Limonite as a Natural Adsorbent for the Removal of Antimony(III) from an Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Adsorption Experiments
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Thermodynamics
2.2.3. Effect of pH on Sb(III) Adsorption
2.2.4. Effects of Ionic Strength and Coexisting Anions on Sb(III) Adsorption
2.3. XPS Characterization
3. Results and Discussion
3.1. Adsorption Kinetics
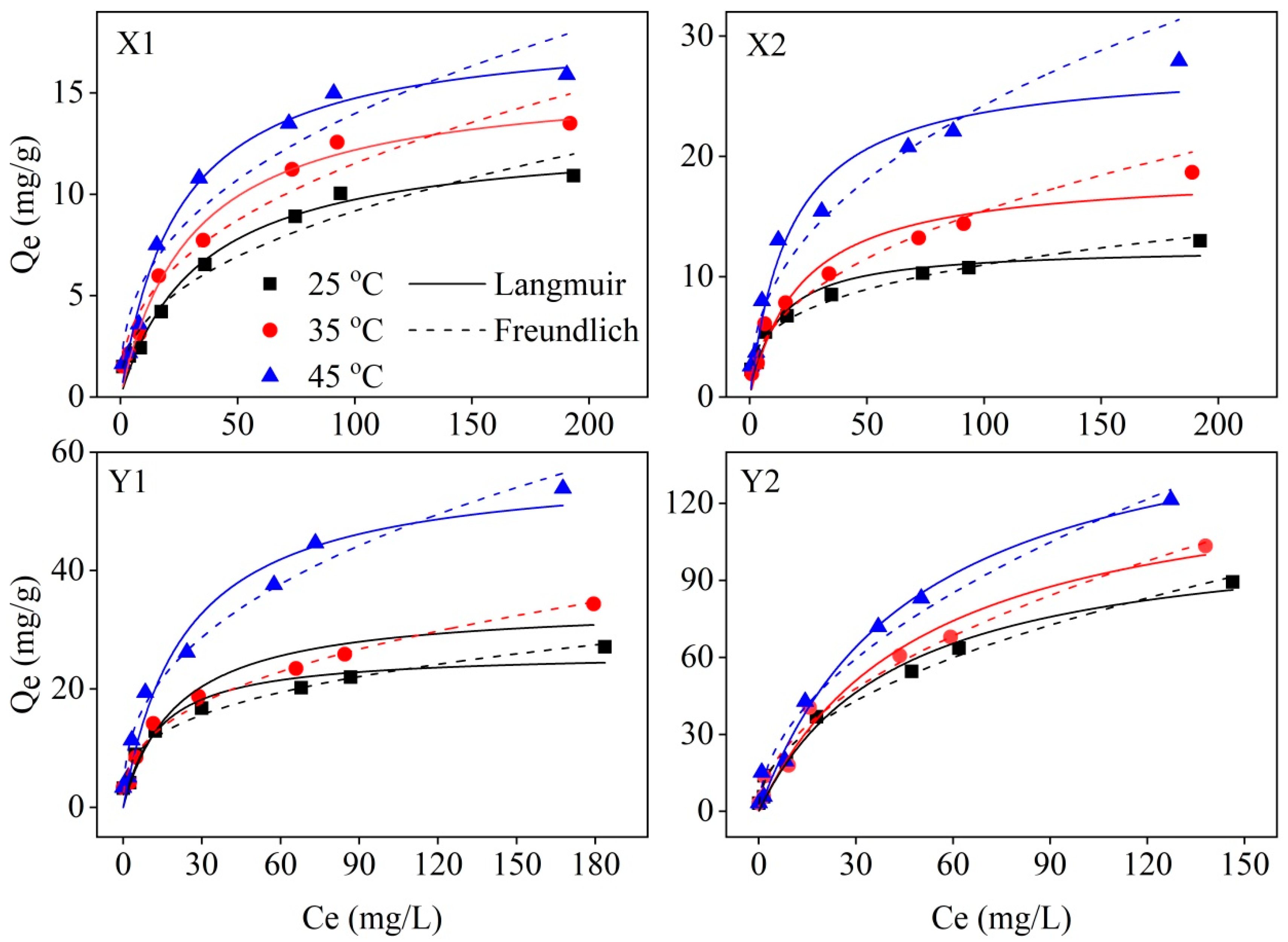
3.2. Adsorption Isotherms and Thermodynamics
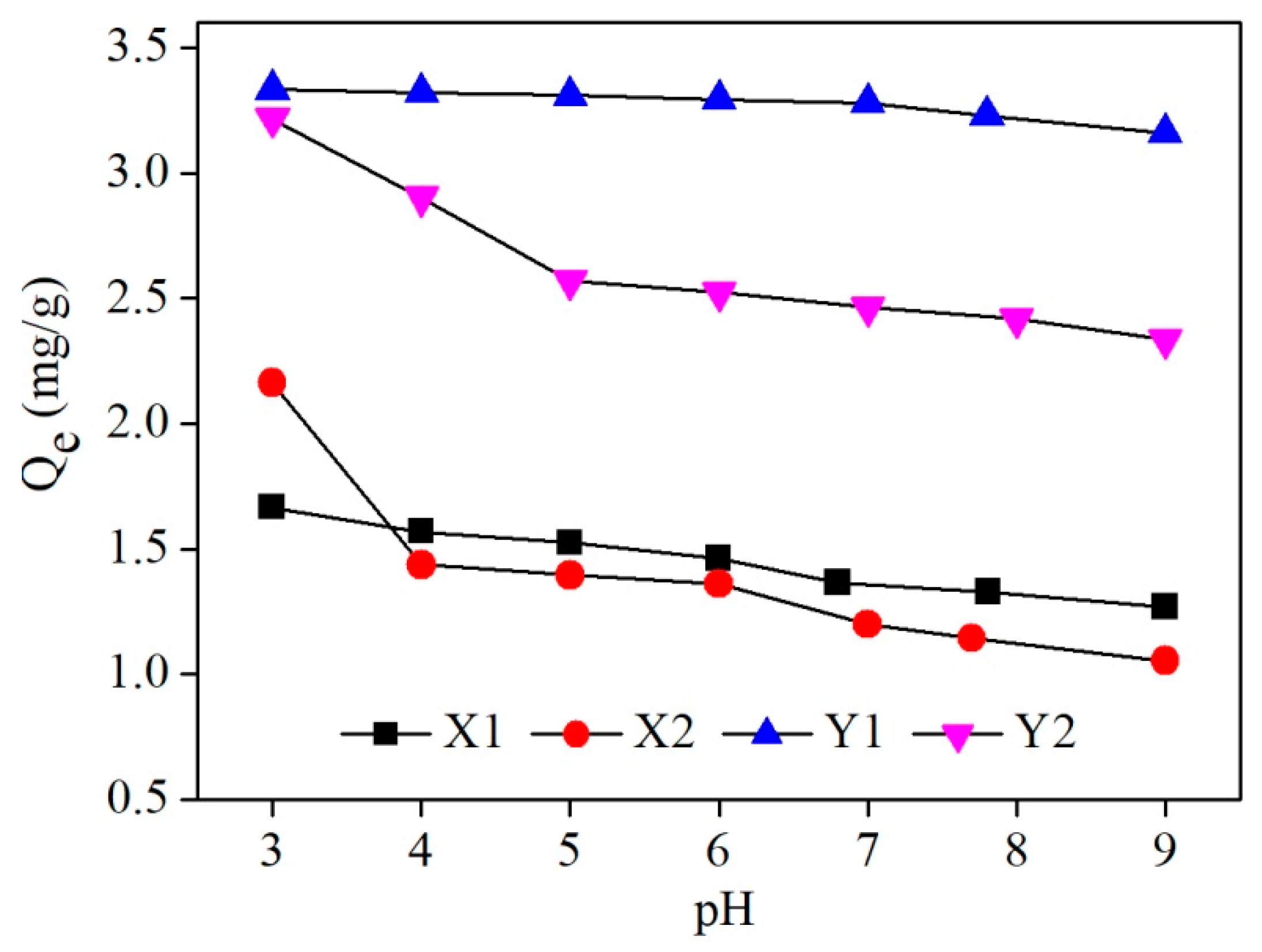
3.3. Effect of Initial Solution pH
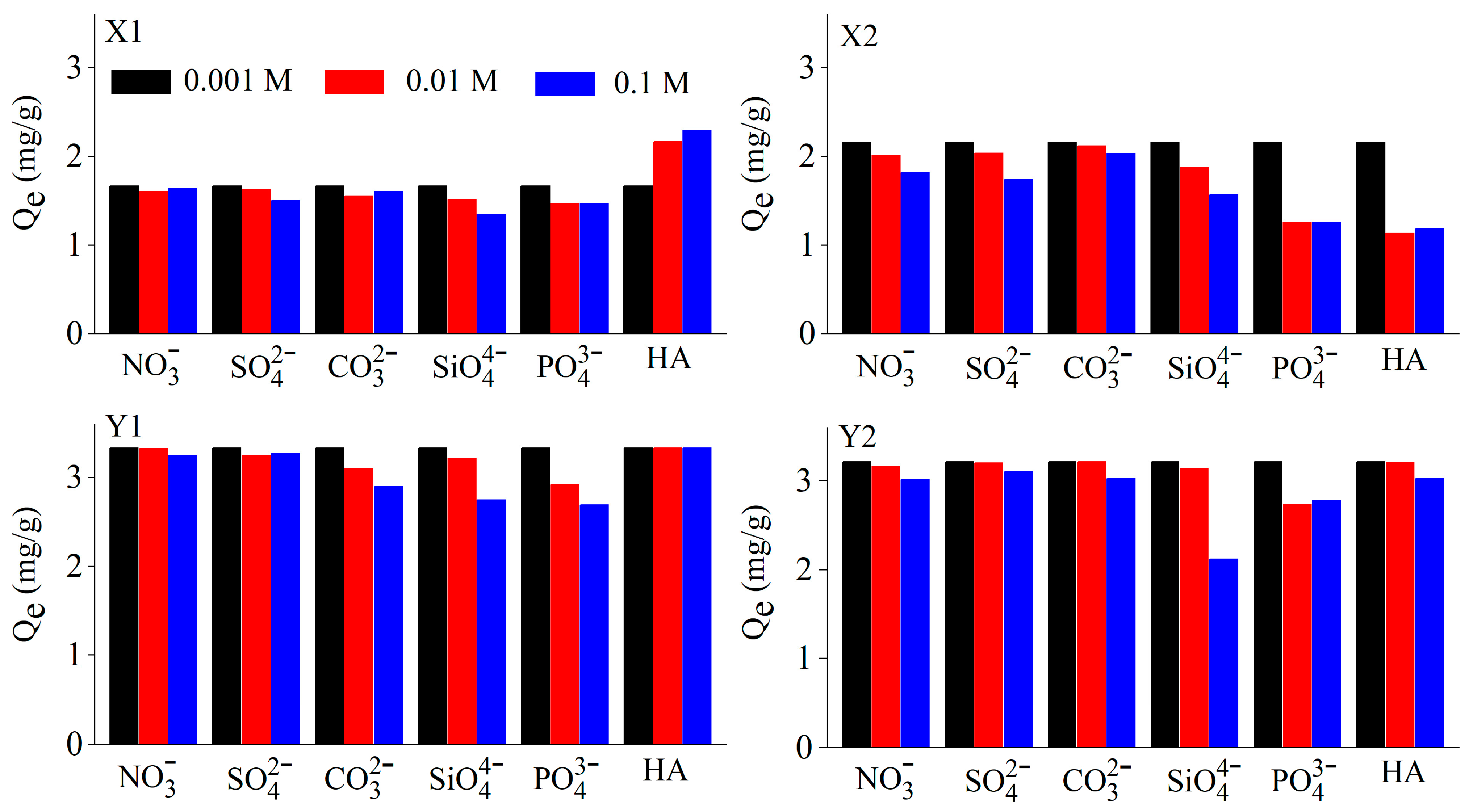
3.4. Effect of Ionic Strength and Coexisting Anions
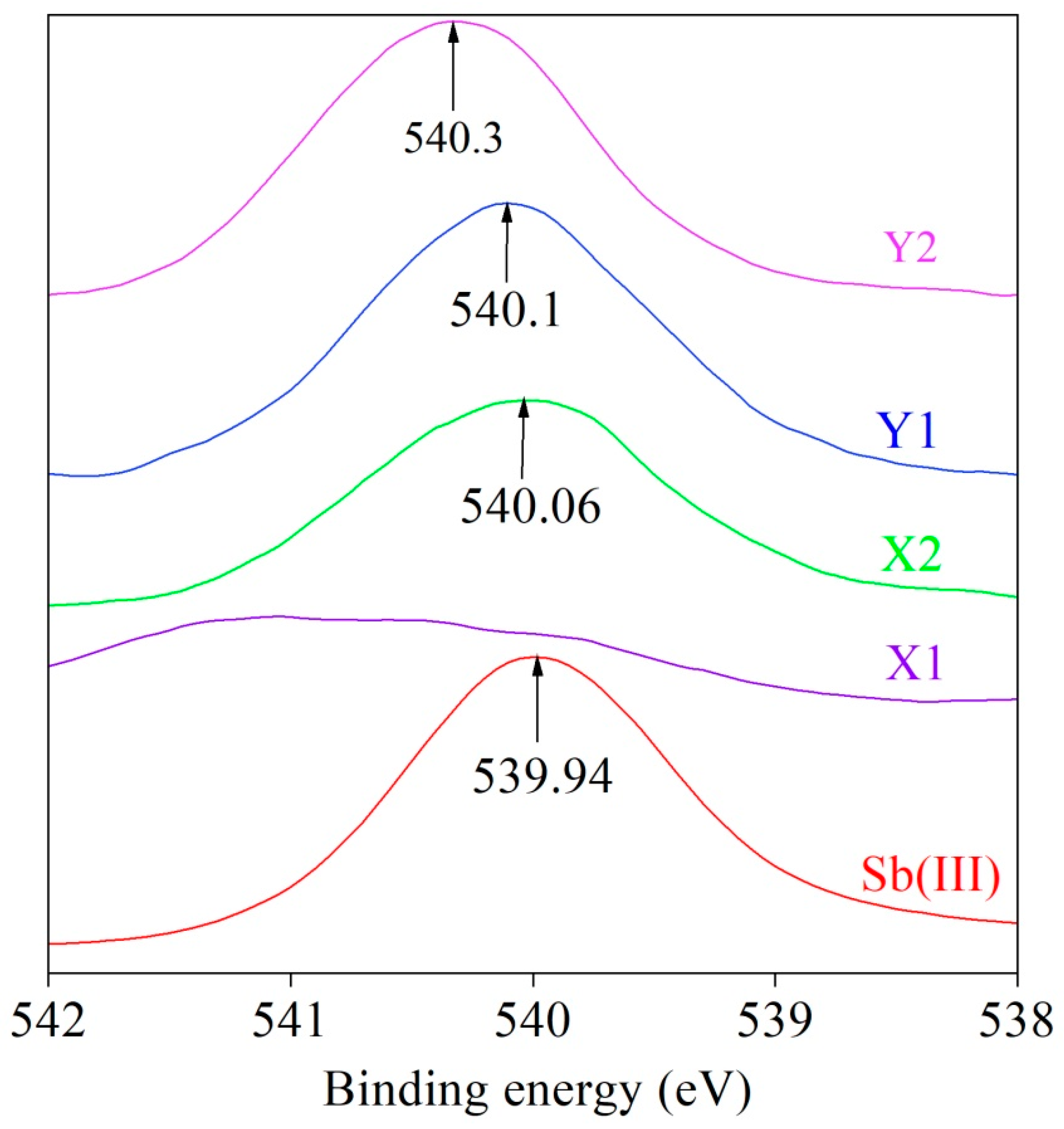
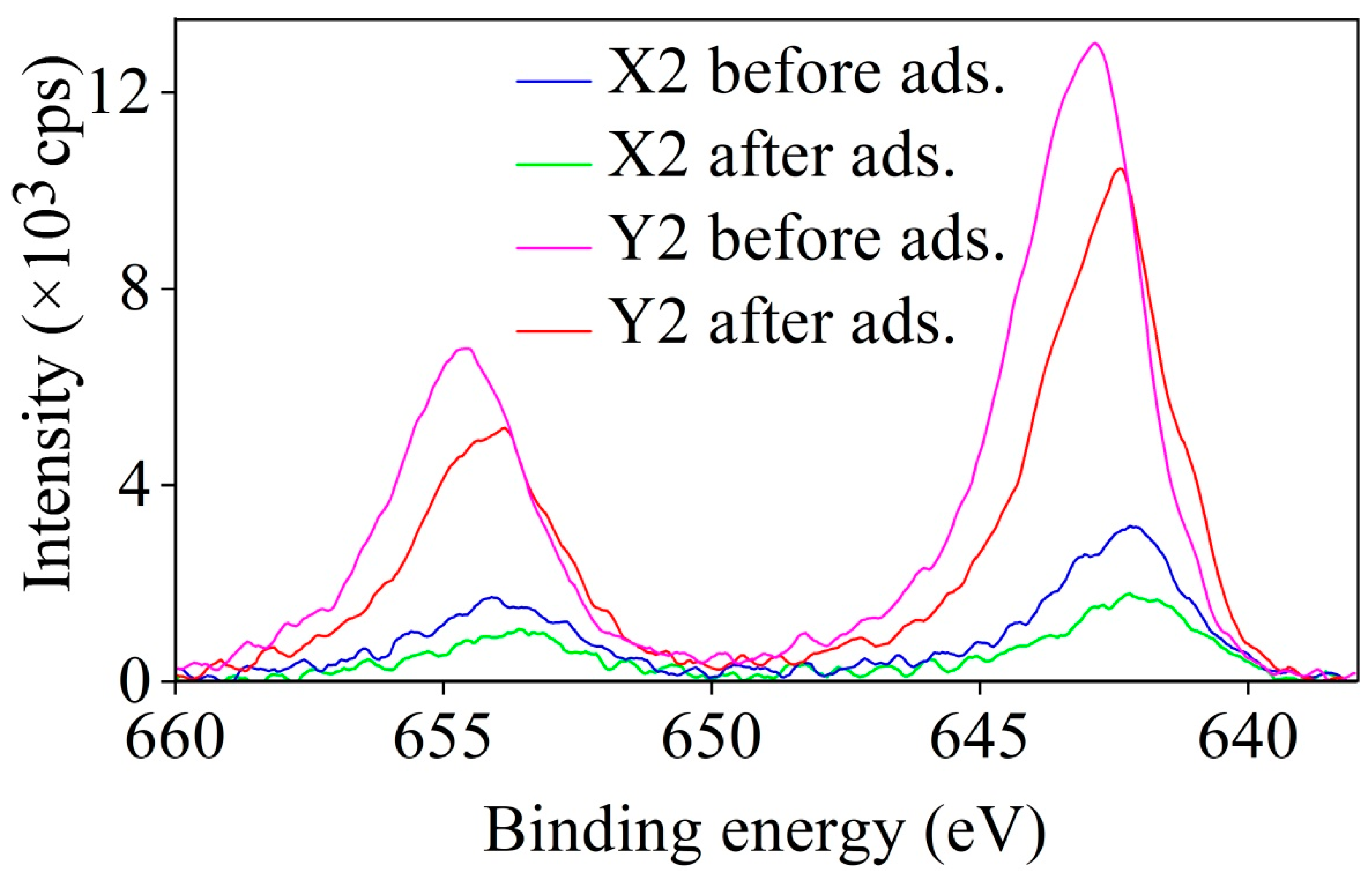
3.5. XPS Results
3.6. Limonite as a Potential Adsorbent and Oxidant for Sb Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
References
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the environment: A review focused on natural waters. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Long, J.; Tan, D.; Deng, S.; Lei, M. Pollution and ecological risk assessment of antimony and other heavy metals in soils from the world’s largest antimony mine area, China. Hum. Ecol. Risk Assess. Int. J. 2017, 24, 679–690. [Google Scholar] [CrossRef]
- Guo, X.; Wu, Z.; He, M. Removal of antimony(V) and antimony(III) from drinking water by coagulation–flocculation–sedimentation (CFS). Water Res. 2009, 43, 4327–4335. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Wang, Q.; He, M.; Wang, Y. Influence of combined pollution of antimony and arsenic on culturable soil microbial populations and enzyme activities. Ecotoxicology 2011, 20, 9–19. [Google Scholar] [CrossRef]
- Warnken, J.; Ohlsson, R.; Welsh, D.T.; Teasdale, P.R.; Chelsky, A.; Bennett, W.W. Antimony and arsenic exhibit contrasting spatial distributions in the sediment and vegetation of a contaminated wetland. Chemosphere 2017, 180, 388–395. [Google Scholar] [CrossRef]
- Reimann, C.; Matschullat, J.; Birke, M.; Salminen, R. Antimony in the environment: Lessons from geochemical mapping. Appl. Geochem. 2010, 25, 175–198. [Google Scholar] [CrossRef]
