Molecular Simulation of Adsorption and Diffusion of Methane and Ethane in Kaolinite Clay under Supercritical Conditions: Effects of Water and Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecular Models
2.2. Simulation Details
3. Results and Discussion
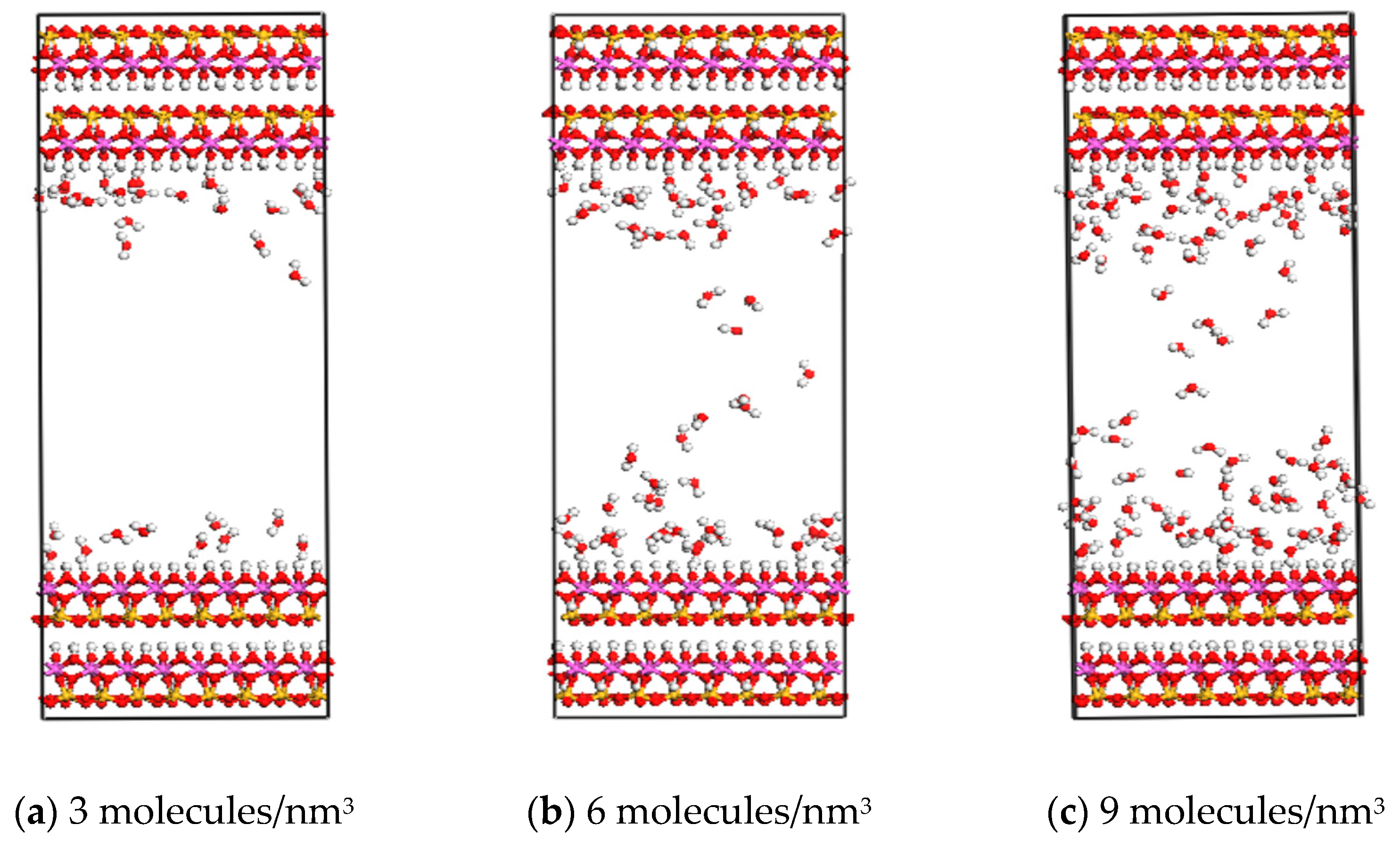
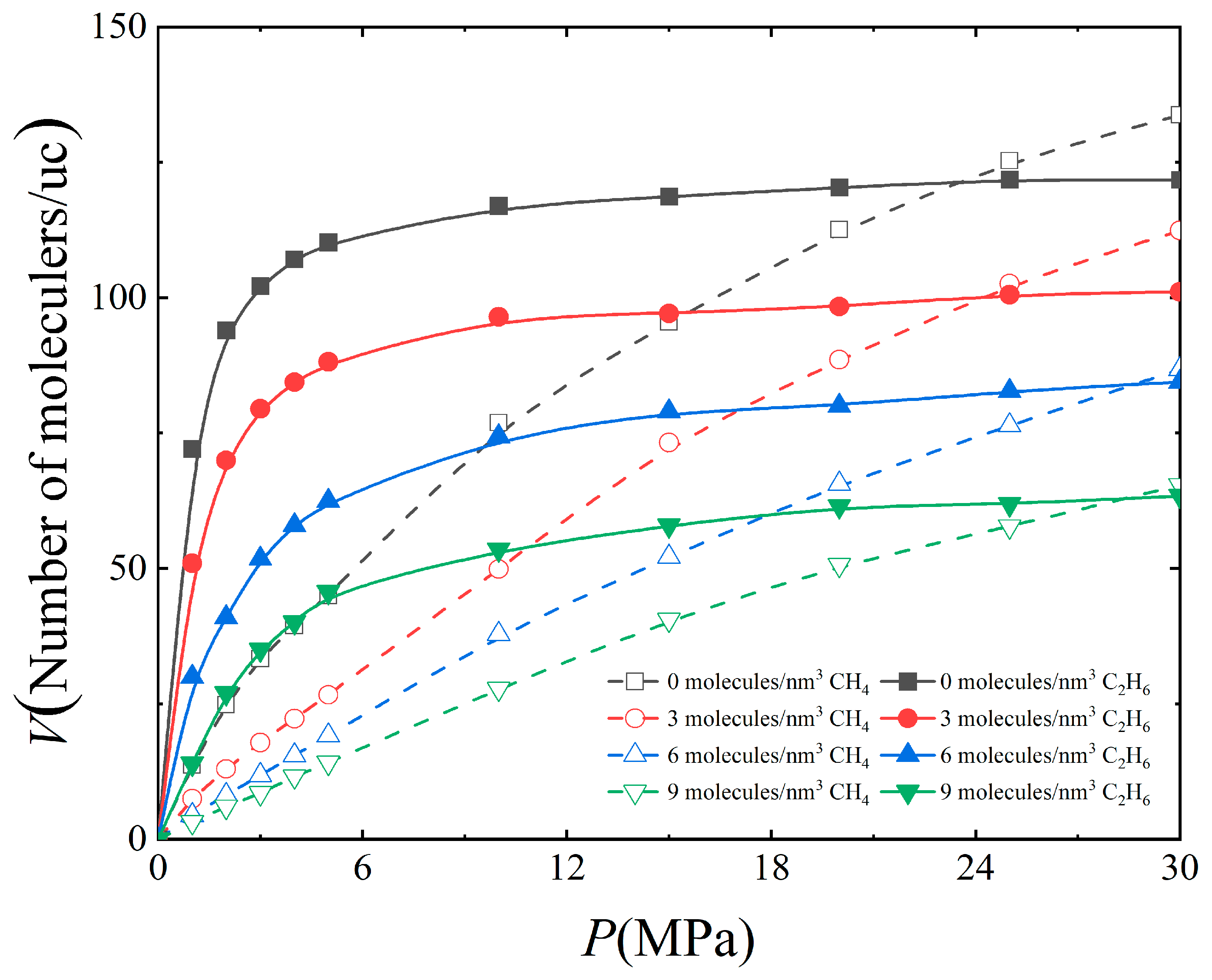
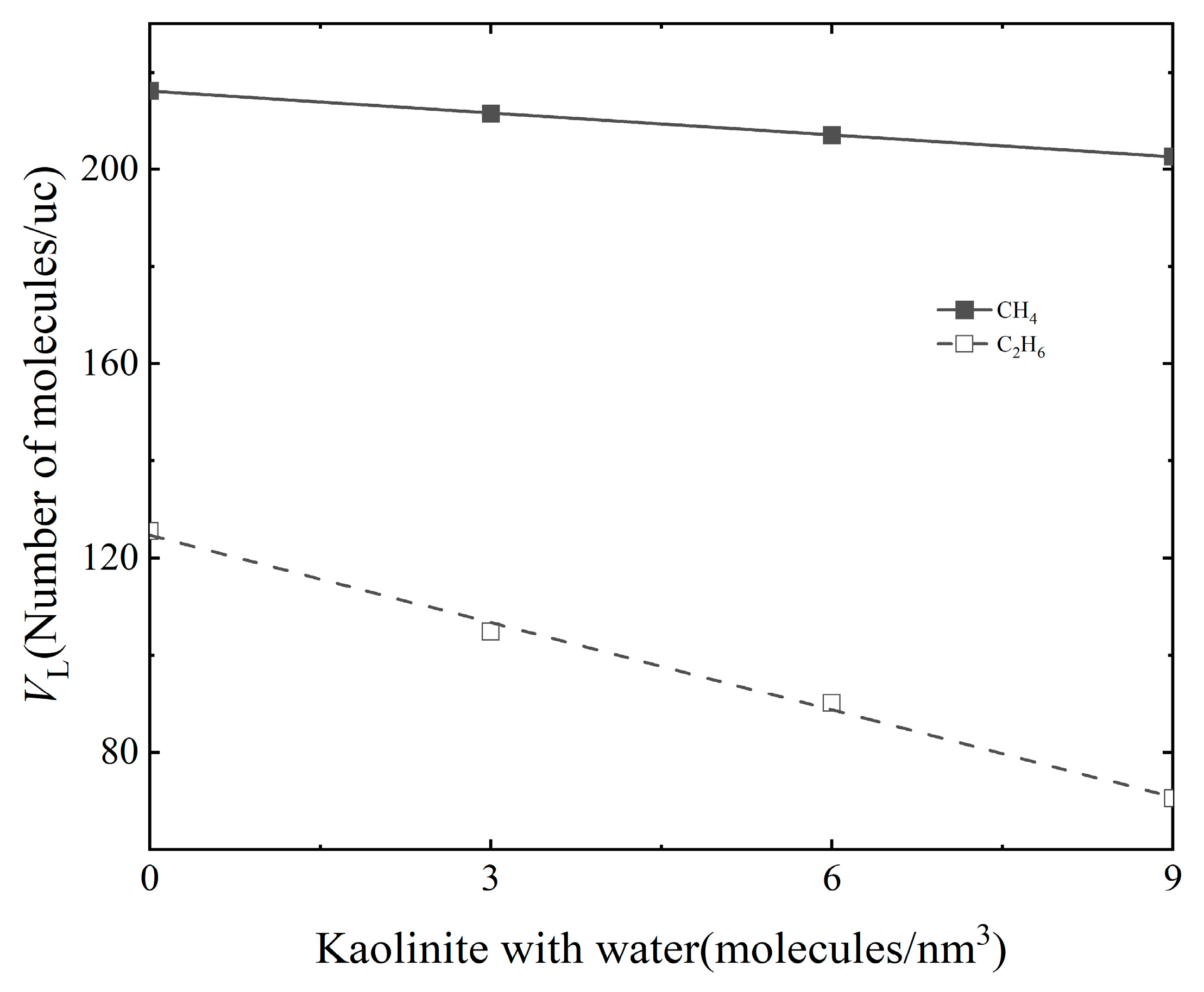
3.1. Effect of Water Content
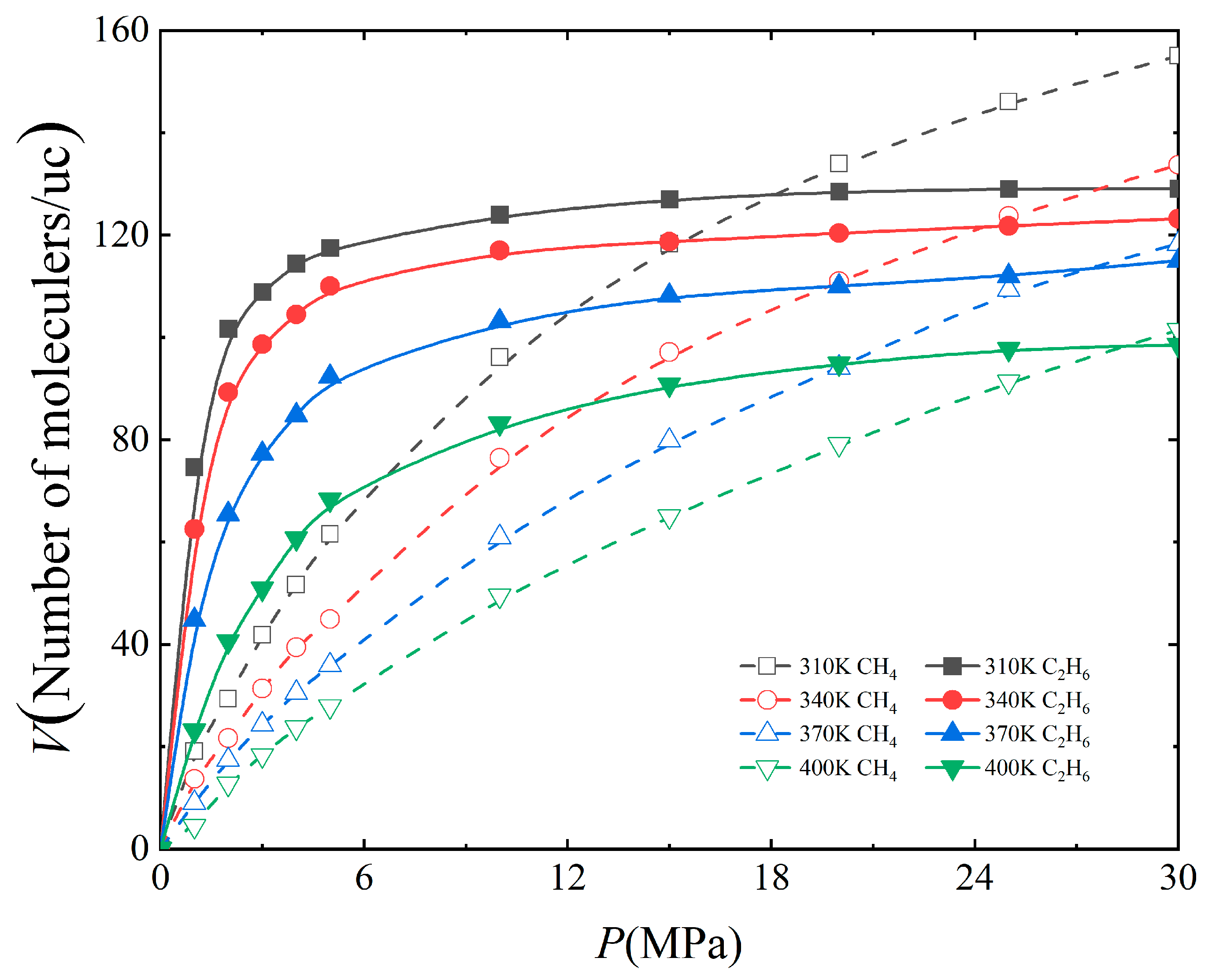
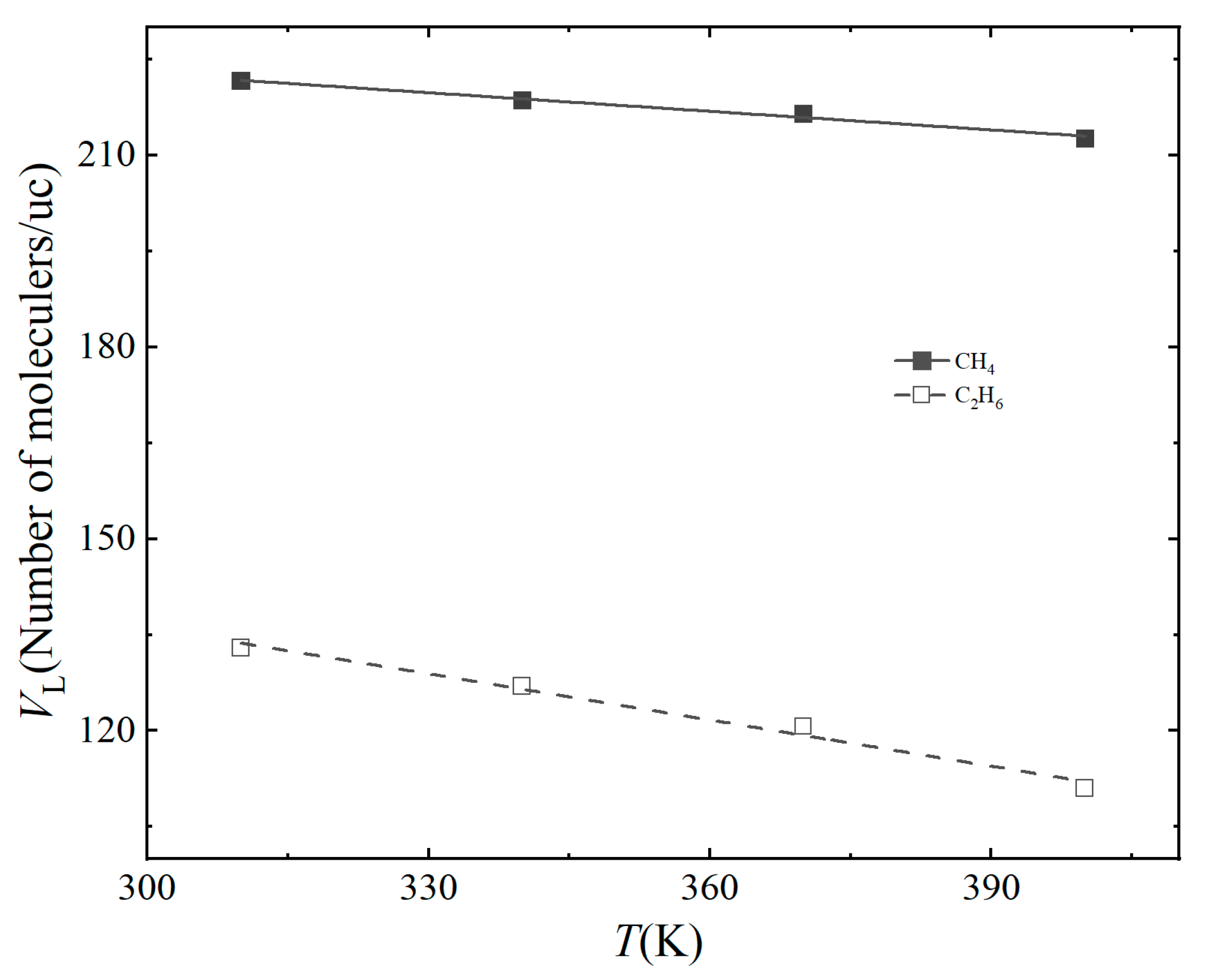
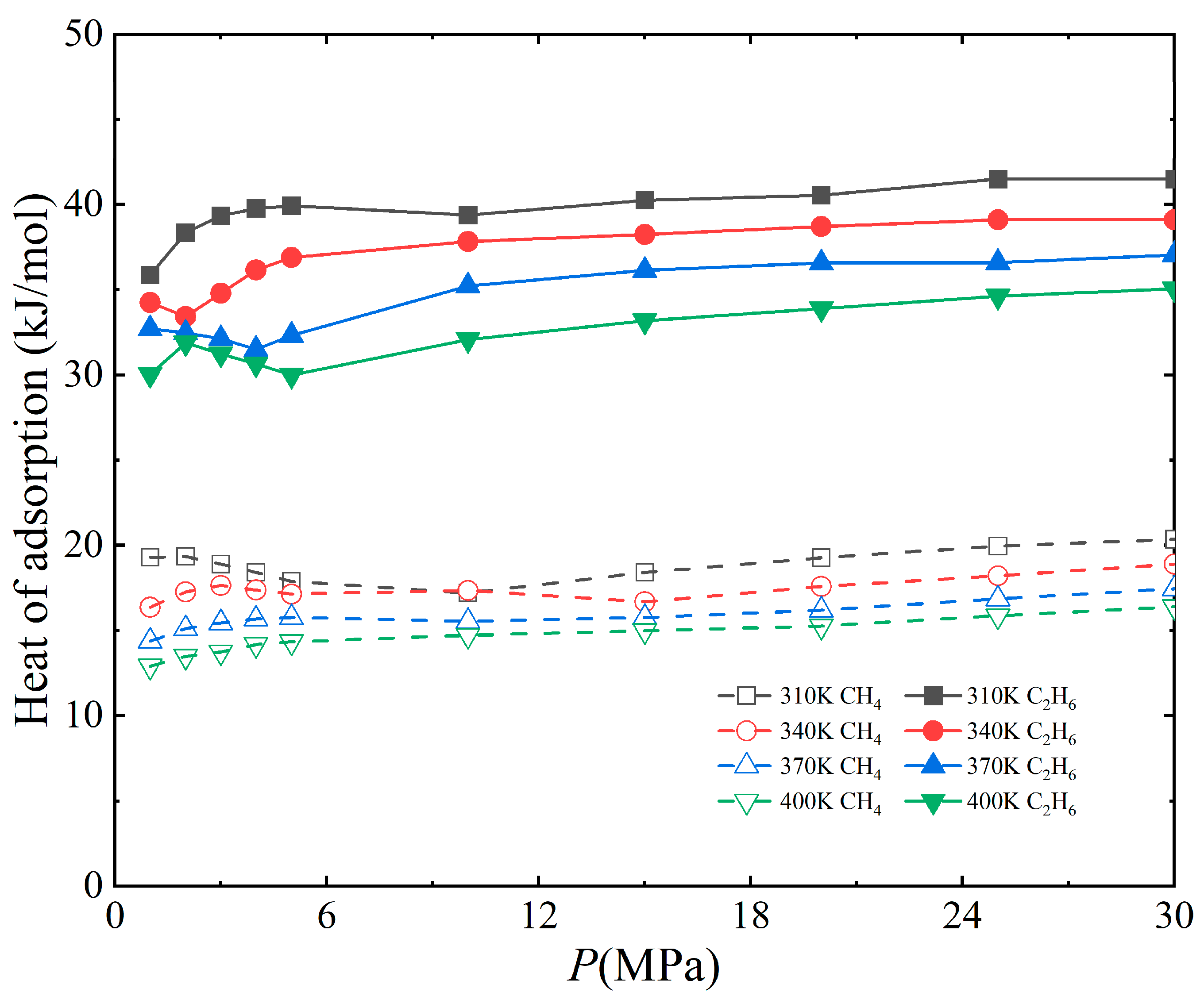
3.2. Effect of Temperature
3.3. Particle Diffusion Coefficient
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Huang, L. Molecular insight into competitive adsorption of methane and carbon dioxide in montmorillonite: Effect of clay structure and water content. Fuel 2019, 239, 32–43. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, K.; Liu, X.J.; Liang, L.X.; Zeng, Q. Molecular simulation of methane adsorption in slit-like quartz pores. RSC Adv. 2016, 6, 110808–110819. [Google Scholar] [CrossRef]
- Wang, T.Y.; Tian, S.C.; Li, G.S.; Zhang, L.Y.; Sheng, M.; Ren, W.X. Molecular simulation of gas adsorption in shale nanopores: A critical review. Renew. Sustain. Energy Rev. 2021, 149, 111391. [Google Scholar] [CrossRef]
- Jin, Z.H.; Firoozabadi, A. Phase behavior and flow in shale nanopores from molecular simulations. Fluid Phase Equilib. 2016, 430, 156–168. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Jha, A.N.; Rogers, H. Natural gas from shale formation–The evolution, evidences and challenges of shale gas revolution in United States. Renew. Sustain. Energy Rev. 2014, 30, 1–28. [Google Scholar] [CrossRef]
