Pressure-Induced Reverse Structural Transition of Calcite at Temperatures up to 873 K and Pressures up to 19.7 GPa
Abstract
:1. Introduction
2. Materials and Methods
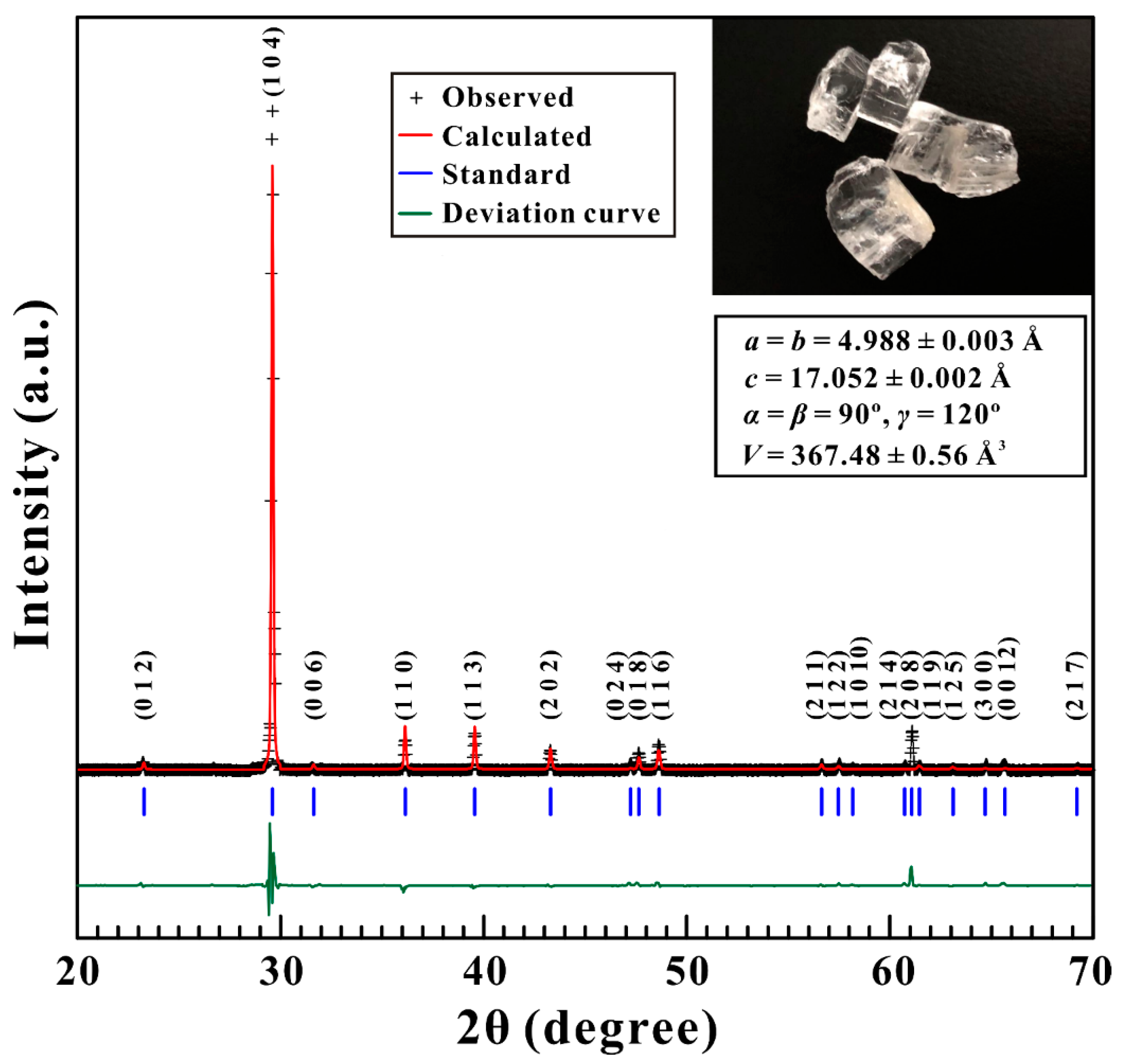
2.1. Sample Preparation and Characterization
2.2. High-Pressure Raman Spectroscopy Measurements
2.3. High-Pressure Electrical Conductivity Measurements
3. Results and Discussion
3.1. High-Pressure Raman Spectroscopy at Room Temperature
3.2. High-Pressure Electrical Conductivity at Ambient Temperature
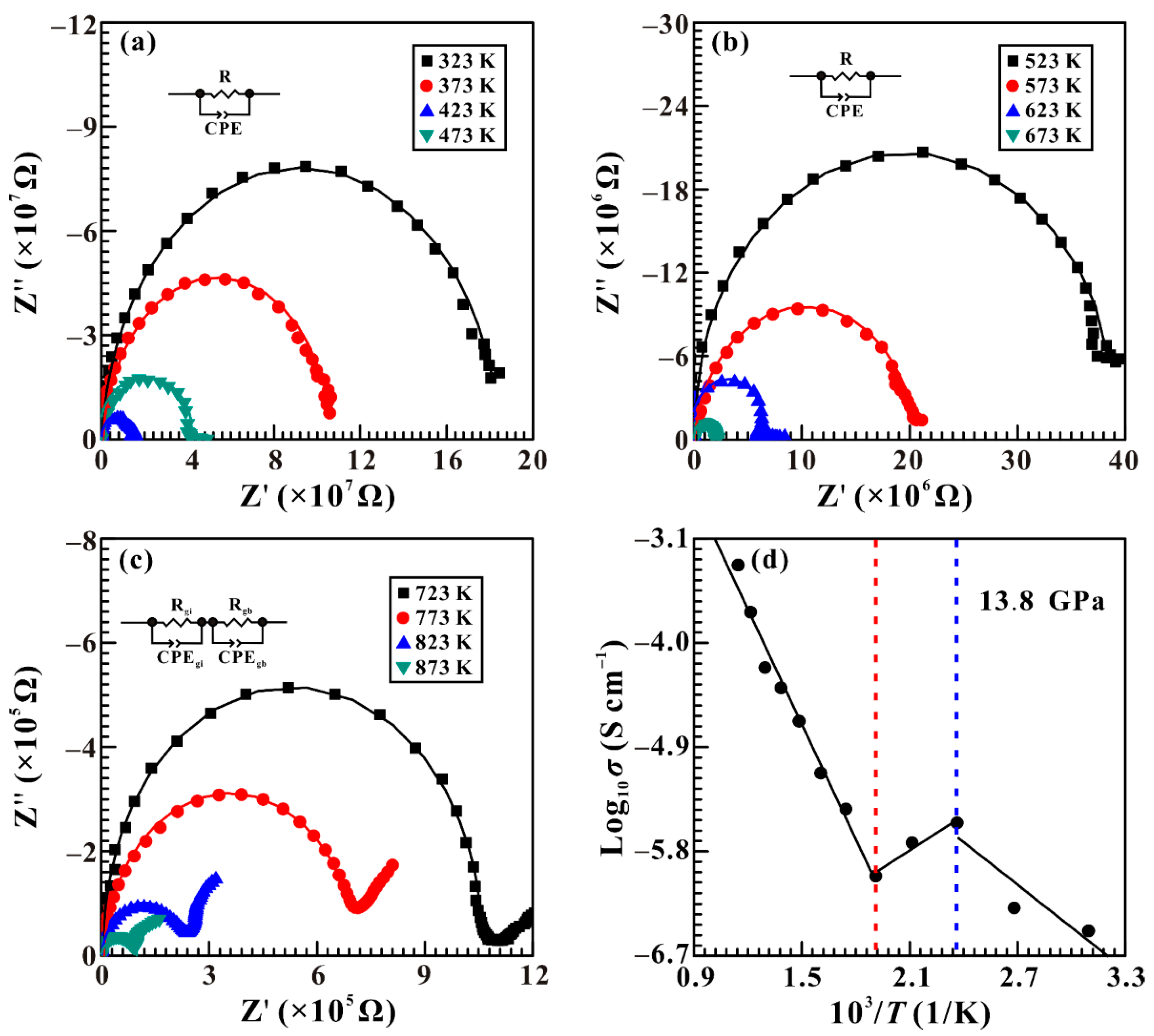
3.3. Electrical Conductivity at High Temperature and High Pressure
4. Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in the Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M.; McDonough, W.F.; Spiegelman, M.; Withers, A.C. Trace element partitioning between garnet lherzolite and carbonatite at 6.6 and 8.6 GPa with applications to the geochemistry of the mantle and of mantle-derived melts. Chem. Geol. 2009, 262, 57–77. [Google Scholar] [CrossRef]
- Doucet, L.S.; Li, Z.X.; El Dien, H.G. Oceanic and super-deep continental diamonds share a transition zone origin and mantle plume transportation. Sci. Rep. 2021, 11, 16958. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ohtani, E.; Litasov, K.D.; Terasaki, H. Solidus of carbonated peridotite from 10 to 20 GPa and origin of magnesiocarbonatite melt in the Earth’s deep mantle. Chem. Geol. 2009, 262, 17–28. [Google Scholar] [CrossRef]
- Litasov, K.D.; Ohtani, E. Solidus and phase relations of carbonated peridotite in the system CaO-Al2O3-MgO-SiO2-Na2O-CO2 to the lower mantle depths. Phys. Earth Planet. Inter. 2009, 177, 46–58. [Google Scholar] [CrossRef]
- Maeda, F.; Ohtani, E.; Kamada, S.; Sakamaki, T.; Hirao, N.; Ohishi, Y. Diamond formation in the deep lower mantle: A high-pressure reaction of MgCO3 and SiO2. Sci. Rep. 2017, 7, 40602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minarik, W.G.; Watson, E.B. Interconnectivity of carbonate melt at low melt fraction. Earth Planet. Sci. Lett. 1995, 133, 423–437. [Google Scholar] [CrossRef]
- Tao, R.B.; Fei, Y.W. Recycled calcium carbonate is an efficient oxidation agent under deep upper mantle conditions. Commun. Earth Environ. 2021, 2, 45. [Google Scholar] [CrossRef]
- Liu, L.G.; Mernagh, T.P. Phase transitions and Raman spectra of calcite at high pressures and room temperature. Am. Mineral. 1990, 75, 801–806. [Google Scholar]
