1. Introduction
The human body engages with its environment in diverse ways, one of which is the interaction between the lungs and the external environment through the act of breathing. The extensive surface area of the lungs, coupled with an incredibly thin air–blood barrier, exposes this vital organ to particles suspended in the inhaled air. The repercussions of this particle–lung interaction can be detrimental to health when inhaled aerosols contain toxic pollutants. Conversely, when inhaled particles consist of therapeutic aerosolized drugs, this interaction can be beneficial for disease treatment. In both scenarios, accurately estimating the dose and pinpointing deposition sites within the respiratory tract stand as fundamental prerequisites for understanding subsequent biological responses. To comprehend these aspects fully, a grasp of the basic physics governing particle motion and engineering principles is essential [
1].
The inhalation of dust particles in underground coal mines poses a series of complications, primarily affecting the small airways in miners’ lungs exposed to such environments [
2]. These complications collectively fall under the category of Coal Mine Dust Lung Diseases (CMDLDs). Among the CMDLDs, Coal Workers’ Pneumoconiosis (CWP) and silicosis are well-known to those in the mining field. Similar diseases, such as mixed dust pneumoconiosis and dust-related diffuse fibrosis, result from inhaling Respirable Coal Mine Dust (RCMD). Moreover, overexposure to RCMD can lead to a more severe form known as Progressive Massive Fibrosis (PMF). Distinguishing PMF from other CMDLDs lies in the size of the opacities it leaves within the lungs, which can range from small (<1 cm) for CWP to larger (>1 cm) for PMF [
3,
4].
Miners diagnosed with CMDLDs often report symptoms such as reduced pulmonary function, shortness of breath, coughing, or sputum production. Diagnosis is typically made through radiographic imaging or pulmonary testing, prompted either by the presentation of respiratory symptoms or by a history of coal dust exposure [
4]. Radiographs of CWP patients reveal lung scarring, predominantly in the lower lung regions and characterized by small, irregular shapes—distinct from PMF, which manifests larger opacities [
5,
6,
7]. This presence of lung opacities in lower lung regions is also recognized as Interstitial Lung Disease (ILD) [
8]. Understanding the deposition of dust within various regions of the respiratory system gains significance due to the existence of Chronic Obstructive Pulmonary Diseases (COPDs), which are associated with regional dust deposition. Emphysema and chronic bronchitis, the most common forms of COPD, pose substantial risks to coal miners. Further research into deposition patterns may shed light on how different sections of the respiratory system are affected [
9].
The deposition of particles within the human respiratory tract is influenced by a combination of biological factors (such as lung morphology and breathing patterns) and physical factors (including fluid dynamics, particle properties, and deposition mechanisms). Current particle deposition models fall into two main categories based on the region of interest within the lung: whole lung models and local scale models. Whole lung models compute particle deposition in individual airways using analytical equations that consider particle deposition efficiencies and specific flow conditions (analytical models). These models require validation through comparison with experimental data from human subjects, and their validation is typically limited to total and regional deposition. However, once the model is validated in some cases available from experiments, this valid model can be used to expand the investigation and evaluate situations that were not examined in the experiment. In contrast, local scale models employ Computational Fluid and Particle Dynamics (CFPD) methods (standard CFD model with particle tracking algorithms) to solve particle transport and deposition equations, providing insights into deposition patterns within specific lung structures [
10].
Airflow within the nasal cavities and oral airways exhibit complexity, including possible transitions to turbulent jet-like flow, recirculating flow, Dean’s flow, vortical flows, large pressure drops, prevailing secondary flows, and merging streams during exhalation. Assumptions underpinning particle transport and deposition include spherical, non-interacting, monodisperse aerosols deposited upon contact with airway surfaces and each other. Such dilute particle suspensions are typically modeled using the Euler–Lagrange approach for micron-sized particles and the Euler–Euler framework for nanoparticles. Micron-sized particles tend to deposit non-uniformly, leading to high concentrations at specific local sites [
8].
This literature review seeks to explore comprehensively the regional deposition of RCMD and the applications of Computational Fluid Dynamics (CFD) models in understanding lung deposition. Given the complexity and breadth of this subject, our objective is to provide an overview of research developments in this domain over time while also offering readers the most recent updates on occupational diseases related to coal dust. The prevalence of dust-related ailments continues to rise, making it crucial to examine innovative approaches, such as the utilization of the Mobile Aerosol Lung Deposition Apparatus (MALDA), which have the potential to revolutionize our understanding of lung deposition and its implications in combating dust-related diseases.
2. Methods of Review
2.1. Literature Search and Study Identification
To identify relevant studies up to September 2022, we conducted a thorough search across electronic databases, including PubMed, Web of Science, Scopus, and Google Scholar. The search terms employed included “RCMD”, “respirable coal mine dust”, “dust exposure”, “respiratory diseases”, “CFD”, “computational fluid dynamics”, “particle lung deposition”, “aerosol”, “airflow”, “validation”, “challenges”, and “benefits”. Additionally, we examined relevant reviews and scrutinized the reference lists of retrieved publications to ensure comprehensive coverage.
2.2. Study Selection and Eligibility Requirements
Following the removal of duplicate records, we assessed the relevance of the identified studies by examining their titles and abstracts. Subsequently, the full texts of potentially relevant studies were evaluated for inclusion based on the following criteria:
The primary focus of the study must be on RCMD regional lung deposition, CFD modeling in relation to particle lung deposition, or related applications.
The study should provide insights into the challenges, advantages, or validation methods of CFD models.
The study must be a peer-reviewed publication in a journal or a conference proceeding.
The study should be available in the English language.
Studies that did not meet these criteria were excluded, as were papers exclusively addressing Metal and Non-Metal (MNM) mining and conducted outside the scope of this review, which primarily covers the USA with occasional comparisons. Any discrepancies in study selection were resolved through discussion and consensus among the review authors.
2.3. Data Extraction and Synthesis
For the included articles, relevant data were extracted and synthesized. In the context of RCMD and dust lung deposition in coal mining, this synthesis encompasses studies published between 2000 and 2022 that specifically address exposure, inhalation, deposition, retention, and particle flow within the respiratory system in US coal mines. For CFD-related material, the synthesis includes study objectives, CFD modeling methods and assumptions, encountered challenges, benefits of employing CFD models, and validation approaches. These components were used to construct the review’s three main sections: challenges of CFD modeling, advantages of CFD for particle lung deposition, and validation of CFD models.
2.4. Limitations of the Review
Several limitations must be acknowledged in this review:
The review’s analysis is restricted to the literature available until September 2022, thereby excluding any developments, new findings, or emerging perspectives in CFD modeling for particle lung deposition beyond that date.
As a literature review, the representativeness of the selected research significantly influences our analysis. Consequently, the conclusions may be subject to bias based on the methodologies, assumptions, and limitations of the included research. Additionally, unpublished or non-peer-reviewed discoveries may have been missed, introducing potential biases.
While we have explored CFD modeling within the context of particle lung deposition, this review does not encompass all pertinent CFD modeling applications and aspects. Variations may exist in fields where CFD modeling is applied, limiting the applicability and generalizability of the discussed challenges, benefits, and validation methods. Furthermore, the study does not offer an exhaustive examination of specific CFD software, which could be a critical factor in model selection and evaluation.
Finally, it is essential to acknowledge that despite efforts to summarize information regarding CFD model challenges, advantages, and validation in the context of particle lung deposition, there may be additional relevant factors and perspectives not covered in this study. These limitations should be considered when interpreting the reported results.