- Pang, W.Y.; He, Y.Q.; Yan, C.H.; Jin, Z.H. Tackling the challenges in the estimation of methane absolute adsorption in kerogen nanoporous media from molecular and analytical approaches. Fuel 2019, 242, 687–698. [Google Scholar] [CrossRef]
- Rexer, T.F.T.; Benham, M.J.; Aplin, A.C.; Thomas, K.M. Methane Adsorption on Shale under Simulated Geological Temperature and Pressure Conditions. Energy Fuels 2013, 27, 3099–3109. [Google Scholar] [CrossRef]
- Hu, K.; Mischo, H. Modeling High-Pressure Methane Adsorption on Shales with a Simplified Local Density Model. ACS Omega 2020, 5, 5048–5060. [Google Scholar] [CrossRef]
- Han, W.C.; Li, A.F.; Memon, A.; Ma, M. Synergetic Effect of Water, Temperature, and Pressure on Methane Adsorption in Shale Gas Reservoirs. ACS Omega 2021, 6, 2215–2229. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.F.; Lin, H.; Zhou, W.; Wang, L.; Zou, J.; Xu, H. High-Pressure Sorption of Methane, Ethane, and Their Mixtures on Shales from Sichuan Basin, China. Energy Fuels 2021, 35, 3989–3999. [Google Scholar] [CrossRef]
- Hao, S.C.; Ren, Z.J. Study on adsorption characteristics of shale gas based on Monte Carlo method. Chin. J. Appl. Mech. 2022, 37, 1314–1320. [Google Scholar] [CrossRef]
- Huang, T.; Liu, D.H.; Sun, J. Simulation on adsorption of methane in micro-pore of clay minerals. Sci. Technol. Eng. 2020, 20, 141–145. [Google Scholar] [CrossRef]
- Huang, L.; Zhou, W.; Xu, H.; Wang, L.; Zou, J.; Zhou, Q. Dynamic fluid states in organic-inorganic nanocomposite: Implications for shale gas recovery and CO2 sequestration. Chem. Eng. J. 2021, 411, 128423. [Google Scholar] [CrossRef]
