Flotation Dephosphorization of High-Phosphorus Oolitic Ore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Test Characterization
3. Results and Discussion
3.1. Experimental Study of Dephosphorization
3.1.1. The Collector Selection Tests
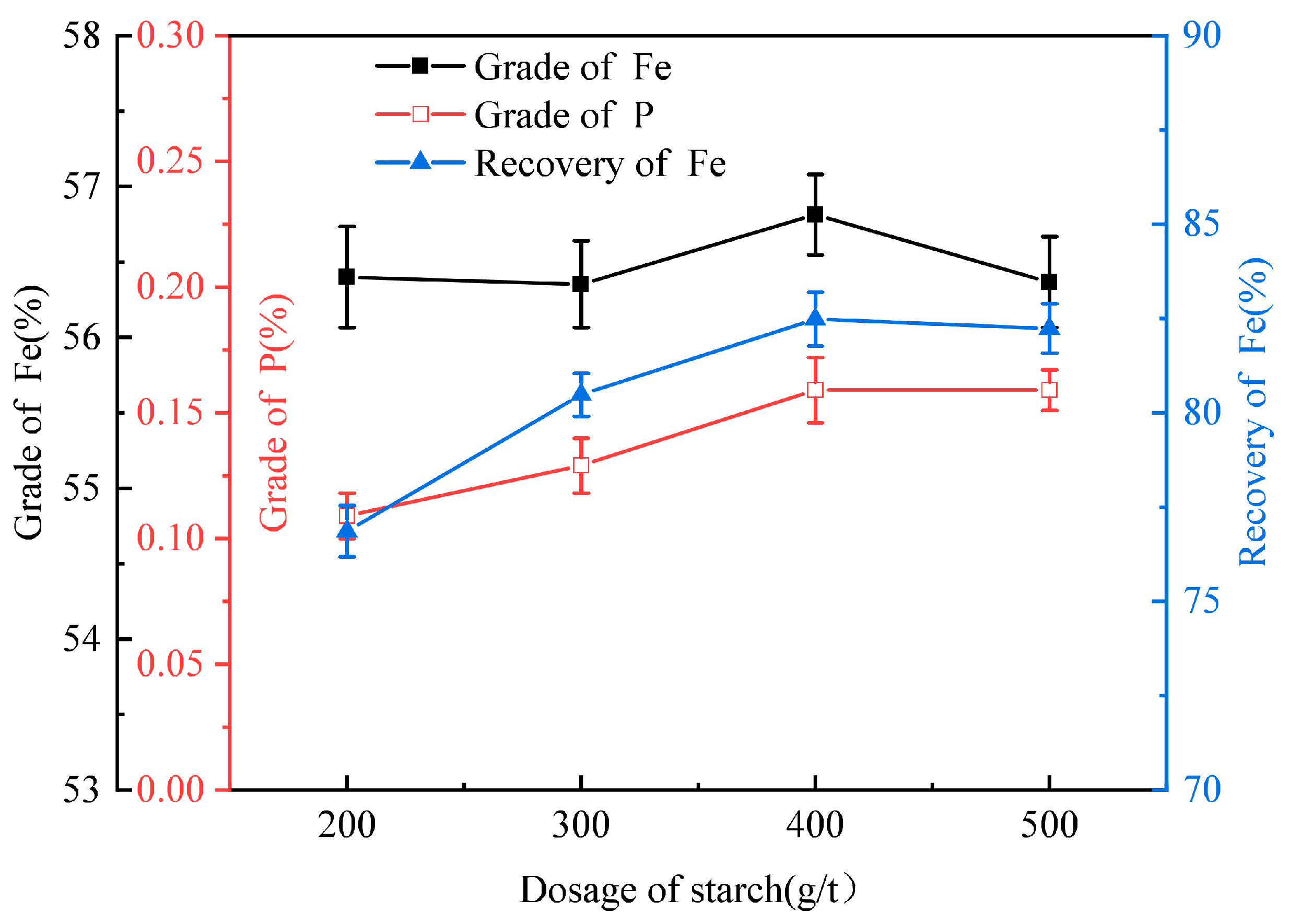
3.1.2. The Starch Dosage Tests
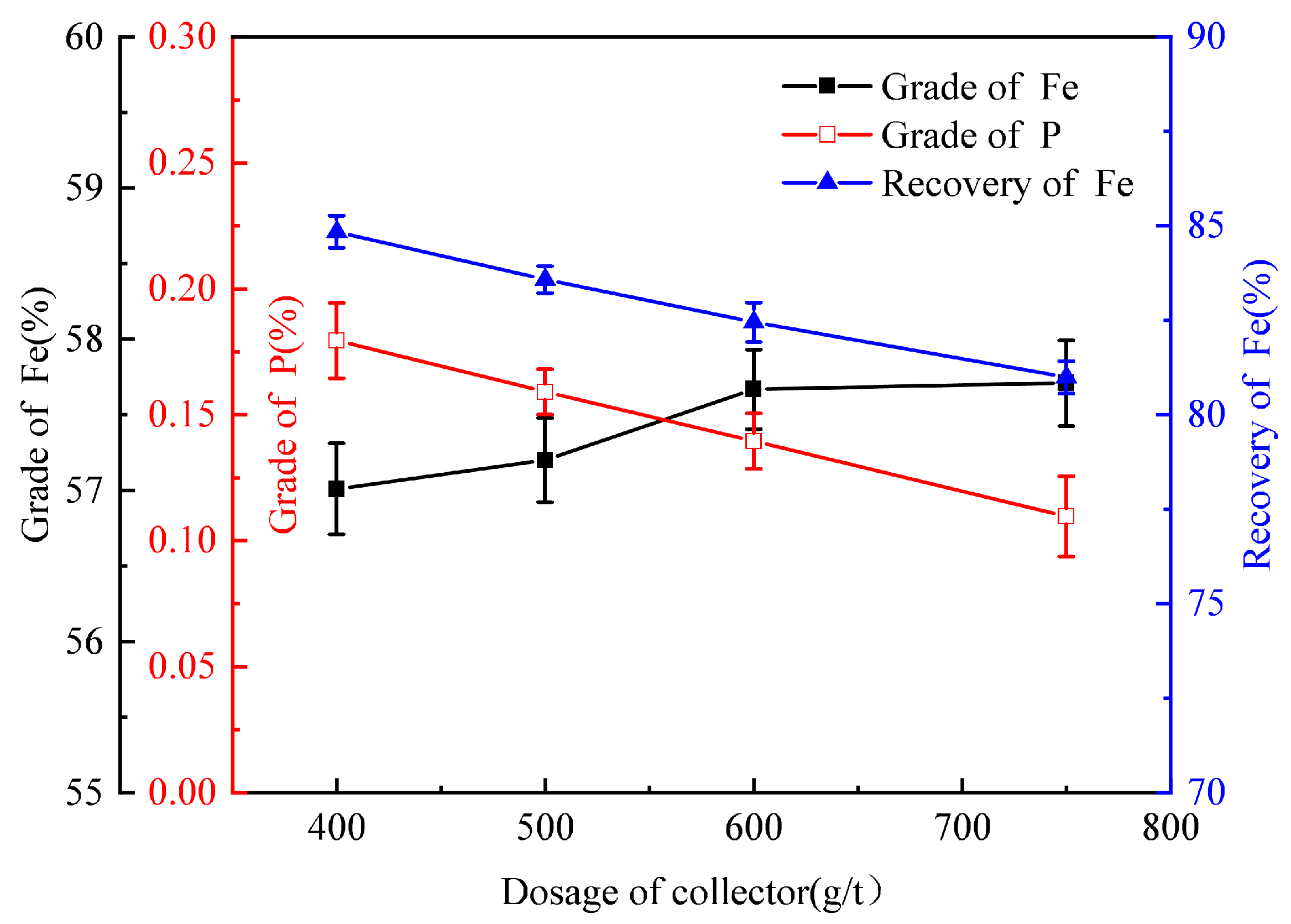
3.1.3. The Collector Dosage Tests
3.2. Flotation Product Characteristics
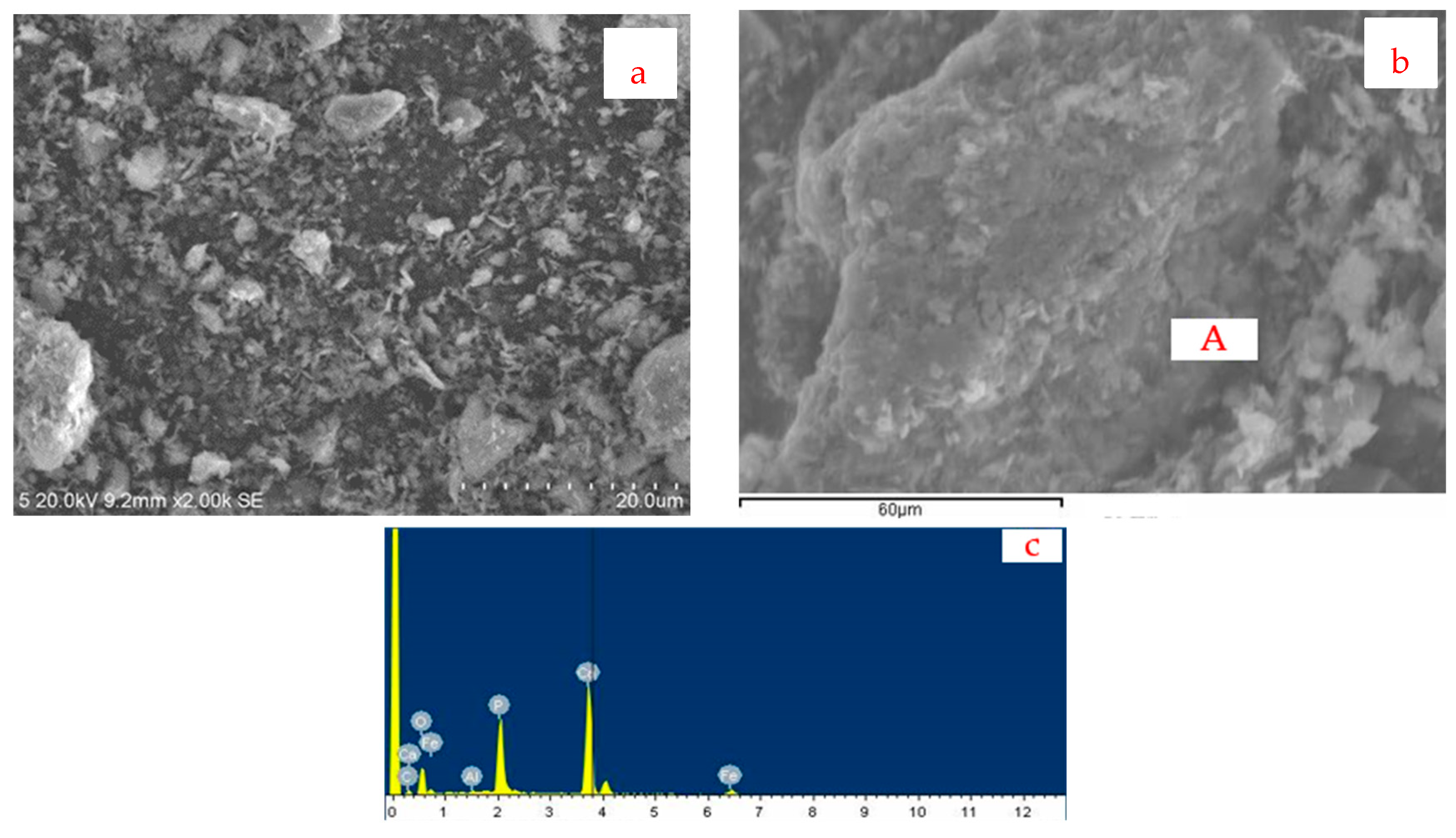
3.2.1. Flotation Tailings
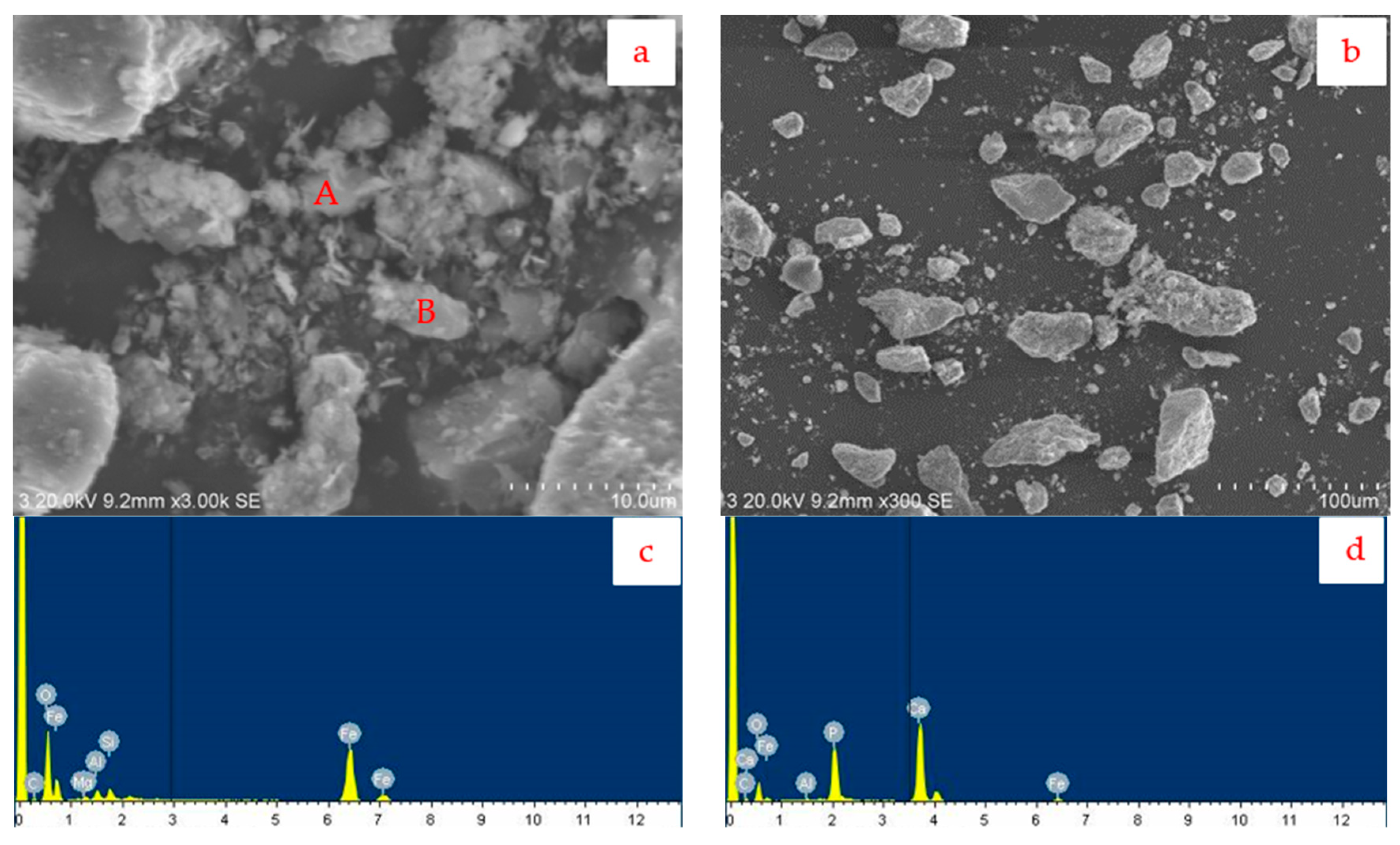
3.2.2. Analysis of Iron Concentrate Products
3.3. Mechanism of Dephosphorization
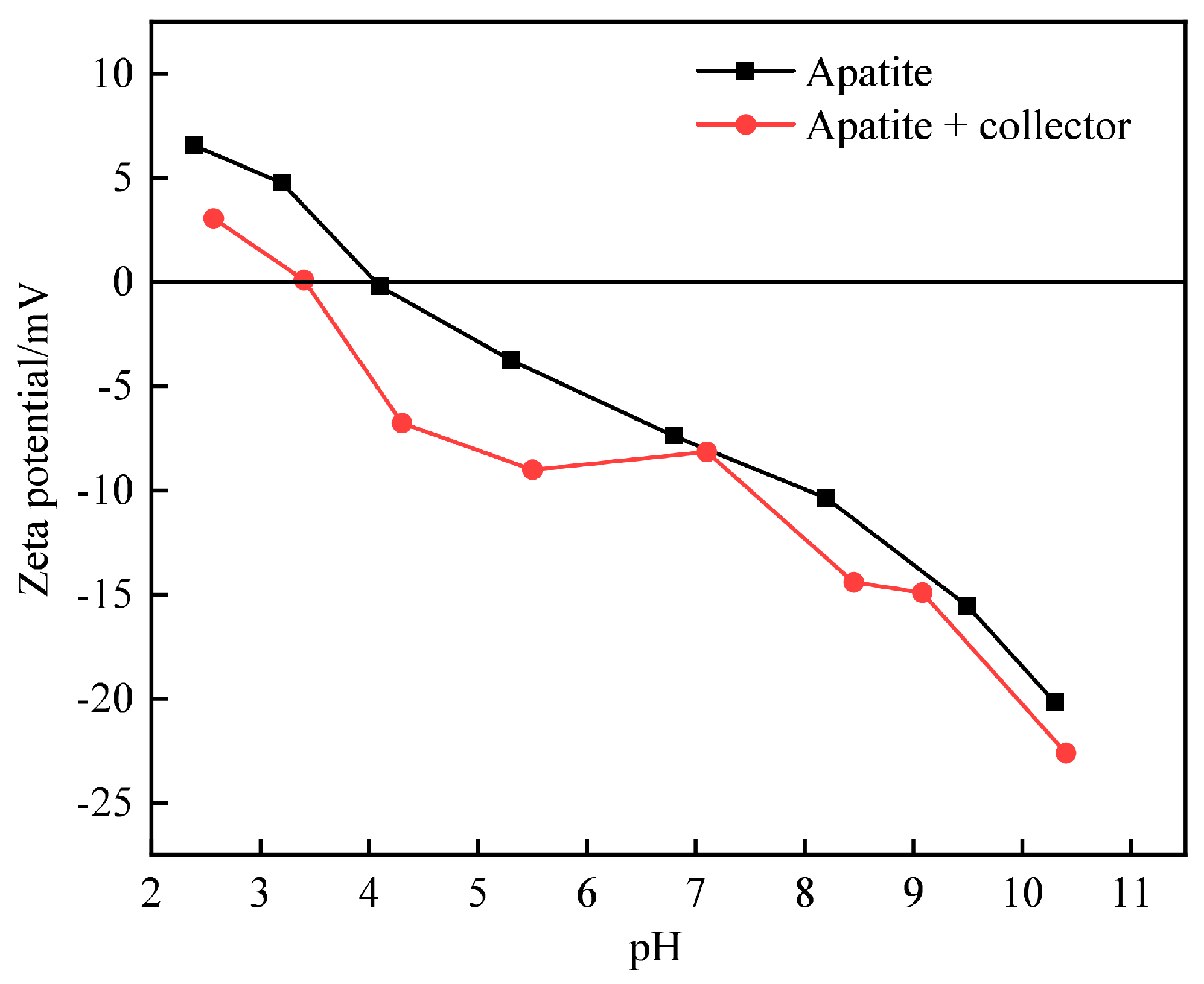
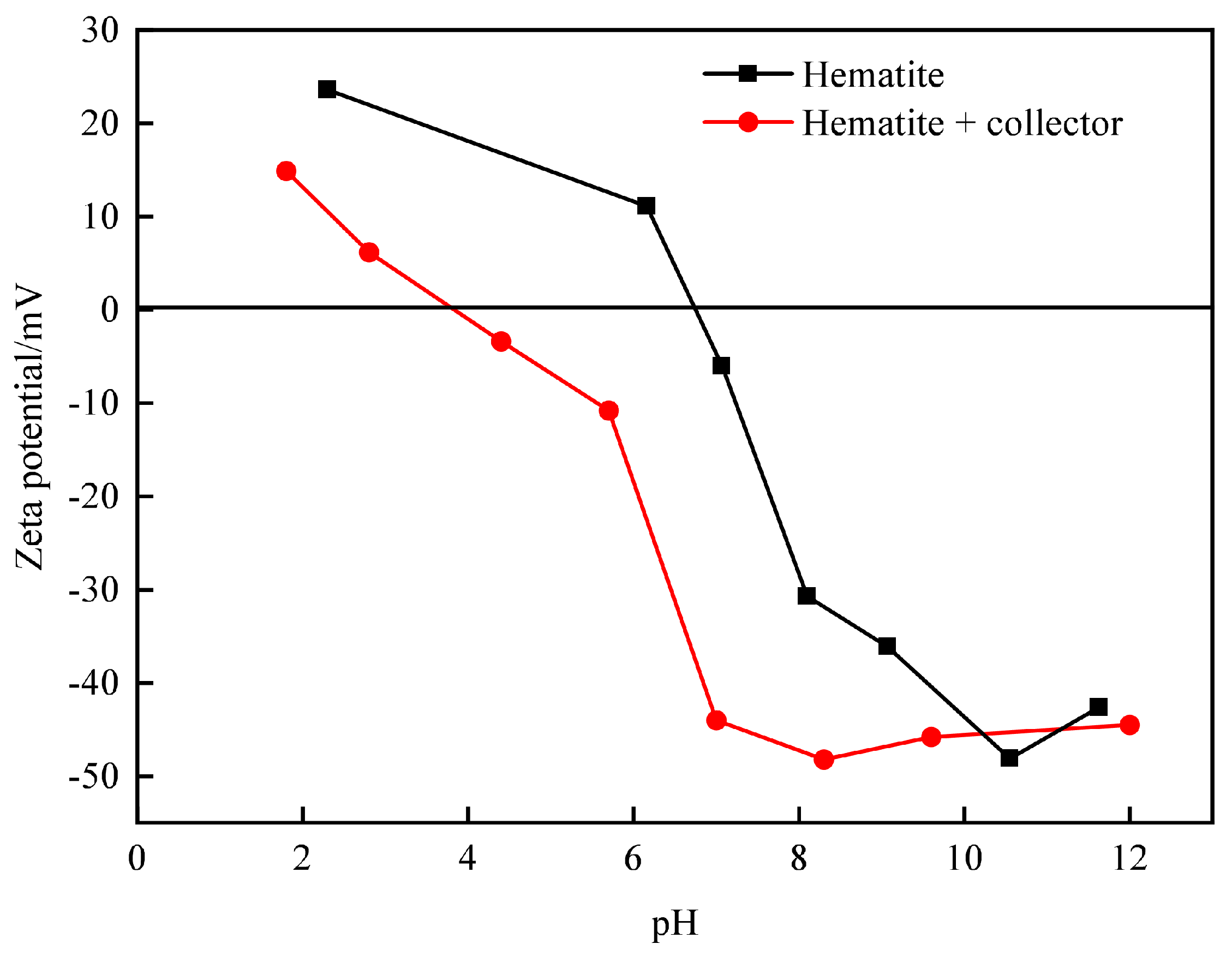
3.3.1. Influence of Floatation Reagents on Zeta Potential of Mineral Surface
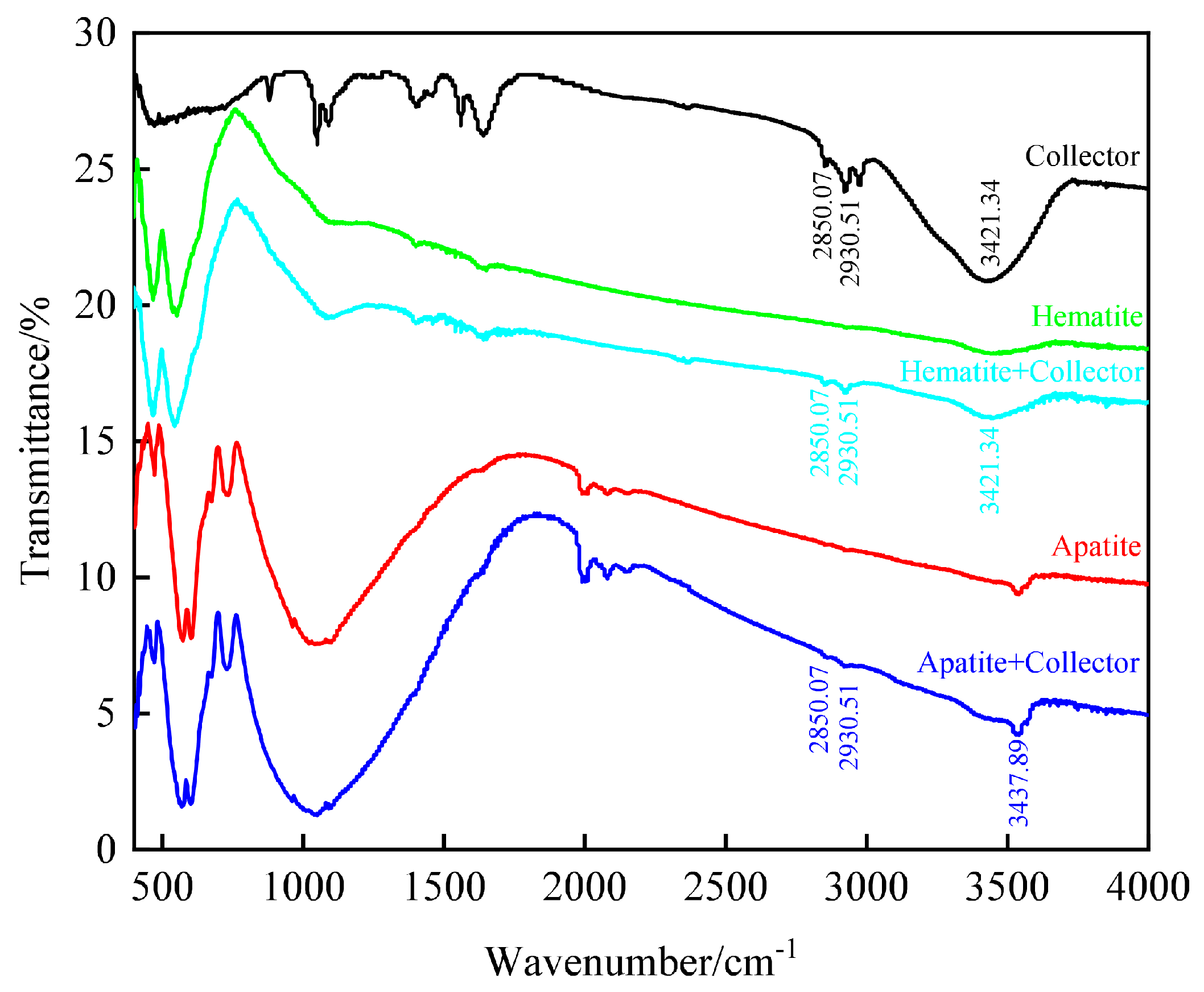
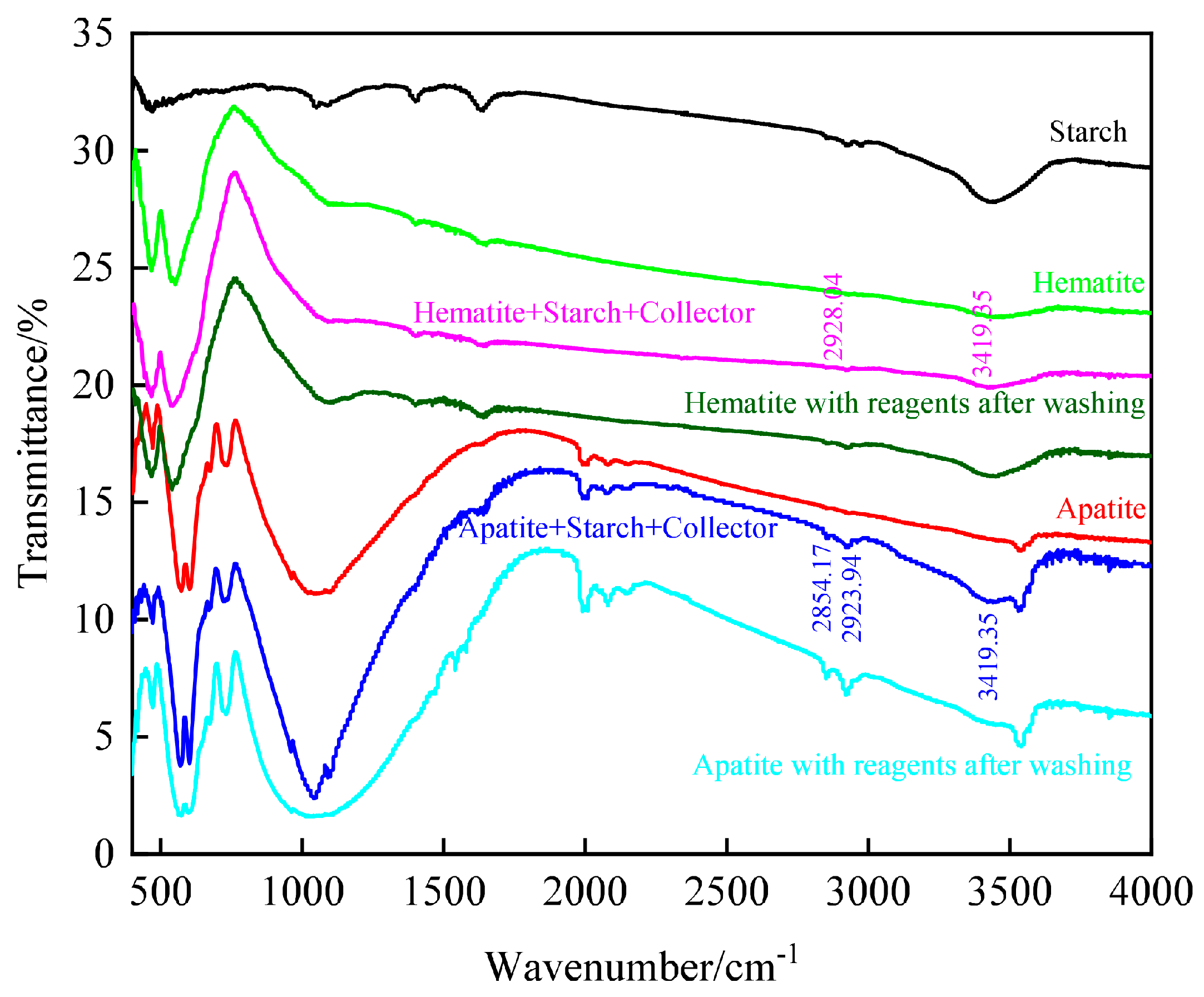
3.3.2. Adsorption Characteristics of Flotation Reagents on Mineral Surface
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Champetier, Y.; Hamdadou, E.; Hamdadou, M. Examples of biogenic support of mineralization in two oolitic iron ores Lorraine (France) and Gara Djebilet (Algeria). Sediment. Geol. 1987, 51, 249–255. [Google Scholar] [CrossRef]
- Abro, M.M.; Pathan, A.G.; Mallah, A.H. Liberation of oolitic hematite grains from iron ore, Dilband Mines Pakistan. Mehran Univ. Res. J. Eng. Technol. 2011, 30, 329–338. [Google Scholar]