3. RCMD Inhalation and Lung Deposition
The journey of inhaled dust particles through the respiratory system is crucial to understanding RCMD inhalation and lung deposition. Beginning at the nostrils or mouth, dust particles enter the respiratory airways, encountering various clearance mechanisms. These mechanisms are found in distinct areas of the respiratory system. The nasal cavity (nasopharynx, larynx, and trachea) contains small hairs and mucus that filter out the largest particles (10 to 6 µm). Moving down the respiratory tract, the tracheobronchial (TB) tree (trachea, bronchi, and smaller bronchioles) is equipped with ciliated columnar and goblet cells, which trap particles in the size range of 6 to 3 µm. Finally, in the alveoli region (smaller bronchiole balloons), alveoli macrophage cells play a pivotal role. Upon contact with dust or harmful particles, the macrophage cells engulf and transport particles to the TB region, where expulsion from the respiratory system occurs through coughing or swallowing [
11,
12]. Lung fluid in this region aids in respiratory processes by moisturizing inhaled air and enhancing macrophage function [
13].
Furthermore, research indicates that these clearance mechanisms, particularly in the alveoli region, can be impaired by prolonged dust exposure and macrophage overload. The retention or accumulation of particles in the lungs depends on the rate of dust particle deposition within the lungs and the efficiency of macrophage clearance. The duration of dust particle exposure directly affects the rate and quantity of particle deposition in the lungs. An increase in unidentified particle deposition in the alveoli prompts the recruitment of additional macrophages to enhance the clearance process, emphasizing the importance of understanding these mechanisms for assessing RCMD inhalation and lung deposition in mining environments [
14,
15].
3.1. Factors Influencing Deposition
Particle physicochemical characteristics (e.g., size, shape, charge), airway physiological parameters (e.g., breathing patterns), and airway geometry collectively influence the deposition of inhaled dust particles [
9,
16,
17].
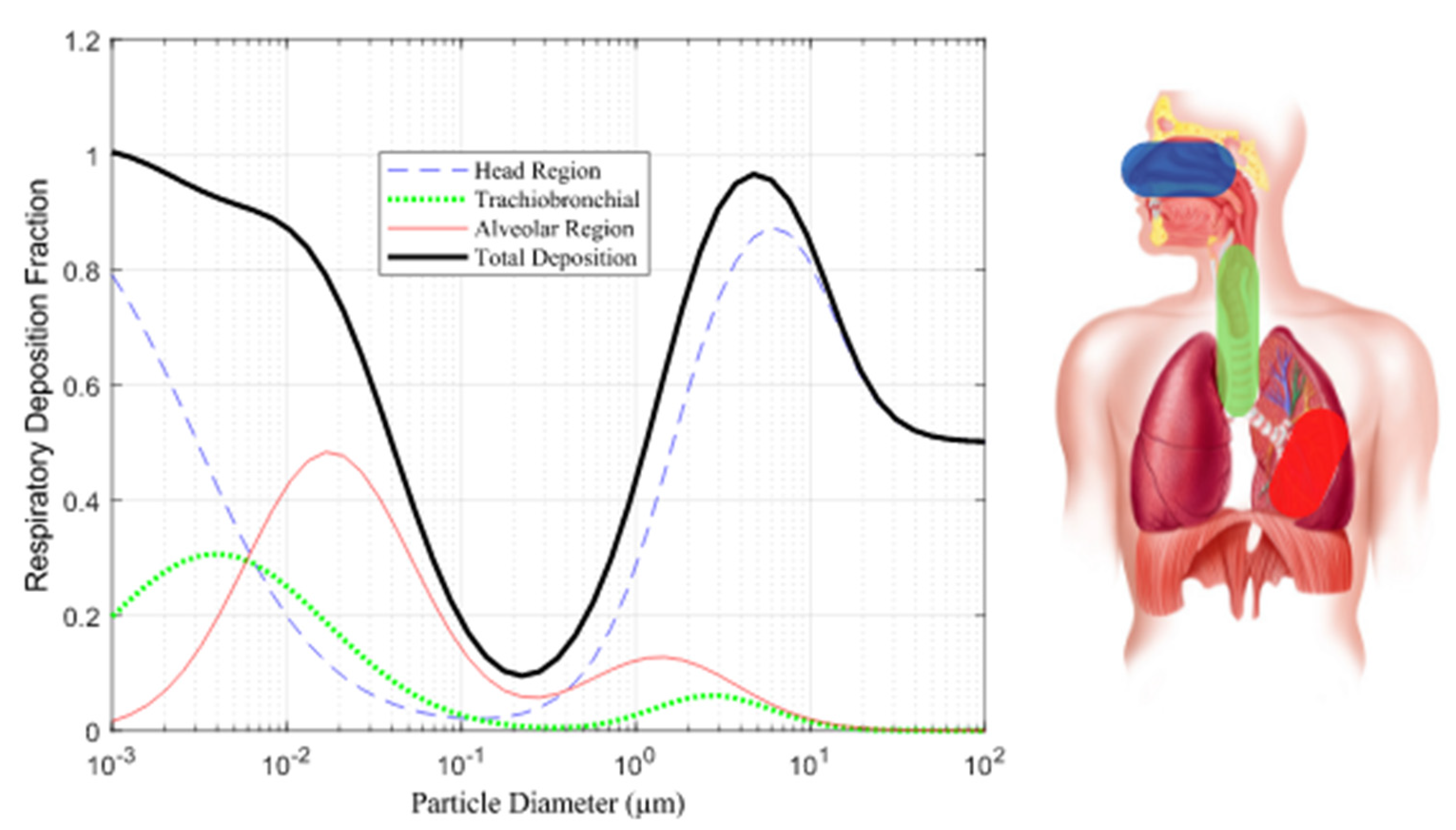
Particle Size: Particle size plays a pivotal role in determining where particles deposit in the respiratory system. Particles in the respirable range (10 nm to 10 µm) tend to deposit differently in three distinct regions: the head, TB, and alveoli regions. Smaller particles (<3 µm) and nano-sized particles (<300 nm) have a higher likelihood of depositing in the alveoli region as Illustrated in
Figure 1 [
18].
Aerodynamic Diameter: The aerodynamic diameter, which relates to particle density and composition, predicts deposition probability in specific respiratory tract regions [
19,
20,
21,
22].
Particle Shape: Particle shape affects interactions with respiratory tissue. Angular particles are more likely to deposit in the airways or lungs [
23].
Electric Charge: Coal dust particles carry electric charge after pulverization, with this property varying by particle type. Electric charge can influence the deposition of very small particles (<4 µm) by allowing them to reach deeper respiratory zones [
24].
3.2. Deposition Mechanisms
Several mechanisms govern dust particle deposition, including inertial impaction, gravitational sedimentation, Brownian diffusion, interception, and turbulent diffusion [
9,
16].
This paper focuses on the main mechanisms based on particle size:
Inertial Impaction: Particles travel at a speed that may cause them to collide with airway surfaces due to changes in airflow direction. Particle size, charge, airflow, and airway geometry influence impaction, particularly in the early respiratory system, where larger particles travel.
Gravitational Sedimentation: Particles gradually settle over time due to gravity, influenced by breath maneuvers, particle aerodynamic diameter, and density. Holding one’s breath increases the likelihood of sedimentation, with heavier particles settling faster.
Brownian Diffusion: Particles of a small enough size experience random trajectories influenced by molecular bombardment, allowing them to follow Brownian motion [
Figure 2]. This phenomenon is also known as diffusion [
13,
14,
25].
Comprehending these factors and mechanisms in conjunction with fluid dynamics enables us to discern the advantages of particle fragmentation into larger sizes, as such particles possess a greater likelihood of early settlement and extraction from the respiratory system, with the converse being true. In scenarios where fine fragmentation prevails, it necessitates the implementation of comprehensive protective measures.
