Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province
Abstract
1. Introduction
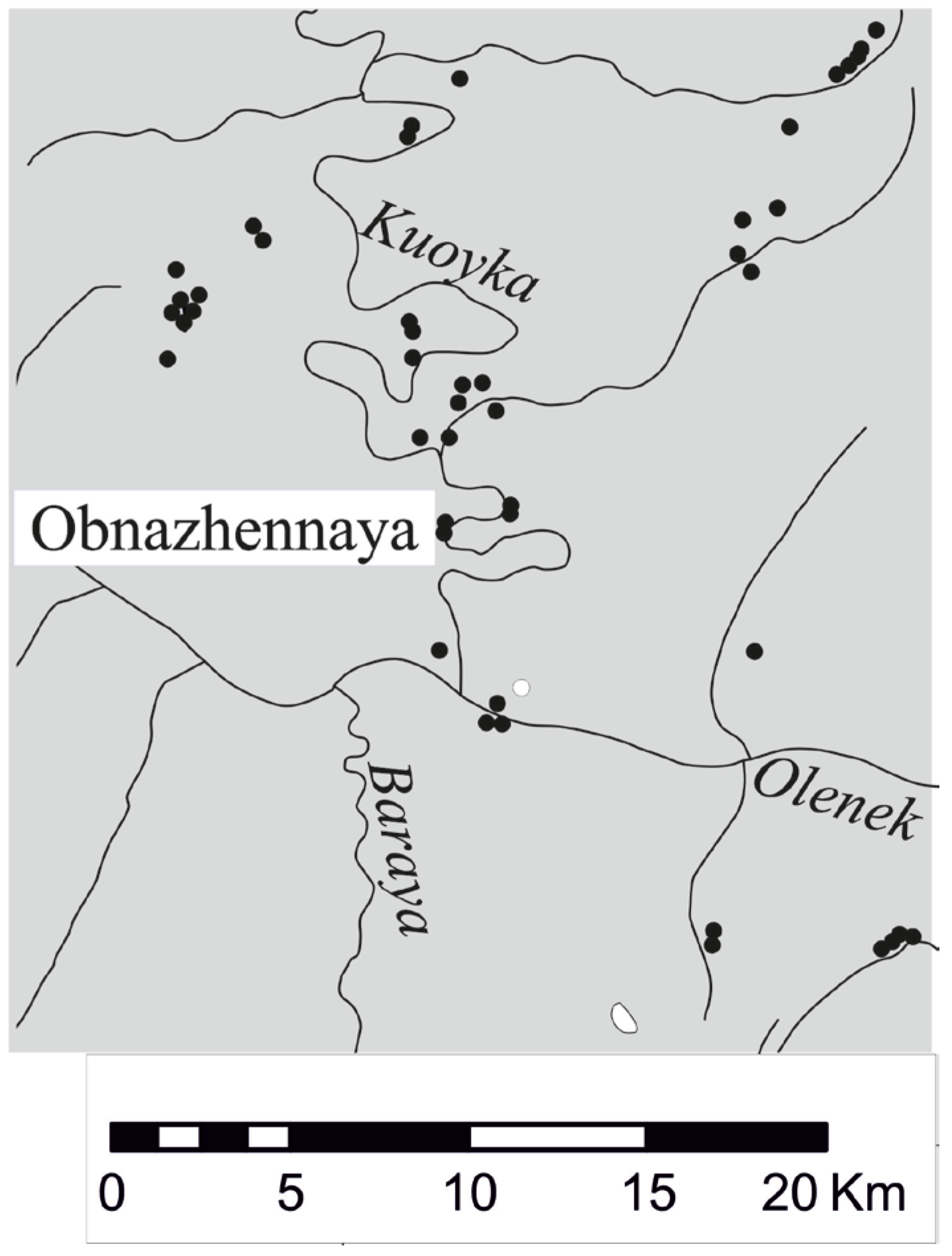
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
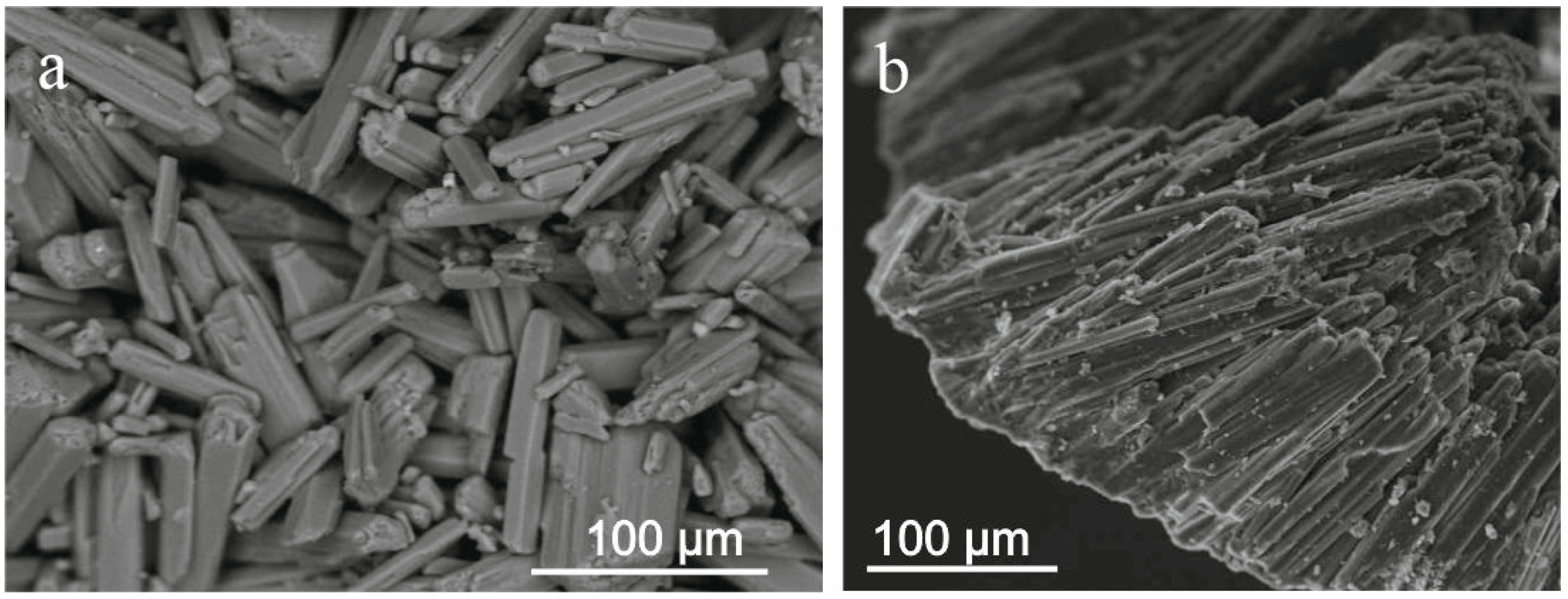
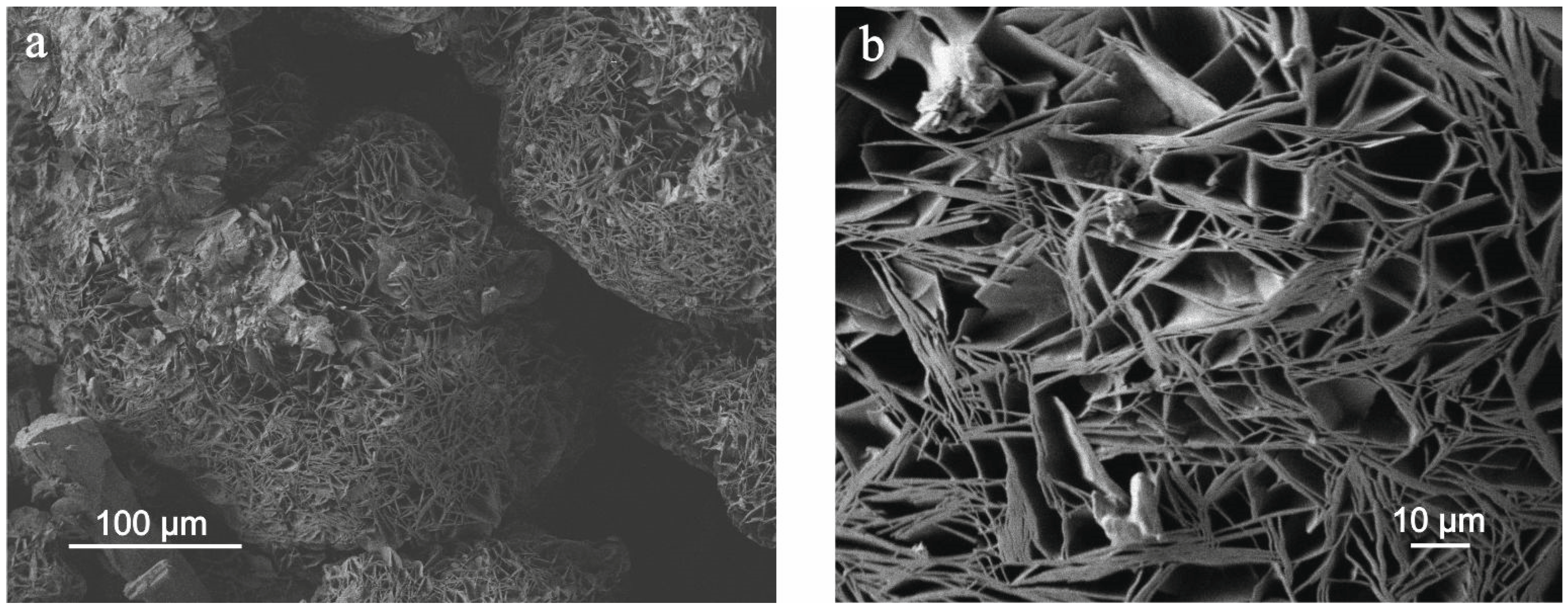
3.1. Micromorphology and Chemical Composition of the Studied Hydrated Magnesium Carbonate Minerals
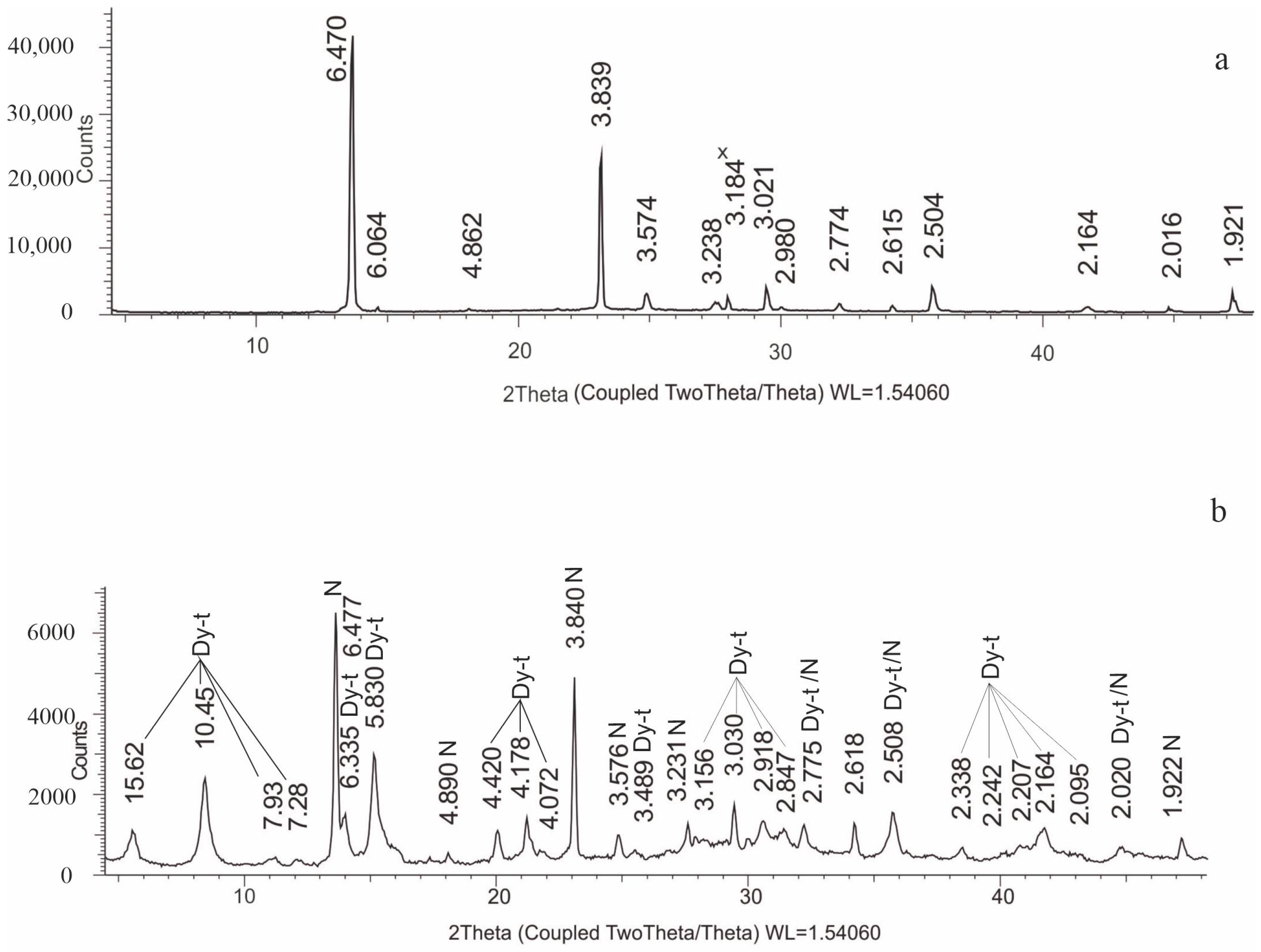
3.2. X-ray Phase Analysis
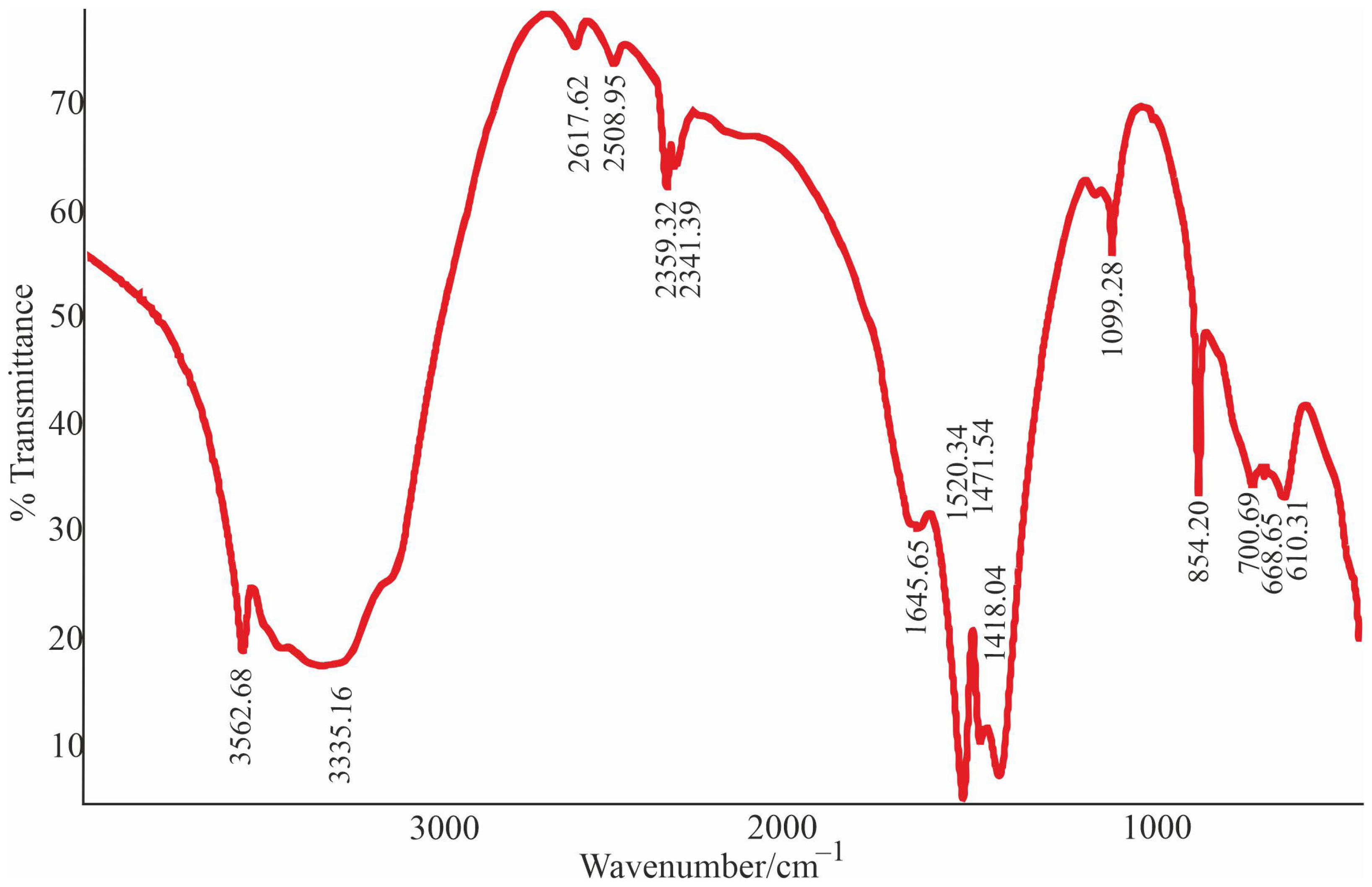
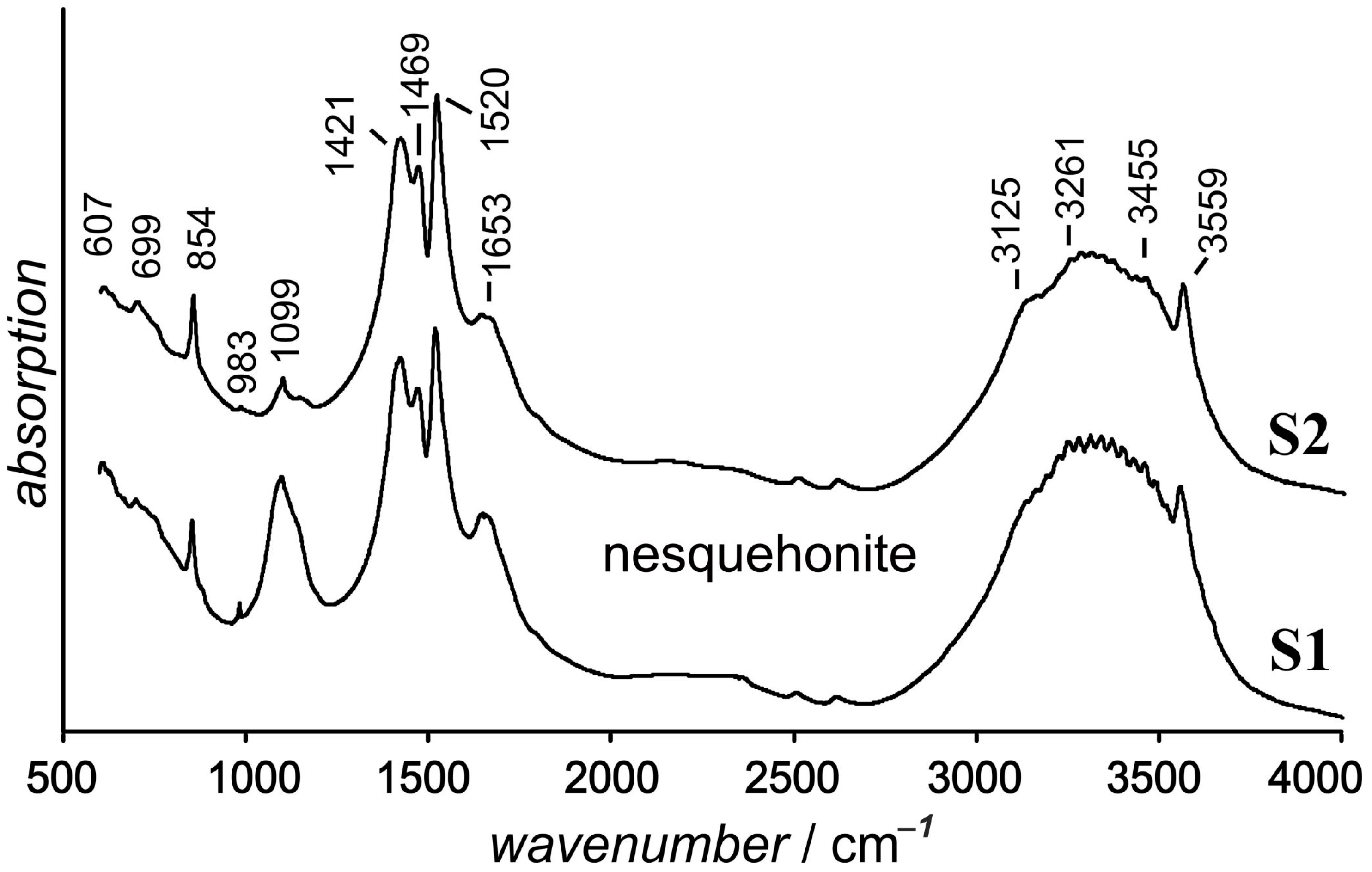
3.3. Thermogravimetric Characteristics and IR Spectra
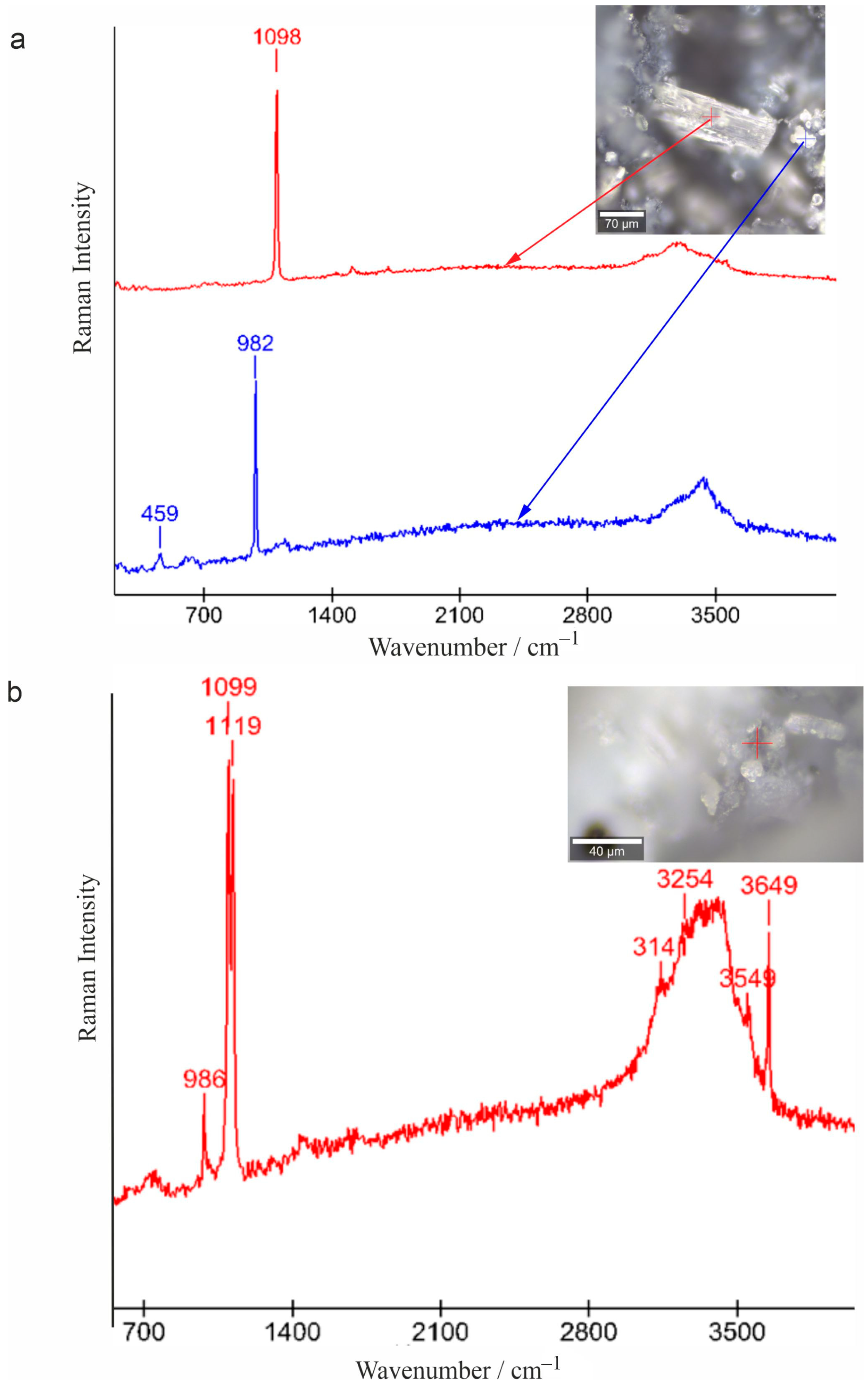
3.4. Raman Spectroscopy
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinchuk, N.N. Postmagmatic Minerals of Kimberlites; Nedra: Moscow, Russia, 2000; p. 538. [Google Scholar]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatskiy, V.S.; Arauho, D.; Griffin, V.L. Carbonate and silicate media of crystallization of fibrous diamonds from placers in the northeast of the Siberian platform. Geol. Geophys. 2011, 52, 1649–1664. [Google Scholar]
- Logvinova, A.M.; Virt, R.; Tomilenko, A.A.; Afanasyev, V.P.; Sobolev, N.V. Features of the phase composition of nanosized inclusions in alluvial diamonds of the Northeastern Siberian platform. Geol. Geophys. 2011, 52, 1634–1648. [Google Scholar]
