Application of Quantum Chemistry in the Study of Flotation Reagents
Abstract
:1. Introduction
1.1. Classification of Flotation Reagents
1.2. Early Exploration of the Structure–Property Relations of Flotation Reagents
1.3. Development of Quantum Chemistry in Mineral Flotation
1.4. Experimental Methods Alongside Theoretical Calculations
1.5. Classification of Mineral–Reagent Interactions
2. Non-Ferrous Metals
2.1. Non-Ferrous Oxide Minerals
2.1.1. Copper Oxide Minerals
2.1.2. Lead Oxide Minerals
2.1.3. Zinc Oxide Minerals
2.1.4. Other Non-Ferrous Oxide Minerals
2.2. Non-Ferrous Sulfide Minerals
2.2.1. Copper Sulfide Minerals
2.2.2. Lead Sulfide Minerals
2.2.3. Zinc Sulfide Minerals
2.2.4. Other Non-Ferrous Sulfide Minerals
3. Ferrous Metals
3.1. Iron Oxide Minerals
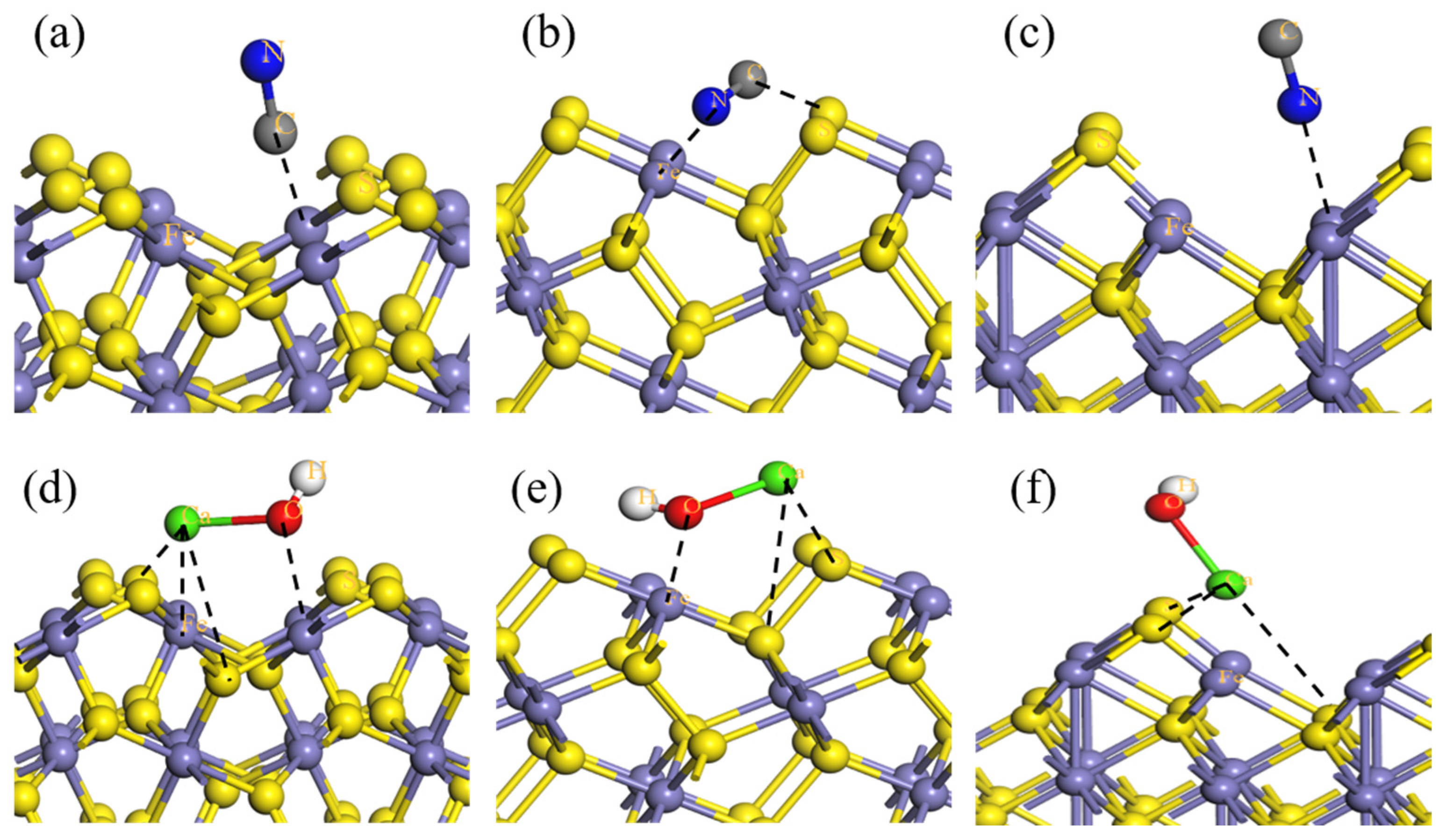
3.2. Iron Sulfide Minerals
3.3. Manganese Oxide Minerals
4. Silicate Minerals
4.1. Quartz
4.2. Kaolinite
4.3. Other Silicate Minerals
5. Flotation of Tungsten and Tin
5.1. Wolframite
5.2. Scheelite
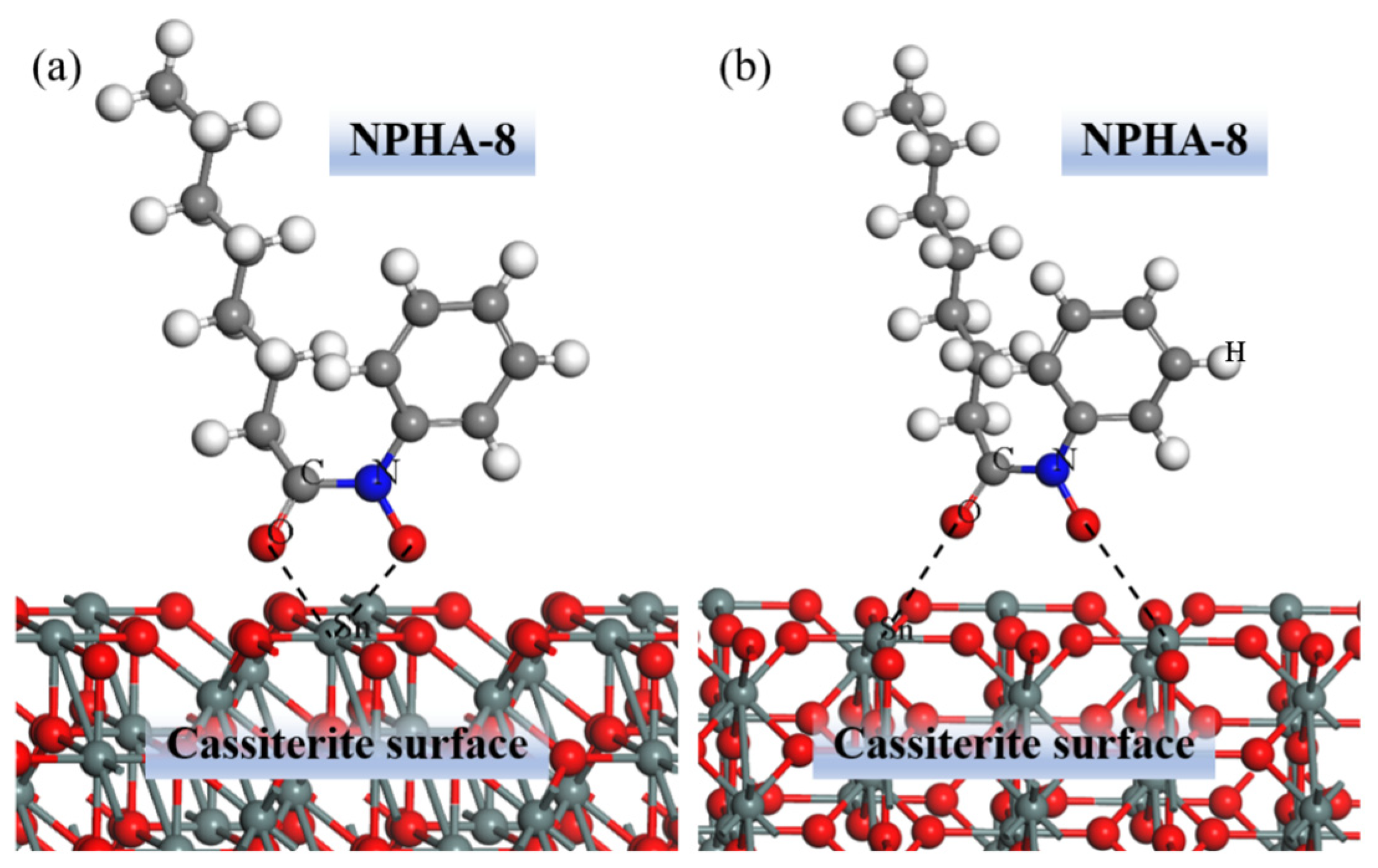
5.3. Cassiterite
6. Calcium-Bearing Minerals
6.1. Fluorite
6.2. Other Calcium-Bearing Minerals
7. Rare Earth Minerals
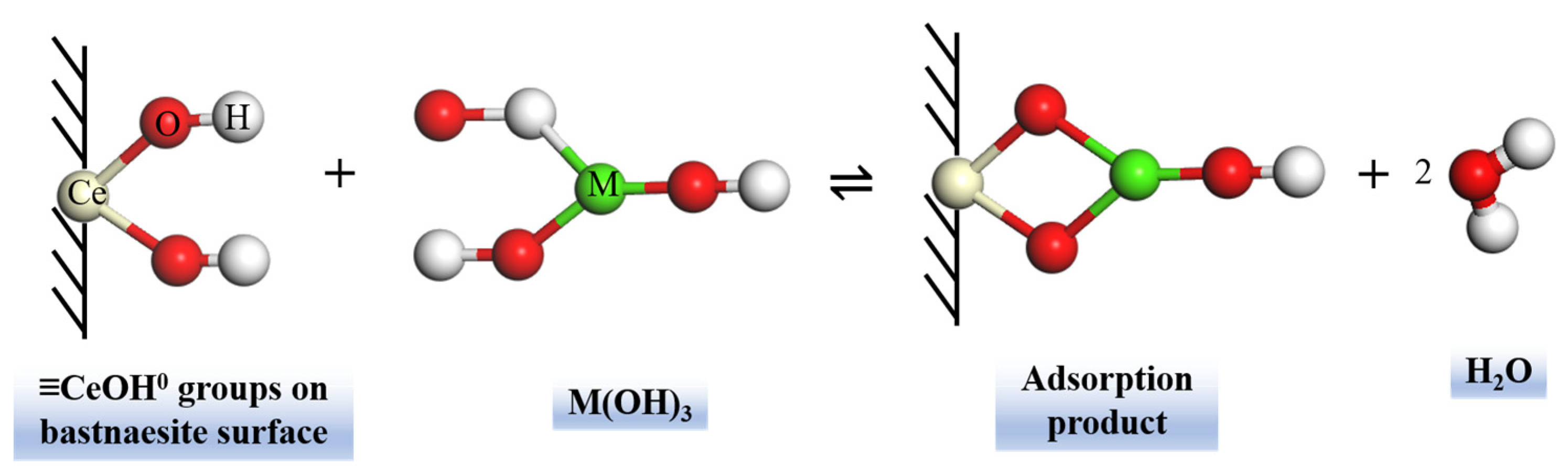
7.1. Bastnaesite
7.2. Other Rare Earth Minerals
8. Other Minerals
8.1. Lithium-Bearing Minerals
8.2. Molybdenum-Bearing Minerals
8.3. Arsenic-Bearing Minerals
8.4. Phosphorus-Bearing Minerals
8.5. Minerals Containing Precious Metals
9. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, W.S.; Asmatulu, E.; Uddin, M.N.; Asmatulu, R. 3—Wet and dry recyclingprocesses. In Recycling and Reusing of Engineering Materials; Khan, W.S., Asmatulu, E., Uddin, M.N., Asmatulu, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–68. [Google Scholar] [CrossRef]
- Duan, F.; Zhu, Y.; Mu, B.; Wang, A. Recent progress and future prospects on aqueous foams stabilized based on clay minerals. Appl. Clay Sci. 2023, 236, 106885. [Google Scholar] [CrossRef]
- Corin, K.; Wiese, J. Investigating froth stability: A comparative study of ionic strength and frother dosage. Miner. Eng. 2014, 66, 130–134. [Google Scholar] [CrossRef]
- Farrokhpay, S. The significance of froth stability in mineral flotation—A review. Adv. Colloid Interface Sci. 2011, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Manono, M.; Corin, K.; Wiese, J. The effect of ionic strength of plant water on foam stability: A 2-phase flotation study. Miner. Eng. 2013, 40, 42–47. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C. Considering specific ion effects on froth stability in sulfidic Cu-Ni-PGM Ore flotation. Minerals 2022, 12, 321. [Google Scholar] [CrossRef]
- Pawlik, M. Fundamentals of froth flotation. ChemTexts 2022, 8, 19. [Google Scholar] [CrossRef]
- Wills, B.A.; Napier-Munn, T. An introduction to the practical aspects of ore treatment and mineral recovery. In Wills’ Mineral Processing Technology; Butterworth-Heinemann: Oxford, UK, 2006; pp. 267–352. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Lu, Y.; Ding, R.; Li, G.; Song, X.; Cao, Y.; Jia, K. Research Progress with Scheelite Flotation Reagents: A Review. Minerals 2023, 13, 1257. [Google Scholar] [CrossRef]
- Song, Z.; Wen, S.; Han, G.; Feng, Q. Recent Progress on Chelating Reagents in Flotation of Zinc Oxide Ores: A Review. Minerals 2023, 13, 1278. [Google Scholar] [CrossRef]
- Taggart, A.; Taylor, T.; Ince, C. Flotation Practice; AIME: New York, NY, USA, 1928; Volume 285. [Google Scholar]
- Marabini, A.M.; Barbaro, M.; Alesse, V. New reagents in sulphide mineral flotation. Int. J. Miner. Process. 1991, 33, 291–306. [Google Scholar] [CrossRef]
- Zhong, X. Structure and performance of flotation reagents—The electronegativity calculation method of the performance of flotation reagents. Nonferrous Met. Extr. Metall. 1975, 4, 44–51+59. (In Chinese) [Google Scholar]
- Zhong, X. Structure and performance of flotation reagents—The CMC calculation method of the performance of flotation reagents. Nonferrous Met. Extr. Metall. 1977, 6, 25–28+65. (In Chinese) [Google Scholar]
- Zhong, X. Structure and performance of flotation reagents—The HLB calculation method of flotation reagents. Nonferrous Met. Extr. Metall. 1977, 7, 36–40. (In Chinese) [Google Scholar]
- Zhong, X. Structure and performance of flotation reagents—The isotonic volume calculation method for the performance of frothers. Nonferrous Met. Extr. Metall. 1977, 8, 42–44+35. (In Chinese) [Google Scholar]
- Zhong, X. Structure and performance of flotation reagents—The molecular geometry and selectivity of flotation reagents. Nonferrous Met. Extr. Metall. 1977, 10, 13–20. (In Chinese) [Google Scholar]
- Wang, D. Structure and performance of flotation reagents—Molecular design of one hundred S-containing organic flotation reagents. Nonferrous Met. Miner. Process. Sect. 1979, 2, 12–26. (In Chinese) [Google Scholar]
- Wang, D. Discussion of the regularity of collection effect of collectors in flotation reagents. Hunan Metall. 1982, 2, 40–48+33. (In Chinese) [Google Scholar]
- Israelachvili, J.N.; Adams, G. Direct measurement of long range forces between two mica surfaces in aqueous KNO3 solutions. Nature 1976, 262, 774–776. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, W.; Xue, Y.; Jiang, Y.; He, B. Application of number of electronic transfer in chemical reaction to study of structure-reactivity relation of flotation reagents. Nonferrous Met. 2001, 53, 19–22. (In Chinese) [Google Scholar]
- Trinajstić, N. New developments in Hückel theory. Int. J. Quantum Chem. 1977, 12, 469–477. [Google Scholar] [CrossRef]
- Wang, D. Foreign application of molecular orbital method to study the theory of flotation reagents. Nonferrous Met. Extr. Metall. 1978, 8, 32–41. (In Chinese) [Google Scholar]
- Chen, J.; Feng, Q.; Lu, Y. Study of flotation reagents using quantum chemical method. Nonferrous Met. 1999, 51, 18–21. (In Chinese) [Google Scholar]
- Chen, J.; Feng, Q.; Lu, Y. Design of functional groups of flotation reagent. Nonferrous Met. 1999, 2, 19–23+18. (In Chinese) [Google Scholar]
- Chen, J.; Feng, Q.; Lu, Y. Calculation for energy of interaction of flotation reagent with mineral surface. Chin. J. Nonferrous Met. 1999, 2, 143–149. (In Chinese) [Google Scholar]
- Wang, J.; Yin, W. Application of acid-base potential scale in structure performance study of flotation reagents. J. Northeast. Univ. Nat. Sci. 2013, 34, 1035–1038+1056. (In Chinese) [Google Scholar]
- Gorelsky, S.I. Ab initio and Semiempirical Methods. In Encyclopedia of Inorganic and Bioinorganic Chemistry; University of Ottawa: Ottawa, ON, Canada, 2012. [Google Scholar] [CrossRef]
- Künne, L. Recent Developments and Applications of Modern Density Functional Theory; University of New Orleans: New Orleans, LA, USA, 1998. [Google Scholar]
- Bhargava, R.N.; Gallagher, D.; Hong, X.; Nurmikko, A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 1994, 72, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chen, Y.; Li, Y.-Q. Quantum-mechanical study of effect of lattice defects on surface properties and copper activation of sphalerite surface. Trans. Nonferrous Met. Soc. China 2010, 20, 1121–1130. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chen, Y.; Li, Y.-Q. Effect of vacancy defects on electronic properties and activation of sphalerite (110) surface by first-principles. Trans. Nonferrous Met. Soc. China 2010, 20, 502–506. [Google Scholar] [CrossRef]
