Research Status and Challenges of High-Purity Quartz Processing Technology from a Mineralogical Perspective in China
Abstract
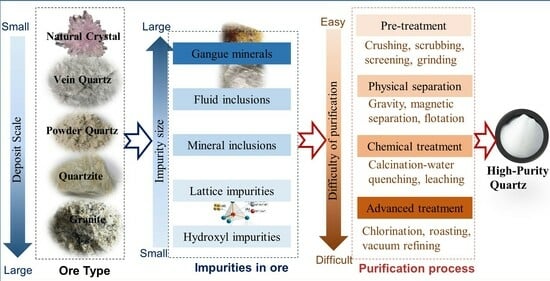
:1. Introduction
2. The Characteristics of Quartz Mineral Resources
2.1. The Reserves of Quartz Mineral Resources in China
2.2. Geological Origins of Quartz Deposits
2.3. Impurities in Quartz Ores
2.3.1. Impurity Elements
2.3.2. Coexisting Gangue Minerals
2.3.3. Fluid Inclusions
2.3.4. Mineral Inclusions
2.4. Structural Impurities in Quartz Minerals
2.4.1. Lattice Impurities
2.4.2. Hydroxyl Impurities
3. Analyzing the Quartz Purification Process from a Mineralogical Perspective
3.1. Common Quartz Purification Techniques
3.1.1. Flotation Separation
- The “with fluoride and acid” method, involves the activation of feldspar using HF acid to enhance the differences in surface properties. Cationic collectors like dodecylamine are then employed to selectively capture the feldspar. However, it is important to note that the use of HF acid carries significant safety risks, and the wastewater generated during this process has a notable environmental impact [35].
- The “without fluoride but with acid” method, entails floating feldspar under strongly acidic conditions, using a combination of cationic and anionic collectors such as dodecylamine and sodium oleate. Currently, this method represents a well-established industrial approach to quartz purification through flotation [36].
- The “without fluoride and without acid” method involves floating feldspar under neutral or alkaline conditions. In a neutral setting, appropriate depressants are employed to remove anionic collectors from the quartz’s surface, inhibiting its flotation. In alkaline conditions, Mg2+ and Ca2+ ions serve as activators, while amine collectors or sodium alkyl sulfonates are used to selectively capture the quartz [37,38].
3.1.2. Acid Leaching
3.1.3. Calcination-Water Quenching
3.1.4. Chlorination Roasting
3.2. Current Status of Research on Quartz Purification Processes
4. Challenges of Quartz Purification Technology
4.1. Selection and Evaluation of Purification Materials
4.2. The Challenges in Purification Technology
4.2.1. The Challenge of Complete Quartz Monomer Liberation
4.2.2. Acid Consumption and Pollution in the Leaching Process
4.2.3. Complexity of the Purification Process
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Lin, X. Research on preparation of High-purified quartz micropowder and quartz glass sand from quartz rock. Non-Met. Mines 2005, 28, 37–39. [Google Scholar]
- The National Bureau of Statistics. Strategic Emerging Industries Classification; National Bureau of Statistics Order No. 23; The National Bureau of Statistics: Beijing, China, 2018.
- Wang, J. Global high purity quartz deposits: Resources distribution and exploitation status. Acta Petrol. Et Mineral. 2021, 1, 131–141. [Google Scholar]
- China Industrial Research Network. Research Report on Comprehensive Investigation and Future Trends Analysis of China’s High-Purity Quartz Sand Industry from 2020 to 2026; China Industrial Research Network: Shanghai, China, 2020. [Google Scholar]
- Götze, J. Chemistry, textures and physical properties of quartz-geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Pan, X.; Li, S.; Li, Y.; Guo, P.; Zhao, X.; Cai, Y. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Z.; Wei, Y.; Liu, B.; Meng, Y.; Qiu, H.; Li, Y. A critical review on the mineralogy and processing for high-grade quartz. Min. Metall. Explor. 2020, 37, 1627–1639. [Google Scholar] [CrossRef]
- Lin, M.; Lei, S.; Pei, Z.; Liu, Y.; Xia, Z.; Xie, F. Application of hydrometallurgy techniques in quartz processing and purification: A review. Metall. Res. Technol. 2018, 115, 303. [Google Scholar] [CrossRef]
- Li, P. Geological characteristics of the non-metallic mineral resource: Powdered quartz deposits. Reg. Gov. 2019, 277, 83–85. [Google Scholar]