- Yuan, X.; Mayanovic, R.A.; Zhang, G. Phase transitions in CaCO3 under hydrous and anhydrous conditions: Implications for the structural transformations of CaCO3 during subduction processes. Am. Mineral. 2021, 106, 1780–1788. [Google Scholar] [CrossRef]
- Suito, K.; Namba, J.; Horikawa, T.; Taniguchi, Y.; Sakural, N.; Kobayashi, M.; Onodera, A.; Shimomura, O.; Kikegawa, T. Phase relations of CaCO3 at high pressure and high temperature. Am. Mineral. 2001, 86, 997–1002. [Google Scholar] [CrossRef]
- Liu, J.; Caracas, R.; Fan, D.W.; Bobocioiu, E.; Zhang, D.Z.; Mao, W.L. High-pressure compressibility and vibrational properties of (Ca,Mn)CO3. Am. Mineral. 2016, 101, 2723–2730. [Google Scholar] [CrossRef]
- Bagdassarov, N.S.; Slutskii, A.B. Phase transformations in calcite from electrical impedance measurements. Phase Transit. 2003, 76, 1015–1028. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.Y.; Jing, C.X.; Dai, L.D.; Yin, C.Y.; Chen, D.M. Electrical conductivity of siderite and its implication for high conductivity anomaly in the slab-mantle wedge interface. Front. Earth Sci. 2022, 10, 985740. [Google Scholar] [CrossRef]
- Mibe, K.; Ono, S. Electrical conductivity of MgCO3 at high pressures and high temperatures. Phys. B Condens. Matter 2011, 406, 2018–2020. [Google Scholar] [CrossRef]
- Ono, S.; Mibe, K. Electrical conductivity of aragonite in the subducted slab. Eur. J. Mineral. 2013, 25, 11–15. [Google Scholar] [CrossRef]
- Ono, S.; Mibe, K. Influence of pressure and temperature on the electrical conductivity of dolomite. Phys. Chem. Minerals. 2015, 42, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.C.; Von Dreele, R.B. General structure analysis system (GSAS). Los Alamos Natl. Lab. Rep. 2004, 748, 86–748. [Google Scholar]
- Ishizawa, N.; Setoguchi, H.; Yanagisawa, K. Structural evolution of calcite at high temperatures: Phase V unveiled. Sci. Rep. 2013, 3, 2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maslen, M.; Streltsov, V.A.; Streltsova, N.R. X-ray study of the electron density in calcite, CaCO3. Acta Cryst. 1993, B49, 636–641. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Dai, L.D.; Wu, L.; Li, H.P.; Hu, H.Y.; Zhuang, Y.K.; Liu, K.X. Evidence of the pressure-induced conductivity switching of yttrium-doped SrTiO3. J. Phys. Condens. Matter 2016, 28, 475501. [Google Scholar] [CrossRef]