- Adedeji, F.A.; Sale, F.R. Characterization and reducibility of Itakpe and Agbaja (Nigerian) iron ores. Clay Miner. 1984, 19, 843–856. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Deutsch, E.R. Magnetic properties of oolitic iron ore on Bell Island, Newfoundland. Earth Planet. Sci. Lett. 1984, 69, 427–441. [Google Scholar] [CrossRef]
- Li, K.Q.; Ni, W.; Zhu, M.; Zheng, M.J.; Li, Y. Iron extraction from oolitic iron ore by a deep reduction process. J. Iron Steel Res. Int. 2011, 18, 9–13. [Google Scholar] [CrossRef]
- Tang, H.Q.; Guo, Z.C.; Zhao, Z.L. Phosphorus removal of high phosphorus iron ore by gas-based reduction and melt separation. J. Iron Steel Res. Int. 2010, 17, 1–6. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, T.C.; Zou, A.H.; Xu, C.Y. Effect of coal levels during direct reduction roasting of high phosphorus oolitic hematite ore in a tunnel kiln. Int. J. Min. Sci. Technol. 2012, 22, 323–328. [Google Scholar] [CrossRef]
- Zhou, W.T.; Han, Y.X.; Sun, Y.S.; Li, Y.J. Strengthening iron enrichment and dephosphorization of high-phosphorus oolitic hematite using high-temperature pretreatment. Int. J. Miner. Metall. Mater. 2020, 27, 443–453. [Google Scholar] [CrossRef]
- Li, W.B.; Liu, D.Q.; Han, Y.X.; Li, Y.J.; Guo, R.N. An innovative study for pretreatment of high-phosphorus oolitic hematite via high-temperature heating: Phase, microstructure, and phosphorus distribution analyses. Adv. Powder Technol. 2023, 34, 103996. [Google Scholar] [CrossRef]
- Delvasto, P.; Valverde, A.; Ballester, A.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Igual, J.M.; García-Balboa, C. Diversity and activity of phosphate bioleaching bacteria from a high-phosphorus iron ore. Hydrometallurgy 2008, 92, 124–129. [Google Scholar] [CrossRef]
