholar] [CrossRef]
- Technical Guidelines for Risk Assessment of Soil Contamination of Land for Construction. HJ 25.3-2019. Available online: https://www.chinesestandard.net/PDF/English.aspx/HJ25.3-2019 (accessed on 25 September 2023).
- Zhang, K.; Liu, S.H.; Wang, S.D.; Zhao, M.Y.; Jia, J.L. Health risk assessment and distribution of VOCs during excavation processes for the remediation of contaminated sites. Hum. Ecol. Risk Assess. 2019, 25, 2073–2088. [Google Scholar] [CrossRef]
- Zhang, K.; Li, H.; Cao, Z.; Shi, Z.; Liu, J. Human health risk assessment and risk source analysis of arsenic in soil from a coal chemical plant in Northwest China. J. Soils Sediments 2019, 19, 2785–2794. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Sun, C.; Sun, T.; Liu, X.; Li, J.; Fang, L.; Fan, Z. Heavy metals in soil of an urban industrial zone in a metropolis: Risk assessment and source apportionment. Stoch. Environ. Res. Risk A 2020, 34, 435–446. [Google Scholar] [CrossRef]
- Schiavo, B.; Meza-Figueroa, D.; Pedroza-Montero, M.; Vidal-Solano, J.; Gonzalez-Grijalva, B.; Navarro-Espinoza, S.; Romero, F.; Hernandez, E.; Gutierrez-Ruiz, M.E.; Ceniceros-Gomez, A.E. In vitro assessment oral and respiratory bioaccessibility of Mn in school dust: Insight of seasonality in a semiarid environment. Appl. Geochem. 2021, 134, 105102. [Google Scholar] [CrossRef]
- Ma, C.; Zheng, R.; Zhao, J.; Han, X.; Wang, L.; Gao, X.; Zhang, C. Relationships between heavy metal concentrations in soils and reclamation history in the reclaimed coastal area of Chongming Dongtan of the Yangtze River Estuary, China. J. Soils Sediments 2015, 15, 139–152. [Google Scholar] [CrossRef]
- Al-Kahtany, K.; Nour, H.E.; Giacobbe, S.; Alharbi, T.; El-Sorogy, A.S. Heavy metal pollution in surface sediments and human health assessment in southern Al-Khobar coast, Saudi Arabia. Mar. Pollut. Bull. 2023, 187, 114508. [Google Scholar] [CrossRef]
- Njayou, M.M.; Ngounouno, A.M.; Ngounouno, I. Trace metal contamination status in soils of the abandoned gold mining district of Bindiba (East Cameroon): Pollution indices assessment, multivariate analysis and; geostatistical approach. J. Environ. Health Sci. 2023, 21, 143–155. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Yang, H.; Yu, J.; Ni, R. Ecological risk assessment based on soil adsorption capacity for heavy metals in Taihu basin, China. Environ. Pollut. 2023, 316, 120608. [Google Scholar] [CrossRef]
- Gurusamy, S.; Thangam, R. Potential health risk assessment of contaminants in soil-like material recovered from landfill mining. Environ. Monit. Assess. 2023, 195, 330. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Song, Z.; Yan, J.; Chen, M.; Yin, J. Human Health Risk Distribution and Safety Threshold of Cadmium in Soil of Coal Chemical Industry Area. Minerals 2021, 11, 678. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Zheng, X.; Qiang, C.; Zhang, X. Human health risk assessment and spatial distribution of fluoride from shallow groundwater in a region of southwest China. J. Water Supply Res. T 2020, 69, 733–747. [Google Scholar] [CrossRef]
- Jiang, P.; Xie, C.; Luo, C.; Meng, W.; Yang, G.; Yu, G.; Gong, Y.; Xu, M.; Wu, T. Distribution and modes of occurrence of heavy metals in opposed multi-burner coal-water-slurry gasification plants. Fuel 2021, 303, 121163. [Google Scholar] [CrossRef]
- Tang, Y.; Yin, R.; Ren, Y.; Zhang, J. Preparation of Sialon Powder from Coal Gasification Slag. J. Wuhan Univ. Technol. 2010, 25, 1044–1046. [Google Scholar] [CrossRef]
- Dalpisol, M.; Serrat, B.M.; Vargas Motta, A.C.; Poggere, G.C.; Bittencourt, S.; Barbosa, J.Z. Zinc, copper and manganese availability in soils treated with alkaline sewage sludge from Parana state (Brazil). Cienc. Agrotecnol. 2017, 41, 81–93. [Google Scholar] [CrossRef]
- Liu, T.; Awasthi, S.K.; Duan, Y.; Zhang, Z.; Awasthi, M.K. Effect of fine coal gasification slag on improvement of bacterial diversity community during the pig manure composting. Bioresour. Technol. 2020, 304, 123024. [Google Scholar] [CrossRef]
- Xiang, Y.; Jiao, Y.; Wang, L. Effect of sludge amino acid-modified magnetic coal gasification slag on plant growth, metal availability, and soil enzyme activity. J. Soil. Water Conserv. 2020, 75, 515–526. [Google Scholar] [CrossRef]