- Dai, L.D.; Wu, L.; Li, H.P.; Hu, H.Y.; Zhuang, Y.K.; Liu, K.X. Pressure-induced phase-transition and improvement of the microdielectric properties in yttrium-doped SrZrO3. Europhys. Lett. 2016, 114, 56003. [Google Scholar] [CrossRef]
- Hong, M.L.; Dai, L.D.; Hu, H.Y.; Zhang, X.Y.; Li, C. High-temperature and high-pressure phase transition of natural barite investigated by Raman spectroscopy and electrical conductivity. Front. Earth Sci. 2022, 10, 864183. [Google Scholar] [CrossRef]
- Hong, M.L.; Dai, L.D.; Hu, H.Y.; Zhang, X.Y.; Li, C.; He, Y. Pressure-induced structural phase transition and metallization of CrCl3 under different hydrostatic environments up to 50.0 GPa. Inorg. Chem. 2022, 61, 4852–4864. [Google Scholar] [CrossRef]
- Hong, M.L.; Dai, L.D.; Hu, H.Y.; Zhang, X.Y.; Li, C.; He, Y. High-pressure structural phase transitions and metallization in layered HfS2 under different hydrostatic environments up to 42.1 GPa. J. Mater. Chem. C 2022, 10, 10541–10550. [Google Scholar] [CrossRef]
- Hu, H.Y.; Dai, L.D.; Sun, W.Q.; Zhuang, Y.K.; Liu, K.X.; Yang, L.F.; Pu, C.; Hong, M.L.; Wang, M.Q.; Hu, Z.M.; et al. Some remarks on the electrical conductivity of hydrous silicate minerals in the earth crust, upper mantle and subduction zone at high temperature and high pressures. Minerals 2022, 12, 161. [Google Scholar] [CrossRef]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Wu, L.; Zhuang, Y.K.; Pu, C.; Yang, L.F. Migration of impurity level reflected in the electrical conductivity variation for natural pyrite at high temperature and high pressure. Phys. Chem. Miner. 2018, 45, 85–92. [Google Scholar] [CrossRef]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Yang, L.F.; Pu, C.; Hong, M.L.; Liu, P.F. Phase transition and metallization of orpiment by Raman spectroscopy, electrical conductivity and theoretical calculation under high pressure. Materials 2019, 12, 784. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.F.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Zhuang, Y.K.; Liu, K.X.; Pu, C.; Hong, M.L. Pressure-induced structural phase transition and dehydration for gypsum investigated by Raman spectroscopy and electrical conductivity. Chem. Phys. Lett. 2018, 706, 151–157. [Google Scholar] [CrossRef]