- Okkenhaug, G.; Zhu, Y.-G.; Luo, L.; Lei, M.; Li, X.; Mulder, J. Distribution, speciation and availability of antimony (Sb) in soils and terrestrial plants from an active Sb mining area. Environ. Pollut. 2011, 159, 2427–2434. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, J.; Zhu, C.; Zhao, D.; Gao, J.; Hao, J.; He, M.; Liu, K.; Wang, K.; Hua, S. A comprehensive global inventory of atmospheric antimony emissions from anthropogenic activities, 1995–2010. Environ. Sci. Technol. 2014, 48, 10235–10241. [Google Scholar] [CrossRef]
- Tandy, S.; Meier, N.; Schulin, R. Use of soil amendments to immobilize antimony and lead in moderately contaminated shooting range soils. J. Hazard. Mater. 2017, 324, 617–625. [Google Scholar] [CrossRef]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony recovery from end-of-life products and industrial process residues: A critical review. J. Sustain. Met. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, D.; Cheng, K.; Lu, L.; Hao, J. Anthropogenic atmospheric emissions of antimony and its spatial distribution characteristics in China. Environ. Sci. Technol. 2012, 46, 3973–3980. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Vink, B. Stability relations of antimony and arsenic compounds in the light of revised and extended Eh-pH diagrams. Chem. Geol. 1996, 130, 21–30. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Rossberg, A.; Vantelon, D.; Xifra, I.; Kretzschmar, R.; Leuz, A.-K.; Funke, H.; Johnson, C.A. Quantitative antimony speciation in shooting-range soils by EXAFS spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 3299–3312. [Google Scholar] [CrossRef]
- He, M.; Wan, H. Distribution, speciation, toxicity and bioavailability of antimony in the environment. Prog. Chem. 2004, 16, 131–135. [Google Scholar]
- Nakamaru, Y.M.; Altansuvd, J. Speciation and bioavailability of selenium and antimony in non-flooded and wetland soils: A review. Chemosphere 2014, 111, 366–371. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Tao, L.; Liu, F.; Zhang, J. Enhanced removal of antimony by acid birnessite with doped iron ions: Companied by the structural transformation. Chemosphere 2019, 226, 834–840. [Google Scholar] [CrossRef]