- Zhu, H.; Ju, Y.; Huang, C.; Chen, F.; Chen, B.; Yu, K. Microcosmic gas adsorption mechanism on clay-organic nanocomposites in a marine shale. Energy 2020, 197, 117256. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Wang, X.; Li, Y.; Wu, K.; Shi, J.; Yang, L.; Feng, D.; Zhang, T.; Yu, P. Water distribution characteristic and effect on methane adsorption capacity in shale clay. Int. J. Coal Geol. 2016, 159, 135–154. [Google Scholar] [CrossRef]
- Li, W.; Pang, X.Q.; Snape, C.; Zhang, B.; Zheng, D.Y.; Zhang, X. Molecular Simulation Study on Methane Adsorption Capacity and Mechanism in Clay Minerals: Effect of Clay Type, Pressure, and Water Saturation in Shales. Energy Fuels 2019, 33, 765–778. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, X.; Liang, L.; Wei, X.; Xu, P. Investigation of the factors influencing methane adsorption on illite. Energy Sci. Eng. 2019, 7, 3317–3331. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Qi, R.; Zeng, Y.; Qin, H.; Ye, H.; Zhang, W. Molecular simulation of adsorption behaviors of methane, carbon dioxide and their mixtures on kerogen: Effect of kerogen maturity and moisture content. Fuel 2018, 211, 159–172. [Google Scholar] [CrossRef]
- Huang, L.; Ning, Z.; Wang, Q.; Zhang, W.; Cheng, Z.; Wu, X.; Qin, H. Effect of organic type and moisture on CO2/CH4 competitive adsorption in kerogen with implications for CO2 sequestration and enhanced CH4 recovery. Appl. Energy 2018, 210, 28–43. [Google Scholar] [CrossRef]
- Bish, D.L. Rietveld refinement of the kaolinite structure at 1.5 K. Clays Clay Miner. 1993, 41, 738–744. [Google Scholar] [CrossRef]
- Li, H.P.; Hu, Y.H.; Wang, D.Z.; Xu, K. Interaction mechanism between cationic surfactant and kaolinite. J. Cent. South Univ. Nat. Sci. Ed. 2004, 35, 228–233. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.S.; Pan, Z.J.; Niu, X.L.; Yu, Y.; Meng, S.Z. Pore structure and its fractal dimensions of transitional shale: A cross-section from east margin of the Ordos Basin, China. Fuel 2019, 241, 417–431. [Google Scholar] [CrossRef]