- Yang, L.F.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Hong, M.L.; Zhang, X.Y.; Liu, P.F. High-pressure investigations on the isostructural phase transition and metallization in realgar with diamond anvil cells. Geosci. Front. 2021, 12, 1031–1037. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Dai, L.D.; Hu, H.Y.; Hong, M.L. Pressure-induced metallic phase transition in gallium arsenide up to 24.3 GPa under hydrostatic conditions. Mod. Phys. Lett. B 2021, 35, 2150460. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Dai, L.D.; Hu, H.Y.; Hong, M.L.; Li, C. Pressure-induced coupled structural-electronic transition in SnS2 under different hydrostatic environments up to 39.7 GPa. RSC Adv. 2022, 12, 2454–2461. [Google Scholar] [CrossRef]
- Zhuang, Y.K.; Dai, L.D.; Wu, L.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Pu, C. Pressure-induced permanent metallization with reversible structural transition in molybdenum disulfide. Appl. Phys. Lett. 2017, 110, 122103. [Google Scholar] [CrossRef]
- Dai, L.D.; Zhuang, Y.K.; Li, H.P.; Wu, L.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Pu, C. Pressure-induced irreversible amorphization and metallization with a structural phase transition in arsenic telluride. J. Mater. Chem. C 2017, 5, 12157–12162. [Google Scholar] [CrossRef]
- Dai, L.D.; Liu, K.X.; Li, H.P.; Wu, L.; Hu, H.Y.; Zhuang, Y.K.; Yang, L.F.; Pu, C.; Liu, P.F. Pressure-induced irreversible metallization accompanying the phase transitions in Sb2S3. Phys. Rev. B 2018, 97, 024103. [Google Scholar] [CrossRef]
- Dai, L.D.; Pu, C.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Hong, M.L. Characterization of metallization and amorphization for GaP under different hydrostatic environments in diamond anvil cell up to 40.0 GPa. Rev. Sci. Instrum. 2019, 90, 066103. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, A.; Shailaja, P.; Nandhini, M.; Sahaya Jude Dhas, S.; Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Chakraborty, S.; Martin Britto Dhas, S.A. Ternary switchable phase transition of CaCO3 by shock waves. Ceram. Int. 2022, 48, 8457–8465. [Google Scholar] [CrossRef]
- Catalli, K.; Williams, Q. A high-pressure phase transition of calcite-III. Am. Mineral. 2005, 90, 1679–1682. [Google Scholar] [CrossRef]
- Hu, H.Y.; Dai, L.D.; Li, H.P.; Hui, K.S.; Sun, W.Q. Influence of dehydration on the electrical conductivity of epidote and implications for high-conductivity anomalies in subduction zones. J. Geophys. Res. Solid Earth 2017, 122, 2751–2762. [Google Scholar] [CrossRef]
