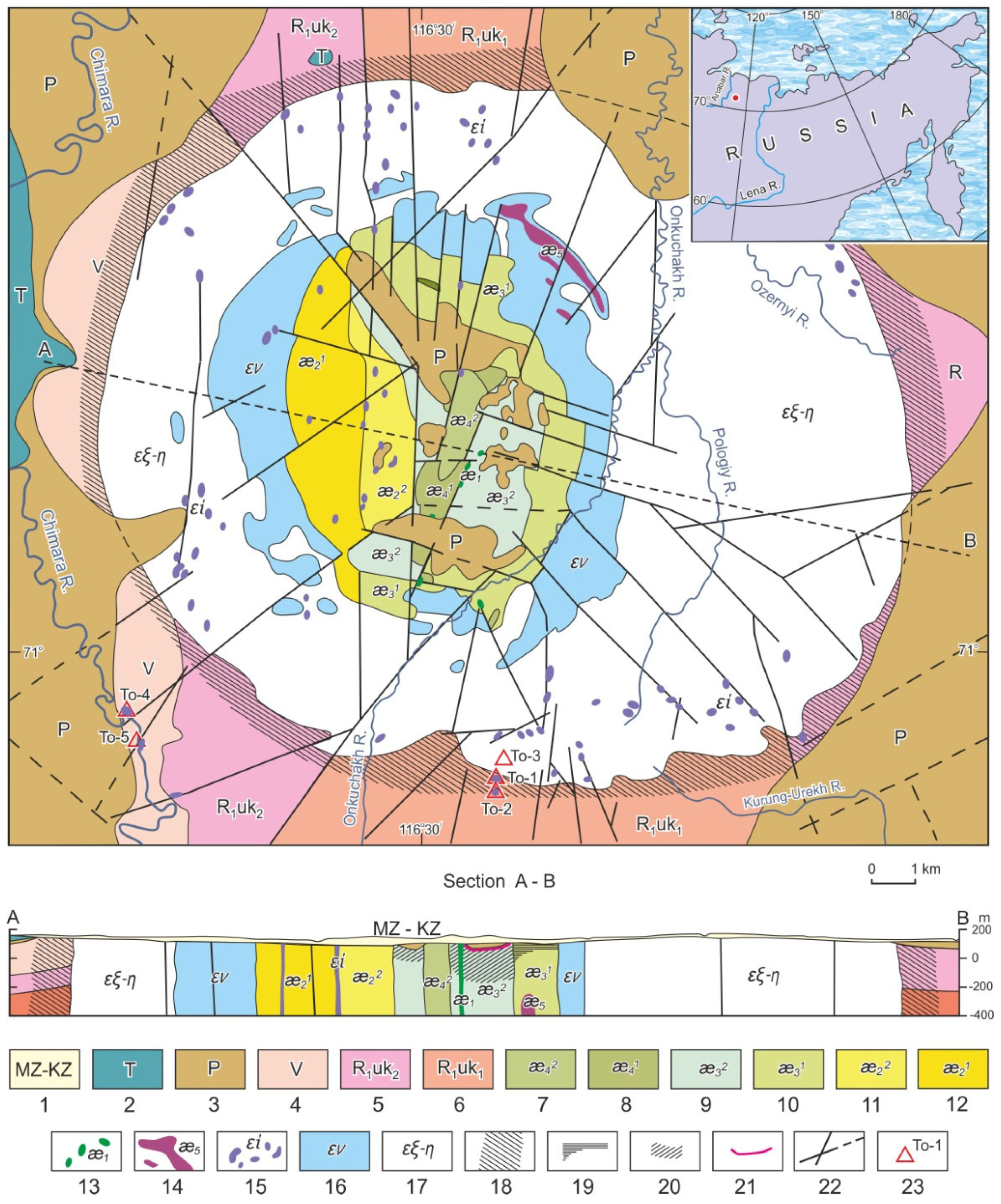
Mineralogical and Geochemical Evidence of Paragenetic Unity of Igneous Silicate and Carbonatite Rocks of the Tomtor Massif in the North-East of the Siberian Platform
Abstract
:1. Introduction
2. Materials and Methods
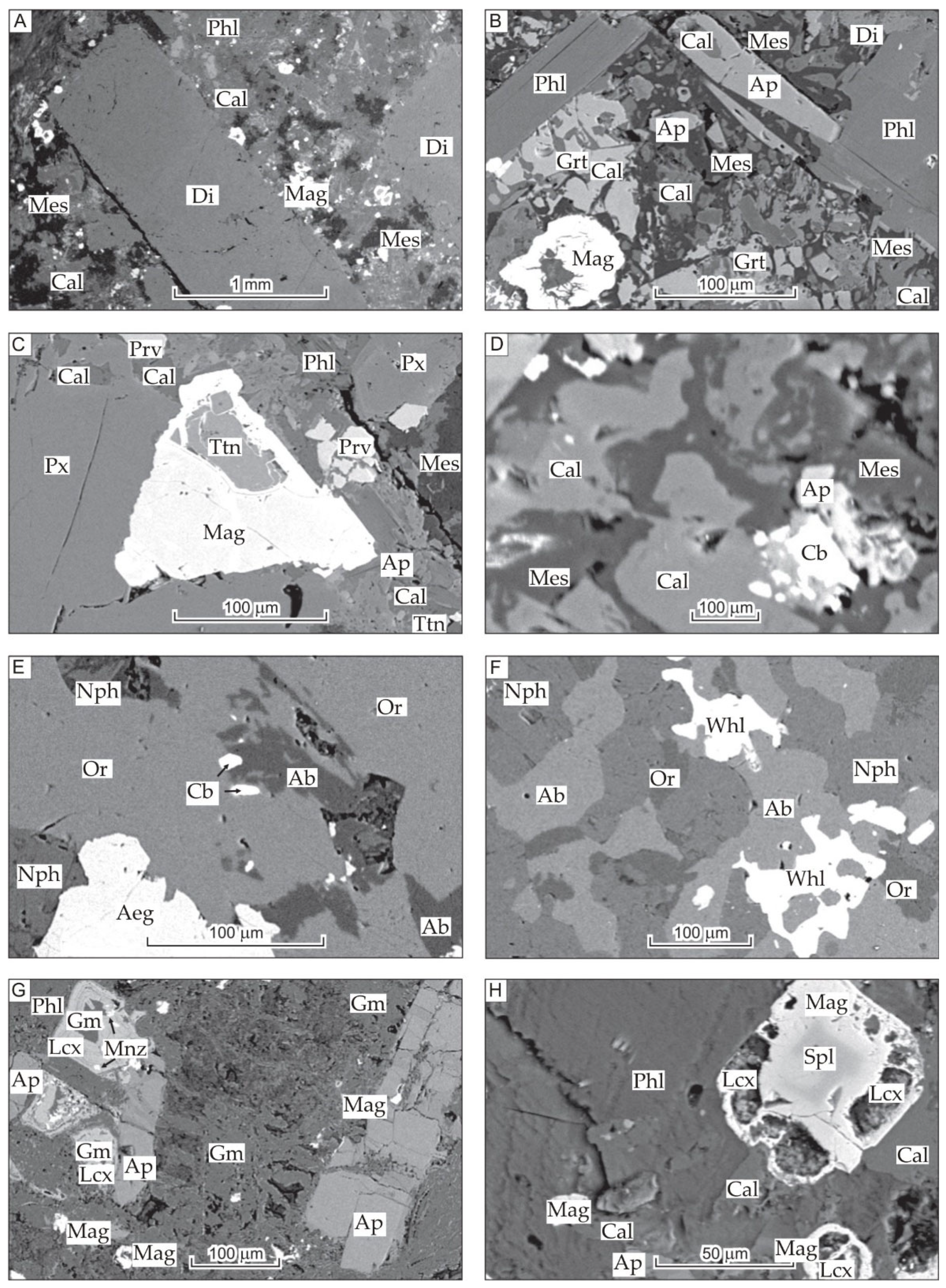
3. Chemical and Mineralogical Features of Tomtor Massif Rocks

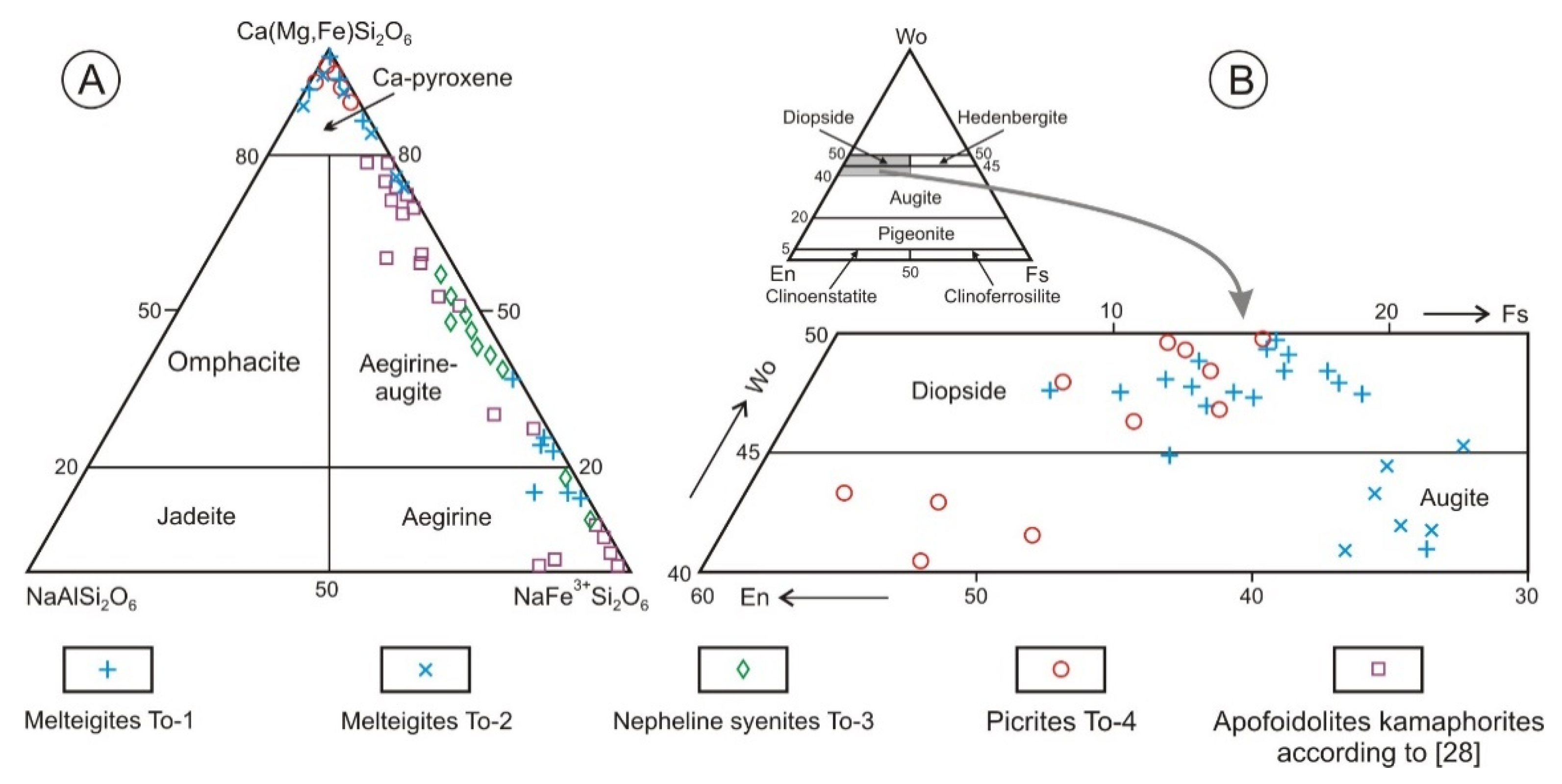
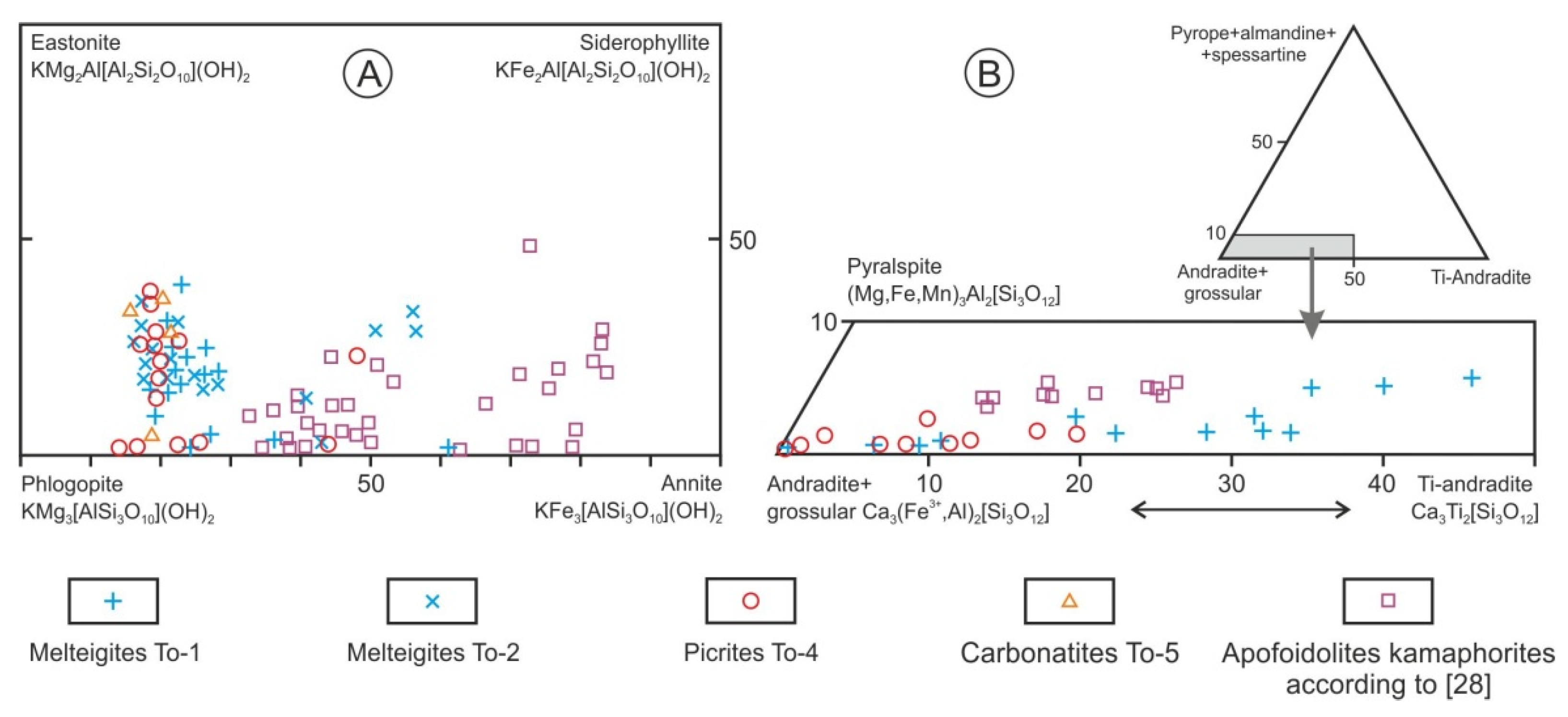
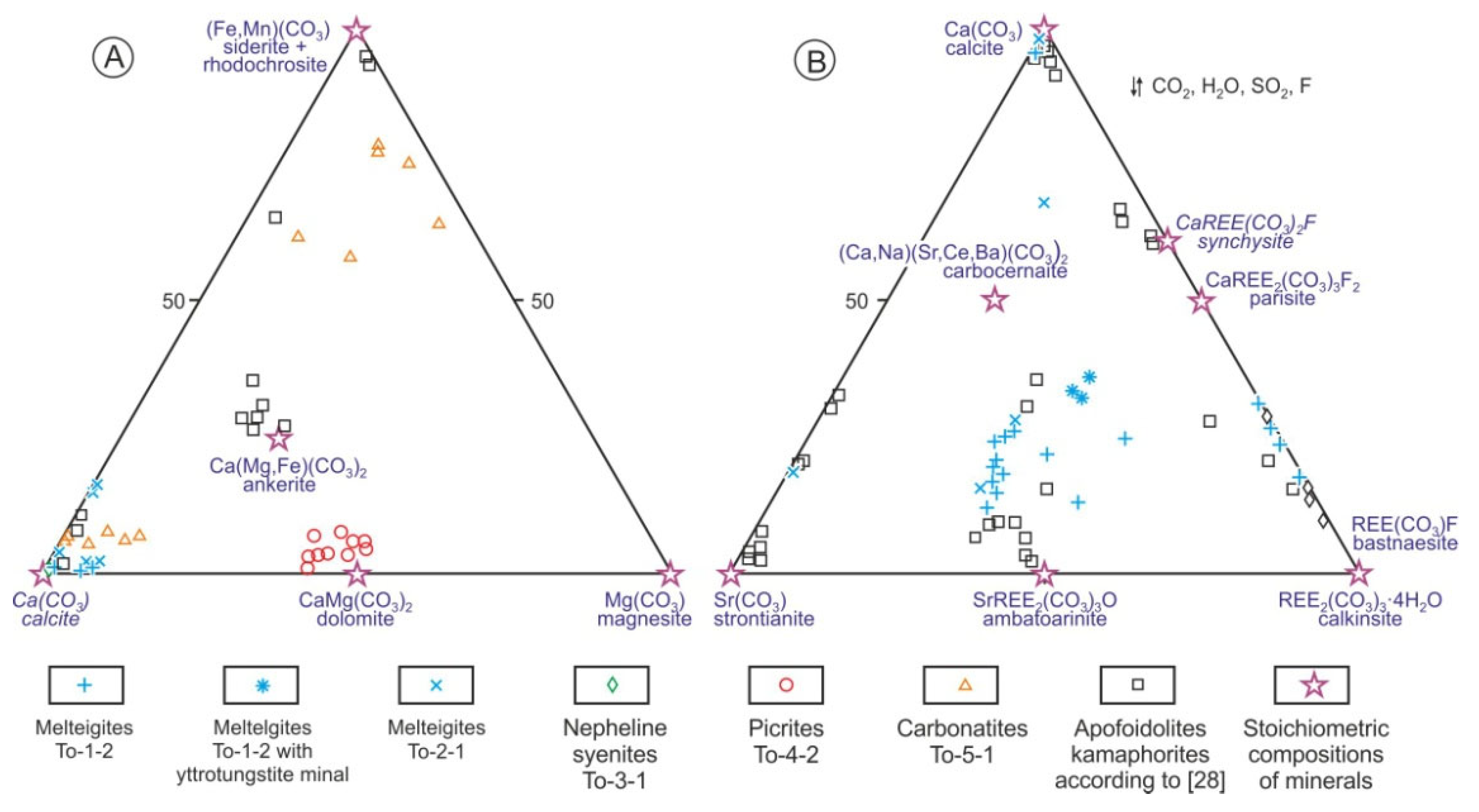
4. Discussion
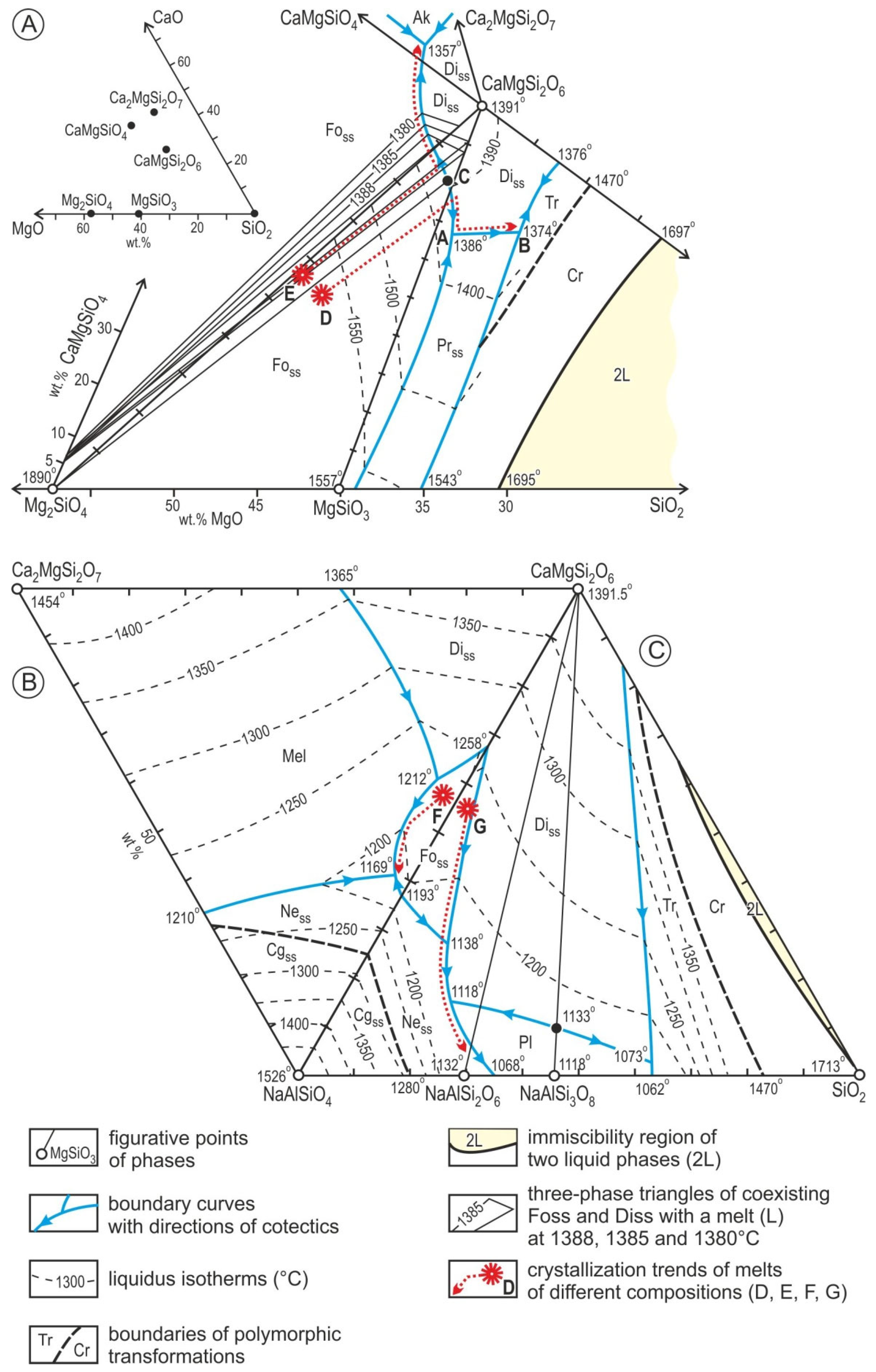
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | P2O5 | H2O+ | CO2 | p.p.p. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| To-1-2 | 33.72 | 3.23 | 11.36 | 7.86 | 3.37 | 0.23 | 7.44 | 16.75 | 3.40 | 2.33 | 0.89 | 4.54 | 3.76 | 0.69 | 99.57 |
