Effects of Sodium Nanoalginate and Lime on Swelling Properties of Expansive Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Sodium Nanoalginate
2.3. Lime
2.4. Experimental Methodology
3. Results and Discussion
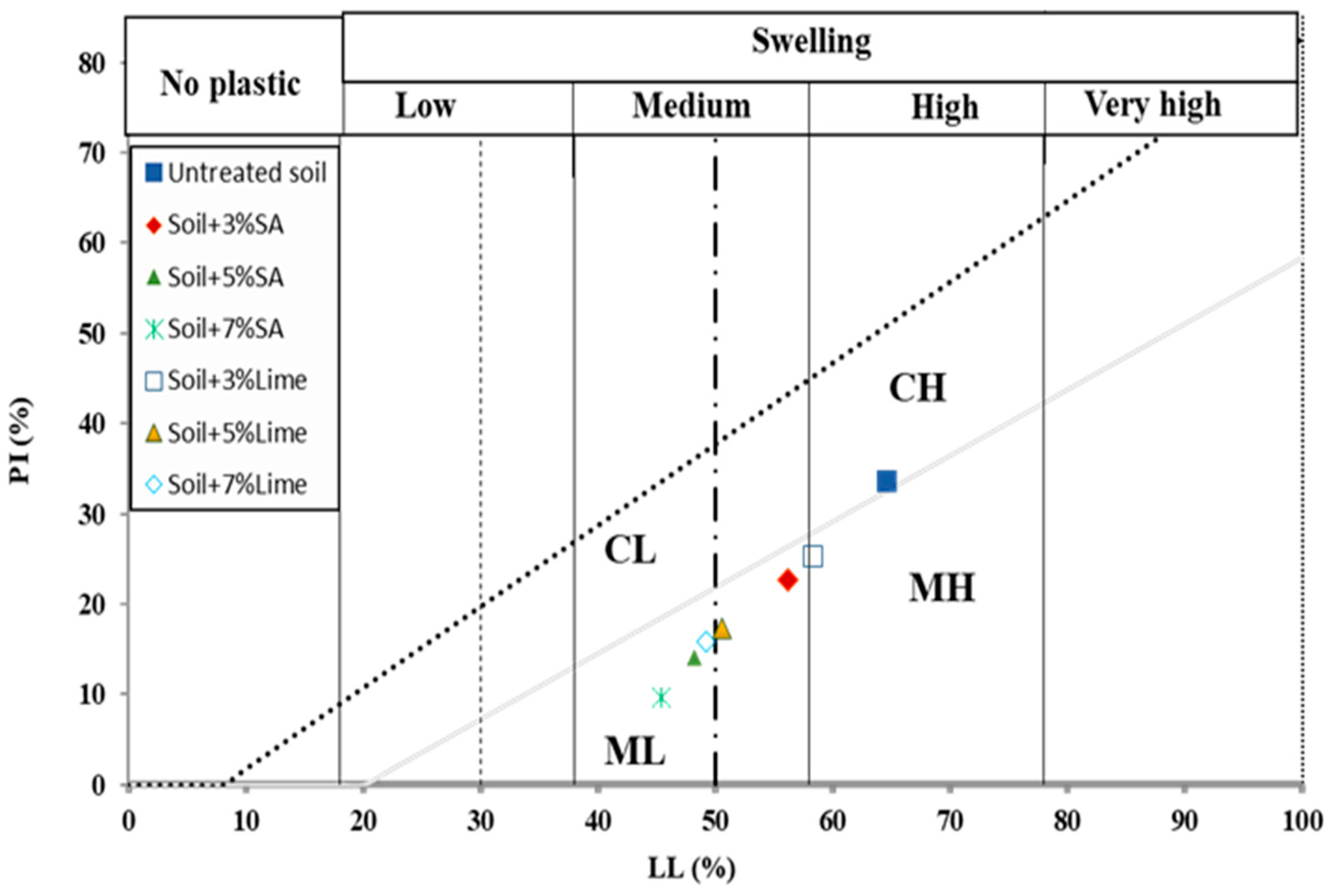
3.1. Atterberg Limit Test
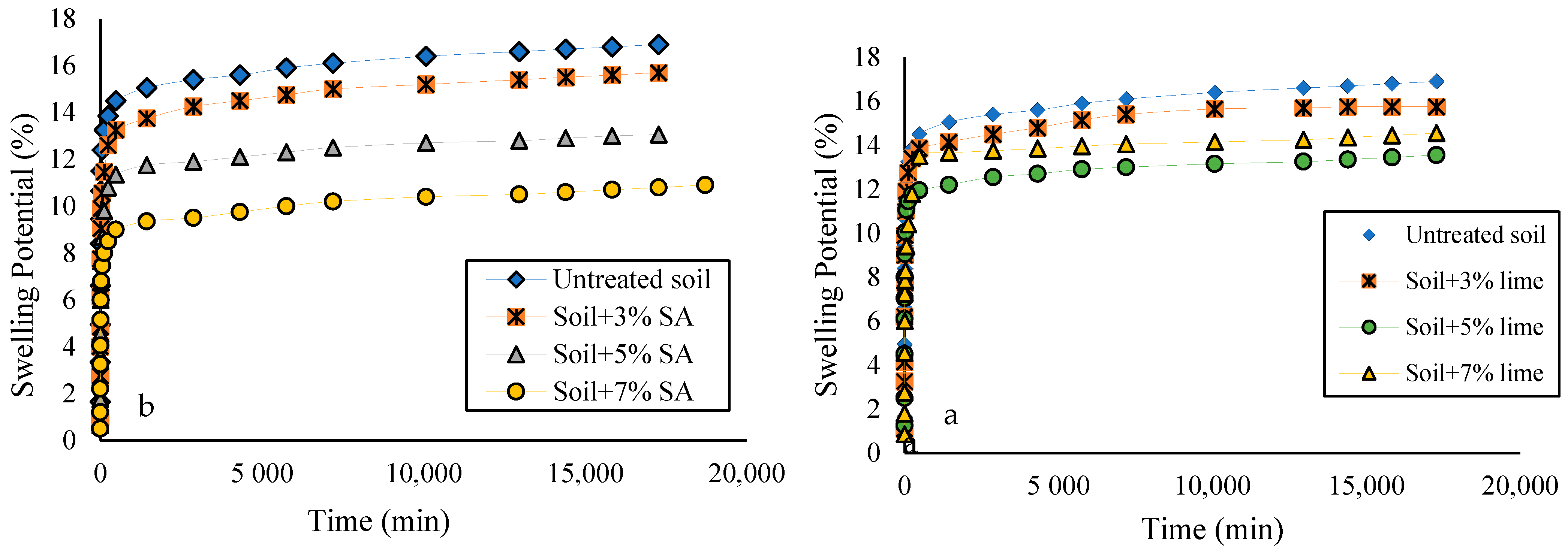
3.2. Free Swelling Test
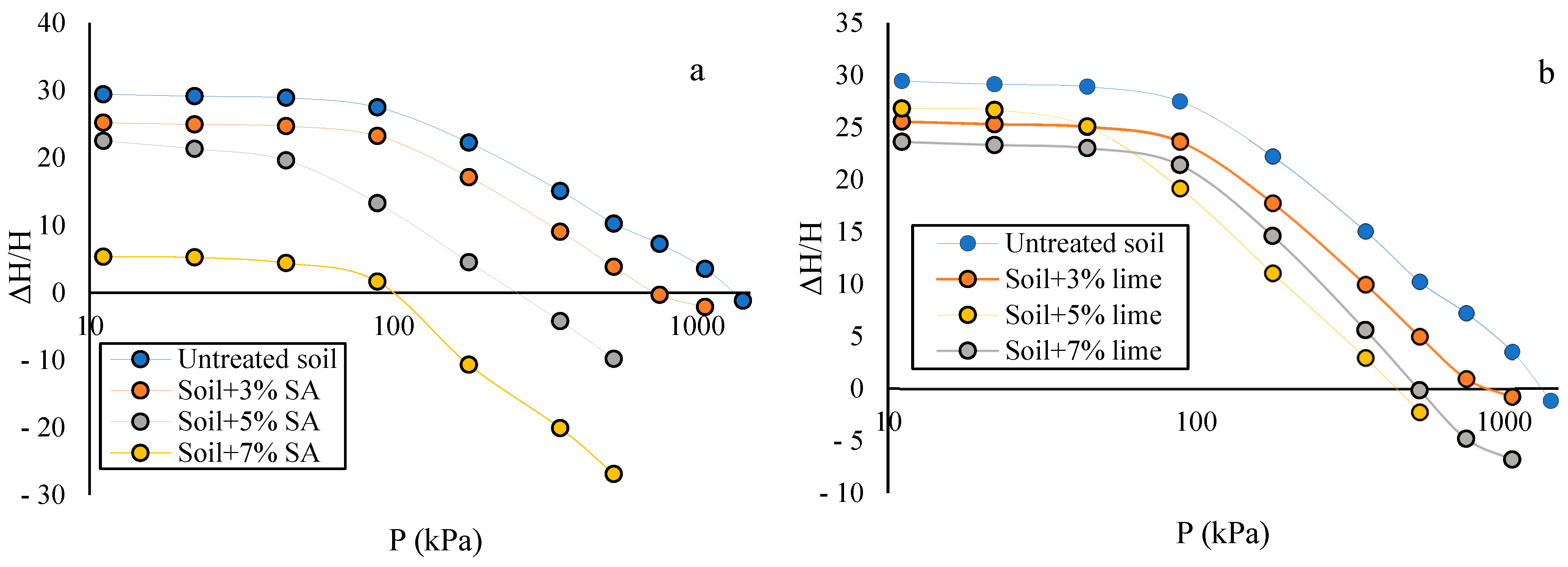
3.3. Swelling Pressure