- Ionov, D.A.; Doucet, L.S.; Xu, Y.; Golovin, A.V.; Oleinikov, O.B. Reworking of Archean mantle in the NE Siberian craton by carbonatite and silicate melt metasomatism: Evidence from a carbonate-bearing, dunite-to websterite xenolith suite from the Obnazhennaya kimberlite. Geochim. Cosmochim. Acta 2018, 224, 132–153. [Google Scholar] [CrossRef]
- Golovin, A.V.; Kamenetsky, V.S. Compositions of kimberlite melts: A review of studies of melt inclusions in kimberlite minerals. Petrology 2023, 31, 115–152. [Google Scholar] [CrossRef]
- Zayakina, N.V.; Oleinikov, O.B.; Vasileva, T.I.; Oparin, N.A. Coalingite from kimberlite breccia of the Manchary pipe, Central Yakutia. Geol. Ore Depos. 2015, 57, 732–736. [Google Scholar] [CrossRef]
- Hansen, L.D.; Dipple, G.M.; Gordon, T.M.; Kellet, D.A. Carbonated serpentinite (listwanite) at Atlin, British Columbia: A geological analogue to carbon dioxide sequestration. Can. Mineral. 2005, 43, 225–239. [Google Scholar] [CrossRef]
- Wilson, S.A.; Raudsepp, M.; Dipple, G.M. Verifying and quantifying carbon fixation in minerals from serpentine-rich mine tailings using the Rietveld method with X-ray powder diffraction data. Am. Mineral. 2006, 91, 1331–1341. [Google Scholar] [CrossRef]