- Delvasto, P.; Ballester, A.; Muñoz, J.A.; González, F.; Blázquez, M.L.; Igual, J.M.; Valverde, A.; García-Balboa, C. Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Miner. Eng. 2009, 22, 1–9. [Google Scholar] [CrossRef]
- Wang, J.C.; Shen, S.B.; Kang, J.H.; Li, H.X.; Guo, Z.C. Effect of ore solid concentration on the bioleaching of phosphorus from high-phosphorus iron ores using indigenous sulfur-oxidizing bacteria from municipal wastewater. Process Biochem. 2010, 45, 1624–1631. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Misra, V.N.; Clough, J.; Muni, R. Dephosphorisation of western Australian iron ore by hydrometallurgical process. Miner. Eng. 1999, 12, 1083–1092. [Google Scholar] [CrossRef]
- Xia, W.T.; Ren, Z.D.; Gao, Y.F. Removal of phosphorus from high phosphorus iron ores by selective HCl leaching method. J. Iron Steel Res. Int. 2011, 18, 1–4. [Google Scholar] [CrossRef]
- Pan, J.; Lu, S.H.; Li, S.W.; Zhu, D.Q.; Guo, Z.Q.; Shi, Y.; Dong, T. A New Route to Upgrading the High-Phosphorus Oolitic Hematite Ore by Sodium Magnetization Roasting-Magnetic Separation-Acid and Alkaline Leaching Process. Minerals 2022, 12, 568. [Google Scholar] [CrossRef]
- Yang, C.C.; Zhu, D.Q.; Pan, J.; Lu, L.M. Simultaneous recovery of iron and phosphorus from a high-phosphorus oolitic iron ore to prepare Fe-P alloy for high-phosphorus steel production. JOM 2017, 69, 1663–1668. [Google Scholar] [CrossRef]