- Chen, J.-H.; Long, X.-H.; Chen, Y. Comparison of Multilayer Water Adsorption on the Hydrophobic Galena (PbS) and Hydrophilic Pyrite (FeS2) Surfaces: A DFT Study. J. Phys. Chem. C 2014, 118, 11657–11665. [Google Scholar] [CrossRef]
- Deng, J.; Lai, H.; Chen, M.; Glen, M.; Wen, S.; Zhao, B.; Liu, Z.; Yang, H.; Liu, M.; Huang, L.; et al. Effect of iron concentration on the crystallization and electronic structure of sphalerite/marmatite: A DFT study. Miner. Eng. 2019, 136, 168–174. [Google Scholar] [CrossRef]
- Ke, B.; Li, Y.; Chen, J.; Zhao, C.; Chen, Y. DFT study on the galvanic interaction between pyrite (100) and galena (100) surfaces. Appl. Surf. Sci. 2016, 367, 270–276. [Google Scholar] [CrossRef]
- October, L.; Manono, M.S.; Corin, K.C.; Schreithofer, N.; Wiese, J.G. The Influence of Specific Ions and Oxyhydroxo Species in Plant Water on the Bubble–Particle Attachment of Pyrrhotite. ACS Omega 2021, 6, 28496–28506. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Ye, G.; Zuo, Q. A DFT-based method to determine the hydrophobicity change mechanism on sphalerite and pyrite surfaces caused by sodium dithionite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127339. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Meng, X.; Ou, P.; Lv, X.; Zhang, L.; Liu, L.; Chen, F.; Qiu, G. Mineralogical phase transformation of Fe containing sphalerite at acidic environments in the presence of Cu2+. J. Hazard. Mater. 2021, 403, 124058. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yao, J.; Yin, W.; Kelebek, S. Molecular dynamics simulation of cetyl phosphate adsorption in flotation of magnesite and pertinent chemical aspects. Minerals 2020, 10, 761. [Google Scholar] [CrossRef]
- Di, Y.; Jiang, A.; Huang, H.; Deng, L.; Zhang, D.; Deng, W.; Wang, R.; Luo, Q.; Chen, S. Molecular Dynamics Simulation Study on the Interactions of Mixed Cationic/Anionic Collectors on Muscovite (001) Surface in Aqueous Solution. Materials 2022, 15, 3816. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, W.; Kelebek, S. Molecular dynamics simulation of magnesite and dolomite in relation to flotation with cetyl phosphate. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125928. [Google Scholar] [CrossRef]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- Paquet, E.; Viktor, H.L. Computational Methods for Ab Initio Molecular Dynamics. Adv. Chem. 2018, 2018, 9839641. [Google Scholar] [CrossRef]
- Mo, P.; Li, C.; Zhao, D.; Zhang, Y.; Shi, M.; Li, J.; Liu, J. Accurate and efficient molecular dynamics based on machine learning and non von Neumann architecture. Npj Comput. Mater. 2022, 8, 107. [Google Scholar] [CrossRef]
- Foucaud, Y.; Badawi, M.; Filippov, L.; Filippova, I.; Lebègue, S. A review of atomistic simulation methods for surface physical-chemistry phenomena applied to froth flotation. Miner. Eng. 2019, 143, 106020. [Google Scholar] [CrossRef]
- Huang, K.; Huang, X.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation. Miner. Eng. 2019, 142, 105895. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Zeng, H. Understanding copper activation and xanthate adsorption on sphalerite by time-of-flight secondary ion mass spectrometry, X-ray photoelectron spectroscopy, and in situ scanning electrochemical microscopy. J. Phys. Chem. C 2013, 117, 20089–20097. [Google Scholar] [CrossRef]
- Lin, X.Y.; Creuzet, F.; Arribart, H. Atomic force microscopy for local characterization of surface acid-base properties. J. Phys. Chem. 1993, 97, 7272–7276. [Google Scholar] [CrossRef]
- Preuss, M.; Butt, H.-J. Measuring the contact angle of individual colloidal particles. J. Colloid Interface Sci. 1998, 208, 468–477. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Rong, G.; Cao, Y.; Liu, J. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Assemi, S.; Nguyen, A.V.; Miller, J.D. Direct measurement of particle–bubble interaction forces using atomic force microscopy. Int. J. Miner. Process. 2008, 89, 65–70. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.V.; Miller, J.D. Hydrophobic Attraction As Revealed by AFM Force Measurements and Molecular Dynamics Simulation. J. Phys. Chem. B 2005, 109, 13112–13118. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wang, J.; Lu, Q.; Hu, W.; Yang, D.; Qiao, C.; Peng, X.; Peng, Q.; Wang, T.; Sun, W.; et al. Surface interaction mechanisms in mineral flotation: Fundamentals, measurements, and perspectives. Adv. Colloid Interface Sci. 2021, 295, 102491. [Google Scholar] [CrossRef]
- Petit, S.; Madejova, J. Chapter 2.7-Fourier Transform Infrared Spectroscopy. In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 213–231. [Google Scholar]
- Shi, Q.; Zhang, G.; Feng, Q.; Deng, H. Effect of solution chemistry on the flotation system of smithsonite and calcite. Int. J. Miner. Process. 2013, 119, 34–39. [Google Scholar] [CrossRef]
- Chelgani, S.C.; Hart, B. TOF-SIMS studies of surface chemistry of minerals subjected to flotation separation–A review. Miner. Eng. 2014, 57, 1–11. [Google Scholar] [CrossRef]
- Wang, H.; Chu, P.K. Chapter 4-Surface Characterization of Biomaterials. In Characterization of Biomaterials; Bandyopadhyay, A., Bose, S., Eds.; Academic Press: Oxford, UK, 2013; pp. 105–174. [Google Scholar]
- Otsuki, A.; Zhao, Y. UV-Vis Study of Mixed Collector Adsorption on Pyrite towards the Better Understanding of the Adsorption Mechanism. Curr. Work. Miner. Process. 2018, 1, 13–20. [Google Scholar] [CrossRef]
- Mikhlin, Y. X-ray photoelectron spectroscopy in mineral processing studies. Appl. Sci. 2020, 10, 5138. [Google Scholar] [CrossRef]
- Fuerstenau, D. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. The Solide Physics of Sulphide Minerals Flotation; Central South University Press: Changsha, China, 2015. [Google Scholar]
- Chen, J.; Xu, Z.; Chen, Y. Electronic Structure and Surfaces of Sulfide Minerals: Density Functional Theory and Applications; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Yang, X.; Liu, S.; Liu, G.; Zhong, H. A DFT study on the structure–reactivity relation of aliphatic oxime derivatives as copper chelating agents and malachite flotation collectors. J. Ind. Eng. Chem. 2017, 46, 404–415. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Liu, G.; Liu, J.; Ma, L.; Niu, X.; Qu, X. A DFT prediction on the chemical reactivity of novel azolethione derivatives as chelating agents: Implications for copper minerals flotation and copper corrosion inhibition. J. Taiwan Inst. Chem. Eng. 2018, 93, 109–123. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Wang, S.; Cao, Z.; Ma, X.; Zhong, H. Structural modification of hydroxamic acid collectors to enhance the flotation performance of malachite and associated mechanism. J. Mol. Liq. 2021, 344, 117959. [Google Scholar] [CrossRef]
- Chen, D.; Liu, M.; Hu, B.; Dong, Y.; Xue, W.; He, P.; Chen, F.; Zhu, J.; Zhang, C. New insights into the promotion mechanism of (NH_4)_2SO_4 in sulfidization flotation: A combined experimental and computational study. Physicochem. Probl. Miner. Process. 2021, 57, 57–70. [Google Scholar]
- Fuerstenau, M.; Olivas, S.; Herrera-Urbina, R.; Han, K. The surface characteristics and flotation behavior of anglesite and cerussite. Int. J. Miner. Process. 1987, 20, 73–85. [Google Scholar] [CrossRef]
- Herrera-Urbina, R.; Sotillo, F.; Fuerstenau, D. Effect of sodium sulfide additions on the pulp potential and amyl xanthate flotation of cerussite and galena. Int. J. Miner. Process. 1999, 55, 157–170. [Google Scholar] [CrossRef]