4. Disease Prevalence, Severity, and Monitoring
As depicted in
Figure 3, there has been a notable shift in the prevalence and severity of CMDLDs, including CWP and PMF, between 2000 and 2022. This change is in contrast to the preceding period, which witnessed a decline primarily attributed to the enactment of the Federal Coal Mine Health and Safety Act of 1969—a pivotal measure aimed at safeguarding miners nationwide [
24,
26,
27]. The escalation in CMDLD prevalence and severity is attributed to various facets of mining, encompassing mine type, tenure, size, geographic location, and extended working hours [
4,
28,
29].
To address this upward trend, the Mine Safety and Health Administration (MSHA) implemented revised exposure limits in the USA, effective as of August 2016. These limits are set at 1.5 milligrams per cubic meter (mg/m
3) for coal dust (30 CFR §70.100 and §71.100), 0.1 mg/m
3 when quartz is present (30 CFR §71.101), and 0.05 mg/m
3 for crystalline silica (29 CFR §1910.1053). It is important to note that slight variations may exist in other countries [
30]. Despite the enhancements made to exposure limits in the United States, the country continues to witness an unacceptably high number of reported cases of CWP.
Comprehensive research conducted across the USA underscores that the prevalence of CWP and its most advanced manifestation, PMF, is notably higher in mines situated in the central Appalachian region. This conclusion is supported by data derived from the federal black lung program and the national surveillance database [
31,
32,
33,
34].
In 2005, the National Institute for Occupational Safety and Health (NIOSH) investigated the progression and severity of CMDLDs. Their findings revealed that 35% of miners with pneumoconiosis had an advanced form of the disease with a higher rate of progression, while 15% had the most severe form, known as PMF. These patterns prompted NIOSH to enhance its surveillance efforts [
29,
31].
5. RCMD Sources and Exposure in Coal Mines
Dust sources in mining activities stem from rock-related processes such as blasting, cutting, transportation, and rock dusting [
35]. Underground coal miners face greater risks compared to surface and metal mine workers due to higher dust concentrations in confined underground environments [
36]. About 3% of airborne mine dust originates from excavations [
37]. From normal operation to wear and tear, mining machinery emits harmful particles with varying chemical compositions [
29]. These particles can linger in the air and enter the deepest parts of the human lung or be removed by the body’s clearance mechanisms [
38].
Approximately 1.7 million U.S. workers, spanning both mining and non-mining sectors, face potential exposure to hazardous dust containing crystalline silica on an annual basis [
39]. Research indicates that miners may develop chronic simple silicosis after roughly 20 years of exposure, with some enduring exposure periods as long as 44 years [
7,
40]. Occupations characterized by high levels of risk encompass roles such as drill operators, roof bolters, and machine operators, among others. Conversely, positions like belt operators and shovel operators entail lower risks [
41,
42]. Notably, roof bolting alone can account for up to 50% of quartz particles in dust, with 20% falling within the respirable size range (<10 µm) [
43,
44]. Workers situated in intake and exhaust areas face heightened risks of inhaling fine particles compared to those stationed at the working face [
45]. Nevertheless, a discernible distinction that provides enhanced protection to individuals in high-risk positions is currently lacking.
Methods for detecting coal and silica dust concentrations in mining operations encompass time-consuming laboratory techniques such as X-ray Diffraction (XRD) and Scanning Electron Microscopy with Energy Disperse X-ray (SEM-EDX). Meanwhile, field-portable devices such as the Fourier-Transform Infrared (FTIR) and Continuous Personal Dust Monitoring (CPDM) furnish essential data without necessitating laboratory assistance. In the context of both surface and underground coal mines, the responsibility for enforcing permissible exposure limits concerning coal dust and silica predominantly falls upon the mining operator, aided by contemporary monitoring devices such as the CPDM. This instrument yields estimations of dust concentrations at the conclusion of each work shift. Regrettably, it exhibits certain limitations, including bulkiness, noise emissions, and an inability to measure silica concentration [
26,
39,
46]. This approach has demonstrated a relative inefficiency in enabling mine operators to make real-time decisions, as opposed to relying solely on end-of-shift data, while also incurring additional fatigue for the mine operator. Consequently, there is a discernible demand for more advanced equipment capable of enhancing precision. Notably, the aforementioned limitations of the CPDM have prompted research into alternative, more convenient solutions [
47,
48].
Continued inhalation of coal dust over time impairs respiratory tract clearance mechanisms, reducing their ability to expel particles and causing lung tissue lesions [
28,
49]. The International Labor Organization (ILO) classifies opacity caused by coal and silica dust inhalation into four categories (0, 1, 2, and 3) with twelve subcategories (0/1, 1/0, 1/1, etc.) [
50]. Progression of pulmonary opacity is linked to higher exposure to silica dust, particularly in the mining of thin coal seams, where more host rock must be moved [
7,
51]. Opacity 1/0+ often advances more rapidly with exposure to quartz dust. Additionally, higher radiologic profusions are associated with ash content in low-rank coal and are influenced by both coal dust and ash content in high-rank coal [
21].
6. RCMD Characterization Techniques
RCMD is typically characterized by mass concentration and mass fraction, but factors such as shape, size, and particle composition also influence its hazard potential [
24]. SEM-EDX, a commonly employed laboratory technique for dust sample characterization, involves the examination of dust particle surfaces through X-ray spectra generated by the interaction between an electron beam and the particle’s surface. However, it is worth noting that, on certain occasions, there is a potential for underestimating coal particles, which may lead to an unnoticed increase in exposure to coal dust due to the mischaracterization of RCMD [
52].
In a study analyzing samples collected from a low-seam mine in central Appalachia (collected at the roof bolter, belt drive, and intake location), the results indicated that particles in the roof bolter and belt drive samples were primarily composed of alumino-silicate, with a presence of carbonaceous content in the roof bolter samples. These particles can be associated with coal mineral micro-agglomerates, which, despite being coal, may not always be classified as such by SEM-EDX. This observation becomes evident when comparing SEM-EDX results with Thermographic Analysis (TGA) results, which often reveal reduced coal values and increased rock dust content. This discrepancy may occur when coal particles are coated with rock dust or Diesel Particulate Matter (DPM) [
11,
51].
7. Computational Fluid Dynamics in Investigating Particle Deposition
In the realm of computational fluid dynamics, numerous attempts have been made to simplify the intricate human respiratory system. These efforts involve focusing on specific respiratory system components or streamlining geometric representations. This section delves into various initiatives within this field.
Within this compiled dataset of references, spanning from 2010 to 2022, a total of 54 references were analyzed for the CFD section. Over the years, the distribution of references has been diverse, with the highest percentage (26.23%) found in 2021. The remaining years each have a percentage of references ranging from 3.28% to 9.84%, illustrating the sustained research interest in this field throughout the past decade. The following bar
Chart 1 visually represents the variation in publication years and their corresponding percentages used in the article.
7.1. Model Geometry
The computational analysis of the complex geometry of the human lung presents considerable challenges and demands a substantial investment of time. Consequently, research in this field frequently employs varying degrees of simplification, influenced by the specific research objectives.
Basic Models: Some studies employ rudimentary models to explore the transport and deposition of microparticles and nanoparticles (1–50 nm) within the bronchial tree during inhalation [
53].
Enhanced Models: In contrast, more sophisticated models, exemplified in
Figure 4, are evident in various studies. These models incorporate additional airway generations and offer a more comprehensive representation of lung dynamics [
54,
55,
56,
57].
The repercussions of these simplifications were explored and compared to actual lung models with their simplified counterparts, emphasizing the differences in material deposition [
58].
Some research endeavors concentrate on specific regions within the respiratory system. For instance, certain studies examine particle deposition within the nasal airway [
59,
60]. Simultaneously, a distinct study delves into the ramifications of wall roughness on deposition rates within the nasal airway, as depicted in
Figure 5 [
61].
To attain a comprehensive understanding of flow patterns and particle deposition, several studies expanded their modeling domain within the human upper airway system, as displayed in
Figure 6 [
62,
63].