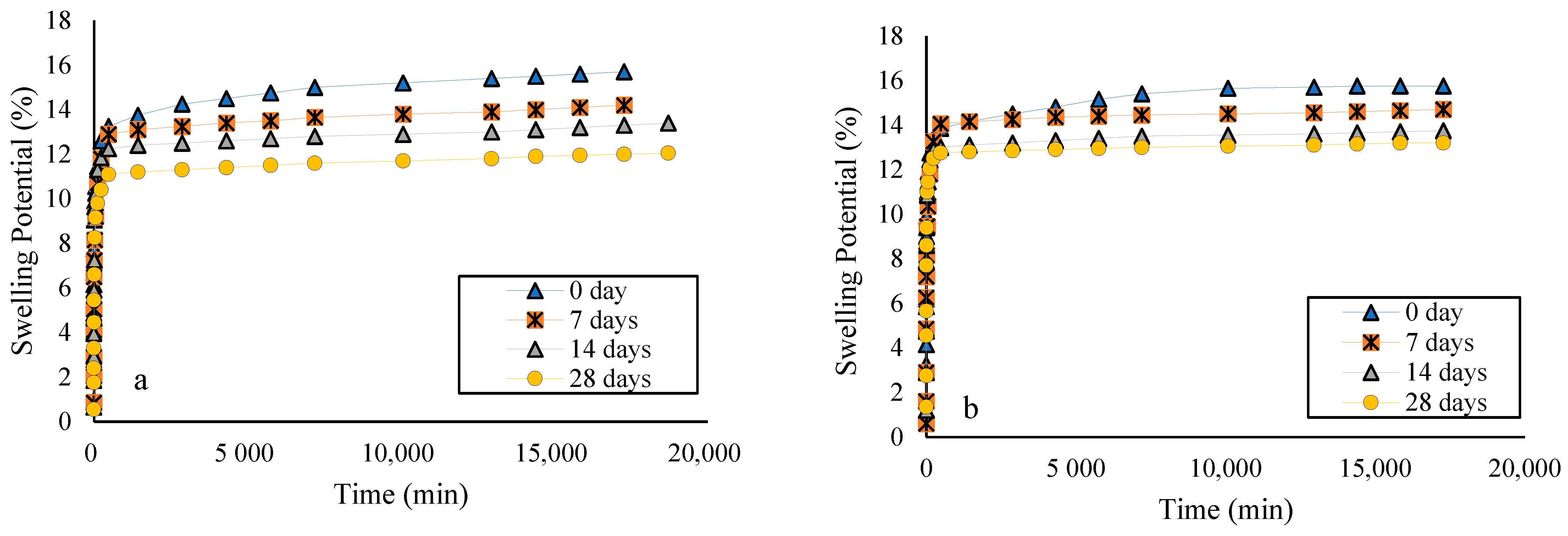
3.4. The Effect of Curing Time on Swelling
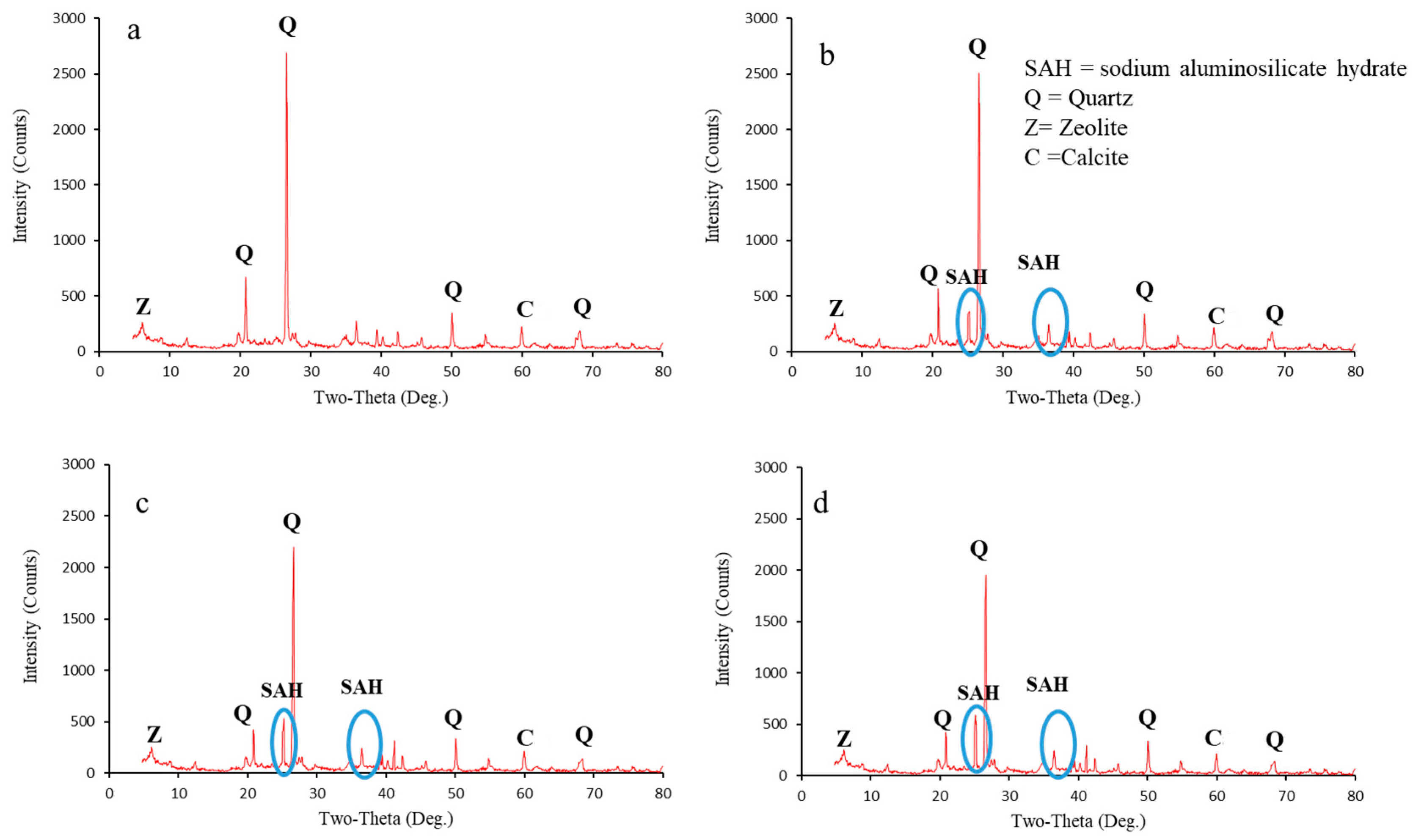
3.5. Microscopic Observations
4. Conclusions
- 1
- Addition of both sodium nanoalginate and lime to the soil resulted in a decreased LL, increased PL, and ultimately decreased the PI of the soil. The use of sodium nanoalginate produced more favorable outcomes compared to lime.