- Xi, J.; He, M.; Lin, C. Adsorption of antimony(III) and antimony(V) on bentonite: Kinetics, thermodynamics and anion competition. Microchem. J. 2011, 97, 85–91. [Google Scholar] [CrossRef]
- Fan, J.-X.; Wang, Y.-J.; Fan, T.-T.; Cui, X.-D.; Zhou, D.-M. Photo-induced oxidation of Sb(III) on goethite. Chemosphere 2014, 95, 295–300. [Google Scholar] [CrossRef]
- Luo, J.; Luo, X.; Crittenden, J.; Qu, J.; Bai, Y.; Peng, Y.; Li, J. Removal of antimonite (Sb(III)) and antimonate (Sb(V)) from aqueous solution using carbon nanofibers that are decorated with zirconium oxide (ZrO2). Environ. Sci. Technol. 2015, 49, 11115–11124. [Google Scholar] [CrossRef]
- Kong, L.; He, M.; Hu, X. Rapid photooxidation of Sb(III) in the presence of different Fe(III) species. Geochim. Cosmochim. Acta 2016, 180, 214–226. [Google Scholar] [CrossRef]
- Dai, C.; Zhou, Z.; Zhou, X.; Zhang, Y. Removal of Sb(III) and Sb(V) from aqueous solutions using nZVI. Water Air Soil Pollut. 2014, 225, 1799. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Sequential and simultaneous adsorption of Sb(III) and Sb(V) on ferrihydrite: Implications for oxidation and competition. Chemosphere 2016, 145, 55–60. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Effective adsorption of antimony(III) from aqueous solutions by polyamide-graphene composite as a novel adsorbent. Chem. Eng. J. 2017, 307, 230–238. [Google Scholar] [CrossRef]
- Wang, H.; Lv, Z.; Song, Y.; Wang, Y.-N.; Zhang, D.; Sun, Y.; Tsang, Y.F.; Pan, X. Adsorptive removal of Sb(III) from wastewater by environmentally-friendly biogenic manganese oxide (BMO) materials: Efficiency and mechanisms. Process Saf. Environ. Prot. 2019, 124, 223–230. [Google Scholar] [CrossRef]
- Yao, S.; Zhu, X.; Wang, Y.; Zhang, D.; Wang, S.; Jia, Y. Simultaneous oxidation and removal of Sb(III) from water by using synthesized CTAB/MnFe2O4/MnO2 composite. Chemosphere 2020, 245, 125601. [Google Scholar] [CrossRef]