- Ma, C.; Feng, A.; Liu, C.; Shao, W.; Zhao, P. Mineralogical Characteristics and Progress in Processing Technology of Raw Materials of High Purity Quartz. Conserv. Util. Miner. Resour. 2019, 39, 48–57. [Google Scholar] [CrossRef]
- Vatalis, K.I.; Charalambides, G.; Benetis, N.P. Market of high purity quartz innovative applications. Procedia Econ. Financ. 2015, 24, 734–742. [Google Scholar] [CrossRef]
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383. [Google Scholar] [CrossRef]
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralisation in kyanite quartzites, Norway. Miner. Depos. 2007, 42, 523–535. [Google Scholar] [CrossRef]
- Larsen, R.B.; Polvé, M.; Juve, G. Granite pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for the formation of high-purity quartz. Nor. Geol. Unders. 2000, 436, 57–66. [Google Scholar]
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, Geochemistry and Genesis of Agate—A Review. Minerals 2020, 10, 1037. [Google Scholar] [CrossRef]
- Wang, M.; Cai, L.; Wen, J.; Li, W.; Yang, X.; Yang, H. The Prospect of Recovering Vanadium, Nickel, and Molybdenum from Stone Coal by Using Combined Beneficiation and Metallurgy Technology Based on Mineralogy Features. Minerals 2022, 13, 21. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Burnard, P. Noble Gases and Halogens in Fluid Inclusions: A Journey Through the Earth’s Crust. In The Noble Gases as Geochemical Tracers. Advances in Isotope Geochemistry; Burnard, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, W.; Wang, D.; Sun, H.; Chen, K.; Yang, B. The role of surface roughness in the wettability and floatability of quartz particles. Appl. Surf. Sci. 2020, 527, 146799. [Google Scholar] [CrossRef]
- Cai, W.; Liu, X.; Zhang, Z.; Gao, J.; Lei, M.; Cui, Q.; Ma, M.; Li, Y.; Song, Y. Genesis of the Yi’nan Tongjing Gold–Copper Skarn Deposit, Luxi District, North China Craton: Evidence from Fluid Inclusions and H–O Isotopes. Minerals 2023, 13, 1348. [Google Scholar] [CrossRef]
- Schmidt-Mumm, A. Low frequency acoustic emission from quartz upon heating from 90 to 610 °C. Phys. Chem. Miner. 1991, 17, 545–553. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Muggerud, A.M.F.; Erbe, A. In situ high temperature spectroscopic study of liquid inclusions and hydroxyl groups in high purity natural quartz. Miner. Eng. 2021, 174, 107238. [Google Scholar] [CrossRef]
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Mineral. Mag. 2009, 73, 569–583. [Google Scholar] [CrossRef]
- Yang, B.; Fu, Y.; Yin, W.; Sheng, Q.; Zhu, Z.; Yin, X. Selective collection performance of an efficient quartz collector and its response to flotation separation of malachite from quartz. Miner. Eng. 2021, 172, 107174. [Google Scholar] [CrossRef]
- Müller, A.; Lennox, P.; Trzebski, R. Cathodoluminescence and micro-structural evidence for crystallisation and deformation processes of granites in the Eastern Lachlan Fold Belt (SE Australia). Contrib. Mineral. Petrol. 2002, 143, 510–524. [Google Scholar] [CrossRef]
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Xia, Z.; Li, W.; Lei, S. Influences of Na2CO3 roasting and H3PO4 hot-pressure leaching on the purification of vein quartz to achieve high-purity quartz. Hydrometallurgy 2023, 218, 106065. [Google Scholar] [CrossRef]
- Stalder, R. OH point defects in quartz—A review. Eur. J. Mineral. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Biró, T.; Kovács, I.J.; Király, E.; Falus, G.; Karátson, D.; Bendő, Z.; Sándorné, J.K. Concentration of hydroxyl defects in quartz from various rhyolitic ignimbrite horizons: Results from unpolarized micro-FTIR analyses on unoriented phenocryst fragments. Eur. J. Mineral. 2016, 28, 313–327. [Google Scholar] [CrossRef]
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Arstad, B.; Muggerud, A.M.F.; Erbe, A. Structural evolution of water and hydroxyl groups during thermal, mechanical and chemical treatment of high purity natural quartz. RSC Adv. 2020, 10, 29018–29030. [Google Scholar] [CrossRef] [PubMed]
- Folstad, M.B.; Yu, H.; Wang, H.; Tangstad, M. Disintegration of Six Different Quartz Types during Heating to 1600 °C. Minerals 2023, 13, 132. [Google Scholar] [CrossRef]