Mouth–Throat and Upper Tracheobronchial Models: Research focusing on regions critical for drug delivery, such as the mouth–throat (MT) and upper tracheobronchial (TB) models, is well-documented [
64,
65,
66,
67,
68]. An innovative Stochastic Individual Path (SIP) modeling approach was introduced to streamline tracheobronchial research [
69,
70].
Patient-Specific Models: Some studies have opted for models reconstructed from actual patient scans to provide an authentic representation of the respiratory system, as illustrated in
Figure 7 [
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81]. The complexity of these realistic models is often dictated by the requisite precision and the scope of the research. Naturally, as these models grow in detail, the computational demands and processing times correspondingly increase.
7.2. Computational Analysis
When analyzing the breadth of computational methodologies utilized across different studies, the significant influence of the computational environment and boundary conditions on the accuracy of results becomes evident [
54]. Delving into specifics, they harnessed fluent to solve the Navier–Stokes equations, factoring in key elements like drag and gravity that impact particle motion. Their assumptions of steady and incompressible flow resonate with the approaches taken by researchers who defined inlet conditions with a fully developed (parabolic) velocity [
82]. In a similar vein, another study anchored their research on Fluent 6.3, focusing on the intricate human upper airway dynamics and incorporating the effects of Brownian motion alongside drag and gravity using the Lagrangian approach [
80].
Moreover, the adoption of varied software platforms and techniques, such as Farhadi’s use of Fluent 14.0 and the Eulerian technique, contrasted with the use of ANSYS’ CFX, Version 15, by others, signifies the versatility researchers find necessary in modeling the intricate behaviors within the lungs [
62,
72]. Notably, one study introduced a distinctive perspective by integrating lung motion modeling into their OPENFOAM CFD simulations [
83]. Concurrently, utilizing ANSYS FLUENT 15, another study cast a spotlight on particle-fluid interactions, ensuring that steady-state, incompressible Newtonian flow was aptly represented [
84]. This spectrum of methodologies, culminating with the employment of the Low Reynolds Number (LRN) k turbulence model by another group, offers a profound reflection of the depth and diversity of approaches in the realm of computational analysis of the respiratory systems [
85].
7.3. Difficulties of CFD Modeling
The realm of CFD modeling, especially when simulating intricate airflow patterns and pollutant dispersion in real environments, is fraught with challenges [
84]. One of the primary hurdles arises from the quality and representativeness of input parameters. For instance, boundary conditions, turbulence models, and material properties play pivotal roles in ensuring the accuracy of the simulations. Yet, acquiring these with high precision remains a formidable task [
54,
55]. Compounding this issue, CFD simulations often resort to simplifications, either by assuming steady-state conditions or overlooking minute turbulence effects, which consequently sews seeds of uncertainty in the results [
58,
66,
67,
72,
77,
86]. The dynamic nature of airflow, characterized by transient behaviors like turbulent flows skirting obstacles or mutable ventilation conditions, adds another layer of complexity [
56,
59,
62,
67,
76,
79,
80,
87]. Even choices made at a technical level—such as the adoption of numerical schemes, meshing techniques, or CFD software—bear consequences on the accuracy and reliability of findings [
57,
60,
64,
65,
72,
74]. Nevertheless, a judicious approach to CFD modeling, which includes a meticulous evaluation of its inherent constraints and uncertainties, can act as a beacon to steer clear of biases, ultimately refining the validity of results [
81].
7.4. Advantages of CFD for Particle Lung Deposition
While CFD modeling poses certain challenges, its benefits, especially in studying airflow patterns and particle dispersion within the lung, are indispensable [
54]. A paramount advantage of CFD is its ability to accurately simulate intricate and evolving airflow patterns within the human respiratory system [
57,
70,
73,
83,
84,
86,
87]. Conducting such detailed simulations through experimental methods could be either practically challenging or ethically questionable. This capability of CFD provides unparalleled insights into the trajectory and settling of pollutants within the lung’s airways [
59,
61,
62,
66,
71,
72].
Furthermore, CFD provides a versatile platform for researchers, allowing them to make modifications to design parameters and simulate varied scenarios, such as changes in ventilation or structural layouts [
55,
64,
68]. This adaptability is pivotal in evaluating the efficiency of various respiratory interventions and understanding the effectiveness of diverse control measures. In a real-world context, CFD modeling is invaluable for decision-makers. It can pinpoint areas of high particle exposure, offer evaluations of different treatment strategies, and project their outcomes [
15,
63,
67,
74,
76,
78,
79].
Collectively, these advantages underscore the immense potential of CFD in delivering a comprehensive, contextual, and practical understanding of particle deposition dynamics within the respiratory system [
54,
56,
60,
62,
75,
80,
82].
7.5. CFD Model Validation
From the reviewed literature, it is apparent that while the significance of CFD model validation is well acknowledged, the methods and metrics used for validation vary across studies [
54,
55,
69,
84].
Several papers have emphasized the importance of comparing CFD outcomes with experimental or real-world data to ascertain model accuracy [
61,
62,
76,
79,
82]. For instance, some studies utilized empirical field observations to identify discrepancies in CFD predictions, ensuring rectification and refined results [
63,
64,
73,
78,
85,
87].
Furthermore, the implications of these validations are multifaceted. They not only enhance the credibility of research conclusions [
57,
58,
70,
75] but also solidify the basis for interpreting CFD-based findings in real-world scenarios [
54,
56,
63,
70]. The process of validation, as highlighted by several sources, aids in unmasking biases and uncertainties, ensuring a comprehensive understanding of the CFD model’s applicability and limitations [
53,
59,
68,
88].
8. Experimental Studies on Particle Deposition Investigations
Describing an ideal experimental setting for particle deposition investigations is challenging due to various influencing factors, such as the aerosol type, particle detector, choice of breathing pattern, and more [
88]. Nonetheless, this section aims to present a comprehensive overview of the diverse efforts made in experimental studies concerning partial lung deposition.
Understanding the precise particulate dose deposited within the human respiratory tract, as measured in terms of number, surface area, and mass, constitutes a crucial element in comprehending the health implications linked to diesel particulate emissions [
89]. As illustrated in
Figure 8, this process entails volunteers inhaling particle-laden air via a unidirectional valve, collecting their exhaled air in a reservoir, and employing a particle sizer to quantify differences in particle size between their inhaled and exhaled air. Notably, this particular experiment involved the participation of ten healthy volunteers. In a parallel study, analogous equipment was utilized to characterize aerosols emanating from eight distinct e-cigarettes, each differing in nicotine content and flavor [
90]. Furthermore, the investigation into experimental particle deposition necessitated the utilization of three distinct mouth–throat models [
64].
An in vitro experiment aimed at investigating deposition in the inhaler, mouth, throat, and tracheobronchial upper TB airways was conducted. This experiment employed a three-way solenoid valve to generate square wave inhalations at flow rates of 37 and 75 L/min for a duration of four seconds while simultaneously maintaining continuous airflow at a constant inhalation flow rate of 30 L/min [
91]. In a separate study, participants adhered to a predetermined schedule involving normal and deep tidal breathing patterns [
92]. To quantify particle numbers, a condensation nuclei counter was utilized, and their size distribution was monitored using a laser spectrometer with high time resolution. Furthermore, this investigation explored the correlation between body plethysmography measurements of pulmonary function and the physical properties of inhaled aerosols, encompassing variations in particle emission both within and between subjects.
Instruments such as the Aerodynamic Particle Sizer (APS) and Droplet Deposition Analysis (DDA) are commonly employed for the measurement of aerosol size distribution within wind tunnels, akin to the one depicted in
Figure 9. This particular wind tunnel design is specifically tailored for use in particle deposition research [
93,
94].