- Kuchar, D.; Fukuta, T.; Onyango, M.; Matsuda, H. Sulfidation treatment of molten incineration fly ashes with Na2S for zinc, lead and copper resource recovery. Chemosphere 2007, 67, 1518–1525. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Deng, J.; Zhao, W. DFT study on the interaction between hydrogen sulfide ions and cerussite (110) surface. Appl. Surf. Sci. 2017, 396, 920–925. [Google Scholar] [CrossRef]
- Herrera-Urbina, R.; Sotillo, F.; Fuerstenau, D. Amyl xanthate uptake by natural and sulfide-treated cerussite and galena. Int. J. Miner. Process. 1998, 55, 113–128. [Google Scholar] [CrossRef]
- Li, J.; Liu, S.; Liu, D.; Liu, R.; Liu, Z.; Jia, X.; Chang, T. Sulfidization mechanism in the flotation of cerussite: A heterogeneous solid-liquid reaction that yields PbCO3/PbS core-shell particles. Miner. Eng. 2020, 153, 106400. [Google Scholar] [CrossRef]
- Liu, R.; Liu, D.; Li, J.; Liu, S.; Liu, Z.; Gao, L.; Jia, X.; Ao, S. Improved understanding of the sulfidization mechanism in cerussite flotation: An XPS, ToF-SIMS and FESEM investigation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124508. [Google Scholar] [CrossRef]
- Marabini, A.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating reagents for flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, F. Investigation on electrochemical properties of cerussite in sulfidation-flotation system. Min. Metall. Eng. 2017, 37, 38–40. (In Chinese) [Google Scholar]
- Tang, X.; Chen, J.; Chen, Y. A density functional based tight binding (DFTB+) study on the sulfidization-xanthate flotation mechanism of cerussite. Appl. Surf. Sci. 2023, 612, 155677. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, M.; Chen, J.; Li, Y.; Zhao, C.; Mu, X. A density functional based tight binding (DFTB+) study on the sulfidization-amine flotation mechanism of smithsonite. Appl. Surf. Sci. 2018, 458, 454–463. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, D.; Feng, Q.; Wen, S.; Chang, W. DFT insights into the electronic properties and adsorption mechanism of HS− on smithsonite (1 0 1) surface. Miner. Eng. 2019, 141, 105846. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Chen, Y.; Zhu, Y. Interaction between smithsonite and carboxyl collectors with different molecular structure in the presence of water: A theoretical and experimental study. Appl. Surf. Sci. 2020, 510, 145410. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, W.; Duan, H.; Wang, X.; Fang, P.; Liu, W.; Zhou, X.; Shen, Y. Design and selection of flotation collectors for zinc oxide minerals based on bond valence model. Miner. Eng. 2021, 160, 106681. [Google Scholar] [CrossRef]
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Zuo, Q.; Yang, J.; Shi, Y.; Wu, D. Use of sodium sulfosalicylate as an activator in hemimorphite sulfidation xanthate flotation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128552. [Google Scholar] [CrossRef]
- Zhong, W.; Yin, W.; Wang, Y.; Yao, J. Selective flotation of magnesite from dolomite using α-chloro-oleate acid as collector. Powder Technol. 2020, 373, 147–151. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, H.; Yin, W.; Yang, B.; Cao, S.; Wang, D.; Kelebek, S. Computational modeling of cetyl phosphate adsorption on magnesite (1 0 4) surface. Miner. Eng. 2021, 171, 107123. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Dai, S.; Sun, W.; Zhou, B. Effect of n-octanol on impurity removal by reverse flotation of magnesite ore. Sci. Rep. 2022, 12, 14990. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, W.; Liu, W.; Dai, S.; Duan, H.; Zhou, S.; Qiu, J. An ion-tolerance collector AESNa for effective flotation of magnesite from dolomite. Miner. Eng. 2021, 170, 106991. [Google Scholar] [CrossRef]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Liu, W.; Li, P.; Shen, Y.; Dai, S. Utilization of a novel bisphosphonic acid surfactant for reverse froth flotation of magnesite and dolomite. Miner. Eng. 2022, 185, 107668. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Liu, W.; Tong, K.; Shen, Y.; Zhao, S.; Zhou, S. Efficient separation of magnesite and quartz using eco-friendly Dimethylaminopropyl lauramide experimental and mechanistic studies. Miner. Eng. 2022, 188, 107814. [Google Scholar] [CrossRef]
- Yao, J.; Sun, H.; Ban, X.; Yin, W. Analysis of selective modification of sodium dihydrogen phosphate on surfaces of magnesite and dolomite: Reverse flotation separation, adsorption mechanism, and density functional theory calculations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126448. [Google Scholar] [CrossRef]
- Cao, Q.; Zou, H.; Chen, X.; Yu, X. Interaction of sulfuric acid with dolomite (104) surface and its impact on the adsorption of oleate anion: A DFT study. Physicochem. Probl. Miner. Process. 2020, 56, 34–42. [Google Scholar]
- Xue, X.; Wang, W.; Fan, H.; Xu, Z.; Pedruzzi, I.; Li, P.; Yu, J. Adsorption behavior of oxalic acid at water–feldspar interface: Experiments and molecular simulation. Adsorption 2019, 25, 1191–1204. [Google Scholar] [CrossRef]
- Liu, G.; Xiao, J.; Zhou, D.; Zhong, H.; Choi, P.; Xu, Z. A DFT study on the structure-reactivity relation of thiophosphorus acids as flotation collectors with sulfide minerals: Implication of surface adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 243–252. [Google Scholar] [CrossRef]
- Weiyong, C.; Jianhua, C.; Yuqiong, L.; Ye, C.; Cuihua, Z. Interactions of xanthate molecule with different mineral surfaces: A comparative study of Fe, Pb and Zn sulfide and oxide minerals with coordination chemistry. Miner. Eng. 2020, 159, 106565. [Google Scholar] [CrossRef]
- Zhao, G.; Peng, J.; Zhong, H.; Wang, S.; Liu, G. Synthesis of novel ether thionocarbamates and study on their flotation performance for chalcopyrite. Minerals 2016, 6, 97. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Xie, S.; Duan, W.; Chen, W. Roles and influences of kerosene on chalcopyrite flotation in MgCl2 solution: EDLVO and DFT Approaches. Minerals 2021, 12, 48. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.; Lu, L.; Zhu, Y.; Han, L.; Ngoepe, P.E. Unravelling the performance of oxycarbonyl-thiocarbamate collectors on chalcopyrite using first-principles calculations and micro-flotation recoveries. Appl. Surf. Sci. 2021, 563, 150332. [Google Scholar] [CrossRef]
- He, J.; Wang, L.; Zhang, C.; Sun, W.; Yin, Z.; Zhang, H.; Chen, D.; Pei, Y. A high throughput screening model of solidophilic flotation reagents for chalcopyrite based on quantum chemistry calculations and machine learning. Miner. Eng. 2022, 177, 107375. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, S.; Xu, Z.; Zhang, C.; He, J.; Zou, J.; Chen, D.; Sun, W. Flotation separation of molybdenite from chalcopyrite using an environmentally-efficient depressant L-cysteine and its adsoption mechanism. Miner. Eng. 2020, 156, 106438. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Wu, M.; Xu, Z.; Tian, M.; Yin, Z.; Sun, W.; Zhang, C. Flotation separation of molybdenite from chalcopyrite using rhodanine-3-acetic acid as a novel and effective depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar] [CrossRef]
- Timbillah, S.; LaDouceur, R.; Das, A.; Young, C.A. Theoretical and experimental investigation of disodium carboxymethyl trithiocarbonate in Cu-Mo flotation. Miner. Eng. 2021, 169, 106943. [Google Scholar] [CrossRef]
- Taheri, B.; Darvishnejad, M.H.; Rezaei, F. Depression Effect of Thioglycolic Acid (TGA) on Flotation Separation of Molybdenite from Copper Sulfides with different Collectors: An Experimental and Theoretical Study. ChemistrySelect 2022, 7, e202200026. [Google Scholar] [CrossRef]