- Wilson, S.A.; Dipple, G.M.; Power, I.M.; Thom, J.M.; Anderson, R.G.; Raudsepp, M.; Gabites, J.E.; Southam, G. Carbon dioxide fixation within mine wastes of ultramafic-hosted ore deposits: Examples from the Clinton Creek and Cassiar chrysotile deposits. Econ. Geol. 2009, 104, 95–112. [Google Scholar] [CrossRef]
- Garanin, V.K.; Posukhova, T.V.; Garanin, K.V. Mineralogy of Diamond Deposits; Max-Press: Moscow, Russia, 2012; p. 329. [Google Scholar]
- Mervine, E.M.; Wilson, S.A.; Power, I.M.; Dipple, G.M.; Turvey, C.C.; Hamilton, J.L.; Vanderzee, S.; Raudsepp, M.; Southam, C.; Matter, J.M.; et al. Potential for offsetting diamond mine carbon emissions through mineral carbonation of processed kimberlite: An assessment of De Beers mine sites in South Africa and Canada. Mineral. Petrol. 2018, 112 (Suppl. S2), 755–765. [Google Scholar] [CrossRef]
- Masanov, A.Y.; Dubovichev, M.A.; Tolstov, A.V.; Anisimova, P.S.; Garanin, K.V.; Dorokhov, A.V.; Baranovskaya, V.B. Offseting potential of GHG emissions of ALROSA Group enterprises due to carbonation of waste kimberliteю Ratsional’noye osvoyeniye nedr. Miner. Min. Conserv. (MMC) 2021, 4, 64–73. [Google Scholar]
- Genth, F.A.; Penfield, S.L. On lansfordite, nesquehonite, a new mineral, and pseudomorphs of nesquehonite after lansfordite. Am. J. Sci. 1890, 139, 121–137. [Google Scholar] [CrossRef][Green Version]
- Pisarsky, B.I.; Konev, A.A. About the find of nesvegonite in Transbaikalia. DAN SSSR 1971, 200, 1423–1425. [Google Scholar]