- Thanabalasingam, P.; Pickering, W.F. Specific sorption of antimony (III) by the hydrous oxides of Mn, Fe, and Al. Water Air Soil Pollut. 1990, 49, 175–185. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W.; Wang, Z. Oxidation of antimony (III) by amorphous iron and manganese oxyhydroxides. Chem. Geol. 2001, 174, 379–387. [Google Scholar] [CrossRef]
- Wang, X.; He, M.; Lin, C.; Gao, Y.; Zheng, L. Antimony(III) oxidation and antimony(V) adsorption reactions on synthetic manganite. Geochemistry 2012, 72, 41–47. [Google Scholar] [CrossRef]
- Fu, L.; Shozugawa, K.; Matsuo, M. Oxidation of antimony (III) in soil by manganese (IV) oxide using X-ray absorption fine structure. J. Environ. Sci. 2018, 73, 31–37. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, R.; Zhao, X.; Qu, J. The mechanism of antimony(III) removal and its reactions on the surfaces of Fe–Mn Binary Oxide. J. Colloid Interface Sci. 2011, 363, 320–326. [Google Scholar] [CrossRef]
- Cai, Y.; Mi, Y.; Zhang, H. Kinetic modeling of antimony(III) oxidation and sorption in soils. J. Hazard. Mater. 2016, 316, 102–109. [Google Scholar] [CrossRef]
- Bai, Y.; Jefferson, W.A.; Liang, J.; Yang, T.; Qu, J. Antimony oxidation and adsorption by in-situ formed biogenic Mn oxide and Fe–Mn oxides. J. Environ. Sci. 2017, 54, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tao, J.; Huang, Q.; Tie, B.; Lei, M.; Yang, Y.; Du, H. Co-adsorption of Cd(II) and Sb(III) by ferrihydrite: A combined XPS and ITC study. J. Soils Sediments 2019, 19, 1319–1327. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Xie, Q.; Xu, L.; Liu, H.; Zhou, Y. Mineralogy and geochemistry of limonite as a weathering product of ilvaite in the Yeshan iron deposit, Tongling, China. Clay. Clay Miner. 2018, 66, 190–207. [Google Scholar] [CrossRef]
- Liu, S.; Xu, L.; Chen, P.; Xie, Q.; Chen, T.; Liu, H. Mineralogy of the limonite ore from the Xinqiao sulfide iron deposit in the Tongling ore concentration area of Anhui Province and its implications. Acta Petrol. Mineral. 2016, 35, 531–542. (In Chinese) [Google Scholar]
- Ambe, S. Adsorption kinetics of antimony(V) ions onto α-Fe2O3 surfaces from an aqueous solution. Langmuir 1987, 3, 489–493. [Google Scholar] [CrossRef]