- Hu, H.Y.; Dai, L.D.; Li, H.P.; Sun, W.Q.; Li, B.S. Effect of dehydrogenation on the electrical conductivity of Fe-bearing amphibole: Implications for high conductivity anomalies in subduction zones and continental crust. Earth Planet. Sci. Lett. 2018, 498, 27–37. [Google Scholar] [CrossRef]
- Hu, H.Y.; Dai, L.D.; Sun, W.Q.; Wang, M.Q.; Jing, C.X. Constraints on fluids in the continental crust from laboratory-based electrical conductivity measurements of plagioclase. Gondwana Res. 2022, 107, 1–12. [Google Scholar] [CrossRef]
- Hong, M.L.; Dai, L.D.; Hu, H.Y.; Zhang, X.Y. Pressure-induced structural phase transitions in natural kaolinite investigated by Raman spectroscopy and electrical conductivity. Am. Mineral. 2022, 107, 385–394. [Google Scholar] [CrossRef]
- Merlini, M.; Hanfland, M.; Crichton, W.A. CaCO3-III and CaCO3-VI, high-pressure polymorphs of calcite: Possible host structures for carbon in the Earth’s mantle. Earth Planet. Sci. Lett. 2012, 333, 265–271. [Google Scholar] [CrossRef]
- Zhao, C.S.; Li, H.P.; Chen, P.F.; Jiang, J.J. Sound velocities across calcite phase transition by Brillouin scattering spectroscopy. Am. Mineral. 2019, 104, 418–424. [Google Scholar] [CrossRef]
- Zhao, X.; Zheng, Z.; Chen, J.Z.; Gao, Y.; Sun, J.H.; Hou, X.; Xiong, M.J.; Mei, S.H. High P-T calcite-aragonite phase transitions under hydrous and anhydrous conditions. Front. Earth Sci. 2022, 10, 907967. [Google Scholar] [CrossRef]
- Trnovcová, V.; Furár, I.; Hanic, F. Influence of technological texture on electrical properties of industrial ceramics. J. Phys. Chem. Solids 2007, 68, 1135–1139. [Google Scholar] [CrossRef]
- Koch-Müller, M.; Jahn, S.; Birkholz, N.; Ritter, E.; Schade, U. Phase transitions in the system CaCO3 at high P and T determined by in situ vibrational spectroscopy in diamond anvil cells and first-principles simulations. Phys. Chem. Miner. 2016, 43, 545–561. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.Q.; Zhang, Q.; Tao, R.B.; Liu, H.; Kono, Y.; Mao, H.K.; Yang, W.G.; Chen, B.; Fei, Y.W. Temperature-induced amorphization in CaCO3 at high pressure and implications for recycled CaCO3 in subduction zones. Nat. Commun. 2019, 10, 1963. [Google Scholar] [CrossRef] [Green Version]
- Bayarjargal, L.; Fruhner, C.J.; Schrodt, N.; Winkler, B. CaCO3 phase diagram studied with Raman spectroscopy at pressures up to 50 GPa and high temperatures and DFT modeling. Phys. Earth Planet. Inter. 2018, 281, 31–45. [Google Scholar] [CrossRef]