- Zhou, W.T.; Lyu, X.J.; Sun, Y.S.; Yuan, S.; Liu, X.; Zhao, Y.Q. An innovative technology for utilization of oolitic hematite via microwave fluidization pretreatment: Separation characteristics and mechanism. J. Cent. South Univ. 2023, 30, 823–833. [Google Scholar] [CrossRef]
- Yehia, A.; Abd El-Halim, S.; Sharada, H.; Fadel, M.; Ammar, M. Application of a fungal cellulase as a green depressant of hematite in the reverse anionic flotation of a high-phosphorus iron ore. Miner. Eng. 2021, 167, 106903. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Pinto, C.L.L.; Valadão, G.E.S.; Viana, P.R.D.M. Floatability studies of wavellite and preliminary results on phosphorus removal from a Brazilian iron ore by froth flotation. Miner. Eng. 2012, 39, 206–212. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zhou, L.L. Increasing iron and reducing phosphorus grades of magnetic-roasted high-phosphorus oolitic iron ore by low-intensity magnetic separation–reverse flotation. Processes 2019, 7, 388. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.F.; Li, J.S.; Feng, J.; Wang, F. Dephosphorization by double-slag process in converter steelmaking. High Temp. Mater. Process. 2018, 37, 625–633. [Google Scholar] [CrossRef]
- Liu, L.W.; Li, G.F.; Li, Y.F.; Zhao, L.B. Dephosphorization behavior of reduced iron and the properties of high-P-containing slag. High Temp. Mater. Process. 2021, 40, 337–344. [Google Scholar] [CrossRef]