- Watkins, R.; Weiss, D.; Dubbin, W.; Peel, K.; Coles, B.; Arnold, T. Investigations into the kinetics and thermodynamics of Sb(III) adsorption on goethite (α-FeOOH). J. Colloid Interface Sci. 2006, 303, 639–646. [Google Scholar] [CrossRef]
- Xi, J.; He, M. Removal of Sb(III) and Sb(V) from aqueous media by goethite. Water Qual. Res. J. 2013, 48, 223–231. [Google Scholar] [CrossRef]
- Shan, C.; Ma, Z.; Tong, M. Efficient removal of trace antimony(III) through adsorption by hematite modified magnetic nanoparticles. J. Hazard. Mater. 2014, 268, 229–236. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Competitive adsorption of As(III), As(V), Sb(III) and Sb(V) onto ferrihydrite in multi-component systems: Implications for mobility and distribution. J. Hazard. Mat. 2017, 330, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Simeonidis, K.; Papadopoulou, V.; Tresintsi, S.; Kokkinos, E.; Katsoyiannis, I.A.; Zouboulis, A.I.; Mitrakas, M. Efficiency of iron-based oxy-hydroxides in removing antimony from groundwater to levels below the drinking water regulation limits. Sustainability 2017, 9, 238. [Google Scholar] [CrossRef]
- Chen, P.; Chen, T.; Xu, L.; Liu, H.; Xie, Q. Mn-rich limonite from the yeshan iron deposit, tongling district, china: A natural nanocomposite. J. Nanosci. Nanotechnol. 2017, 17, 6931–6935. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Yang, Y.; Wei, H.; Laipan, M.; Zhu, R.; He, H.; Hochella, M., Jr. Coupled redox cycling of Fe and Mn in the environment: The complex interplay of solution species with Fe- and Mn-(oxyhydr)oxide crystallization and transformation. Earth-Sci. Rev. 2022, 232, 104105. [Google Scholar] [CrossRef]
- Tighe, M.; Lockwood, P.; Wilson, S. Adsorption of antimony(v) by floodplain soils, amorphous iron(iii) hydroxide and humic acid. J. Environ. Monit. 2005, 7, 1177–1185. [Google Scholar] [CrossRef]
- Kolbe, F.; Weiss, H.; Morgenstern, P.; Wennrich, R.; Lorenz, W.; Schurk, K.; Stanjek, H.; Daus, B. Sorption of aqueous antimony and arsenic species onto akaganeite. J. Colloid Interface Sci. 2011, 357, 460–465. [Google Scholar] [CrossRef]