- Yang, B.; Huang, P.; An, Q. An efficient chalcopyrite depressant for Cu-Mo separation and its interaction mechanism: Adsorption configuration and DFT calculations. J. Mol. Liq. 2022, 345, 118171. [Google Scholar] [CrossRef]
- Pan, C.; Wei, X.; Zhang, X.; Xu, Y.; Xu, P.; Luo, Y. 2-((5-Mercapto-1, 3, 4-thiadiazol-2-yl) thio) acetic acid as a novel chalcopyrite depressant for selective flotation separation of molybdenite from chalcopyrite. Miner. Eng. 2022, 183, 107625. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, L.; Luo, A.; Xiong, W.; Chen, J. Interface adsorption of 5-amino-1, 3, 4-thiadiazole-2-thiol on chalcopyrite surface as flotation depressant in Cu/Mo separation. Appl. Surf. Sci. 2023, 611, 155703. [Google Scholar] [CrossRef]
- Wu, J.; Ma, W.; Wang, X.; Jiao, F.; Qin, W. The effect of galvanic interaction between chalcopyrite and pyrite on the surface chemistry and collector adsorption: Flotation and DFT study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125377. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.; Lu, L.; Xiong, W.; Zhu, Y.; Han, L.; Ngoepe, P.E. Adsorption mechanisms and effects of thiocarbamate collectors in the separation of chalcopyrite from pyrite minerals: DFT and experimental studies. Miner. Eng. 2022, 176, 107318. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Sun, W.; Chen, D.; Chen, J.; Wang, R.; Han, M.; Zhang, C. The effects of hydroxyl on selective separation of chalcopyrite from pyrite: A mechanism study. Appl. Surf. Sci. 2023, 608, 154963. [Google Scholar] [CrossRef]
- Liu, R.; Xu, R.; Wang, L.; Jiang, F.; Jin, J.; Gao, Z.; Tang, H.; Sun, W. 3-Mercaptopropionic/3-Mercaptoisobutyric Acids Used as Novel Selective Depressants for Improved Flotation of Chalcopyrite from Galena. Minerals 2020, 10, 258. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, W.; Lu, L.; Qian, Z.; Zhu, Y.; Mkhonto, P.P.; Zheng, Y.; Han, L.; Ngoepe, P.E. A novel synthetic polymer depressant for the flotation separation of chalcopyrite and galena and insights into its interfacial adsorption mechanism. Sep. Purif. Technol. 2021, 279, 119658. [Google Scholar] [CrossRef]
- Porento, M.; Hirva, P. A theoretical study on the interaction of sulfhydryl surfactants with a covellite (0 0 1) surface. Surf. Sci. 2004, 555, 75–82. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, M.; Tang, L.; Zheng, S.; Fu, Y.; Sheng, Q.; Yin, W. Flotation separation mechanism for secondary copper sulfide minerals and pyrite using novel collector ethyl isobutyl xanthogenic acetate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 634, 128010. [Google Scholar] [CrossRef]
- Botero, Y.L.; Canales-Mahuzier, A.; Serna-Guerrero, R.; López-Valdivieso, A.; Benzaazoua, M.; Cisternas, L.A. Physical-chemical study of IPETC and PAX collector’s adsorption on covellite surface. Appl. Surf. Sci. 2022, 602, 154232. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Long, X.; Guo, J. First-principle theory on electronic structure of copper sulfides. J. Cent. South Univ. 2011, 42, 3612–3617. [Google Scholar]
- Zhao, C.-H.; Chen, J.-H.; Wu, B.-Z.; Long, X.-H. Density functional theory study on natural hydrophobicity of sulfide surfaces. Trans. Nonferrous Met. Soc. China 2014, 24, 491–498. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Y.; Zhong, H.; Wang, S.; Liu, G.; Zhao, G. A novel surfactant S-benzoyl-N, N-diethyldithiocarbamate synthesis and its flotation performance to galena. Appl. Surf. Sci. 2016, 365, 342–351. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, X.; Huang, K.; Wang, S.; Cao, Z.; Zhong, H. Synthesis, flotation performance and adsorption mechanism of 3-(ethylamino)-N-phenyl-3-thioxopropanamide onto galena/sphalerite surfaces. J. Ind. Eng. Chem. 2019, 77, 416–425. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, Y.; Huang, Y.; Chen, L.; Wang, M.; Zhang, Y. Synthesis of trimethylacetyl thiobenzamide and its flotation separation performance of galena from sphalerite. Appl. Surf. Sci. 2021, 569, 151055. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Khoso, S.A.; Wang, L.; Liu, Y.; Ge, P.; Tian, M.; Sun, W. A reagent scheme for galena/sphalerite flotation separation: Insights from first-principles calculations. Miner. Eng. 2021, 167, 106885. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Z.; Zheng, G.; Zhu, Y.; Wu, W. The design of a macromolecular depressant for galena based on DFT studies and its application. Miner. Eng. 2017, 112, 50–56. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, S.; Xie, Q. Theoretical study of 2-mercaptobenzimidazole derivatives as chelating collectors in flotation separation of galena and pyrite. Int. J. Min. Sci. Technol. 2013, 23, 619–623. [Google Scholar] [CrossRef]
- Chen, J.; Lan, L.; Chen, Y. Computational simulation of adsorption and thermodynamic study of xanthate, dithiophosphate and dithiocarbamate on galena and pyrite surfaces. Miner. Eng. 2013, 46, 136–143. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, T.; Xu, B.; Li, Q.; Zhong, H.; Yang, Y. Selective flotation of galena using a novel collector S-benzyl-N-ethoxycarbonyl thiocarbamate: An experimental and theoretical investigation. J. Mol. Liq. 2021, 330, 115643. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, J.; Liu, Z.; Chen, J. Selective enhancement of jamesonite flotation using Aerophine 3418A/DDTC mixture. Miner. Eng. 2023, 191, 107934. [Google Scholar] [CrossRef]
- LI, Y.; LONG, Q.; CHEN, J. Molecular structures and activity of organic depressants for marmatite, jamesonite and pyrite flotation. Trans. Nonferrous Met. Soc. China 2010, 20, 1993–1999. [Google Scholar]
- Liu, R.; Sun, W.; Hu, Y.; Wang, D. New collectors for the flotation of unactivated marmatite. Miner. Eng. 2010, 23, 99–103. [Google Scholar] [CrossRef]
- Porento, M.; Hirva, P. Effect of copper atoms on the adsorption of ethyl xanthate on a sphalerite surface. Surf. Sci. 2005, 576, 98–106. [Google Scholar] [CrossRef]
- Long, X.; Chen, J.; Chen, Y. Adsorption of ethyl xanthate on ZnS (110) surface in the presence of water molecules: A DFT study. Appl. Surf. Sci. 2016, 370, 11–18. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Deng, J.; Chen, X.; Feng, Q. DFT study of ethyl xanthate interaction with sphalerite (1 1 0) surface in the absence and presence of copper. Appl. Surf. Sci. 2014, 311, 258–263. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Chen, X.; Bai, S.; Liu, D.; Cao, Q. DFT computation of Cu adsorption on the S atoms of sphalerite (1 1 0) surface. Miner. Eng. 2013, 46, 1–5. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite–Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Qin, W.; Jiao, F.; Sun, W.; Wang, X.; Liu, B.; Wang, J.; Zeng, K.; Wei, Q.; Liu, K. Effects of sodium salt of N, N-dimethyldi-thiocarbamate on floatability of chalcopyrite, sphalerite, marmatite and its adsorption properties. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 181–192. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, B.; Feng, J.; Jia, F. Evaluation of, 1-diphosphonic acid as an efficient and low-toxic sphalerite depressant in the selective flotation of galena from sphalerite. J. Clean. Prod. 2021, 329, 129612. [Google Scholar] [CrossRef]