- Lin, W.H.; Jiao, S.Q.; Zhou, K.X.; Sun, J.K.; Feng, X.M.; Liu, Q. A review of multi-phase slag refining for dephosphorization in the steelmaking process. Front. Mater. 2020, 7, 602522. [Google Scholar] [CrossRef]
- Xiao, M.; Qiu, X.Y.; Lan, Q.F. Study on the new technology of reduction roasting, magnetic separation and melting of high phosphorus oolitic hematite. Multipurp. Util. Miner. Resour. 2020, 5, 87–91. [Google Scholar]
- Zhou, J.D.; Bi, X.G.; Wu, J.; Huang, Z.C.; Wen, Z.J.; Xiong, W.; Li, Y.B.; Jin, Y. Experimental study on rational technology of dephosphorization pretreatment for high phosphorus content hot iron. Chin. J. Process Eng. 2011, 11, 50–55. [Google Scholar]
- Yoshida, K.; Yamazaki, I.; Tozaki, Y.; Aoki, N.; Yoshiyama, J.I.; Arai, K. Development of effective refining process consisting of both hot metal pretreatment and decarburization in two top and bottom blown converters. Tetsu-to-Hagané 1990, 76, 1817–1822. [Google Scholar] [CrossRef] [PubMed]
- Ina, M. Metallurgical characteristics of LD type hot metal pretreatment. CAMP-ISIJ 1991, 4, 1154. [Google Scholar]
- Wakamatsu, S. Dephosphorization at hot metal pretreatment in a BOF vessel. Curr. Adv. Mater. Process. 1996, 9, 864. [Google Scholar]
- Su, F.W.; Rao, K.H.; Forssberg, K.S.E.; Samskog, P.O. The influence of temperature on the kinetics of apatite flotation from magnetite fines. Int. J. Miner. Process. 1998, 54, 131–145. [Google Scholar] [CrossRef]