- Yang, F.; Fang, X.H.; Zeng, F.G.; Zhang, Y.Y. Simulation of adsorption of lead and cadmium on kaolinite surface. Multipurp. Util. Miner. Resour. 2020, 225, 196–202. [Google Scholar] [CrossRef]
- Tang, J.P.; Sun, S.J.; Bao, S.Y. Molecular simulation of methane adsorption on kaolinite. Pet. Geol. Recovery Effic. 2019, 26, 43–49. [Google Scholar] [CrossRef]
- Abbaspour, M. Computation of some thermodynamics, transport, structural properties, and new equation of state for fluid methane using two-body and three-body intermolecular potentials from molecular dynamics simulation. J. Mol. Liq. 2011, 161, 30–35. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Robinson, D.B.; Peng, D.Y.; Chung, Y.K. The development of the Peng–Robinson equation and its application to phase equilibrium in a system containing methanol. Fluid Phase Equilib. 1985, 24, 25–41. [Google Scholar] [CrossRef]
- Gotzias, A.; Heiberg-Andersen, H.; Kainourgiakis, M.; Steriotis, T. Grand canonical Monte Carlo simulations of hydrogen adsorption in carbon cones. Appl. Surf. Sci. 2010, 256, 5226–5231. [Google Scholar] [CrossRef]
- Liu, X.Q.; He, X.; Qiu, N.X.; Yang, X.; Tian, Z.Y.; Li, M.J.; Xue, Y. Molecular simulation of CH4, CO2, H2O and N2 molecules adsorption on heterogeneous surface models of coal. Appl. Surf. Sci. 2016, 389, 894–905. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, J.T.; Kang, T.H. Effect of water on methane adsorption on the kaolinite (001) surface based on molecular simulations. Appl. Surf. Sci. 2018, 439, 792–800. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fang, X.H.; Zeng, F.G. Molecular simulation of methane adsorption in kaolinite slit. Multipurp. Util. Miner. Resour. 2019, 1, 130–134+113. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, T.H.; Kang, J.T.; Song, Z.P. Molecular simulation of competitive adsorption of C2H6 and CH4 on kaolinite surface. J. Taiyuan Univ. Technol. 2019, 50, 297–302. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Wang, S.; Feng, Q.H.; Javadpour, F.; Hu, Q.H.; Wu, K.L. Competitive adsorption of methane and ethane in montmorillonite nanopores of shale at supercritical conditions: A grand canonical Monte Carlo simulation study. Chem. Eng. J. 2019, 355, 76–90. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, Q.B. Molecular simulations of competitive adsorption behavior between CH4-C2H6 in K-illite clay at supercritical conditions. Fuel 2020, 260, 116358. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76, 137–152. [Google Scholar] [CrossRef]
- Xu, J.T. Study on the influence of temperature on methane adsorption characteristics of soft and hard anthracite based on molecular simulation method. Saf. Coal Mines 2022, 53, 158–165. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.K.; Xu, J.T. Study on the influence of water on methane adsorption characteristics of soft and hard anthracite based on molecular simulation method. Saf. Coal Mines 2022, 53, 20–27. [Google Scholar] [CrossRef]
- Sharma, A.; Luxami, V.; Paul, K. Purine-benzimidazole hybrids: Synthesis, single crystal determination and in vitro evaluation of antitumor activities. Eur. J. Med. Chem. 2015, 93, 414–422. [Google Scholar] [CrossRef]
- Hao, X.S.; Jia, Z.H.; Zhang, L.L.; Wang, X.; Zhang, J.; Zhou, L.X.; Yan, Y.G. Molecular Insight into the Methane Occurrence inside a Shale Nanochannel with Formation Water. Energy Fuels 2023, 37, 1207–1215. [Google Scholar] [CrossRef]