- Zhang, S.; Deng, Z.; Xie, X.; Tong, X. Study on the depression mechanism of calcium on the flotation of high-iron sphalerite under a high-alkalinity environment. Miner. Eng. 2021, 160, 106700. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, X.; Feng, Q.; Wen, S. Activation mechanism of lead ion in the flotation of stibnite. Miner. Eng. 2018, 119, 173–182. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Yuan, Z.; Xu, X.; Song, Z. AFM and DFT study of depression of hematite in oleate-starch-hematite flotation system. Appl. Surf. Sci. 2019, 480, 749–758. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Sun, W.; Chen, D.; Li, S.; Han, M.; Yu, H.; Zhang, C. Selective adsorption mechanism of dodecylamine on the hydrated surface of hematite and quartz. Sep. Purif. Technol. 2021, 275, 119137. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Yang, S.; Liu, C.; Xu, Y. Investigations on the reverse flotation of quartz from hematite using carboxymethyl chitosan as a depressant. Powder Technol. 2021, 393, 109–115. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Zhao, Q.; Shen, Y.; Wang, X.; Wang, B.; Peng, X. Design and flotation performance of a novel hydroxy polyamine surfactant based on hematite reverse flotation desilication system. J. Mol. Liq. 2020, 301, 112428. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Zhao, Q.; Peng, X.; Wang, B.; Zhou, S.; Zhao, L. Investigating the performance of a novel polyamine derivative for separation of quartz and hematite based on theoretical prediction and experiment. Sep. Purif. Technol. 2020, 237, 116370. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, B.; Duan, H.; Peng, X.; Chen, X.; Zhao, Q. Novel hydroxy polyamine surfactant N-(2-hydroxyethyl)-N-dodecyl-ethanediamine: Its synthesis and flotation performance study to quartz. Miner. Eng. 2019, 142, 105894. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, W.; Zhang, C.; He, J.; Chen, D.; Zhu, Y. Adsorption performance and mechanism of the commonly used collectors with Oxygen-containing functional group on the ilmenite surface: A DFT study. J. Mol. Liq. 2022, 346, 117829. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Adsorption of α-hydroxyoctyl phosphonic acid to ilmenite/water interface and its application in flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 67–73. [Google Scholar] [CrossRef]
- Li, L.; Zhang, C.; Yuan, Z.; Liu, Z.; Li, C. Selectivity of benzyl hydroxamic acid in the flotation of ilmenite. Front. Chem. 2019, 7, 886. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Z.; Hu, L.; Ji, F.; Zhang, Y.; Huang, T. Flotation performance measurement and the DFT study of salicylhydroxamic acid as a collector in niobite flotation, Advances in Energy and Environment Research. In Proceedings of the International Conference on Advances in Energy and Environment Research (ICAEER2016), Guangzhou, China, 12–14 August 2016; CRC Press: Boca Raton, FL, USA, 2017; p. 147. [Google Scholar]
- Rath, S.S.; Sinha, N.; Sahoo, H.; Das, B.; Mishra, B.K. Molecular modeling studies of oleate adsorption on iron oxides. Appl. Surf. Sci. 2014, 295, 115–122. [Google Scholar] [CrossRef]
- Han, G.; Su, S.; Huang, Y.; Peng, W.; Cao, Y.; Liu, J. An insight into flotation chemistry of pyrite with isomeric xanthates: A combined experimental and computational study. Minerals 2018, 8, 166. [Google Scholar] [CrossRef]
- Yang, X.; Albijanic, B.; Liu, G.; Zhou, Y. Structure–activity relation of xanthates with different hydrophobic groups in the flotation of pyrite. Miner. Eng. 2018, 125, 155–164. [Google Scholar] [CrossRef]
- Kumar, D.; Srinivasan, S.G.; Jain, V.; Rai, B. Understanding flotation processes at the atomic scale using density functional theory–A case study on adsorption of 2-Mercaptobenzothiazole on chalcopyrite and pyrite surfaces. Appl. Surf. Sci. 2022, 579, 152112. [Google Scholar] [CrossRef]
- Mkhonto, P.P.; Zhang, X.; Lu, L.; Xiong, W.; Zhu, Y.; Han, L.; Ngoepe, P.E. Design, synthesis and investigating the interaction of novel s-triazine collector with pyrite surface: A DFT-D3+ U and experimental studies. Surf. Interfaces 2023, 38, 102820. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, D.; Chen, J.; Li, Y.; Chen, Y.; Li, W. The interaction of cyanide with pyrite, marcasite and pyrrhotite. Miner. Eng. 2016, 95, 131–137. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.; Li, Y.; Li, W. DFT study of interactions between calcium hydroxyl ions and pyrite, marcasite, pyrrhotite surfaces. Appl. Surf. Sci. 2015, 355, 577–581. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Kang, D.; Guo, J. Depression of pyrite in alkaline medium and its subsequent activation by copper. Miner. Eng. 2012, 26, 64–69. [Google Scholar] [CrossRef]
- Cao, Q.; Yan, W.; Wen, S.; Liu, D.; Li, Y. New insights into pyrite-hydrogen peroxide interactions during froth flotation: Experimental and DFT study. Physicochem. Probl. Miner. Process. 2023, 59, 157409. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, T.; Wang, S.; Zhong, H. Study on a novel hydroxamic acid as the collector of rhodochrosite. Physicochem. Probl. Miner. Process. 2018, 54, 428–439. [Google Scholar]
- Huang, Z.; Zhong, H.; Wang, S.; Xia, L.; Zou, W.; Liu, G. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1, 2-bis (dimethyl-dodecyl-ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Liu, A.; Fan, J.; Fan, M. Quantum chemical calculations and molecular dynamics simulations of amine collector adsorption on quartz (0 0 1) surface in the aqueous solution. Int. J. Miner. Process. 2015, 134, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, S.; Guo, Z.; Sun, W.; Zhang, C. Selective separation mechanism of hematite from quartz by anionic reverse flotation: Implications from surface hydroxylation. Appl. Surf. Sci. 2023, 614, 156056. [Google Scholar] [CrossRef]
- Fomasiero, D.; Ralston, J. Cu (II) and Ni (II) activation in the flotation of quartz, lizardite and chlorite. Int. J. Miner. Process. 2005, 76, 75–81. [Google Scholar] [CrossRef]
- Jie, Z.; Weiqing, W.; Jing, L.; Yang, H.; Qiming, F.; Hong, Z. Fe (III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Luo, X.; Lin, Q.; Wang, Y.; Tian, M.; Lai, H.; Bai, S.; Zhou, Y. New insights into the activation mechanism of calcium species to quartz: ToF-SIMS and AFM investigation. Miner. Eng. 2020, 153, 106398. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Ge, P.; Sun, W.; Tang, H.; Hu, W. Activation mechanisms of quartz flotation with calcium ions and cationic/anionic mixed collectors under alkalescent conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127771. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Effects of metal ions on the flotation separation of spodumene from feldspar and quartz. Miner. Eng. 2021, 168, 106931. [Google Scholar] [CrossRef]
- Luo, A.; Chen, J. Effect of hydration and hydroxylation on the adsorption of metal ions on quartz surfaces: DFT study. Appl. Surf. Sci. 2022, 595, 153553. [Google Scholar] [CrossRef]
- Gong, G.; Liu, J.; Han, Y.; Zhu, Y. An atomic scale investigation of the adsorption of sodium oleate on Ca2+ activated quartz surface. Physicochem. Probl. Miner. Process. 2019, 55, 426–436. [Google Scholar]
- Wang, Y.; Khoso, S.A.; Luo, X.; Tian, M. Understanding the depression mechanism of citric acid in sodium oleate flotation of Ca2+-activated quartz: Experimental and DFT study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, Z.; Liu, W.; Tian, P. Effect of TIPA/TEA combined grinding aid on the behavior of quartz flotation in DDA system. Powder Technol. 2022, 406, 117570. [Google Scholar] [CrossRef]
- Monte, M.B.D.M.; Pimentel, D.A.; de Albuquerque, M.D.D.F.; Neumann, R.; Silva, L.A.; Correia, J.C.; Uliana, A. Synergism of mixed cationic collectors in the flotation of quartz unveiled by AFM, solution chemistry and quantum chemical calculations. J. Mol. Liq. 2023, 376, 121397. [Google Scholar] [CrossRef]