The assessment of oscillatory flow velocity in computed tomography (CT)-scanned extra-thoracic airway (ETA) was carried out using the particle image velocimetry (PIV) method in a study [
94]. To address transparency issues in their model, optical index matching was employed [
81].
The direction of deposited carbon fibers and particle deposition fractions was examined using a lung model featuring a single horizontal bifurcation under various steady breathing conditions [
95]. Micrographs were employed to determine the orientation of the deposited fibers, as shown in
Figure 10.
Researchers developed a specialized MALDA to assess the pulmonary deposition of ultrafine 3D printing particles. The MALDA comprises replicas of various human airway sections, including the mouth, throat, trachea, and the 11th bifurcation generation of bronchiolar airways. Numerous pulmonary deposition studies were conducted in the laboratory using the MALDA in conjunction with a desktop 3D printer to measure the deposition of ultrafine 3D printing particles in distinct lung generations [
95].
Figure 11 provides an illustration of this setup.
9. Discussion
The literature extensively discusses the recent surge in cases of CMDLD and its potential correlation with evolving mining practices. This proposition suggests that miners may have encountered varying quantities and characteristics of the same particulate matter due to changes and advancements in mining technologies. As the mining industry evolves, new technologies are integrated to enhance extraction efficiency, which can influence the size and nature of particles miners are exposed to. Several aspects of mining operations have undergone modifications, potentially leading to increased exposure levels, particularly driven by the exploration of thin coal mines in response to demand pressures.
However, it is essential to emphasize that particle size remains a crucial factor in determining regional deposition within the respiratory tract and lungs. The majority of particles posing the highest health risks to miners fall within the fine to very fine category, with a size of less than 3 µm. These small particles can penetrate the alveoli region of the respiratory system, making them particularly hazardous. Therefore, understanding particle deposition mechanisms and developing strategies to prevent it in these critical anatomical regions is of utmost importance.
Furthermore, the advancement of CFD holds promise for more accurate assessments of particle deposition within the lung. Still, the choice of an appropriate CFD model requires careful consideration due to its significant influence on computational time. The size of the model directly affects solver time estimates for flow characterization within the lung. Researchers have explored various approaches, some focusing on specific lung regions, while others use mathematically simplified models to reduce computational demands. Additionally, the accuracy of the computational solver depends on thoughtful choices of boundary conditions and turbulence models.
It is worth noting that, despite the availability of CFD tools, experimental validation remains a crucial aspect of research. Validation is necessary to confirm the reliability of CFD solutions before engaging in broader computational investigations. While there is no one-size-fits-all validation setup, its specifics should be tailored to the chosen CFD model and associated boundary conditions.
In summary, recommended action for future studies is listed as follows:
Prioritize quantifying the impact of modern mining technologies on particulate attributes and consequent exposure.
Collaborative endeavors can work towards establishing a comprehensive database delineating particle deposition mechanisms across varying sizes.
A consortium of CFD experts can be envisioned to curate guidelines for model selection, ensuring both computational efficiency and result accuracy.
Standardization initiatives should be championed to foster a universally accepted CFD validation protocol.
This discussion highlights the critical importance of thorough model validation in ensuring the precision and reliability of CFD simulations. Proper validation substantiates CFD models based on robust scientific principles, ensuring their accuracy in reproducing real-world phenomena. Neglecting model validation can lead to erroneous predictions, potentially resulting in ill-informed decisions or actions based on flawed simulations.
10. Conclusions
The escalating incidence of confirmed CMDLDs is a persistent concern warranting a comprehensive investigation. This research has illuminated the extensive body of work dedicated to understanding the exposure, deposition, and clearance mechanisms of hazardous dust particles responsible for CMDLDs. Despite efforts within mining operations to mitigate coal dust exposure, the rise in miners afflicted with lung damage remains puzzling. Existing literature suggests a connection between CMDLD cases and evolving mining practices, but the precise causal factors behind this trend remain unclear.
Particle size emerges as a critical determinant when examining regional deposition within the respiratory tract and lungs. The majority of hazardous particles fall within the fine to very fine range, measuring less than 3 µm, and are capable of penetrating the alveoli region. Therefore, prioritizing particle deposition simulations is crucial to unraveling the complexities of particle transport within the deepest recesses of the respiratory system and exploring strategies for mitigation.
Furthermore, the advancement of CFD holds promise for more accurate assessments of lung particle deposition. However, choosing an appropriate CFD model is crucial, as it affects computational time. Various approaches have been explored, but the accuracy of the computational solver depends on judicious choices of boundary conditions and turbulence models.
It is essential to emphasize that, despite the availability of CFD tools, experimental validation remains indispensable. Validation ensures the reliability of CFD solutions and should be tailored to the chosen model and boundary conditions.
In conclusion, this review underscores the critical significance of thorough model validation to ensure the precision and reliability of CFD simulations. Proper validation substantiates CFD models based on robust scientific principles, ensuring their faithfulness in reproducing real-world phenomena. Neglecting model validation can lead to erroneous predictions, potentially resulting in ill-informed decisions based on flawed simulations.
Specifically, this study highlights the implications of using validated versus invalidated CFD models. It demonstrates that validated CFD models can offer insights into complex flow patterns and particle dispersion while underscoring the risks of relying on inadequately validated models, including potential errors and uncertainties. To maintain the integrity of findings and mitigate the risks associated with invalidated models, researchers and practitioners must diligently assess the validity of CFD models in their investigations.
Future research in CFD modeling should focus on developing standardized validation techniques, exploring novel CFD model applications, and addressing emerging challenges and opportunities. Establishing robust validation practices and reducing uncertainties associated with CFD modeling demand further investigation. Continued research and development in this field will undoubtedly enhance the precision and reliability of CFD modeling and its practical applications.