| To-2-1 | 31.70 | 2.73 | 11.79 | 5.19 | 4.83 | 0.32 | 5.75 | 15.65 | 4.84 | 2.91 | 1.26 | 5.71 | 5.95 | 0.46 | 99.09 |
| To-3-1 | 49.50 | 0.76 | 19.37 | 4.74 | 1.41 | 0.21 | 1.47 | 3.07 | 7.42 | 3.48 | 0.06 | 5.50 | 2.33 | 0.18 | 99.50 |
| To-4-2 | 31.25 | 2.87 | 8.38 | 10.29 | 5.82 | 0.18 | 15.18 | 11.12 | 0.63 | 2.57 | 1.22 | 4.45 | 5.37 | 0.65 | 99.98 |
| To-5-1 | 14.12 | 1.86 | 5.17 | 6.71 | 7.18 | 0.34 | 4.99 | 29.23 | 0.21 | 0.82 | 1.25 | 2.60 | 24.96 | 0.50 | 99.94 |
| Sample | Analysis | SiO2 | TiO2 | Al2O3 | Fe2O3 * | FeO | MnO | MgO | CaO | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diopside (12-183; 283), augite (23-2; 20-10; 287), aegirine (11-27; 4-7) | ||||||||||||
| To-1-2 | 12-183 | 44.93 | 2.74 | 6.57 | 1.23 | 6.09 | 0.09 | 13.02 | 24.43 | 0.48 | nd | 99.58 |
| To-1-2 | 23-2 | 46.78 | 2.64 | 2.66 | 4.70 | 13.11 | 0.10 | 8.51 | 20.18 | 1.82 | nd | 100.50 |
| To-1-2 | 11-27 | 49.91 | 1.72 | 1.66 | 30.08 | 0.00 | 0.08 | 1.76 | 2.71 | 12.41 | nd | 100.32 |
| To-2-1 | 20-10 | 49.59 | 1.76 | 2.05 | nd | 15.83 | 0.52 | 9.58 | 21.09 | 0.10 | nd | 100.52 |
| To-4-2 | 283 | 51.74 | 0.89 | 0.65 | 2.31 | 6.01 | 0.30 | 12.76 | 24.09 | 0.90 | nd | 99.64 |
| To-4-2 | 287 | 50.49 | 1.62 | 2.65 | 2.59 | 5.43 | 0.21 | 13.79 | 20.62 | 1.22 | nd | 98.63 |
| To-3-1 | 4-7 | 51.41 | 0.23 | 0.40 | 17.70 | 8.75 | 0.12 | 2.21 | 12.65 | 6.86 | nd | 100.33 |
| Phlogopite (11-5; 3-7; 23-10), annite (11-11; 18-183), muscovite (20-3) | ||||||||||||