- Sun, W.; Wang, Y.; Li, H.; Hu, Y. Nature of (001) and (00) faces and flocculation flotation of kaolinite. Trans. Nonferrous Met. Soc. China 2003, 13, 968–971. (In Chinese) [Google Scholar]
- Hu, Y.; Wei, S.; Hao, J.; Miller, J.; Fa, K. The anomalous behavior of kaolinite flotation with dodecyl amine collector as explained from crystal structure considerations. Int. J. Miner. Process. 2005, 76, 163–172. [Google Scholar] [CrossRef]
- Liu, C.; Feng, A.; Guo, Z.; Cao, X.; Hu, Y. Dynamics simulation of tertiary amines adsorbing on kaolinite (001) plane. Trans. Nonferrous Met. Soc. China 2011, 21, 1874–1879. [Google Scholar] [CrossRef]
- Wang, F.; Zhan, G.; Jiang, Y.; Guo, J.; Yin, Z.; Feng, R. Theoretical evaluation of flotation performance of carboxyl hydroxamic acids with different number of polar groups on the surfaces of diaspore (010) and kaolinite (001). J. Mol. Model. 2013, 19, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Gong, J.; Liu, Y.; Qiao, E. Substituent Effects in Kaolinite Flotation Using Dodecylamine: Experiment and DFT Study. Processes 2023, 11, 703. [Google Scholar] [CrossRef]
- Peng, C.; Zhong, Y.; Min, F. Adsorption of alkylamine cations on montmorillonite (001) surface: A density functional theory study. Appl. Clay Sci. 2018, 152, 249–258. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, W.; Zhuo, Q.; Han, Y. Interaction mechanism of organic carboxylate with kaolinite and montmorillonite: A density functional theory study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126047. [Google Scholar] [CrossRef]
- Laskowski, J.; Liu, Q.; O’connor, C. Current understanding of the mechanism of polysaccharide adsorption at the mineral/aqueous solution interface. Int. J. Miner. Process. 2007, 84, 59–68. [Google Scholar] [CrossRef]
- Manono, M.; Corin, K.; Wiese, J. The effect of the ionic strength of process water on the interaction of talc and CMC: Implications of recirculated water on floatable gangue depression. Minerals 2019, 9, 231. [Google Scholar] [CrossRef]
- Manono, M.S.; Corin, K.C.; Wiese, J.G. The Behavior of Gangue During the Flotation of a Sulfidic PGM-Bearing Ore in Response to Various Monovalent and Divalent Ions in Process Water. Front. Chem. 2020, 8, 79. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Chen, J.; Zhang, G.; Xia, Y.; Zhu, B.; Zhou, H. Insights into the adsorption performance and mechanism of hydrated Ca ion on talc (0 0 1) basal surface from DFT calculation. Int. J. Min. Sci. Technol. 2022, 32, 887–896. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Chen, J.; Zhang, G.; Xia, Y.; Zhu, B.; Zhou, H. Mechanism insights into the hydrated Al ion adsorption on talc (001) basal surface: A DFT study. Surf. Interfaces 2022, 30, 101973. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Q.; Gao, X.; Hu, Y. Understanding the differential depression of tetrasodium glutamate diacetate on the separation of specularite and chlorite: Experimental and DFT study. Miner. Eng. 2020, 159, 106629. [Google Scholar] [CrossRef]
- Wang, R.; Sun, W.; Han, H.; Sun, W.; Wei, Z.; Peng, J. Al-caustic starch coordination compounds: A new depressant for fine calcite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129268. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Sun, W.; Han, H. The inhibiting effect of Pb-starch on chlorite flotation and its adsorption configuration based on DFT computation. Appl. Surf. Sci. 2023, 610, 155482. [Google Scholar] [CrossRef]
- He, J.; Han, H.; Zhang, C.; Xu, Z.; Yuan, D.; Chen, P.; Sun, W.; Hu, Y. Novel insights into the surface microstructures of lead (II) benzohydroxamic on oxide mineral. Appl. Surf. Sci. 2018, 458, 405–412. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, Q.; Wu, Y.; He, J. Quantum chemistry assisted screening of zircon flotation collectors. Miner. Eng. 2022, 189, 107892. [Google Scholar] [CrossRef]
- Li, B.; Zhang, G.; Liu, D.; Chen, J. Selective alteration mechanisms of sodium tripolyphosphate towards serpentine: Implications for flotation of pyrite from serpentine. J. Mol. Liq. 2022, 368, 120687. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Zhong, H. Study on the role of a hydroxamic acid derivative in wolframite flotation: Selective separation and adsorption mechanism. Appl. Surf. Sci. 2021, 550, 149223. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, X.; Lu, Y.; Wang, S.; Zhong, H. Insights into the selective adsorption mechanism of a multifunctional thioether-containing hydroxamic acid on separation of wolframite from fluorite. Powder Technol. 2021, 380, 421–429. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W. Flotation separation of wolframite from calcite using a new trisiloxane surfactant as collector. Int. J. Min. Sci. Technol. 2023, 33, 379–387. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W. Selective separation of wolframite from calcite by froth flotation using a novel amidoxime surfactant: Adsorption mechanism and DFT calculation. Miner. Eng. 2022, 185, 107716. [Google Scholar] [CrossRef]
- Huang, H.; Qiu, T.; Ren, S.; Qiu, X. Research on flotation mechanism of wolframite activated by Pb (II) in neutral solution. Appl. Surf. Sci. 2020, 530, 147036. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Zhong, H. Study on the activation of scheelite and wolframite by lead nitrate. Minerals 2015, 5, 247–258. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Lyu, F.; Sun, W.; Khoso, S.A.; Zhang, C.; Liu, R.; Wang, L.; Gao, J. Adsorption mechanism of propyl gallate as a flotation collector on scheelite: A combined experimental and computational study. Miner. Eng. 2019, 133, 19–26. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.; Cao, J.; Gui, X.; Xing, Y.; Zhang, C.; Zou, J. Enhanced electronic effect improves the collecting efficiency of benzohydroxamic acid for scheelite flotation. Miner. Eng. 2020, 152, 106308. [Google Scholar] [CrossRef]
- Ni, C.; Xie, Y.; Liu, C.; Han, Z.; Shen, H.; Ran, W.; Xie, W.; Liang, Y. Exploring the separation mechanism of Gemini surfactant in scheelite froth flotation at low temperatures: Surface characterization, DFT calculations and kinetic simulations. Sep. Purif. Technol. 2023, 305, 122358. [Google Scholar] [CrossRef]
- Yin, W.; Wang, J.; Sun, Z. Structure–activity relation and mechanisms of reagents used in scheelite flotation. Rare Met. 2015, 34, 882–887. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Liu, C.; Ni, C.; Yao, J.; Chang, Z.; Wang, Z.; Zeng, G.; Luo, X.; Yang, L.; Ren, Z.; Shao, P. Hydroxypropyl amine surfactant: A novel flotation collector for efficient separation of scheelite from calcite. Miner. Eng. 2021, 167, 106898. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W. Adsorption of trisiloxane surfactant for selective flotation of scheelite from calcite at room temperature. Langmuir 2022, 38, 9010–9020. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, S.; Zhong, H. Optimization of conventional hydroxamic acid for cassiterite flotation: Application of structural modification under principle of isomerism. Miner. Eng. 2021, 167, 106901. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Ou, L.; Zhang, Y.; Chen, J. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025. [Google Scholar] [CrossRef]
- Qi, J.; Liu, S.; Dong, Y.; Liu, G. Revealing the role of dithiocarbamate ester group in hydroxamic acid flotation of cassiterite with in situ AFM, DFT and XPS. Appl. Surf. Sci. 2022, 604, 154521. [Google Scholar] [CrossRef]