- Pan, H.; Ritter, J.A.; Balbuena, P.B. Examination of the approximations used in determining the isosteric heat of adsorption from the Clausius− Clapeyron equation. Langmuir 1998, 14, 6323–6327. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, X.; Zhang, K.W.; Zeng, F.H.; Wang, Z.H. Investigation of dynamical properties of methane in slit-like quartz pores using molecular simulation. RSC Adv. 2018, 8, 33798–33816. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ning, Z.F.; Zhang, R.; Zhao, H.W. Adsorption isothermal process of methane on shale. Int. J. Coal Sci. Technol. 2014, 39, 1327–1332. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Liu, Y.; Li, Q.B.; Cheng, Q.L.; Cai, S.Y. Transport Property of Methane and Ethane in K-Illite Nanopores of Shale: Insights from Molecular Dynamic Simulations. Energy Fuels 2020, 34, 1710–1719. [Google Scholar] [CrossRef]
- Li, A.; Han, W.; Fang, Q.; Memon, A.; Ma, M. Experimental investigation of methane adsorption and desorption in water-bearing shale. Capillarity 2020, 3, 45–55. [Google Scholar] [CrossRef]
- Collell, J.; Galliero, G.; Gouth, F.O.; Montel, F.O.; Pujol, M.; Ungerer, P.; Yiannourakou, M. Molecular simulation and modelisation of methane/ethane mixtures adsorption onto a microporous molecular model of kerogen under typical reservoir conditions. Microporous Mesoporous Mater. 2014, 197, 194–203. [Google Scholar] [CrossRef]
- Hui, W.; Long, S. Experimental study on isothermal adsorption of coalbed methane under different temperature conditions. Shanxi Coal 2018, 38, 71–75. [Google Scholar] [CrossRef]