Author Contributions
Conceptualization, E.M. and A.A.; methodology, A.A.; validation, P.R.; formal analysis, P.R.; investigation, E.M.; resources, W.-C.S.; writing—original draft preparation, E.M.; writing—review and editing, A.A.; supervision, P.R.; project administration, P.R.; funding acquisition, P.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the National Institute for Occupational Safety and Health (NIOSH) [Contract #: 75D30119C06390]. The views, opinions, and recommendations expressed herein are solely those of the authors and do not imply any endorsement by NIOSH.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Acknowledgments
The authors would like to acknowledge Bio-render for using their tool to generate some of the figures.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle transport and deposition: Basic physics of particle kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Gordon, T.; Price, O.; Asgharian, B. Thoracic and respirable particle definitions for human health risk assessment. Part. Fibre Toxicol. 2013, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zare Naghadehi, M.; Sereshki, F.; Mohammadi, F. Pathological study of the prevalence of silicosis among coal miners in Iran: A case history. Atmos. Environ. 2014, 83, 1–5. [Google Scholar] [CrossRef]
- Perret, J.L.; Plush, B.; Lachapelle, P.; Hinks, T.S.; Walter, C.; Clarke, P.; Irving, L.; Brady, P.; Dharmage, S.C.; Stewart, A. Coal mine dust lung disease in the modern era. Respirology 2017, 22, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Colinet, J.F. The Impact of Black Lung and a Methodology for Controlling Respirable Dust. Min. Met. Explor. 2020, 37, 1847–1856. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Dehbandi, R.; Mohammadyan, M.; Aarabi, M.; Dominguez, A.O.; Kelly, F.J.; Khodabakhshloo, N.; Rahman, M.M.; Naidu, R. Physico-chemical properties and reactive oxygen species generation by respirable coal dust: Implication for human health risk assessment. J. Hazard. Mater. 2021, 405, 124185. [Google Scholar] [CrossRef]
- Beer, C.; Kolstad, H.A.; Søndergaard, K.; Bendstrup, E.; Heederik, D.; Olsen, K.E.; Omland, Ø.; Petsonk, E.; Sigsgaard, T.; Sherson, D.L.; et al. A systematic review of occupational exposure to coal dust and the risk of interstitial lung diseases. Eur. Clin. Respir. J. 2017, 4, 1264711. [Google Scholar] [CrossRef]
- Laney, A.S.; Weissman, D.N. Respiratory diseases caused by coal mine dust. J. Occup. Environ. Med. 2014, 56 (Suppl. 10), S18–S22. [Google Scholar] [CrossRef]
- Hofmann, W. Modelling inhaled particle deposition in the human lung—A review. J. Aerosol Sci. 2011, 42, 693–724. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Airflow and Particle Transport in the Human Respiratory System. Annu. Rev. Fluid Mech. 2010, 42, 301–334. [Google Scholar] [CrossRef]
- Shekarian, Y. An Investigation of the Effects of Mining Parameters on the Prevalence of Coal Worker’s Pneumoconiosis (CWP) Risks Among the US Coal Miners; New Mexico Institute of Mining and Technology: Socorro, NM, USA, 2020. [Google Scholar]
- Kuempel, E.D.; Attfield, M.D.; Vallyathan, V.; Lapp, N.L.; Hale, J.M.; Smith, R.J.; Castranova, V. Pulmonary inflammation and crystalline silica in respirable coal mine dust: Dose-response. J. Biosci. 2003, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; Peters, J.I.; Williams, R.O., 3rd. Influence of particle size on regional lung deposition—What evidence is there? Int. J. Pharm. 2011, 406, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kuempel, E.D.; Tran, C.L.; Smith, R.J.; Bailer, A.J. A biomathematical model of particle clearance and retention in the lungs of coal miners. II. Evaluation of variability and uncertainty. Regul. Toxicol. Pharmacol. 2001, 34, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Piglione, M.C.; Fontana, D.; Vanni, M. Simulation of particle deposition in human central airways. Eur. J. Mech.—B/Fluids 2012, 31, 91–101. [Google Scholar] [CrossRef]
- Rahimi, E.; Shekarian, Y.; Shekarian, N.; Roghanchi, P. Investigation of respirable coal mine dust (RCMD) and respirable crystalline silica (RCS) in the U.S. underground and surface coal mines. Sci. Rep. 2023, 13, 1767. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Su, W.-C. Deposition of Particles in Human Mouth–Throat Replicas and a USP Induction Port. J. Aerosol Med. Pulm. Drug Deliv. 2015, 28, 147–155. [Google Scholar] [CrossRef]
- Schatzel, S.J. Identifying sources of respirable quartz and silica dust in underground coal mines in southern West Virginia, western Virginia, and eastern Kentucky. Int. J. Coal Geol. 2009, 78, 110–118. [Google Scholar] [CrossRef]
- Sellaro, R.; Sarver, E.; Baxter, D. A Standard Characterization Methodology for Respirable Coal Mine Dust Using SEM-EDX. Resources 2015, 4, 939–957. [Google Scholar] [CrossRef]
- Deng, Q.; Deng, L.; Miao, Y.; Guo, X.; Li, Y. Particle deposition in the human lung: Health implications of particulate matter from different sources. Environ. Res. 2019, 169, 237–245. [Google Scholar] [CrossRef]
- Sarver, E.; Keles, C.; Rezaee, M. Beyond conventional metrics: Comprehensive characterization of respirable coal mine dust. Int. J. Coal Geol. 2019, 207, 84–95. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Prediction of Aerosol Deposition in the Human Respiratory Tract via Computational Models: A Review with Recent Updates. Atmosphere 2020, 11, 137. [Google Scholar] [CrossRef]
- Xie, Y.-S.; Fan, G.-X.; Dai, J.-W.; Song, X.-B. New Respirable Dust Suppression Systems for Coal Mines. J. China Univ. Min. Technol. 2007, 17, 321–325. [Google Scholar] [CrossRef]
- Shekarian, Y.; Rahimi, E.; Rezaee, M.; Su, W.-C.; Roghanchi, P. Respirable Coal Mine Dust: A Review of Respiratory Deposition, Regulations, and Characterization. Minerals 2021, 11, 696. [Google Scholar] [CrossRef]
- Kodros, J.K.; Volckens, J.; Jathar, S.H.; Pierce, J.R. Ambient Particulate Matter Size Distributions Drive Regional and Global Variability in Particle Deposition in the Respiratory Tract. GeoHealth 2018, 2, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Wang, X.; Chow, J.C.; Watson, J.G.; Peik, B.; Nasiri, V.; Riemenschnitter, K.B.; Elahifard, M. Review of Respirable Coal Mine Dust Characterization for Mass Concentration, Size Distribution and Chemical Composition. Minerals 2021, 11, 426. [Google Scholar] [CrossRef]
- Cohen, R.A.C. Is the increasing prevalence and severity of coal workers’ pneumoconiosis in the United States due to increasing silica exposure? Occup. Environ. Med. 2010, 67, 649–650. [Google Scholar] [CrossRef]
- Suarthana, E.; Laney, A.S.; Storey, E.; Hale, J.M.; Attfield, M.D. Coal workers’ pneumoconiosis in the United States: Regional differences 40 years after implementation of the 1969 Federal Coal Mine Health and Safety Act. Occup. Environ. Med. 2011, 68, 908–913. [Google Scholar] [CrossRef]
- Shekarian, Y.; Rahimi, E.; Shekarian, N.; Rezaee, M.; Roghanchi, P. An analysis of contributing mining factors in coal workers’ pneumoconiosis prevalence in the United States coal mines, 1986–2018. Int. J. Coal Sci. Technol. 2021, 8, 1227–1237. [Google Scholar] [CrossRef]
- Lu, C.; Dasgupta, P.; Cameron, J.; Fritschi, L.; Baade, P. A systematic review and meta-analysis on international studies of prevalence, mortality and survival due to coal mine dust lung disease. PLoS ONE 2021, 16, e0255617. [Google Scholar] [CrossRef]
- Petsonk, E.L.; Rose, C.; Cohen, R. Coal mine dust lung disease. New lessons from old exposure. Am. J. Respir. Crit. Care Med. 2013, 187, 1178–1185. [Google Scholar] [CrossRef]