- Sun, Q.; Lu, Y.; Wang, S.; Zhong, H. A novel surfactant 2-(benzylthio)-acetohydroxamic acid: Synthesis, flotation performance and adsorption mechanism to cassiterite, calcite and quartz. Appl. Surf. Sci. 2020, 522, 146509. [Google Scholar] [CrossRef]
- Gong, G.; Han, Y.; Liu, J.; Zhu, Y.; Li, Y.; Yuan, S. In situ investigation of the adsorption of styrene phosphonic acid on cassiterite (110) surface by molecular modeling. Minerals 2017, 7, 181. [Google Scholar] [CrossRef]
- Gong, G.; Liu, J.; Han, Y. Comprehensive investigation of the adsorption of 2-carboxyethylphenylphosphinic acid on cassiterite. Sep. Sci. Technol. 2021, 56, 2194–2203. [Google Scholar] [CrossRef]
- Tian, M.; Khoso, S.A.; Wang, L.; Sun, W.; Zhang, C.; Hu, Y. Selective separation behavior and its molecular mechanism of cassiterite from quartz using cupferron as a novel flotation collector with a lower dosage of Pb2+ ions. Appl. Surf. Sci. 2019, 486, 228–238. [Google Scholar] [CrossRef]
- He, J.; Zhou, Q.; Chen, S.; Tian, M.; Zhang, C.; Sun, W. Interfacial microstructures and adsorption mechanisms of benzohydroxamic acid on Pb2+-activated cassiterite (1 1 0) surface. Appl. Surf. Sci. 2021, 541, 148506. [Google Scholar] [CrossRef]
- Gong, G.; Wang, P.; Liu, J.; Han, Y.; Zhu, Y. Effect and mechanism of Cu (II) on flotation separation of cassiterite from fluorite. Sep. Purif. Technol. 2020, 238, 116401. [Google Scholar] [CrossRef]
- Gong, G.; Liu, J.; Han, Y.; Zhu, Y. Experimental and density functional theory studies of the effects and mechanisms of Cu2+ on flotation separation of cassiterite from fluorite. J. Mol. Liq. 2021, 322, 114907. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Zhao, L.; Wang, X. Flotation performance and selective adsorption mechanism of novel hydroxamic acid on the separation of fluorite from barite. Miner. Eng. 2021, 171, 107101. [Google Scholar] [CrossRef]
- Lv, L.; Duan, H.; Liu, W.; Yue, T. Flotation separation of fluorite from calcite using bis hydroxamic acid collector. Miner. Eng. 2022, 187, 107803. [Google Scholar] [CrossRef]
- Tao, L.; Sun, W.; Wang, J. Selective separation of fluorite from calcite using saponified tridecanoic acid as a novel collector. Miner. Process. Extr. Metall. Rev. 2023, 1–13. [Google Scholar] [CrossRef]
- Miao, Z.; Tao, L.; Wang, J.; Jiang, Z.; Peng, T.; Sun, W.; Gao, Z. Selective separation of fluorite from scheelite using N-decanoylsarcosine sodium as a novel collector. Minerals 2022, 12, 855. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Chen, R.; Wang, Y.; Deng, J.; Luo, X. Flotation separation of scheelite from fluorite using sodium polyacrylate as inhibitor. Minerals 2017, 7, 102. [Google Scholar] [CrossRef]
- He, J.; Sun, W.; Zeng, H.; Fan, R.; Hu, W.; Gao, Z. Unraveling roles of lead ions in selective flotation of scheelite and fluorite from atomic force microscopy and first-principles calculations. Miner. Eng. 2022, 179, 107424. [Google Scholar] [CrossRef]
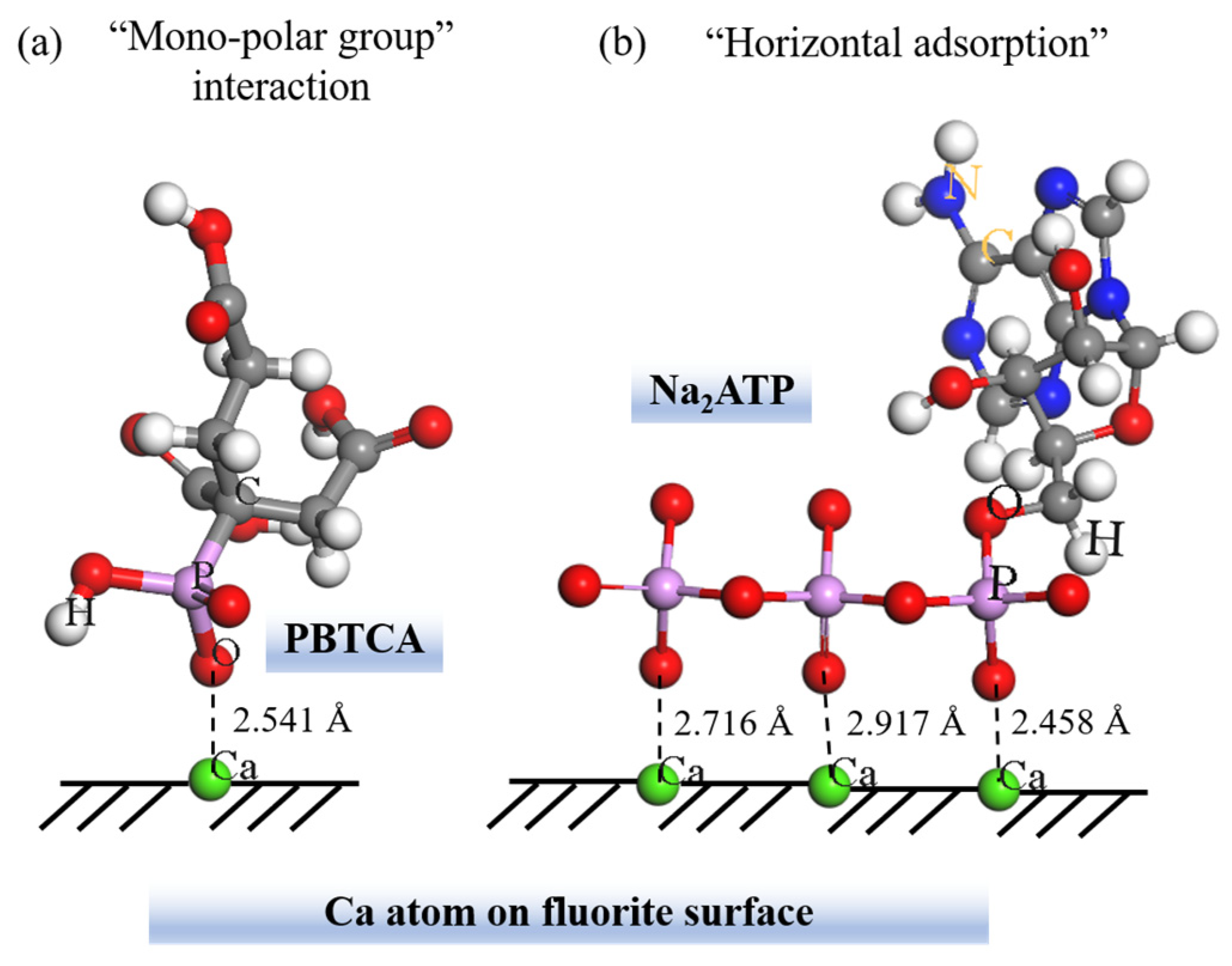
- Wang, X.; Liu, J.; Zhu, Y.; Li, Y. Adsorption and depression mechanism of an eco-friendly depressant PBTCA on fluorite surface for the efficient separation of cassiterite from fluorite. Miner. Eng. 2021, 171, 107124. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, Y.; Li, Y. The application and mechanism of high-efficiency depressant Na2ATP on the selective separation of cassiterite from fluorite by direct flotation. Miner. Eng. 2021, 169, 106963. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Li, Y.; Wang, Y.; Luo, X. Flotation separation of scheelite from calcite using sodium polyacrylate as depressant. Physicochem. Probl. Miner. Process. 2018, 54, 505–516. [Google Scholar]
- Wang, Y.; He, G.; Abudukade, D.; Li, K.; Guo, T.; Li, S.; Xiao, Z.; Wang, J.; Nie, S. Selective inhibition of sodium tripolyphosphate on calcite in the process of magnesite flotation. J. Mol. Liq. 2022, 345, 117412. [Google Scholar] [CrossRef]
- Jin, D.; Sun, R.; Wang, G.; Deng, J.; Zhang, X. Flotation separation of fluorite and calcite using anhydrous glucose and aluminum sulfate as a combined depressant. Appl. Surf. Sci. 2023, 624, 157089. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Xu, H.; Miller, J. Lauryl phosphate adsorption in the flotation of Bastnaesite,(Ce, La) FCO3. J. Colloid Interface Sci. 2017, 490, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Wanhala, A.K.; Doughty, B.; Bryantsev, V.S.; Wu, L.; Mahurin, S.M.; Jansone-Popova, S.; Cheshire, M.C.; Navrotsky, A.; Stack, A.G. Adsorption mechanism of alkyl hydroxamic acid onto bastnäsite: Fundamental steps toward rational collector design for rare earth elements. J. Colloid Interface Sci. 2019, 553, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Yang, X.; Qi, J.; Liu, G.; Qin, J. A comparative investigation into floatability of bastnaesite with three di/trialkyl phosphate surfactants. J. Rare Earths 2021, 39, 1442–1449. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. A century of research leading to understanding the scientific basis of selective mineral flotation and design of flotation collectors. Min. Metall. Explor. 2019, 36, 3–20. [Google Scholar]
- Zhang, X.; Du, H.; Wang, X.; Miller, J. Surface chemistry aspects of bastnaesite flotation with octyl hydroxamate. Int. J. Miner. Process. 2014, 133, 29–38. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Kuzmina, O.; Waters, K.E. Surface chemistry considerations in the flotation of bastnäsite. Miner. Eng. 2014, 66, 119–129. [Google Scholar] [CrossRef]
- Fuerstenau, D. The adsorption of hydroxamate on semi-soluble minerals. Part I: Adsorption on barite, Calcite and Bastnaesite. Colloids Surf. 1983, 8, 103–119. [Google Scholar]
- Yao, X.; Yu, X.; Zeng, Y.; Mao, L.; Xie, H.; Liu, S.; He, G.; Huang, Z.; Wang, H.; Liu, Z. Behavior and Mechanism of a Novel Hydrophobic Collector in the Flotation of Bastnaesite. Minerals 2022, 12, 817. [Google Scholar] [CrossRef]
- Duan, H.; Liu, W.; Wang, X.; Gu, X.; Sun, W.; Peng, X.; Yue, H. Investigation on flotation separation of bastnaesite from calcite and barite with a novel surfactant: Octylamino-bis-(butanohydroxamic acid). Sep. Purif. Technol. 2021, 256, 117792. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhou, Z.; Gao, Z.; Hu, Y.; Sun, W. Hydroxyethylidene-1, 1-diphosphonic acid used as pH-dependent switch to depress and activate fluorite flotation I: Depressing behavior and mechanism. Chem. Eng. Sci. 2020, 214, 115369. [Google Scholar] [CrossRef]
- Coveney, P.V.; Davey, R.; Griffin, J.L.; He, Y.; Hamlin, J.D.; Stackhouse, S.; Whiting, A. A new design strategy for molecular recognition in heterogeneous systems: A universal crystal-face growth inhibitor for barium sulfate. J. Am. Chem. Soc. 2000, 122, 11557–11558. [Google Scholar] [CrossRef]
- Davey, R.; Black, S.; Bromley, L.; Cottier, D.; Dobbs, B.; Rout, J. Molecular design based on recognition at inorganic surfaces. Nature 1991, 353, 549–550. [Google Scholar] [CrossRef]
- Cao, S.; Cao, Y.; Ma, Z.; Liao, Y. The adsorption mechanism of Al (III) and Fe (III) ions on bastnaesite surfaces. Physicochem. Probl. Miner. Process. 2019, 55, 97–107. [Google Scholar]
- Sarvaramini, A.; Azizi, D.; Larachi, F. Hydroxamic acid interactions with solvated cerium hydroxides in the flotation of monazite and bastnäsite—Experiments and DFT study. Appl. Surf. Sci. 2016, 387, 986–995. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic− molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, F.; Zhang, Y.; Zhang, B.; Yang, S.; Luo, X. Insights into the floatability between spodumene and albite from crystal chemistry standpoint. Int. J. Min. Sci. Technol. 2022, 32, 1329–1339. [Google Scholar] [CrossRef]