- Yu, K.P.; Yu, Y.F.; Xu, X.Y. Separation behavior and mechanism of hematite and collophane in the presence of collector RFP-138. Trans. Nonferr. Met. Soc. China 2013, 23, 501–507. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Gong, W.Q.; Yan, W. Application of a novel cationic collector in reverse flotation beneficiation of oolitic hematite ore. Int. Symp. Proj. Manag. 2010, 25. [Google Scholar]
- Ji, J. Study on dephosphorization technology for high-phosphorus iron ore. Min. Metall. 2003, 12, 33–37. [Google Scholar]
- Guo, W.; Sun, Y.S.; Yang, Y.H. Research progress of dephosphorization of high phosphorus oolitic hematite. Multipurp. Util. Miner. Resour. 2014, 6, 15–19. [Google Scholar]
- Yang, Y.H.; Zhang, Y.S.; Liu, Y.C. Research on Iron-increasing and impurities-reducing of high phosphorus oolitic hematite ore in east Chongqing. Multipurp. Util. Miner. Resour. 2013, 6, 22–25. [Google Scholar]
- Weissenborn, P.K.; Warren, L.J.; Dunn, J.G. Optimisation of selective flocculation of ultrafine iron ore. Int. J. Miner. Process. 1994, 42, 191–213. [Google Scholar] [CrossRef]
- Lima, N.P.; Silva, K.; Souza, T.; Filippov, L. The Characteristics of Iron Ore Slimes and Their Influence on The Flotation Process. Minerals 2020, 10, 675. [Google Scholar] [CrossRef]
- Wang, M.T.; Zhang, G.F.; Chen, Y.F.; Zhao, L. Effect of surface oxidization on quartz slime coating in the sulfidization-amine flotation of smithsonite. Miner. Eng. 2022, 188, 107847. [Google Scholar] [CrossRef]
- Jia, W.H.; Jiang, F.; Zhu, H.L.; Xu, L.; Qin, W.Q. Mitigating the negative effects of feldspar slime on spodumene flotation using mixed anionic/cationic collector. Miner. Eng. 2021, 168, 106813. [Google Scholar] [CrossRef]