- Hess, N.J.; Ghose, S.; Exarhos, G.J. Raman spectroscopy at simultaneous high pressure and temperature: Phase relations of CaCO3 and the lattice dynamics of the calcite CaCO3(II) phase transition. In Recent Trends in High Pressure Research; Proceedings of the X IIIth AIRAPT International Conference on High Pressure Science and Technology, Bangalore, India, 7–11 October 1991; Singh, A.K., Ed.; US Department of Energy: Oak Ridge, TN, USA, 1991; pp. 236–241. [Google Scholar]
- Komabayashi, T.; Omori, S.; Maruyama, S. Petrogenetic grid in the system MgO-SiO2-H2O up to 30 GPa, 1600 °C: Applications to hydrous peridotite subducting into the Earth’s deep interior. J. Geophys. Res. Solid Earth 2004, 109, B03206. [Google Scholar] [CrossRef] [Green Version]








| Pressure Range (GPa) | Mode (cm−1) | Pressure Coefficient (cm−1 GPa−1) | |
|---|---|---|---|
| CaCO3-I phase | 0.5–1.6 GPa | T | 2.07 |
| L | 1.83 | ||
| v4 | 11.51 | ||
| v1 | 16.14 | ||
| CaCO3-II phase | 1.6–2.2 GPa | M1 | 8.50 |
| M2 | 22.83 | ||
| M3 | 0.17 | ||
| M4 | 42.03 | ||
| v4 | 32.67 | ||
| v1 | 16.50 | ||
| CaCO3-III phase | 2.2–16.8 GPa | M5 | 2.00 |
| M1 | 1.38 | ||
| M2 | 1.02 | ||
| M3 | 1.61 | ||
| M6 | 2.36 | ||
| M7 | 2.28 | ||
| M8 | 3.89 | ||
| M9 | 5.09 | ||
| M10 | 0.87 | ||
| M4 | 1.19 | ||
| v4 | 1.21 | ||
| M11 | –0.39 | ||
| M12 | 1.46 | ||
| v1 | 1.84 | ||
| CaCO3-VI phase | 16.8–19.4 GPa | M5 | 0.15 |
| M1 | 0.50 | ||
| M2 | 0.46 | ||
| M3 | 0.76 | ||
| M6 | 1.08 | ||
| M13 | 2.81 | ||
| M7 | 2.31 | ||
| M8 | 1.62 | ||
| M9 | 1.08 | ||
| M10 | 0.65 | ||
| M4 | 0.77 | ||
| M11 | 0.88 | ||
| v1 | 0.57 |
| Pressure Range (GPa) | Mode (cm−1) | Pressure Coefficient (cm−1 GPa−1) | |
|---|---|---|---|
| CaCO3-VI phase | 18.3–5.4 GPa | M5 | 0.20 |
| M1 | 0.33 | ||
| M2 | 0.73 | ||
| M3 | 1.34 | ||
| M6 | 0.87 | ||
| M13 | 1.35 | ||
| M7 | 1.54 | ||
| M8 | 1.70 | ||
| M9 | 2.47 | ||
| M10 | 0.35 | ||
| M4 | 0.50 | ||
| M11 | 0.04 | ||
| v1 | 1.18 | ||
| CaCO3-III phase | 5.4–1.5 GPa | M5 | 1.63 |
| M1 | 1.33 | ||
| M2 | 1.23 | ||
| M3 | 2.87 | ||
| M6 | 4.73 | ||
| M7 | 6.37 | ||
| M8 | 4.83 | ||
| M9 | 8.87 | ||
| M10 | 2.30 | ||
| M4 | 3.27 | ||
| v4 | 3.30 | ||
| M11 | –0.70 | ||
| M12 | 2.23 | ||
| v1 | 3.27 | ||
| CaCO3-II phase | 1.5–0.4 GPa | M1 | 7.48 |
| M2 | 6.93 | ||
| M3 | 8.27 | ||
| M4 | 9.73 | ||
| v4 | 10.26 | ||
| v1 | 5.59 | ||
| CaCO3-I phase | 0.4 GPa−1 atm | T | 3.62 |
| L | 1.21 | ||
| v4 | 4.12 | ||