- Zhou, H.; Xie, F.; Luo, X.; Zhang, B.; Yang, S.; Zhang, Y. Difference of solidophilic atoms in collectors: Enlightenments for flotation separation of spodumene and feldspar. Appl. Surf. Sci. 2023, 615, 156363. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, X.; Yu, F.; Miller, J.D. States of coadsorption for oleate and dodecylamine at selected spodumene surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 313–321. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, Y.; Zhou, H.; Xie, F.-X.; Yang, Z.; Zhang, B.; Luo, C. Flotation separation of spodumene and albite with activation of calcium ion hydrolysate components. Rare Met. 2022, 41, 3919–3931. [Google Scholar] [CrossRef]
- Yongbing, Z.; Hepeng, Z.; Yijun, C.; Xianping, L.; Fanxin, X.; Boyuan, Z.; Siqi, Y. Activation mechanism of calcium hydrolysate on the spodumene surface and its effect on the adsorption of collector. Miner. Eng. 2021, 174, 107221. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z.; Zhang, L. First-principle investigation on mechanism of Ca ion activating flotation of spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Wang, J.; Xie, Z. Investigation on different behavior and mechanism of Ca (II) and Fe (III) adsorption on spodumene surface. Physicochem. Probl. Miner. Process. 2014, 50, 535–550. [Google Scholar]
- Tian, M.; Gao, Z.; Khoso, S.A.; Sun, W.; Hu, Y. Understanding the activation mechanism of Pb2+ ion in benzohydroxamic acid flotation of spodumene: Experimental findings and DFT simulations. Miner. Eng. 2019, 143, 106006. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; He, G.; Shen, L.; Chen, X.; Shuai, S.; Li, F.; Wang, H.; Liu, R.; Zhang, S. Adsorption Mechanism of Amidoxime Collector on the Flotation of Lepidolite: Experiment and DFT Calculation. Langmuir 2022, 38, 15858–15865. [Google Scholar] [CrossRef]
- Wu, J.; Yang, B.; Song, S.; Quintana, M.; Jia, F.; Tian, X. The efficient recovery of molybdenite fines using a novel collector: Flotation performances, adsorption mechanism and DFT calculation. Miner. Eng. 2022, 188, 107848. [Google Scholar] [CrossRef]
- Wu, J.; Feng, J.; Yang, B.; Martin, R.; Song, S.; Quintana, M.; Jia, F.; Tian, X. The anisotropic adsorption of potassium cetyl phosphate on molybdenite surface and its implication for improving the flotation of molybdenite fines. J. Mol. Liq. 2023, 378, 121616. [Google Scholar] [CrossRef]
- Wan, H.; Yi, P.; Song, X.; Luukkanen, S.; Qu, J.; Yang, W.; Li, H.; Bu, X. Role of improving molybdenite flotation by using aromatic hydrocarbon collector in high-calcium water: A multiscale investigation. Miner. Eng. 2023, 191, 107984. [Google Scholar] [CrossRef]
- Sun, L.; Cao, Y.; Li, L.; Zeng, Q. Adsorption Characteristics and Mechanism of Calcium Ions on Different Molybdenite Surfaces via Experiments and DFT Simulations. Separations 2021, 8, 107. [Google Scholar] [CrossRef]
- Sun, X.; Wu, B.; Deng, J.; Qiu, H.; Hu, M.; Cai, J.; Jin, X.; Xu, H. Synergistic Mechanism of Combined Inhibitors on the Selective Flotation of Arsenopyrite and Pyrite, ACS omega 2022, 7, 6302–6312.
- Sun, X.; Wu, B.; Qiu, H.; Chen, J.; Hu, M.; Hu, K. Interaction of a novel depressant m-nitrobenzoate with arsenopyrite surface: DFT and experimental studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129613. [Google Scholar] [CrossRef]
- Dong, W.; Liu, R.; Wang, C.; Zhu, X.; Xie, Z.; Sun, W. Insight into selective depression of sodium thioglycallate on arsenopyrite flotation: Adsorption mechanism and constructure. J. Mol. Liq. 2023, 377, 121480. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, Q.; Liu, R.; Luo, Y.; Xie, Z.; Cao, Z.; Sun, W. Surface modification with hydroxyl calcium ions strengthen CMC selectively depress arsenopyrite: Bridging adsorption mechanism and application in Cu-As separation. Appl. Surf. Sci. 2023, 618, 156642. [Google Scholar] [CrossRef]
- Yekeler, M.; Yekeler, H. Molecular modeling study on the relative stabilities of the flotation products for arsenic-containing minerals: Dixanthogens and arsenic (III) xanthates. J. Colloid Interface Sci. 2005, 284, 694–697. [Google Scholar] [CrossRef]
- Gordeijev, J.; Hirva, P. Theoretical studies on the interaction of oleoyl sarcosine with the surface of apatite. Surf. Sci. 1999, 440, 321–326. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.; Zhao, Q.; Liu, W.; Tong, K.; Zhao, P. Development and utilization of a novel hydrogen bonding enhanced collector in the separation of apatite from quartz. Miner. Eng. 2022, 180, 107477. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Mao, S.; Li, L.; Ke, B.; Zhang, Q. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (0 0 1) surface: A first-principles calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
- Du, W.; Li, X.; Zhang, Q. DFT study of coadsorption of fatty acid and kerosene on fluorapatite (001) surface. Physicochemical Problems of Mineral Processing 2023, 59. [Google Scholar] [CrossRef]
- Zou, H.; Cao, Q.-B.; Liu, D.-W.; Chen, X.-M.; Jiao, Y. Flotation features of fluorapatite with ricinoleic acid: The role of hydrogen bonds between collectors. Chem. Pap. 2021, 75, 1949–1958. [Google Scholar] [CrossRef]
- Eskanlou, A.; Huang, Q.; Foucaud, Y.; Badawi, M.; Romero, A.H. Effect of Al3+ and Mg2+ on the flotation of fluorapatite using fatty-and hydroxamic-acid collectors–A multiscale investigation. Appl. Surf. Sci. 2022, 572, 151499. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Hou, J. Behavior and mechanism of collophane and dolomite separation using alkyl hydroxamic acid as a flotation collector. Physicochem. Probl. Miner. Process. 2016, 52, 155–169. [Google Scholar]
- Yekeler, H.; Yekeler, M. Reactivities of some thiol collectors and their interactions with Ag (+1) ion by molecular modeling. Appl. Surf. Sci. 2004, 236, 435–443. [Google Scholar] [CrossRef]
- Wei, T.; Tang, X.; Chen, Y.; Chen, J.; Feng, Y. The interaction mechanism and collecting performance of three sulfydryl collectors with Au (1 0 0) surface: A study combining DFT and molecular dynamics. J. Mol. Liq. 2023, 382, 121957. [Google Scholar] [CrossRef]
- Liu, W.; Miller, J.D.; Sun, W.; Hu, Y. Analysis of the selective flotation of elemental gold from pyrite using diisobutyl monothiophosphate. Minerals 2022, 12, 1310. [Google Scholar] [CrossRef]
- Hugosson, H.W.; Eriksson, O.; Jansson, U.; Ruban, A.V.; Souvatzis, P.; Abrikosov, I.A. Surface energies and work functions of the transition metal carbides. Surf. Sci. 2004, 557, 243–254. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Chen, J.; Chen, Y.; Krivovichev, S.V. Application of Quantum Chemistry in the Study of Flotation Reagents. Minerals 2023, 13, 1487. https://doi.org/10.3390/min13121487
Tang X, Chen J, Chen Y, Krivovichev SV. Application of Quantum Chemistry in the Study of Flotation Reagents. Minerals. 2023; 13(12):1487. https://doi.org/10.3390/min13121487
Chicago/Turabian StyleTang, Xiaoqin, Jianhua Chen, Ye Chen, and Sergey V. Krivovichev. 2023. "Application of Quantum Chemistry in the Study of Flotation Reagents" Minerals 13, no. 12: 1487. https://doi.org/10.3390/min13121487
APA StyleTang, X., Chen, J., Chen, Y., & Krivovichev, S. V. (2023). Application of Quantum Chemistry in the Study of Flotation Reagents. Minerals, 13(12), 1487. https://doi.org/10.3390/min13121487