| To-1-2 | 11-5 | 39.34 | nd | 11.40 | 1.61F ** | 12.28 | nd | 20.16 | nd | nd | 10.44 | 95.22 |
| To-1-2 | 11-11 | 37.73 | nd | 11.04 | nd | 28.38 | nd | 9.95 | nd | nd | 9.80 | 96.90 |
| To-4-2 | 3-7 | 37.13 | 6.32 | 15.12 | nd | 8.52 | nd | 19.80 | nd | nd | 9.18 | 96.07 |
| To-4-2 | 18-183 | 40.80 | 0.93 | 11.03 | nd | 20.25 | 0.35 | 12.21 | 0.54 | 0.34 | 8.86 | 95.30 |
| To-5-1 | 23-10 | 37.25 | 5.08 | 16.72 | nd | 9.78 | nd | 19.59 | nd | nd | 8.98 | 97.40 |
| To-3-1 | 20-3 | 45.90 | nd | 36.02 | nd | nd | nd | nd | nd | 3.89 | 7.30 | 93.11 |
| Mesolite (14-1), natrolite (23-5; 7-1), chlorite (26-1), montmorillonite (19-2) | ||||||||||||
| To-1-2 | 14-1 | 41.65 | nd | 31.19 | nd | nd | nd | nd | 5.19 | 11.31 | nd | 89.33 |