- Brasili, J.; Fox, K.; Badamo, D.; Berghe, G.; Khanal, R.; Singh, R. Molecular dynamics simulation of shale gas confined inside slit-like calcite 104 nanopore. Mol. Simul. 2019, 45, 104–110. [Google Scholar] [CrossRef]
- Owusu, J.P.; Karalis, K.; Prasianakis, N.I.; Churakov, S.V. Mobility of Dissolved Gases in Smectites under Saturated Conditions: Effects of Pore Size, Gas Types, Temperature, and Surface Interaction. J. Phys. Chem. C 2022, 126, 17441–17455. [Google Scholar] [CrossRef]
- Spera, M.M.; Franco, L.M. Surface and confinement effects on the self-diffusion coefficients for methane-ethane mixtures within calcite nanopores. Fluid Phase Equilib. 2020, 522, 112740. [Google Scholar] [CrossRef]
- Sharma, A.; Namsani, S.; Singh, J.K. Molecular simulation of shale gas adsorption and diffusion in inorganic nanopores. Mol. Simul. 2015, 41, 414–422. [Google Scholar] [CrossRef]





| Substance | Atoms | |||
|---|---|---|---|---|
| kaolinite | Si | 155.992 | 4.27 | +1.25 |
| Al | 155.992 | 4.39 | +1.05 | |
| O | 48.15624 | 3.4046 | −0.85 | |
| H | 7.64864 | 3.195 | +0.28 | |
| CH4 | C | 33.24 | 3.5 | −0.24 |
| H | 15.109 | 2.5 | +0.06 | |
| C2H6 | C | 33.24 | 3.5 | −0.18 |
| H | 15.109 | 2.5 | +0.06 | |
| H2O | H | 0 | 0 | +0.4238 |
| O | 78.18 | 3.166 | −0.8476 |
| Gas | Water Content (Molecules/nm3) | VL (Molecules/uc) | PL (Mpa) | R2 |
|---|---|---|---|---|
| CH4 | 0 | 216.2 | 18.44 | 0.995 |
| 3 | 211.5 | 39.22 | 0.997 | |
| 6 | 207.1 | 46.47 | 0.996 | |
| 9 | 202.7 | 62.07 | 0.998 | |
| C2H6 | 0 | 125.5 | 0.67 | 0.985 |
| 3 | 104.8 | 0.95 | 0.986 | |
| 6 | 90.1 | 2.21 | 0.996 | |
| 9 | 70.5 | 3.13 | 0.992 |
| Gas | Temperature (K) | VL (Molecules/uc) | PL (Mpa) | R2 |
|---|---|---|---|---|
| CH4 | 310 K | 221.6 | 13.06 | 0.996 |
| 340 K | 218.6 | 17.50 | 0.998 | |