- Giester, G.; Lengauer, C.; Rieck, B. The crystal structure of nesquehonite, MgCO3·3H2O, from Lavrion, Greece. Mineral. Petrol. 2000, 70, 153–163. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K.; Serafimova, U.K.; Sergeeva, S.V. Chlorartinite Mg2(CO3)ClOH 3H2O—A new mineral of volcanic exhalations. ZVMO 1998, 2, 55–59. [Google Scholar]
- Cesnokov, B.; Kotrly, M.; Nisanbajev, T. Brennende Abraumhalden und Aufschlüsse im Tscheljabinsker Kohlenbecken-eine reiche Mineralienküche. Mineralien-Welt 1998, 9, 54–63. [Google Scholar]
- Mazurov, M.P.; Grishina, S.N.; Istomin, V.E.; Titov, A.T. Metasomatism and Ore Formation at Contacts of Dolerite with Saliferous Rocks in the Sedimentary Cover of the Southern Siberian Platform. Geol. Ore Depos. 2007, 49, 271–284. [Google Scholar] [CrossRef]
- Pavlovsky, A.B.; Pechenkin, I.G.; Lugovskaya, I.G. Geological and Industrial Types of Mineral Deposits; Tin. Study Guide; VIMS: Moscow, Russia, 2015. [Google Scholar]
- Raade, G. Dypingite, a new hydrous basic carbonate of magnesium from Norvay. Am. Mineral. 1970, 55, 1457–1465. [Google Scholar]
- Brakhfogel, F.F. Geological Aspects of Kimberlite Magmatism in the North-Eastern Siberian Platform; Yakutian Branch of SO AN USSR Press: Yakutsk, Russia, 1984; p. 128. [Google Scholar]