- Dapiaggi, M.; Pagliari, L.; Pavese, A.; Sciascia, L.; Merli, M.; Francescon, F. The formation of silica high temperature polymorphs from quartz: Influence of grain size and mineralising agents. J. Eur. Ceram. Soc. 2015, 35, 4547–4555. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Wu, J.; Wu, D.; Wei, K.; Ma, W. Effect of quartz crystal structure transformations on the removal of iron impurities. Hydrometallurgy 2021, 204, 105715. [Google Scholar] [CrossRef]
- Campione, M.; Malaspina, N.; Frezzotti, M.L. Threshold size for fluid inclusion decrepitation. JGR Solid Earth 2015, 120, 7396–7402. [Google Scholar] [CrossRef]
- Du, X.; Liang, C.; Hou, D.; Sun, Z.; Zheng, S. Scrubbing and Inhibiting Coagulation Effect on the Purification of Natural Powder Quartz. Minerals 2019, 9, 140. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R.A. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Wang, Y.; Khoso, S.A.; Luo, X.; Tian, M. Understanding the depression mechanism of citric acid in sodium oleate flotation of Ca2+-activated quartz: Experimental and DFT study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Kou, J.; Sun, C. Synergy Effect between Sodium Oleate and Alcohol Ethoxylates on the Reverse Flotation of Quartz. Minerals 2023, 13, 93. [Google Scholar] [CrossRef]
- Zhong, L. Study on Purifying Preparation and Mechanism of Ultra-Pure Quartz. Ph.D. thesis, Wuhan University of Technology, Wuhan, China, 2015. [Google Scholar]
- Lee, S.O.; Tran, T.; Jung, B.H.; Kim, S.J.; Kim, M.J. Dissolution of iron oxide using oxalic acid. Hydrometallurgy 2007, 87, 91–99. [Google Scholar] [CrossRef]
- Veglio, F.; Passariello, B.; Barbaro, M.; Plescia, P.; Marabini, A.M. Drum leaching tests in iron removal from quartz using oxalic and sulphuric acids. Int. J. Miner. Process. 1998, 54, 183–200. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, X.; Huang, H.; Zhou, L.; Xiong, T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process. 2012, 114, 30–34. [Google Scholar] [CrossRef]
- Lin, M.; Pei, Z.; Lei, S. Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals 2017, 7, 161. [Google Scholar] [CrossRef]
- Kenneth, B.L.; Robert, D.J.; Asheville, N.C. Purified Quartz and Process for Purifying Quartz. U.S. Patent 5037625, 6 August 1991. [Google Scholar]
- Zhong, T.; Yu, W.; Shen, C.; Wu, X. Research on preparation and characterisation of high-purity silica sands by purification of quartz vein ore from dabie mountain. Silicon 2021, 14, 4723–4729. [Google Scholar] [CrossRef]
- Dang, C. Experimental Study of Processing 4N8 Standard Grade High-Purity Quartz by Vein Quartz. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2014. [Google Scholar]
- Yuan, X.; Wu, X.; Du, G.; Wen, R.; Bu, C. Purifying technology of preparing high purity quartz sands from vein quartz ore. China Powder Sci. Technol. 2013, 19, 52–57. [Google Scholar]
- Lei, S.; Pei, Z.; Zhong, L.; Ma, Q.; Huang, D.; Yang, Y. Study on the technology and mechanism of reverse flotation and hot pressing leaching with vein quartz. Non-Met. Mines 2014, 2, 40–43. [Google Scholar]
- Tan, J.; Wang, L.; Yang, L. Study of quartz sandstone refinement in Muchuan, Sichuan province. Guizhou Geol. 2010, 27, 312–316. [Google Scholar]
- Wang, B.; Li, D. Research on purification of Leshan quartz sand by De-ironing. China Non-Met. Min. Ind. J. 2013, 3, 24–27. [Google Scholar]
- Yang, F.; Li, L.; Li, K.; Zhou, X. Study on process mineralogy of quartz sandstone in Helan Mountain of Ningxia. China Non-Met. Min. Ind. J. 2020, 1, 13–16. [Google Scholar]
- Wu, Z.; Liu, X.; Wang, L.; Zheng, S. Study on processing and purification technology of powder quartz in Jiangxi. China Non-Met. Min. Ind. J. 2011, 4, 21–23. [Google Scholar]
- Tian, H.; Huang, L.; Tian, Z. A Method for Producing 4N and above SiO2 Quartz Sand from Granite Pegmatite as Raw Material. Chinese Invention Patent 202210778924.9, 4 October 2023. [Google Scholar]