| v1 | 3.72 |
| Phase Transition | Pressure (GPa) | Method | Reference |
|---|---|---|---|
| CaCO3-I to CaCO3-II phases | 1.6 | Raman spectroscopy | This study |
| 1.6 | Raman spectroscopy | [9] | |
| 1.6 | Raman spectroscopy | [10] | |
| 1.4 | Raman spectroscopy | [11] | |
| 1.7 | Brillouin spectroscopy | [45] | |
| 1.5 | Synchrotron X-ray diffraction | [44] | |
| CaCO3-II to CaCO3-III phases | 2.3 | Electrical conductivity | This study |
| 2.2 | Raman spectroscopy | This study | |
| 2.0 | Raman spectroscopy | [9] | |
| 2.0 | Raman spectroscopy | [10] | |
| 2.0 | Raman spectroscopy | [12] | |
| 2.1 | Raman spectroscopy | [11] | |
| 2.2 | Brillouin spectroscopy | [45] | |
| 2.5 | Synchrotron X-ray diffraction | [44] | |
| CaCO3-III to CaCO3-VI phases | 15.5 | Electrical conductivity | This study |
| 16.8 | Raman spectroscopy | This study | |
| 16.0 | Raman spectroscopy | [12] | |
| 15.5 | Infrared spectroscopy | [39] | |
| 15.0 | Synchrotron X-ray diffraction | [44] |
| P (GPa) | T (K) | Phase | σ0 (S/cm) | ΔH (eV) | R2 |
|---|---|---|---|---|---|
| 10.5 | 323–573 | CaCO3-III | 5.89 × 101 | 5.48 ± 0.91 | 87.57 |
| 10.5 | 723–873 | CaCO3-VI | 2.04 × 102 | 10.26 ± 2.71 | 82.08 |
| 12.5 | 323–473 | CaCO3-III | 8.91 × 10−5 | 1.62 ± 0.27 | 92.07 |
| 12.5 | 623–873 | CaCO3-VI | 3.24 × 10−2 | 5.90 ± 0.42 | 97.54 |
| 13.8 | 323–423 | CaCO3-III | 1.73 × 10−3 | 2.44 ± 1.01 | 70.46 |
| 13.8 | 523–873 | CaCO3-VI | 1.77 × 100 | 6.55 ± 0.36 | 97.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Dai, L.; Hu, H.; Li, C. Pressure-Induced Reverse Structural Transition of Calcite at Temperatures up to 873 K and Pressures up to 19.7 GPa. Minerals 2023, 13, 188. https://doi.org/10.3390/min13020188
Zhang X, Dai L, Hu H, Li C. Pressure-Induced Reverse Structural Transition of Calcite at Temperatures up to 873 K and Pressures up to 19.7 GPa. Minerals. 2023; 13(2):188. https://doi.org/10.3390/min13020188
Chicago/Turabian StyleZhang, Xinyu, Lidong Dai, Haiying Hu, and Chuang Li. 2023. "Pressure-Induced Reverse Structural Transition of Calcite at Temperatures up to 873 K and Pressures up to 19.7 GPa" Minerals 13, no. 2: 188. https://doi.org/10.3390/min13020188
APA StyleZhang, X., Dai, L., Hu, H., & Li, C. (2023). Pressure-Induced Reverse Structural Transition of Calcite at Temperatures up to 873 K and Pressures up to 19.7 GPa. Minerals, 13(2), 188. https://doi.org/10.3390/min13020188