- 2
- The addition of different concentrations of sodium nanoalginate and lime reduced soil swelling, with sodium nanoalginate demonstrating superior results compared to lime. It is worth noting that increasing the percentage of the sodium nanoalginate additive led to higher values of free swelling and swelling pressure. However, with more than 5% of lime, the soil’s free swelling and swelling pressure decreased.
- 3
- Increasing the curing time decreased the soil’s free swelling and swelling pressure when treated with both agents. With the increasing curing time, sodium nanoalginate performed better compared to lime.
- 4
- The XRD tests showed that sodium nanoalginate reduced soil swelling by filling pores, binding particles, and altering the mineral composition. Lime addition to the soil reduced quartz and calcite peaks, forming new compounds, such as calcite, nicolite, CSH, and CAH.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mousavi, F.; Atarodi, A.; Abdi, E. Optimization of Polymer Materials Performance on Improving Mechanical Properties of Fine-Grained Soils of Forest. Road Mater. Pavement Des. 2023, 1–23. [Google Scholar] [CrossRef]
- Mousavi, F.; Abdi, E.; Rahimi, H. Effect of Polymer Stabilizer on Swelling Potential and CBR of Forest Road Material. KSCE J. Civ. Eng. 2014, 18, 2064–2071. [Google Scholar] [CrossRef]
- Torfi, S.; Khayat, N.; Horpibulsuk, S. Sustainable Stabilization of Compacted Clay Using Sodium Alginate for Subgrade Application. Int. J. Geosynth. Gr. Eng. 2021, 7, 82. [Google Scholar] [CrossRef]
- Mousavi, F.; Avatefi Hemmat, M.; Abdi, E.; Norouzi, A. The Effect of Polymer Materials on the Stabilization of Forest Road Subgrade. Int. J. For. Eng. 2021, 32, 235–245. [Google Scholar] [CrossRef]
- Mousavi, F.; Abdi, E. Unconfined Compression Strength of Polymer Stabilized Forest Soil Clay. Geotech. Geol. Eng. 2022, 40, 4095–4107. [Google Scholar] [CrossRef]
- Cho, H.J.; Preston, D.J.; Zhu, Y.; Wang, E.N. Nanoengineered Materials for Liquid–Vapour Phase-Change Heat Transfer. Nat. Rev. Mater. 2016, 2, 16092. [Google Scholar] [CrossRef]
- Latif, D.O.; Rifa’i, A.; Suryolelono, K.B. Chemical Characteristics of Volcanic Ash in Indonesia for Soil Stabilization: Morphology and Mineral Content. GEOMATE J. 2016, 11, 2606–2610. [Google Scholar] [CrossRef]
- Arab, M.G.; Mousa, R.A.; Gabr, A.R.; Azam, A.M.; El-Badawy, S.M.; Hassan, A.F. Resilient Behavior of Sodium Alginate–Treated Cohesive Soils for Pavement Applications. J. Mater. Civ. Eng. 2019, 31, 4018361. [Google Scholar] [CrossRef]
- Ahn, S.; Park, G.; Yoon, H.; Han, J.-H.; Jung, J. Evaluation of Soil–Structure Interaction in Structure Models via Shaking Table Test. Sustainability 2021, 13, 4995. [Google Scholar] [CrossRef]
- Thangaraj, R.; Thenmozhi, R. Sustainable Concrete Using High Volume Fly Ash from Thermal Power Plants. Ecol. Environ. Conserv. 2013, 19, 461–466. [Google Scholar]
- Soltani, A.; Taheri, A.; Khatibi, M.; Estabragh, A.R. Swelling Potential of a Stabilized Expansive Soil: A Comparative Experimental Study. Geotech. Geol. Eng. 2017, 35, 1717–1744. [Google Scholar] [CrossRef]
- Tabarsa, A.; Latifi, N.; Meehan, C.L.; Manahiloh, K.N.; Rosales, J.; Agrela, F.; Marcobal, J.R.; Diaz-López, J.L.; Cuenca-Moyano, G.M.; Caballero, Á.; et al. Laboratory Investigation and Field Evaluation of Loess Improvement Using Nanoclay–A Sustainable Material for Construction. Constr. Build. Mater. 2018, 36, 3058. [Google Scholar] [CrossRef]
- Yazdandoust, F.; Yasrobi, S.S. Effect of Cyclic Wetting and Drying on Swelling Behavior of Polymer-Stabilized Expansive Clays. Appl. Clay Sci. 2010, 50, 461–468. [Google Scholar] [CrossRef]