- Liu, G.; Ma, Y.; Liu, L.; Zhang, H.; Zhu, L.; Guo, L.; Cao, F. Study on deep impurity removal technology of a granite pegmatite-type high-purity quartz in Altay Region of Xinjiang. Conserv. Util. Miner. Resour. 2022, 42, 7. [Google Scholar]
- Zhao, J.; Zhang, C.; Zhang, S. Study on the utilization of high-purity quartz minerals in the Dongqinling granite pegmatite. Bull. Mineral. Petrol. Geochem. 2022, 41, 1305–1308. [Google Scholar]
- Tuncuk, A.; Akcil, A. Iron removal in production of purified quartz by hydrometallurgical process. Int. J. Miner. Process. 2016, 153, 44–50. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zuo, Q.; Li, J.; Ban, B.; Chen, J. Purification mechanism of quartz sand by combination of microwave heating and ultrasound assisted acid leaching treatment. Silicon 2021, 13, 531–541. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, Y.; Jeong, S.; Chae, Y.; Ko, K. Acid leaching purification and neutron activation analysis of high purity silicas. J. Radioanal. Nucl. Chem. 2009, 282, 629–633. [Google Scholar] [CrossRef]
- Taxiarchou, M.; Panias, D.; Douni, I.; Paspaliaris, I.; Kontopoulos, A. Removal of iron from silica sand by leaching with oxalic acid. Hydrometallurgy 1997, 46, 215–227. [Google Scholar] [CrossRef]
- Zhou, Y. Study on refining quartz powder by leaching in HF acid solution. J. Mineral. Pet. 2005, 25, 23–26. [Google Scholar]
- Buttress, A.J.; Rodriguez, J.M.; Ure, A.; Ferrari, R.S.; Dodds, C.; Kingman, S.W. Production of high purity silica by microfluidic-inclusion fracture using microwave pre-treatment. Miner. Eng. 2019, 131, 407–419. [Google Scholar] [CrossRef]
- Zhao, D. Research on Removing Tiny Fluid Inclusions for Preparation of High Purity Quartz Sand. Master’s Thesis, South China University of Technology, Guangzhou, China, 2014. [Google Scholar]
- Li, J.; Li, X.; Qin, S.; Zhang, Z.; Du, F. Further purification of industrial quartz by much milder conditions and a harmless method. Environ. Sci. Technol. 2010, 44, 7673–7677. [Google Scholar] [CrossRef]
- Du, J. Purification Methods Applied in the Production of High-Purity Quartz Sand for Quartz Glass Raw Materials. Chinese Patent CN101337767, 29 September 2010. [Google Scholar]
- Wang, W.; Cong, J.; Deng, J.; Weng, X.; Lin, Y.; Huang, Y.; Peng, T. Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment. Minerals 2018, 8, 149. [Google Scholar] [CrossRef]
- Zhang, D. Experimental Study of 5N High-Purity Quartz Processing by Vein Quartz. Master’s Thesis, Chengdu University of Technology, Chengdu, China, 2016. [Google Scholar]
- Martello, E.D.; Bernardis, S.; Larsen, R.B.; Tranell, G.; Di Sabatino, M.; Arnberg, L. Electrical fragmentation as a novel route for the refinement of quartz raw materials for trace mineral impurities. Powder Technol. 2012, 224, 209–216. [Google Scholar] [CrossRef]








| Ore Type | Ore Genesis | Deposit Scale | Mineralogical Features | Application Areas | Resource Distribution/Extraction Status |
|---|---|---|---|---|---|
| Natural Crystal | Slow growth in crystal caves | Small | High purity, high transparency | Optical instruments, electronics | Low resource reserves, difficult to mine |
| Vein Quartz | Hydrothermal filling along fault fractures | Medium/small | Well-crystallized | Glass, metallurgy | Many mining locations, low reserves |
| Quartz Sandstone | Lacustrine deposition | Large/medium | Comprising intricate cementing materials | Glass, ceramics, building materials | Concentrated in southern China, easy to mine |
| Quartz Sand | Alluvial deposition | Large/medium | Lack of natural grain shape | Glass, construction, casting molds | Large reserves, favorable mining |
| Powder Quartz | Weathering residual | Large/medium | Extremely fine particle size | Metallurgy, glass, cement, ceramics | Found in Jiangxi, Guizhou, Hunan |
| Quartzite | Sedimentary deposits altered by metamorphism | Large/very large | Blocky structure | Refractories, silicon alloys, glass | Large reserves |
| Granite Quartz | Slow crystallization in deep magma | Large/very large | Large grains, higher impurity | Underdeveloped utilization | Widely distributed |