| To-1-2 | 23-5 | 46.62 | nd | 27.78 | nd | nd | nd | nd | 1.95 | 14.71 | nd | 91.06 |
| To-4-2 | 7-1 | 47.52 | nd | 27.34 | nd | nd | nd | nd | 0.32 | 16.42 | nd | 91.60 |
| To-5-1 | 26-1 | 33.91 | nd | 15.61 | nd | 13.42 | nd | 22.59 | nd | nd | 1.63 | 87.16 |
| To-5-1 | 19-2 | 54.69 | nd | 18.12 | nd | 9.76 | nd | 3.62 | 1.55 | nd | 0.88 | 88.62 |
| Nepheline (19-1), orthoclase (5-5), albite (5-7), hyalophane (5-12; 16-4) | ||||||||||||
| To-3-1 | 19-1 | 43.29 | nd | 32.84 | nd | nd | nd | nd | nd | 15.54 | 5.97 | 97.64 |
| To-3-1 | 5-5 | 64.25 | nd | 19.81 | nd | nd | nd | nd | nd | 1.29 | 14.75 | 100.10 |
| To-3-1 | 5-7 | 68.51 | nd | 20.20 | nd | nd | nd | nd | nd | 11.67 | 0.28 | 100.66 |
| To-3-1 | 5-12 | 47.31 | nd | 30.73 | 10.95BaO ** | nd | nd | nd | 1.18 | 7.52 | 1.08 | 98.77 |
| To-3-1 | 16-4 | 43.81 | nd | 28.27 | 19.84BaO ** | nd | nd | nd | nd | 4.05 | 2.20 | 98.17 |
| Andradite (14-5; 5-10), schorlomite (14-12; 10-8) | ||||||||||||
| To-1-2 | 14-5 | 34.86 | 3.24 | 2.23 | 26.21 | nd | nd | nd | 34.37 | nd | nd | 100.91 |
| To-1-2 | 14-12 | 33.36 | 12.56 | 2.21 | 18.29 | nd | nd | nd | 33.17 | nd | nd | 99.59 |
| To-4-2 | 5-10 | 34.16 | 2.95 | 14.01 | 12.17 | nd | nd | 1.84 | 35.01 | nd | nd | 100.14 |
| To-4-2 | 10-8 | 34.29 | 6.20 | nd | 26.01 | nd | nd | nd | 33.50 | nd | nd | 100.00 |
| Sample | Analysis | CaO | SrO | WO3 | Ce2O3 | La2O3 | Nd2O3 | Total | Mineral |
|---|---|---|---|---|---|---|---|---|---|