- Azzam, W.R. Reduction of the Shrinkage–Swelling Potential with Polymer Nanocomposite Stabilization. J. Appl. Polym. Sci. 2012, 123, 299–306. [Google Scholar] [CrossRef]
- Soltani, A.; Deng, A.; Taheri, A.; O’Kelly, B.C. Intermittent Swelling and Shrinkage of a Highly Expansive Soil Treated with Polyacrylamide. J. Rock Mech. Geotech. Eng. 2022, 14, 252–261. [Google Scholar] [CrossRef]
- Soltani, A.; Taheri, A.; Deng, A.; O’Kelly, B.C. Improved Geotechnical Behavior of an Expansive Soil Amended with Tire-Derived Aggregates Having Different Gradations. Minerals 2020, 10, 923. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Lu, Y.; Feng, Q.; Zhang, F.; Qi, C.; Wei, J.; Kanungo, D.P. Effect of Polyvinyl Acetate Stabilization on the Swelling-Shrinkage Properties of Expansive Soil. Int. J. Polym. Sci. 2017, 2017, 8128020. [Google Scholar] [CrossRef]
- Alazigha, D.P.; Indraratna, B.; Vinod, J.S.; Heitor, A. Mechanisms of Stabilization of Expansive Soil with Lignosulfonate Admixture. Transp. Geotech. 2018, 14, 81–92. [Google Scholar] [CrossRef]
- Cabalar, A.F.; Awraheem, M.H.; Khalaf, M.M. Geotechnical Properties of a Low-Plasticity Clay with Biopolymer. J. Mater. Civ. Eng. 2018, 30, 4018170. [Google Scholar] [CrossRef]
- Singh, S.P.; Das, R. Geo-Engineering Properties of Expansive Soil Treated with Xanthan Gum Biopolymer. Geomech. Geoengin. 2020, 15, 107–122. [Google Scholar] [CrossRef]
- Fernandez, M.T.; INTECIN, U.B.A.; Argentina Codevilla, M.; Argentina Pique, T.M.; ITPN, U.B.A.; Argentina Manzanal, D. Polymer Applications to Control Soil Expansion. MH 2016, 96, 80. [Google Scholar]
- ZUMRAWI, M.M.E.; Mohammed, A.E. Effects of Poly Vinyl Acetate on Characteristics of Expansive Soil. J. Mater. Eng. Struct. JMES 2019, 6, 167–176. [Google Scholar]
- Peng, Y.; Xiao, D.; Yu, G.; Feng, Y.; Li, J.; Zhao, X.; Tang, Y.; Wang, L.; Zhang, Q. Effect of an Eco-Friendly o/w Emulsion Stabilized with Amphiphilic Sodium Alginate Derivatives on Lambda-Cyhalothrin Adsorption–Desorption on Natural Soil Minerals. J. Environ. Sci. 2019, 78, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Li, Y.; Huang, W.; Armwood, C.; Amini, F.; Li, L. Mechanical Behaviors of Hydrogel-Impregnated Sand. Constr. Build. Mater. 2019, 207, 174–180. [Google Scholar] [CrossRef]
- Fatehi, H.; Bahmani, M.; Noorzad, A. Strengthening of Dune Sand with Sodium Alginate Biopolymer. In Proceedings of the Eighth International Conference on Case Histories in Geotechnical Engineering, American Society of Civil Engineers, Reston, VA, USA, 21 March 2019; pp. 157–166. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar Gum: Processing, Properties and Food Applications—A Review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil PH and Soil Acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar]
- Loeppert, R.; Inskeep, W. Ron—Methods of Soil Analyses; SSSA/ASA: Madison, WI, USA, 1996; pp. 639–664. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bower, C.A. Fixation of Ammonium in Difficultly Exchangeable Form under Moist Conditions by Some Soils of Semiarid Regions. Soil Sci. 1950, 70, 375–384. [Google Scholar] [CrossRef]
- Mousavi, F.; Abdi, E.; Borz, S.A. Forest Road Subgrade Improvement by Lime and Sodium Nanoalginate Used as Stabilizers for Clay Soils. Forests 2023, 14, 1332. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Baraskar, V.V.; Mallikarjun Setty, C.; Sa, B. Interpenetrating Polymer Network Matrices of Sodium Alginate and Carrageenan for Controlled Drug Delivery Application. Fibers Polym. 2011, 12, 352–358. [Google Scholar] [CrossRef]
- ASTM Annual Book of ASTM Standards—Natural Building Stones. Soil and Rock, Part 19. In Annual Book of ASTM Standard, Soil and Rock; American Society for Testing and Materials: West Conshohocken, PA, USA, 1980.