- Zhu, D.; Miao, S.; Xue, B.; Jiang, Y.; Wei, C. Effect of Coal Gasification Fine Slag on the Physicochemical Properties of Soil. Water Air Soil. Pollut. 2019, 230, 155. [Google Scholar] [CrossRef]
- Marove, C.A.; Tangviroon, P.; Tabelin, C.B.; Igarashi, T. Leaching of hazardous elements from Mozambican coal and coal ash. J. Afr. Earth Sci. 2020, 168, 103861. [Google Scholar] [CrossRef]
- Wang, F.; Shen, Z.; Al-Tabbaa, A. PC-based and MgO-based binders stabilised/solidified heavy metal-contaminated model soil: Strength and heavy metal speciation in early stage. Geotechnique 2018, 68, 1025–1030. [Google Scholar] [CrossRef]
- Zhan, B.J.; Poon, C.S.; Shi, C.J. CO2 curing for improving the properties of concrete blocks containing recycled aggregates. Cem. Concr. Comp. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Ullah, H.; Hussain, Q.; Al-Wabel, M.I.; Ahmad, M.; Hussain, A.; Riaz, M.; Ok, Y.S.; Kong, J. Adsorption and thermodynamic mechanisms of manganese removal from aqueous media by biowaste-derived biochars. J. Mol. Liq. 2018, 266, 373–380. [Google Scholar] [CrossRef]
- Jovanovic, M.; Arcon, I.; Kovac, J.; Tusar, N.N.; Obradovic, B.; Rajic, N. Removal of manganese in batch and fluidized bed systems using beads of zeolite a as adsorbent. Microporous Mesoporous Mater. 2016, 226, 378–385. [Google Scholar] [CrossRef]
- Lan, S.; Qin, Z.J.; Wang, X.M.; Yan, Y.P.; Tang, Y.D.; Feng, X.H.; Zhang, Q. Kinetics of Mn(II) adsorption and catalytic oxidation on the surface of ferrihydrite. Sci. Total Environ. 2021, 791, 148225. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.X.; Yuan, P.; Yue, Y.Y.; Bai, Z.S.; Zhu, H.B.; Wang, T.H.; Bao, X.J. Selective adsorption of Co(II)/Mn(II) by zeolites from purified terephthalic acid wastewater containing dissolved aromatic organic compounds and metal ions. Sci. Total Environ. 2020, 698, 134287. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xia, W.; Lu, P. Study on adsorption characteristics of biochar on heavy metals in soil. Korean J. Chem. Eng. 2017, 34, 1867–1873. [Google Scholar] [CrossRef]

| Heavy Metal | Sampling Location | Standard Value | Leaching Concentration | Coefficient of Variation % |
|---|---|---|---|---|
| Species Total mercury | slag pool | 0.05 | 0.0007 ± 0.0005 c | 71.43 |
| coarse slag yard | 0.00060 ± 00003 c | 5.00 | ||
| filter slagging site | 0.0137 ± 0.0114 b | 83.21 | ||
| fine slag yard | 0.0206 ± 0.0008 a | 3.88 | ||
| Total arsenic | slag pool | 0.5 | 0.0062 ± 0.0046 b | 74.19 |
| coarse slag yard | 0.0008 ± 0.0000 b | 0.00 | ||
| filter slagging site | 0.0297 ± 0.0281 a | 94.61 | ||
| fine slag yard | 0.0063 ± 0.0000 b | 0.00 | ||
| Total zinc | slag pool | 2 | 0.0000 ± 0.0000 c | - |
| coarse slag yard | 0.3165 ± 0.0144 a | 4.55 | ||
| filter slagging site | 0.0064 ± 0.0170 c | 265.63 | ||
| fine slag yard | 0.2943 ± 0.0140 b | 4.76 | ||
| Fluoride | slag pool | 10 | 0.5750 ± 0.3504 b | 60.94 |
| coarse slag yard | 0.2840 ± 0.0070 c | 2.46 | ||
| filter slagging site | 0.9030 ± 0.3698 a | 40.95 | ||
| fine slag yard | 0.1100 ± 0.0047 c | 4.27 | ||
| Total manganese | slag pool | 2 | 0.0353 ± 0.0487 c | 137.96 |
| coarse slag yard | 2.4490 ± 0.1480 a | 6.04 | ||
| filter slagging site | 0.0380 ± 0.0562 c | 147.89 | ||
| fine slag yard | 0.4945 ± 0.0126 b | 2.55 |
| Category | Total Mercury | Growth Rate % | Total Arsenic | Growth Rate % | Total Zinc | Growth Rate % | Fluoride | Growth Rate % | Total Manganese | Growth Rate % |
|---|---|---|---|---|---|---|---|---|---|---|
| Soil | 1.08 | - | 7.32 | - | 42.34 | - | 35.74 | - | 578.25 | - |
| 15% CGCS | 2.01 | 86 | 8.22 | 12 | 49.48 | 17 | 37.42 | 5 | 808.66 | 40 |
| 15% CGFS | 2.02 | 87 | 8.04 | 10 | 59.66 | 41 | 41.12 | 15 | 676.16 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Song, S.; Zhao, J.; Li, X.; Liu, C. Land Reclamation Using Typical Coal Gasification Slag in Xinjiang: A Full-Cycle Environmental Risk Study. Minerals 2023, 13, 1263. https://doi.org/10.3390/min13101263
Zhang K, Song S, Zhao J, Li X, Liu C. Land Reclamation Using Typical Coal Gasification Slag in Xinjiang: A Full-Cycle Environmental Risk Study. Minerals. 2023; 13(10):1263. https://doi.org/10.3390/min13101263
Chicago/Turabian StyleZhang, Kai, Shuang Song, Jiangang Zhao, Xiaonan Li, and Changyong Liu. 2023. "Land Reclamation Using Typical Coal Gasification Slag in Xinjiang: A Full-Cycle Environmental Risk Study" Minerals 13, no. 10: 1263. https://doi.org/10.3390/min13101263