| To-1-2 | 29-2 | 3.66 | nd | nd | 29.17 | 20.25 | 7.84 | 60.92 | Calkinsite |
| To-1-2 | 20-2 | 6.43 | 11.75 | nd | 20.84 | 12.62 | 4.70 | 56.34 | Ambatoarinite |
| To-1-2 | 27-2 | 5.62 | 18.01 | nd | 21.51 | 12.36 | 7.61 | 65.11 | Ambatoarinite |
| To-1-2 | 22-6 | 5.60 | nd | 20.00 | 20.90 | 10.96 | 5.47 | 62.93 | La-Ce-tungstite |
| To-1-2 | 23-8 | 6.44 | nd | 18.70 | 22.31 | 13.80 | 5.38 | 66.63 | La-Ce-tungstite |
| To-2-1 | 2-2 | 20.32 | 9.06 | nd | 19.25 | 8.71 | nd | 57.34 | Carbocerianite |
| To-2-1 | 3-4 | 6.17 | 16.79 | nd | 27.84 | 13.31 | nd | 64.11 | Ambatoarinite |
| To-2-1 | 20-7 | 3.17 | 19.57 | nd | 19.01 | 10.21 | 8.79 | 60.75 | Ambatoarinite |
| To-3-1 | 3-7 | 1.59 | nd | nd | 31.22 | 22.37 | nd | 55.18 | Calkinsite |