- Suman, S.K.; Kumar, S. Reverse flotation studies on iron ore slime by the synergistic effect of cationic collectors. Sep. Sci. Technol. 2020, 55, 1702–1714. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Araujo, A.C.; Viana, P.R.M.; Peres, A.E.C. Reagents in iron ores flotation. Miner. Eng. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Sun, N.; Wang, G.D.; Ge, P.; Sun, W.; Xu, L.H.; Tang, H.H.; Wang, L. Selective flotation of quartz from feldspar using hydroxypropyl starch as depressant. Miner. Eng. 2023, 195, 108022. [Google Scholar] [CrossRef]
- Cheng, Q.; Mei, G.J.; Xu, W.; Yuan, Q.Z. Flotation of quartz using imidazole ionic liquid collectors with different counterions. Miner. Eng. 2022, 180, 107491. [Google Scholar] [CrossRef]
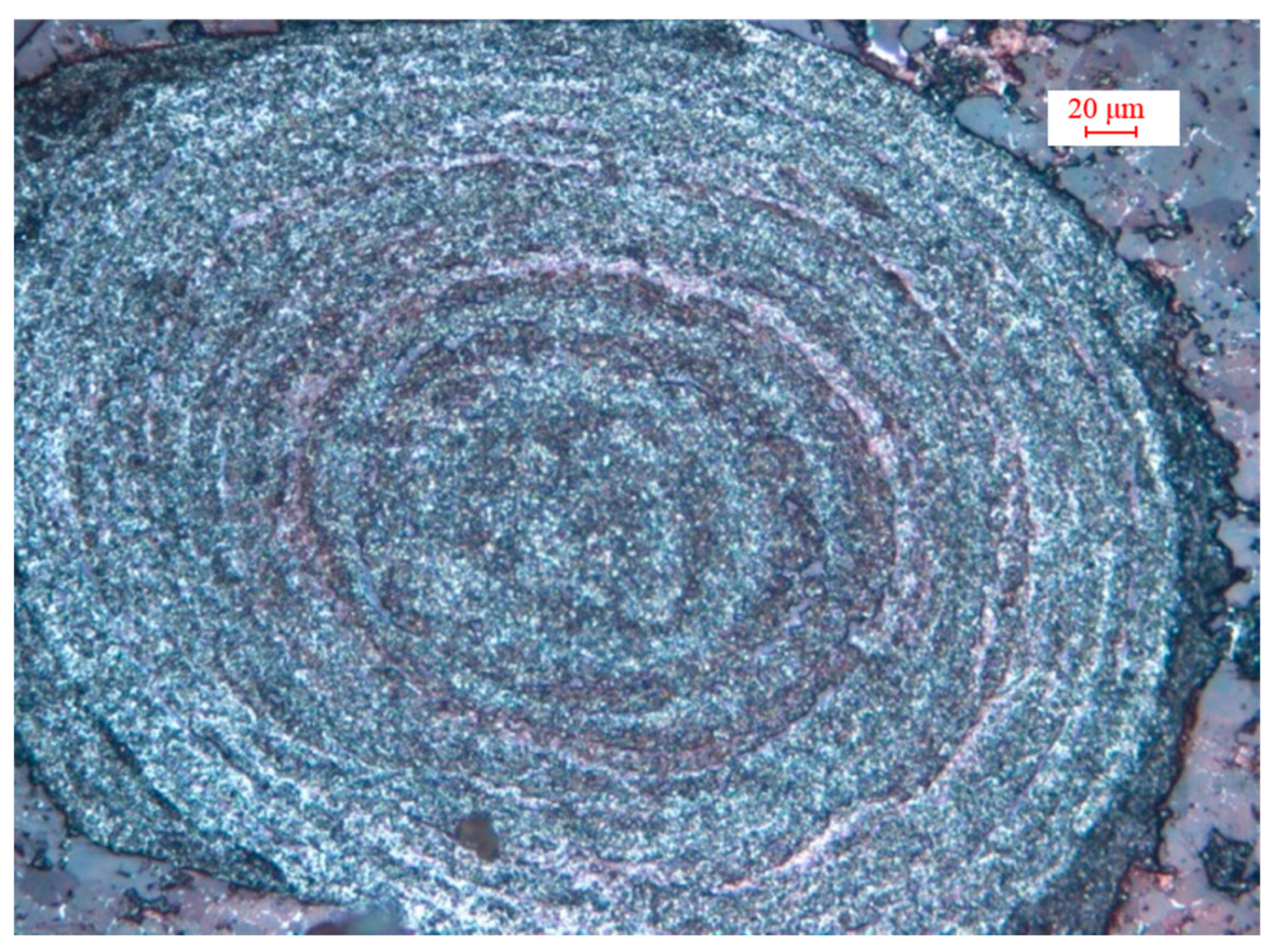

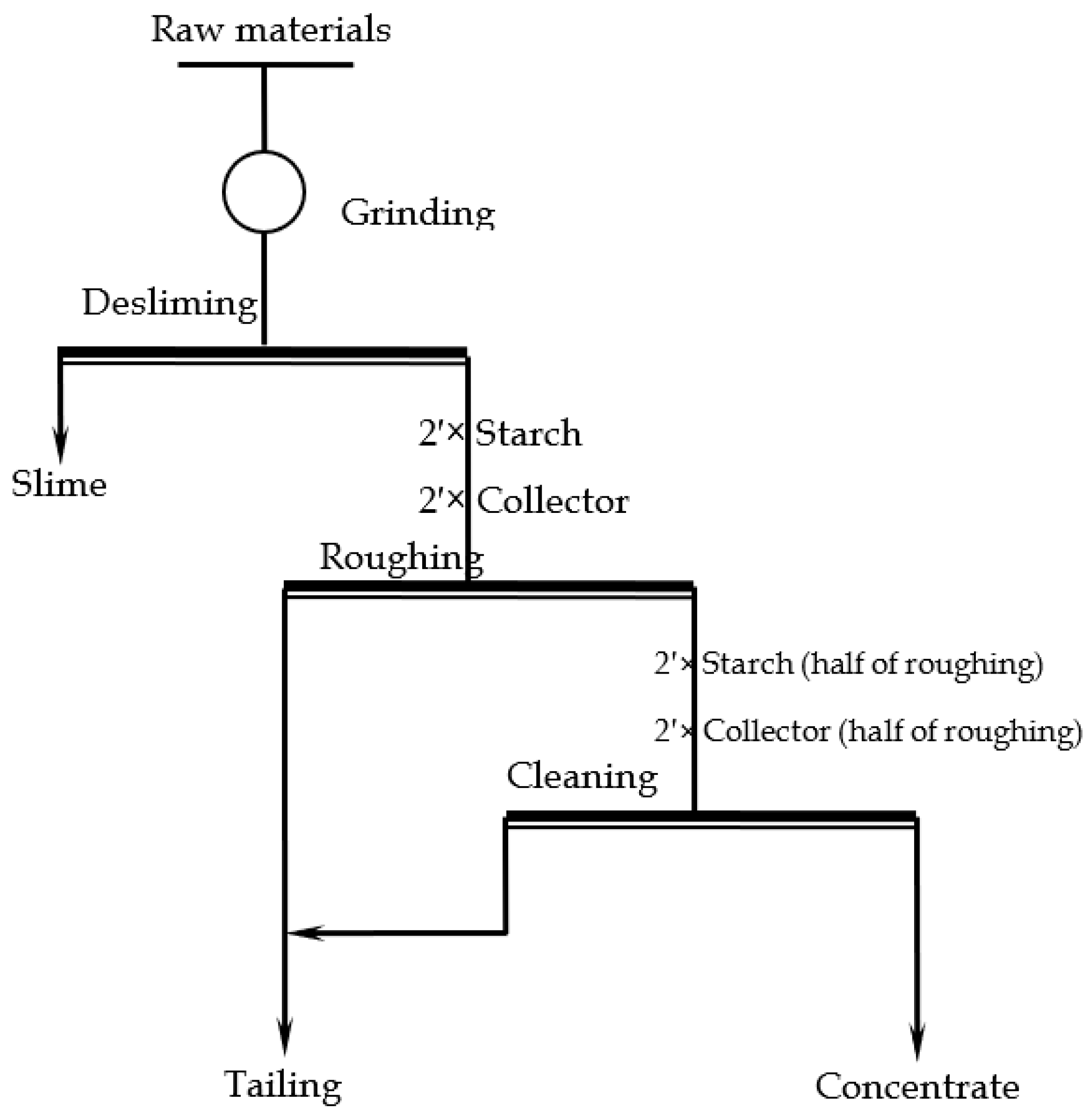
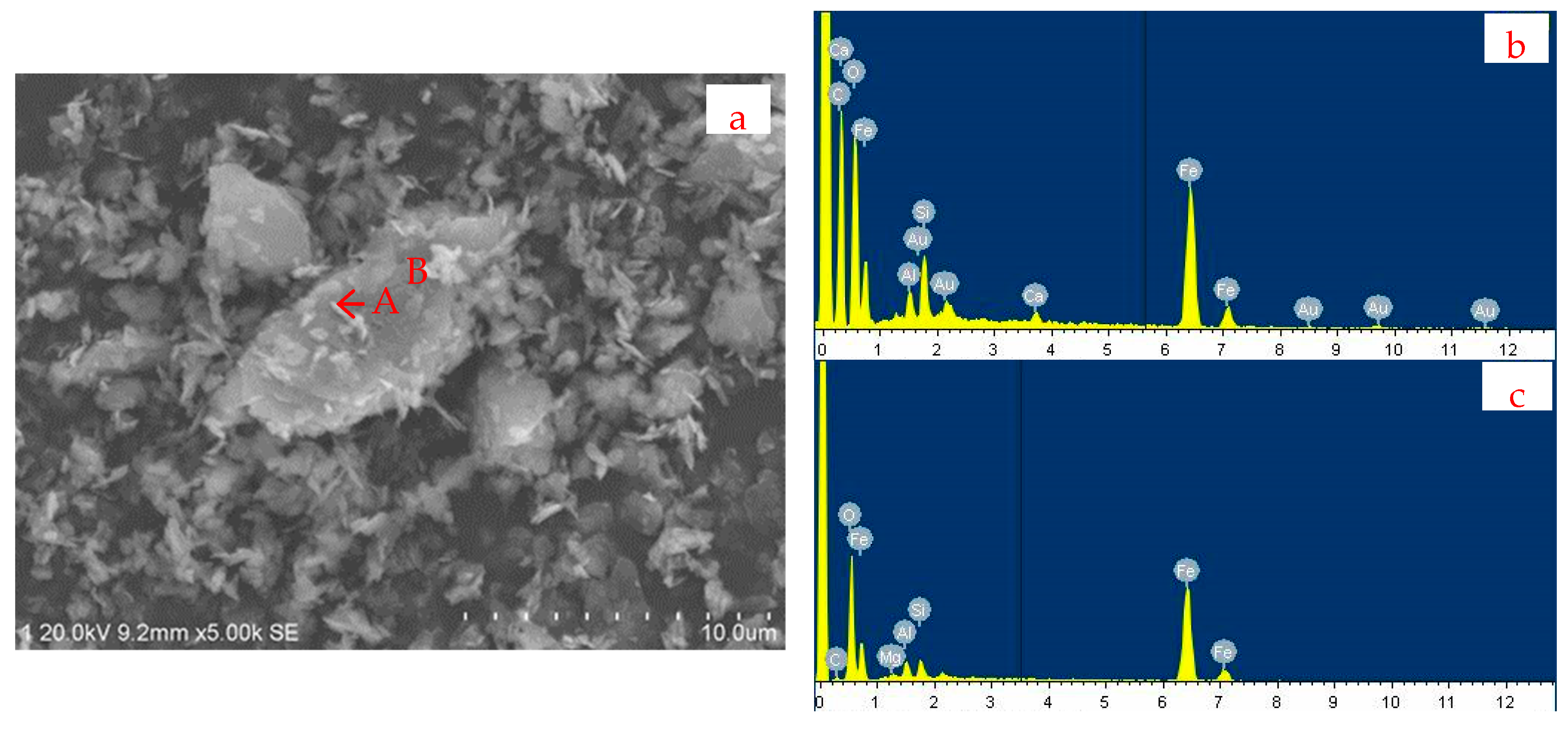

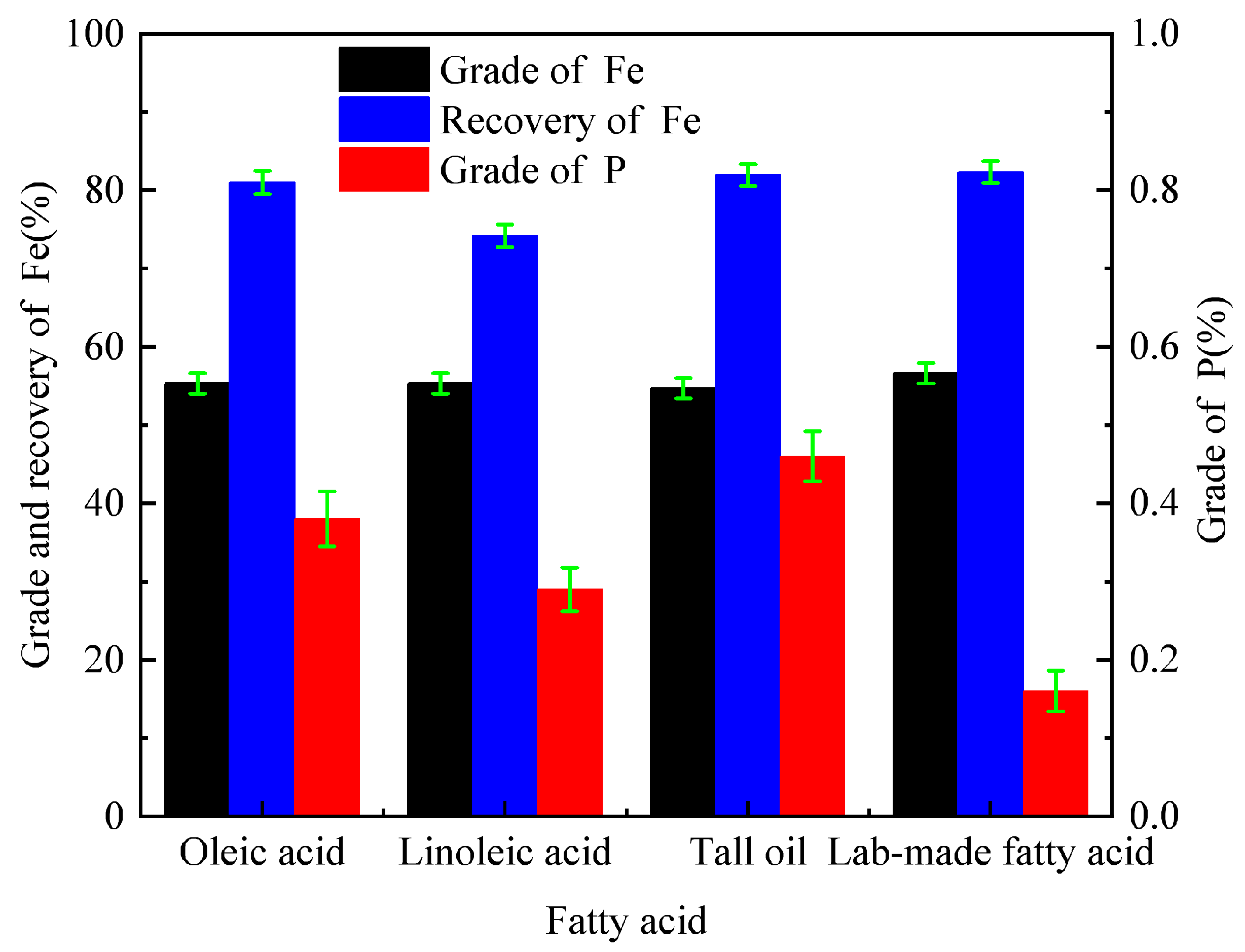

| Fetotal | FeO | Fe2O3 | P | S | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|
| 48.80 | 1.70 | 67.83 | 1.32 | 0.028 | 15.22 | 4.77 | 2.58 | 0.54 | 0.25 | 0.085 |
| Iron Phase | Fe in Magnetite | Fe in Siderite | Fe in Hematite or Limonite | Fe in Iron Pyrite | Fe in Iron Metasilicate | Fe in Iron Sulfate | Total Fe |
|---|---|---|---|---|---|---|---|
| Content | 0.24 | 0.54 | 44.45 | 0.06 | 3.52 | 0.11 | 48.92 |
| Distribution | 0.49 | 1.10 | 90.86 | 0.12 | 7.20 | 0.22 | 100.00 |