- Vithanage, M.; Rajapaksha, A.U.; Dou, X.; Bolan, N.S.; Yang, J.E.; Ok, Y.S. Surface complexation modeling and spectroscopic evidence of antimony adsorption on iron-oxide-rich red earth soils. J. Colloid Interface Sci. 2013, 406, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Cai, Y.; Li, L.; Zhang, H. Kinetic modeling of pH-dependent antimony(V) sorption and transport in iron oxide-coated sand. Chemosphere 2015, 138, 758–764. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption-from theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Romero-González, J.; Peralta-Videa, J.R.; Rodríguez, E.; Ramirez, S.L.; Gardea-Torresdey, J.L. Determination of thermodynamic parameters of Cr(VI) adsorption from aqueous solution onto Agave lechuguilla biomass. J. Chem. Thermody. 2005, 37, 343–347. [Google Scholar] [CrossRef]
- Leuz, A.K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to goethite: influence on Sb(III) oxidation and mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, J.S.; Hiemstra, T.; Van Riemsdijk, W.H. Phosphate and sulfate adsorption on goethite: Single anion and competitive adsorption. Geochim. Cosmochim. Acta 1997, 61, 2389–2396. [Google Scholar] [CrossRef]
- Wang, L.; Wan, C.-L.; Zhang, Y.; Lee, D.-J.; Liu, X.; Chen, X.-F.; Tay, J.-H. Mechanism of enhanced Sb(V) removal from aqueous solution using chemically modified aerobic granules. J. Hazard. Mater. 2015, 284, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Bang, S.; Korfiatis, G.P. Effects of silicate, sulfate, and carbonate on arsenic removal by ferric chloride. Water Res. 2000, 34, 1255–1261. [Google Scholar] [CrossRef]
- Gu, C.; Wang, Z.; Kubicki, J.D.; Wang, X.; Zhu, M. X-ray absorption spectroscopic quantification and speciation modeling of sulfate adsorption on ferrihydrite surfaces. Environ. Sci. Technol. 2016, 50, 8067–8076. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.; Liu, R.; Qu, J. Removal of phosphate from water by a Fe–Mn binary oxide adsorbent. J. Colloid Interface Sci. 2009, 335, 168–174. [Google Scholar] [CrossRef]
- Brechbühl, Y.; Christl, I.; Elzinga, E.J.; Kretzschmar, R. Competitive sorption of carbonate and arsenic to hematite: Combined ATR-FTIR and batch experiments. J. Colloid Interface Sci. 2012, 377, 313–321. [Google Scholar] [CrossRef]