| Purification Method | Principle | Main Impurities Separated | Characteristics |
|---|---|---|---|
| Scrubbing | Mutual particle friction | Adhered fine clay and oxide films | Simple operation, low purification efficiency |
| Gravity Separation | Differences in mineral density | Heavy minerals like zircon, garnet, and epidote | Ore loss in the process |
| Magnetic Separation | Differences in mineral magnetism | Magnetic minerals such as magnetite, hematite, and pyrrhotite | Requires multiple stages of strong magnetic fields |
| Flotation | Differences in mineral surface properties | Silicate minerals like mica, feldspar, and iron minerals | Relies on the flotation reagent system |
| Calcination-Water Quenching | Volume expansion during quartz phase transition | Rupture and exposure of inclusion impurities | Enhances acid leaching purification |
| Leaching | Differential solubility in acids | Impurity elements like Fe, Al, Cr, Ti | Acidic nature with corrosive properties |
| Chlorination Roasting | Generation of chemical gradients with chlorine gas | Impurity elements within the lattice | Limited processing capacity, and gaseous chloride emissions |
| Types of Ores | Location | SiO2 of Raw Ore/% | Feed Size/mm | Purification Process | SiO2 of Purified Product/% | References |
|---|---|---|---|---|---|---|
| Vein quartz | Anhui Dabie Mountain | 99.06 | 0.074–0.1 | Crushing–grinding–calcination–water quenching–flotation–leaching | >99.99 | [45] |
| Sichuan province | 99.95 | <0.38 | Crushing–calcination–water quenching–leaching | >99.99 | [46] | |
| Anhui province | 99.67 | <0.29 | Calcination–water quenching–flotation–leaching–washing | >99.9 | [47] | |
| Sichuan province | 99.96 | <0.1 | Crushing–screening–magnetic separation–flotation–high–temperature high–pressure leaching | >99.99 | [48] | |
| Quartz sandstone | Sichuan Muchuan county | 93.42 | 0.1–0.2 | Crushing–scrubbing–grinding–magnetic separation–leaching | >99.9 | [49] |
| Sichuan Leshan | 97.08 | <0.074 | Crushing–grinding–washing–leaching–roasting–water quenching secondary leaching | >99 | [50] | |
| Ningxia Helanshan | 98.48 | <0.096 | Calcination–water quenching–grinding scrubbing–magnetic separation–leaching | >99.9 | [51] | |
| Quartzite | Jiangxi Tonggu | 97.44 | 0.106–0.425 | Crushing–screening–grinding–magnetic separation–leaching | >99 | [1] |
| Powder quartz | Jiangxi Province | 97.90 | <0.04 | Screening–magnetic separation–flotation–leaching–calcination | >99.9 | [52] |
| Granite/pegmatite | Gansu Akse County | Quartz content ~25% | 0.096–0.21 | Crushing–roasting–water quenching–magnetic separation–color sorting–high–temperature high–pressure leaching–flotation–heavy liquid separation–high–temperature chlorination–high–temperature oxidation | >99.99 | [53] |
| Xinjiang Altai | 84.45 | <0.425 | Crushing–gravity separation–magnetic separation–flotation–calcination–water quenching–leaching | >99.99 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Tang, C.; Ni, W.; Yuan, J.; Zhou, Y.; Liu, X. Research Status and Challenges of High-Purity Quartz Processing Technology from a Mineralogical Perspective in China. Minerals 2023, 13, 1505. https://doi.org/10.3390/min13121505
Zhang R, Tang C, Ni W, Yuan J, Zhou Y, Liu X. Research Status and Challenges of High-Purity Quartz Processing Technology from a Mineralogical Perspective in China. Minerals. 2023; 13(12):1505. https://doi.org/10.3390/min13121505
Chicago/Turabian StyleZhang, Ruiyang, Chunhua Tang, Wen Ni, Jing Yuan, Yu Zhou, and Xiaolong Liu. 2023. "Research Status and Challenges of High-Purity Quartz Processing Technology from a Mineralogical Perspective in China" Minerals 13, no. 12: 1505. https://doi.org/10.3390/min13121505
APA StyleZhang, R., Tang, C., Ni, W., Yuan, J., Zhou, Y., & Liu, X. (2023). Research Status and Challenges of High-Purity Quartz Processing Technology from a Mineralogical Perspective in China. Minerals, 13(12), 1505. https://doi.org/10.3390/min13121505