| 370 K | 216.5 | 26.94 | 0.998 | |
| 400 K | 212.6 | 33.26 | 0.999 | |
| C2H6 | 310 K | 132.9 | 0.66 | 0.988 |
| 340 K | 126.9 | 0.86 | 0.978 | |
| 370 K | 120.6 | 1.69 | 0.984 | |
| 400 K | 110.96 | 3.35 | 0.995 |
| 10−8 m2·s−1 | 0 Molecules/nm3 | 3 Molecules/nm3 | 6 Molecules/nm3 | 9 Molecules/nm3 |
|---|---|---|---|---|
| C2H6 | 2.84 | 2.60 | 2.26 | 1.64 |
| CH4 | 9.91 | 9.73 | 8.92 | 8.74 |
| 10−8 m2·s−1 | 310 K | 340 K | 370 K | 400 K |
|---|---|---|---|---|
| C2H6 | 1.89 | 2.84 | 3.87 | 5.59 |
| CH4 | 7.50 | 9.91 | 13.2 | 16.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-Y.; Liu, D.-M.; Hu, H.-K.; Bo, H.-F.; Zhang, Z.-X. Molecular Simulation of Adsorption and Diffusion of Methane and Ethane in Kaolinite Clay under Supercritical Conditions: Effects of Water and Temperature. Minerals 2023, 13, 1269. https://doi.org/10.3390/min13101269
Li D-Y, Liu D-M, Hu H-K, Bo H-F, Zhang Z-X. Molecular Simulation of Adsorption and Diffusion of Methane and Ethane in Kaolinite Clay under Supercritical Conditions: Effects of Water and Temperature. Minerals. 2023; 13(10):1269. https://doi.org/10.3390/min13101269
Chicago/Turabian StyleLi, De-Yang, Dong-Mei Liu, Hong-Kui Hu, Hui-Feng Bo, and Zhan-Xin Zhang. 2023. "Molecular Simulation of Adsorption and Diffusion of Methane and Ethane in Kaolinite Clay under Supercritical Conditions: Effects of Water and Temperature" Minerals 13, no. 10: 1269. https://doi.org/10.3390/min13101269
APA StyleLi, D.-Y., Liu, D.-M., Hu, H.-K., Bo, H.-F., & Zhang, Z.-X. (2023). Molecular Simulation of Adsorption and Diffusion of Methane and Ethane in Kaolinite Clay under Supercritical Conditions: Effects of Water and Temperature. Minerals, 13(10), 1269. https://doi.org/10.3390/min13101269