- Terrier, C.; Chatelon, J.; Roger, J.; Berjoan, R.; Dubois, C. Analysis of antimony doping in tin oxide thin films obtained by the sol-gel method. J. Sol-Gel Sci. Technol. 1997, 10, 75–81. [Google Scholar] [CrossRef]
- Da Fonseca, B.T.; D’Elia, E.; Siqueira, J.M.; De Oliveira, S.M.; Castro, K.L.D.; Ribeiro, E.S. Study of the characteristics, properties and characterization of new SiO2/TiO2/Sb2O5 ternary oxide obtained by the sol-gel process. J. Mater. Sci. Mater. Electron. 2018, 29, 2159–2169. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Wu, F.; Cao, Z.; Wang, S.; Zhong, H. Novel and green metallurgical technique of comprehensive utilization of refractory limonite ores. J. Clean. Prod. 2018, 171, 831–843. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Han, Y.; Li, Y.; Gao, P. Iron recovery from refractory limonite ore using suspension magnetization roasting: A pilot-scale study. J. Clean. Prod. 2020, 261, 121221. [Google Scholar] [CrossRef]
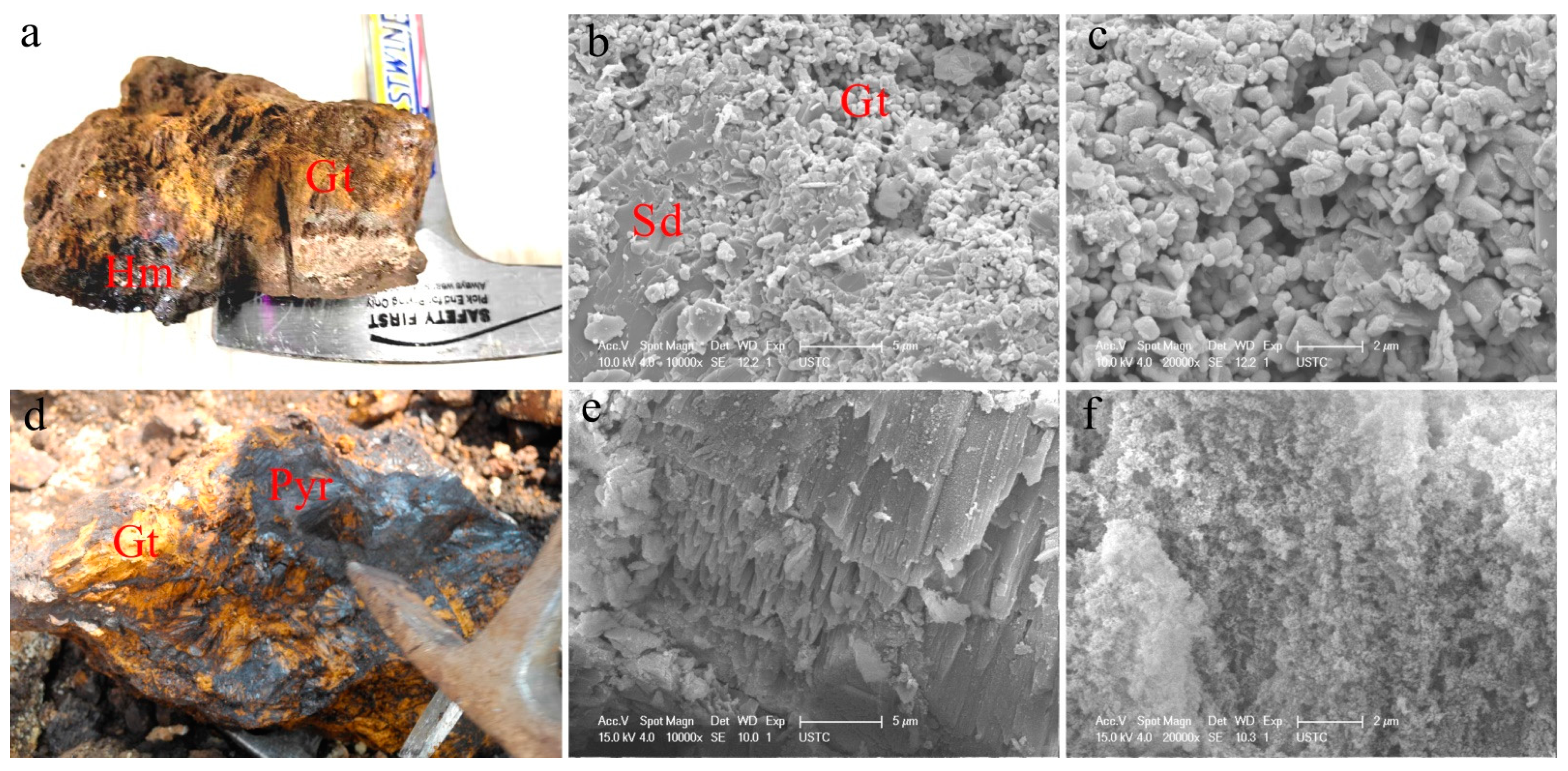



| Sample ID | Fe2O3 | SiO2 | Al2O3 | MnO | pHPZC b | SA c | Mineral Assemblage |
|---|---|---|---|---|---|---|---|
| X1 | 83.79 | 2.39 | 1.42 | − a | 6.9 | 13.7 | goethite, hematite, and quartz |
| X2 | 66.26 | 19.01 | 5.47 | 3.56 | 4.0 | 12.8 | goethite, hematite, quartz, illite, and pyrolusite |
| Y1 | 80.01 | 1.75 | 1.73 | 0.49 | 7.1 | 124.8 | goethite, hematite, and amorphous silica |
| Y2 | 68.08 | 7.02 | 2.33 | 19.24 | 4.1 | 171.7 | goethite, hematite, Mn-goethite, groutite, ramsdellite, pyrolusite, quartz, and residual ivalite |
| Sample | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| ID | qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 L/(mg·min) | R2 |
| X1 | 1.475 | 0.223 | 0.899 | 1.525 | 0.242 | 0.956 |
| X2 | 2.008 | 0.117 | 0.880 | 2.104 | 0.084 | 0.959 |
| Y1 | 3.162 | 0.106 | 0.970 | 3.280 | 0.059 | 0.994 |
| Y2 | 3.134 | 0.557 | 0.996 | 3.154 | 0.842 | 0.998 |
| Sample ID | T | Freundlich Parameters | Langmuir Parameters | ||||
|---|---|---|---|---|---|---|---|
| KF | n | R2 | KL | Qmax | R2 | ||
| X1 | 298.15 | 1.424 | 2.47 | 0.957 | 0.030 | 12.995 | 0.980 |
| 308.15 | 1.822 | 2.50 | 0.953 | 0.033 | 15.839 | 0.985 | |