- Zaitsev, A.I.; Smelov, A.P. Isotope Geochronology of Kimberlite Rock Formation of the Yakutsk Province; Ofset: Yakutsk, Russia, 2010; p. 108. [Google Scholar]
- Milashev, V.A.; Shulgina, N.I. New data on the age of kimberlites of the Siberian platform. DAN USSR 1959, 126, 1320–1322. [Google Scholar]
- Malkov, B.A.; Gustomesov, V.A. Jurassic fauna in the kimberlites of the Olenek uplift and the age of kimberlite volcanism in the northeast of the Siberian platform. DAN USSR 1976, 229, 435–438. [Google Scholar]
- Malkov, B.A.; Silin, Y.I.; Tsovbun, Y.M. Radiological evidence of xenogenicity of “porphyry inclusions” of olivine, pyrope, chromdiopside in kimberlites. Rep. USSR Acad. Sci. 1979, 245, 927–929. [Google Scholar]
- Kostrovitsky, S.I.; Admakin, L.A. The discovery of xenolith wood in a kimberlite pipe Exposed. Geol. Geophys. 1991, 11, 82–84. [Google Scholar]
- Grinenko, V.S.; Yuganova, L.A.; Truschelev, A.N.; Malanin, Y.A.; Smetannikova, L.I.; Knyazev, V.G.; Prokopiev, A.V.; Kazakova, G.G.; Protopopov, R.I. State Geological Map of the Russian Federation. Scale 1:1,000,000 (Third Generation); Sheet R-51–Jarjan. Explanatory Note; Publishing House of St. Petersburg Kartfabriki VSEGEI: St. Petersburg, Russia, 2012; p. 379. [Google Scholar]