- Blackley, D.J.; Halldin, C.N.; Laney, A.S. Continued Increase in Prevalence of Coal Workers’ Pneumoconiosis in the United States, 1970–2017. Am. J. Public Health 2018, 108, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Adeyemi, O.; Arif, A.A. Estimating mortality from coal workers’ pneumoconiosis among Medicare beneficiaries with pneumoconiosis using binary regressions for spatially sparse data. Am. J. Ind. Med. 2022, 65, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Almberg, K.S.; Halldin, C.N.; Blackley, D.J.; Laney, A.S.; Storey, E.; Rose, C.S.; Go, L.H.T.; Cohen, R.A. Progressive Massive Fibrosis Resurgence Identified in U.S. Coal Miners Filing for Black Lung Benefits, 1970–2016. Ann. Am. Thorac. Soc. 2018, 15, 1420–1426. [Google Scholar] [CrossRef]
- Stacey, P.; Thorpe, A.; Roberts, P.; Butler, O. Determination of respirable-sized crystalline silica in different ambient environments in the United Kingdom with a mobile high flow rate sampler utilising porous foams to achieve the required particle size selection. Atmos. Environ. 2018, 182, 51–57. [Google Scholar] [CrossRef]
- Brodny, J.; Tutak, M. Exposure to Harmful Dusts on Fully Powered Longwall Coal Mines in Poland. Int. J. Environ. Res. Public Health 2018, 15, 1846. [Google Scholar] [CrossRef]
- Trechera, P.; Querol, X.; Lah, R.; Johnson, D.; Wrana, A.; Williamson, B.; Moreno, T. Chemistry and particle size distribution of respirable coal dust in underground mines in Central Eastern Europe. Int. J. Coal Sci. Technol. 2022, 9, 3. [Google Scholar] [CrossRef]
- Baron, P.A.; Rice, F.L.; Key-Schwartz, R.; Bartley, D.; Schlecht, P. Health Effects of Occupational Exposure to Respirable Crystalline Silica; National Institute for Occupational Safety and Health: Cincinnati, OH, USA, 2002; Volume 145. [Google Scholar]
- Pollard, K.M. Silica, Silicosis, and Autoimmunity. Front. Immunol. 2016, 7, 97. [Google Scholar] [CrossRef]
- Chekan, G.; Colinet, J.; Grau, R. Silica Dust Sources in Underground Metal/Nonmetal Mines—Two Case Studies. In Proceedings of the SME Annual Meeting, Phoenix, Arizona, 1 January 2002. [Google Scholar]
- Pandey, J.K.; Agarwal, D.; Gorain, S.; Dubey, R.K.; Vishwakarma, M.K.; Mishra, K.K.; Pal, A.K. Characterisation of respirable dust exposure of different category of workers in Jharia Coalfields. Arab. J. Geosci. 2017, 10, 183. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, Y. Development of a roof bolter drilling control process to reduce the generation of respirable dust. Int. J. Coal Sci. Technol. 2021, 8, 199–204. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, Y.; McQuerrey, J. Experimental study on effects of drilling parameters on respirable dust production during roof bolting operations. J. Occup. Environ. Hyg. 2018, 15, 143–151. [Google Scholar] [CrossRef]
- Shangguan, Y.; Zhuang, X.; Querol, X.; Li, B.; Li, J.; Moreno, N.; Trechera, P.; Sola, P.C.; Uzu, G. Mineralogical and geochemical variations from coal to deposited dust and toxicity of size-segregated respirable dust in a blasting mining underground coal mine in Hunan Province, South China. Int. J. Coal Geol. 2021, 248, 103863. [Google Scholar] [CrossRef]
- Ainsworth, S.M. Infrared Analysis of Respirable Coal Mine Dust for Quartz: Thirty-Five Years. J. ASTM Int. 2005, 2, 12231. [Google Scholar] [CrossRef]
- Pan, L.; Golden, S.; Assemi, S.; Sime, M.F.; Wang, X.; Gao, Y.; Miller, J. Characterization of Particle Size and Composition of Respirable Coal Mine Dust. Minerals 2021, 11, 276. [Google Scholar] [CrossRef]
- Miller, A.L.; Drake, P.L.; Murphy, N.C.; Cauda, E.G.; LeBouf, R.F.; Markevicius, G. Deposition Uniformity of Coal Dust on Filters and Its Effect on the Accuracy of FTIR Analyses for Silica. Aerosol Sci. Technol. 2013, 47, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Hajizadehmotlagh, M.; Paprotny, I. Miniaturized Wearable Respirable Dust Monitor (WEARDM) for Underground Coal Mines: Designs and Experimental Evaluation. In Proceedings of the 2019 IEEE SENSORS, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar]
- Gregoratto, D.; Bailey, M.R.; Marsh, J.W. Modelling particle retention in the alveolar-interstitial region of the human lungs. J. Radiol. Prot. 2010, 30, 491–512. [Google Scholar] [CrossRef]
- Cohen, R.A.; Petsonk, E.L.; Rose, C.; Young, B.; Regier, M.; Najmuddin, A.; Abraham, J.L.; Churg, A.; Green, F.H.Y. Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am. J. Respir. Crit. Care Med. 2016, 193, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sarver, E.; Keleş, Ç.; Afrouz, S.G. Particle size and mineralogy distributions in respirable dust samples from 25 US underground coal mines. Int. J. Coal Geol. 2021, 247, 103851. [Google Scholar] [CrossRef]
- Gonzalez, J.; Keles, C.; Sarver, E. On the Occurrence and Persistence of Coal-Mineral Microagglomerates in Respirable Coal Mine Dust. Min. Metall. Explor. 2022, 39, 271–282. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, W.; Zhou, X.; Jin, B.; Sun, B. CFD–DEM simulation of particle transport and deposition in pulmonary airway. Powder Technol. 2012, 228, 309–318. [Google Scholar] [CrossRef]
- Feng, Y.; Kleinstreuer, C. Micron-particle transport, interactions and deposition in triple lung-airway bifurcations using a novel modeling approach. J. Aerosol Sci. 2014, 71, 1–15. [Google Scholar] [CrossRef]
- Islam, M.S.; Saha, S.C.; Gemci, T.; Yang, I.A.; Sauret, E.; Ristovski, Z.; Gu, Y.T. Euler-Lagrange Prediction of Diesel-Exhaust Polydisperse Particle Transport and Deposition in Lung: Anatomy and Turbulence Effects. Sci. Rep. 2019, 9, 12423. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Feng, Y.; Zhong, W.; Sun, B.; Tao, F. Numerical investigation of particle deposition in a triple bifurcation airway due to gravitational sedimentation and inertial impaction. Powder Technol. 2018, 323, 284–293. [Google Scholar] [CrossRef]
- Kim, Y.H.; Tong, Z.B.; Chan, H.K.; Yang, R.Y. CFD modelling of air and particle flows in different airway models. J. Aerosol Sci. 2019, 134, 14–28. [Google Scholar] [CrossRef]
- Gao, N.; Niu, J.; He, Q.; Zhu, T.; Wu, J. Using RANS turbulence models and Lagrangian approach to predict particle deposition in turbulent channel flows. Build. Environ. 2012, 48, 206–214. [Google Scholar] [CrossRef]
- Lambert, A.R.; O’Shaughnessy, P.; Tawhai, M.H.; Hoffman, E.A.; Lin, C.L. Regional deposition of particles in an image-based airway model: Large-eddy simulation and left-right lung ventilation asymmetry. Aerosol Sci. Technol. 2011, 45, 11–25. [Google Scholar] [CrossRef]
- Schroeter, J.D.; Garcia, G.J.; Kimbell, J.S. Effects of Surface Smoothness on Inertial Particle Deposition in Human Nasal Models. J. Aerosol Sci. 2011, 42, 52–63. [Google Scholar] [CrossRef]
- Xi, J.; Si, X.; Kim, J.W.; Berlinski, A. Simulation of airflow and aerosol deposition in the nasal cavity of a 5-year-old child. J. Aerosol Sci. 2011, 42, 156–173. [Google Scholar] [CrossRef]
- Farhadi Ghalati, P.; Keshavarzian, E.; Abouali, O.; Faramarzi, A.; Tu, J.; Shakibafard, A. Numerical analysis of micro- and nano-particle deposition in a realistic human upper airway. Comput. Biol. Med. 2012, 42, 39–49. [Google Scholar] [CrossRef]
- Hindle, M.; Longest, P.W. Evaluation of enhanced condensational growth (ECG) for controlled respiratory drug delivery in a mouth-throat and upper tracheobronchial model. Pharm. Res. 2010, 27, 1800–1811. [Google Scholar] [CrossRef]
- Longest, P.W.; Tian, G.; Walenga, R.L.; Hindle, M. Comparing MDI and DPI aerosol deposition using in vitro experiments and a new stochastic individual path (SIP) model of the conducting airways. Pharm. Res. 2012, 29, 1670–1688. [Google Scholar] [CrossRef]