| Products | Yield | Grade | Recovery | ||
|---|---|---|---|---|---|
| Fetotal | P | Fetotal | P | ||
| Desilting | 89.71 | 49.10 | 1.340 | 89.75 | 89.99 |
| Slime | 10.29 | 48.92 | 1.300 | 10.25 | 10.01 |
| Feeding | 100.00 | 49.08 | 1.336 | 100.00 | 100.00 |
| Minerals | Quartz | Collophanite Aggregate | Hematite Limonite Aggregate | Silicate Mineral Aggregate | Sulfide | Carbonate | Manganese Oxide | Total |
|---|---|---|---|---|---|---|---|---|
| Contents | 4.09 | 80.44 | 10.39 | 2.13 | 0.04 | 2.88 | 0.02 | 100.00 |
| Fetotal | P | S | SiO2 | Al2O3 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| 58.86 | 0.24 | 0.03 | 6.29 | 4.39 | 0.95 | 0.54 | 0.17 | 0.05 |
| Iron Phase | Fe in Magnetite | Fe in Siderite | Fe in Hematite or Limonite | Fe in Iron Metasilicate | Fe in Iron Sulfate | Total Fe |
|---|---|---|---|---|---|---|
| Content | 0.31 | 0.45 | 57.89 | 0.096 | 0.067 | 58.81 |
| Distribution | 0.53 | 0.77 | 98.43 | 0.16 | 0.11 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhang, Y.; Zou, K.; Zhang, F. Flotation Dephosphorization of High-Phosphorus Oolitic Ore. Minerals 2023, 13, 1485. https://doi.org/10.3390/min13121485
Chen C, Zhang Y, Zou K, Zhang F. Flotation Dephosphorization of High-Phosphorus Oolitic Ore. Minerals. 2023; 13(12):1485. https://doi.org/10.3390/min13121485
Chicago/Turabian StyleChen, Chao, Yushu Zhang, Kai Zou, and Feilong Zhang. 2023. "Flotation Dephosphorization of High-Phosphorus Oolitic Ore" Minerals 13, no. 12: 1485. https://doi.org/10.3390/min13121485