- Kornilova, V.P.; Nikolaev, L.I. Petrography and chemical nature of kimberlite and comagmatic rocks of the Kuoyka field. In Kimberlite and Basite Magmatism of the Olenek Uplift Area. Yakutsk; Ofset: Yakutsk, Russia, 1980; pp. 92–106. [Google Scholar]
- Smith, B.S.; Nowicki, T.E.; Russell, J.K.; Webb, K.; Mitchell, R.H.; Hetman, C.; vA Robey, J. A Glossary of Kimberlite and Related Terms (Part 1, Part 2, Part 3); Scott-Smith Petrology Inc.: Vancouver, BC, Canada, 2017. [Google Scholar]
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase transitions in the MgCO2H2O system and the thermal decomposition of dypingite, Mg3 (CO3)4(OH)2·5H2O: Implications for geosequestration of carbon dioxide. Chem. Geol. 2013, 40, 59–67. [Google Scholar] [CrossRef]
- Jauffret, G.; Morrison, J.; Glasser, F.P. On the thermal decomposition of nesquehonite. J. Therm. Anal. Calorim. 2015, 122, 601–609. [Google Scholar] [CrossRef]
- Skliros, V.; Anagnostopoulou, A.; Tsakiridis, P.; Perraki, M. Mineralogical and spectroscopic study of nesquehonite synthesized by reaction of gaseous CO2 with Mg chloride solution. Bull. Geol. Soc. Greece 2016, 50, 2009–2017. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Martens, W.N.; Nothdurft, L.; Duong, L.V.; Webb, G.E. Low temperature synthesis and characterization of nesquehonite. J. Mater. Sci. Lett. 2003, 22, 825–829. [Google Scholar] [CrossRef]