- Zhang, Z.; Kleinstreuer, C. Laminar-to-turbulent fluid–nanoparticle dynamics simulations: Model comparisons and nanoparticle-deposition applications. Int. J. Numer. Methods Biomed. Eng. 2011, 27, 1930–1950. [Google Scholar] [CrossRef]
- Awadalla, M.; Miyawaki, S.; Abou Alaiwa, M.H.; Adam, R.J.; Bouzek, D.C.; Michalski, A.S.; Fuld, M.K.; Reynolds, K.J.; Hoffman, E.A.; Lin, C.-L.; et al. Early Airway Structural Changes in Cystic Fibrosis Pigs as a Determinant of Particle Distribution and Deposition. Ann. Biomed. Eng. 2014, 42, 915–927. [Google Scholar] [CrossRef]
- Koullapis, P.G.; Kassinos, S.C.; Bivolarova, M.P.; Melikov, A.K. Particle deposition in a realistic geometry of the human conducting airways: Effects of inlet velocity profile, inhalation flowrate and electrostatic charge. J. Biomech. 2016, 49, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Kleinstreuer, C.; Hyun, S. Size-change and deposition of conventional and composite cigarette smoke particles during inhalation in a subject-specific airway model. J. Aerosol Sci. 2012, 46, 34–52. [Google Scholar] [CrossRef]
- Longest, P.W.; Tian, G.; Khajeh-Hosseini-Dalasm, N.; Hindle, M. Validating Whole-Airway CFD Predictions of DPI Aerosol Deposition at Multiple Flow Rates. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Inthavong, K.; Choi, L.-T.; Tu, J.; Ding, S.; Thien, F. Micron particle deposition in a tracheobronchial airway model under different breathing conditions. Med. Eng. Phys. 2010, 32, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Sturm, R. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thorac. Cancer 2010, 1, 141–152. [Google Scholar] [CrossRef]
- Mead-Hunter, R.; King, A.J.C.; Larcombe, A.N.; Mullins, B.J. The influence of moving walls on respiratory aerosol deposition modelling. J. Aerosol Sci. 2013, 64, 48–59. [Google Scholar] [CrossRef]
- Soni, B.; Aliabadi, S. Large-scale CFD simulations of airflow and particle deposition in lung airway. Comput. Fluids 2013, 88, 804–812. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Kleinstreuer, C. Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part I: Theory and model validation. Comput. Biol. Med. 2016, 79, 193–204. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Kleinstreuer, C.; Sadikot, R.T. Computationally efficient analysis of particle transport and deposition in a human whole-lung-airway model. Part II: Dry powder inhaler application. Comput. Biol. Med. 2017, 84, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Gemci, T.; Yang, I.A.; Gu, Y.T. Ultrafine particle transport and deposition in a large scale 17-generation lung model. J. Biomech. 2017, 64, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lintermann, A.; Schröder, W. Simulation of aerosol particle deposition in the upper human tracheobronchial tract. Eur. J. Mech.—B/Fluids 2017, 63, 73–89. [Google Scholar] [CrossRef]
- Kadota, K.; Imanaka, A.; Shimazaki, M.; Takemiya, T.; Kubo, K.; Uchiyama, H.; Tozuka, Y. Effects of inhalation procedure on particle behavior and deposition in the airways analyzed by numerical simulation. J. Taiwan Inst. Chem. Eng. 2018, 90, 44–50. [Google Scholar] [CrossRef]
- Poorbahrami, K.; Oakes, J.M. Regional flow and deposition variability in adult female lungs: A numerical simulation pilot study. Clin. Biomech. 2019, 66, 40–49. [Google Scholar] [CrossRef]
- Koullapis, P.G.; Stylianou, F.S.; Sznitman, J.; Olsson, B.; Kassinos, S.C. Towards whole-lung simulations of aerosol deposition: A model of the deep lung. J. Aerosol Sci. 2020, 144, 105541. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, W.; Tom, J.; Kleinstreuer, C.; Feng, Y.; He, X. Experimental-computational study of fibrous particle transport and deposition in a bifurcating lung model. Particuology 2016, 28, 102–113. [Google Scholar] [CrossRef]
- Garcia, G.J.; Schroeter, J.D.; Kimbell, J.S. Olfactory deposition of inhaled nanoparticles in humans. Inhal. Toxicol. 2015, 27, 394–403. [Google Scholar] [CrossRef]
- Pourmehran, O.; Rahimi-Gorji, M.; Gorji-Bandpy, M.; Gorji, T.B. Simulation of magnetic drug targeting through tracheobronchial airways in the presence of an external non-uniform magnetic field using Lagrangian magnetic particle tracking. J. Magn. Magn. Mater. 2015, 393, 380–393. [Google Scholar] [CrossRef]
- Tian, G.; Longest, P.W.; Su, G.; Walenga, R.L.; Hindle, M. Development of a stochastic individual path (SIP) model for predicting the tracheobronchial deposition of pharmaceutical aerosols: Effects of transient inhalation and sampling the airways. J. Aerosol Sci. 2011, 42, 781–799. [Google Scholar] [CrossRef]
- Löndahl, J.; Möller, W.; Pagels, J.H.; Kreyling, W.G.; Swietlicki, E.; Schmid, O. Measurement techniques for respiratory tract deposition of airborne nanoparticles: A critical review. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 229–254. [Google Scholar] [CrossRef]
- Islam, M.S.; Saha, S.C.; Sauret, E.; Gemci, T.; Gu, Y.T. Pulmonary aerosol transport and deposition analysis in upper 17 generations of the human respiratory tract. J. Aerosol Sci. 2017, 108, 29–43. [Google Scholar] [CrossRef]
- Kolanjiyil, A.V.; Kleinstreuer, C. Computational analysis of aerosol-dynamics in a human whole-lung airway model. J. Aerosol Sci. 2017, 114, 301–316. [Google Scholar] [CrossRef]
- Rissler, J.; Swietlicki, E.; Bengtsson, A.; Boman, C.; Pagels, J.; Sandström, T.; Blomberg, A.; Löndahl, J. Experimental determination of deposition of diesel exhaust particles in the human respiratory tract. J. Aerosol Sci. 2012, 48, 18–33. [Google Scholar] [CrossRef]
- Manigrasso, M.; Buonanno, G.; Fuoco, F.C.; Stabile, L.; Avino, P. Aerosol deposition doses in the human respiratory tree of electronic cigarette smokers. Environ. Pollut. 2015, 196, 257–267. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, J.; Cheng, Y.S. Comparison of deposition in the USP and physical mouth-throat models with solid and liquid particles. J. Aerosol Med. Pulm. Drug Deliv. 2011, 24, 277–284. [Google Scholar] [CrossRef]
- Schwarz, K.; Biller, H.; Windt, H.; Koch, W.; Hohlfeld, J.M. Characterization of exhaled particles from the healthy human lung--a systematic analysis in relation to pulmonary function variables. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 371–379. [Google Scholar] [CrossRef]
- Johnson, G.R.; Morawska, L.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Chao, C.Y.H.; Wan, M.P.; Li, Y.; Xie, X.; Katoshevski, D.; et al. Modality of human expired aerosol size distributions. J. Aerosol Sci. 2011, 42, 839–851. [Google Scholar] [CrossRef]
- Aboelezz, A.; Hassanalian, M.; Roghanchi, P. Design and Manufacturing of Dust Tunnel for Respirable Dust Characterization Investigations. In Proceedings of the AIAA AVIATION 2021 FORUM, Virtual Event, 2–6 August 2021. [Google Scholar]
- Zhu, Z.; Ju, Y.; Zhang, C. In-Vitro Experimental Modeling of Oscillatory Respiratory Flow in a CT-Scanned OSAHS Tract. Appl. Sci. 2020, 10, 7979. [Google Scholar] [CrossRef]
- Su, W.-C.; Chen, Y.; Xi, J. Estimation of the deposition of ultrafine 3D printing particles in human tracheobronchial airways. J. Aerosol Sci. 2020, 149, 105605. [Google Scholar] [CrossRef]
- Sweeney, L.M.; Parker, A.; Haber, L.T.; Tran, C.L.; Kuempel, E.D. Application of Markov chain Monte Carlo analysis to biomathematical modeling of respirable dust in US and UK coal miners. Regul. Toxicol. Pharmacol. 2013, 66, 47–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).