| 318.15 | 2.393 | 2.61 | 0.928 | 0.041 | 18.344 | 0.991 | |
| X2 | 298.15 | 2.816 | 3.38 | 0.980 | 0.086 | 12.442 | 0.940 |
| 308.15 | 2.158 | 2.34 | 0.973 | 0.046 | 18.758 | 0.951 | |
| 318.15 | 3.432 | 2.35 | 0.955 | 0.058 | 27.727 | 0.960 | |
| Y1 | 298.15 | 5.381 | 3.18 | 0.975 | 0.076 | 26.187 | 0.948 |
| 308.15 | 4.901 | 2.65 | 0.980 | 0.049 | 34.335 | 0.950 | |
| 318.15 | 7.566 | 2.55 | 0.975 | 0.043 | 58.008 | 0.967 | |
| Y2 | 298.15 | 8.609 | 2.11 | 0.988 | 0.024 | 110.970 | 0.978 |
| 308.15 | 8.362 | 1.95 | 0.984 | 0.020 | 136.451 | 0.974 | |
| 318.15 | 10.297 | 1.94 | 0.978 | 0.021 | 165.500 | 0.984 | |
| Sample ID | T (K) | K0 (L/g) | ΔH (kJ/mol) | ΔS (J/(mol·k)) | ΔG (kJ/mol) |
|---|---|---|---|---|---|
| X1 | 298 | 1.17 | 11.58 | 40.20 | −0.40 |
| 308 | 1.39 | 11.58 | 40.36 | −0.85 | |
| 318 | 1.57 | 11.58 | 40.16 | −1.19 | |
| X2 | 298 | 4.95 | 21.67 | 80.01 | −3.96 |
| 308 | 2.66 | 21.67 | 78.51 | −2.51 | |
| 318 | 8.58 | 21.67 | 86.01 | −5.68 | |
| Y1 | 298 | 35.87 | −22.83 | −46.85 | −8.87 |
| 308 | 29.08 | −22.83 | −46.10 | −8.63 | |
| 318 | 20.09 | −22.83 | −46.85 | −7.93 | |
| Y2 | 298 | 28.79 | 0.79 | 30.57 | −8.32 |
| 308 | 76.71 | 0.79 | 38.64 | −11.11 | |
| 318 | 29.37 | 0.79 | 30.60 | −8.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Zhou, Y.; Chen, Y.; Xie, Q.; Chen, T. Limonite as a Natural Adsorbent for the Removal of Antimony(III) from an Aqueous Solution. Minerals 2023, 13, 1494. https://doi.org/10.3390/min13121494
Luo H, Zhou Y, Chen Y, Xie Q, Chen T. Limonite as a Natural Adsorbent for the Removal of Antimony(III) from an Aqueous Solution. Minerals. 2023; 13(12):1494. https://doi.org/10.3390/min13121494
Chicago/Turabian StyleLuo, Haicui, Yuefei Zhou, Yan Chen, Qiaoqin Xie, and Tianhu Chen. 2023. "Limonite as a Natural Adsorbent for the Removal of Antimony(III) from an Aqueous Solution" Minerals 13, no. 12: 1494. https://doi.org/10.3390/min13121494
APA StyleLuo, H., Zhou, Y., Chen, Y., Xie, Q., & Chen, T. (2023). Limonite as a Natural Adsorbent for the Removal of Antimony(III) from an Aqueous Solution. Minerals, 13(12), 1494. https://doi.org/10.3390/min13121494