- Coleyshaw, E.; Crump, G.; Griffith, W. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2231–2239. [Google Scholar] [CrossRef]
- Morgan, B.; Wilson, S.A.; Madsen, I.C.; Gozukara, Y.M.; Habsuda, J. Increased thermal stability of nesquehonite (MgCO3•3H2O) in the presence of humidity and CO2: Implications for lowtemperature O2 storage. Int. J. Greenh. Gas Control 2015, 39, 366–376. [Google Scholar] [CrossRef]
- Ferrini, V.; De Vito, C.; Mignardi, S. Synthesis of nesquehonite by reaction of gaseous CO2 with Mg chloride solution: Its potential role in the sequestration of carbon dioxide. J. Hazard. Mater. 2009, 168, 832–837. [Google Scholar] [CrossRef]
- Hopkinson, L.; Rutt, K.; Cressey, G. The transformation of nesquehonite to hydromagnesite in the system CaO-MgO-H2O-CO2: An experimental spectroscopic study. J. Geol. 2008, 116, 387–400. [Google Scholar] [CrossRef]
- Hopkinson, L.; Kristova, P.; Rutt, K.; Cressey, G. Phase transitions in the systemMgO-CO2-H2O during CO2 degassing of Mg-bearing solutions. Geochim. Cosmochim. Acta 2012, 76, 1–13. [Google Scholar] [CrossRef]
- Hales, M.C.; Frost, R.L.; Martens, W.N. Thermo-Raman spectroscopy of synthetic nesquehonite—implication for the geosequestration of greenhouse gases. J. Raman Spectrosc. 2008, 39, 1141–1149. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Infrared and infrared emission spectroscopy of nesquehonite Mg(OH)(HCO3)•2H2O-implications for the formula of nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Lanas, J.; Alvarez, J.I. Dolomitic lime: Thermal decomposition of nesquehonite. Thermochim. Acta 2004, 421, 123–132. [Google Scholar] [CrossRef]





| Mineral | MgO, % | CaO, % | K2O, % | SO2, % |
|---|---|---|---|---|
| Nesquehonite | 28.98 | - | - | - |
| Dypingite | 43.68 | 0.37 | 1.31 | 1.56 |
| 48.26 | 0.33 | - | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugapeva, S.S.; Oleinikov, O.B.; Zayakina, N.V. Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province. Minerals 2023, 13, 1363. https://doi.org/10.3390/min13111363
Ugapeva SS, Oleinikov OB, Zayakina NV. Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province. Minerals. 2023; 13(11):1363. https://doi.org/10.3390/min13111363
Chicago/Turabian StyleUgapeva, Sargylana S., Oleg B. Oleinikov, and Nadezhda V. Zayakina. 2023. "Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province" Minerals 13, no. 11: 1363. https://doi.org/10.3390/min13111363
APA StyleUgapeva, S. S., Oleinikov, O. B., & Zayakina, N. V. (2023). Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province. Minerals, 13(11), 1363. https://doi.org/10.3390/min13111363