- Holtz, W.G.; Gibbs, H.J. Engineering Properties of Expansive Clays. Trans. Am. Soc. Civ. Eng. 1956, 121, 641–663. [Google Scholar] [CrossRef]
- Snethen, D.R.; Johnson, L.D.; Patrick, D.M. An Evaluation of Expedient Methodology for Identification of Potentially Expansive Soils; United States, Federal HIghway Administration, Office of Research and Development: Fairfax, VA, USA, 1977. [Google Scholar]
- McCormack, D.E. Foundations on Expansive Soils; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands, 1976; Volume 40, ISBN 9780444601667. [Google Scholar]
- Kong, R.; Zhang, F.; Wang, G.; Peng, J. Stabilization of Loess Using Nano-SiO2. Materials 2018, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.S.A.; Latifi, N.; Meehan, C.L.; Manahiloh, K.N. Sustainable Improvement of Tropical Residual Soil Using an Environmentally Friendly Additive. Geotech. Geol. Eng. 2017, 35, 2613–2623. [Google Scholar] [CrossRef]
- Jalal, F.-E.-; Xu, Y.; Jamhiri, B.; Memon, S.A. On the Recent Trends in Expansive Soil Stabilization Using Calcium-Based Stabilizer Materials (CSMs): A Comprehensive Review. Adv. Mater. Sci. Eng. 2020, 2020, 1–23. [Google Scholar] [CrossRef]
- Moen, D.E.; Richardson, J.L. Ultrasonic Dispersion of Soil Aggregates Stabilized by Polyvinyl Alcohol and T403-Glyoxal Polymers. Soil Sci. Soc. Am. J. 1984, 48, 628–631. [Google Scholar] [CrossRef]
- Richardson, J.L.; Gunnerson, W.T.; Giles, J.F. Influence of in Situ Two-Phase Polymers on Aggregate Stabilization in Various Textured North Dakota Soils. Can. J. soil Sci. 1987, 67, 209–213. [Google Scholar] [CrossRef]
- Amadi, A.A.; Okeiyi, A. Use of Quick and Hydrated Lime in Stabilization of Lateritic Soil: Comparative Analysis of Laboratory Data. Int. J. Geo-Eng. 2017, 8, 3. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.M.; Axelos, M.A. V Physico-Chemical Properties and Rheology of Alginate Gel Beads Formed with Various Divalent Cations. Polym. Gels Networks 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Beetham, P.; Dijkstra, T.; Dixon, N.; Fleming, P.; Hutchison, R.; Bateman, J. Lime Stabilisation for Earthworks: A UK Perspective. Proc. Inst. Civ. Eng. Improv. 2015, 168, 81–95. [Google Scholar] [CrossRef]
- Bakhshizadeh, A.; Khayat, N.; Horpibulsuk, S. Surface Stabilization of Clay Using Sodium Alginate. Case Stud. Constr. Mater. 2022, 16, e01006. [Google Scholar] [CrossRef]
- Jha, A.K.; Sivapullaiah, P. V Lime Stabilization of Soil: A Physico-Chemical and Micro-Mechanistic Perspective. Indian Geotech. J. 2020, 50, 339–347. [Google Scholar] [CrossRef]
- Jahandari, S.; Tao, Z.; Saberian, M.; Shariati, M.; Li, J.; Abolhasani, M.; Kazemi, M.; Rahmani, A.; Rashidi, M. Geotechnical Properties of Lime-Geogrid Improved Clayey Subgrade under Various Moisture Conditions. Road Mater. Pavement Des. 2022, 23, 2057–2075. [Google Scholar] [CrossRef]




| Properties | Amount | Properties | Amount | Properties | Amount |
|---|---|---|---|---|---|
| Gs (gr/cm3) | 2.85 | Na+ (meq/L) | 0.39 | LL (%) | 64.3 |
| SO42− | 1.9 | Ca2+ (meq/L) | 1.7 | PL (%) | 30.8 |
| CaCO3 (%) | 0.74 | Mg2+ (meq/L) | 2.8 | SL (%) | 21.4 |
| CEC (cmol/kg) | 39.09 | CL− (meq/L) | 0.8 | PI (%) | 33.73 |
| EC (ds/m) | 121.2 | (meq/L) | 0 | Maximum dry density (kN/m3) | 13.5 |
| OC (%) | 1.68 | HCO32− | 1.88 | Optimum water content (%) | 24 |
| pH | 4.8 | K+ (meq/L) | 0.9 | Clay (%) | 87 |
| SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | CaO (%) | Na2O (%) | MgO (%) | K2O (%) | TiO2 (%) | MnO (%) | P2O5 (%) |
| 52.502 | 17.734 | 10.965 | 0.398 | 0.295 | 1.587 | 2.291 | 1.185 | 0.061 | 0.049 |
| LOI (%) | Cl (ppm) | S (ppm) | As (ppm) | Ba (ppm) | Ce (ppm) | Co (ppm) | Cr (ppm) | Cu (ppm) | Nb (ppm) |
| 2 | N | 30 | 644 | 51 | 25 | 178 | N | 10 | 2 |
| Pb (ppm) | Rb (ppm) | Sr (ppm) | V (ppm) | Y (ppm) | Zr (ppm) | Zn (ppm) | Mo (ppm) | Ni (ppm) | Pb (ppm) |
| 36 | 139 | 84 | 145 | 47 | 203 | 167 | 31 | 36 | 139 |
| Property | Value |
|---|---|
| Chemical formula | (C6H7O6Na)n |
| pH | 5.5–7.5 for a 1% aqueous solution (at 25 °C) |
| Matter insoluble in water | 1% |
| Arsenic (As) | <3 ppm |
| Lead (Pb) | <10 ppm |
| Sulphated ash | 22.6 |
| Sulphur (S) | <0.02% |
| Phosphor (P) | <0.02% |
| Molecular weight | 216 g/mol |
| Dynamic viscosity | 12 CPS |
| Appearance | Cream to pale yellowish brown powder |
| Property | Value |
|---|---|
| Chemical formula | CaO |
| pH | 12.8 |
| Matter insoluble in water | Chemical reaction, converts to calcium hydroxide |
| Gs | 3.34 g/cm3 |
| Melting point | 2613 °C |
| Appearance | White powder |
| Soil Sample | Important Minerals | 2θ (angle) |
|---|---|---|
| Control soil | Calcium carbonate (CaCO3) | 19.5°, 24.5°, 40° |
| Zeolite | 7.5° | |
| Quartz (silicon dioxide (SiO2)) | 21°, 28.5°, 50°, 68.5° | |
| Soil sample mixed with sodium nanoalginate | SAH | 25°, 36.5° |
| Soil sample mixed with lime | CSH, CAH | 33°, 37°, 39°, 75° |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousavi, F.; Abdi, E.; Taheri, A. Effects of Sodium Nanoalginate and Lime on Swelling Properties of Expansive Soils. Minerals 2023, 13, 1515. https://doi.org/10.3390/min13121515
Mousavi F, Abdi E, Taheri A. Effects of Sodium Nanoalginate and Lime on Swelling Properties of Expansive Soils. Minerals. 2023; 13(12):1515. https://doi.org/10.3390/min13121515
Chicago/Turabian StyleMousavi, Fatemeh, Ehsan Abdi, and Abbas Taheri. 2023. "Effects of Sodium Nanoalginate and Lime on Swelling Properties of Expansive Soils" Minerals 13, no. 12: 1515. https://doi.org/10.3390/min13121515