| To-3-1 | 15-3 | 1.78 | nd | nd | 31.33 | 17.96 | 6.96 | 58.03 | Calkinsite |
| To-3-1 | 15-7 | 3.26 | nd | nd | 28.78 | 13.86 | 5.69 | 51.59 | Calkinsite |
| Sample | Analysis | TiO2 | Al2O3 | Cr2O3 | Fe2O3 | FeO | MnO | MgO | Total |
|---|---|---|---|---|---|---|---|---|---|
| To-4-l | 9-116 | 3.54 | 6.03 | 45.76 | 11.58 | 24.86 | 0.40 | 6.86 | 99.03 |
| To-4-1 | 3-116 | 3.58 | 6.20 | 42.14 | 16.17 | 21.18 | 0.48 | 9.18 | 98.93 |
| To-4-l | 16-116 | 0.55 | 11.71 | 35.46 | 21.47 | 20.00 | 0.36 | 8.60 | 98.15 |
| To-4-l | 18-116 | 7.45 | 5.36 | 30.58 | 19.67 | 28.31 | 0.49 | 6.58 | 98.44 |
| To 4-1 | 22-116 | 6.89 | 7.79 | 18.64 | 33.13 | 18.86 | 0.48 | 12.51 | 98.30 |
| To-4-1 | 285-183 | 9.19 | 6.36 | 7.56 | 36.86 | 31.97 | 0.94 | 4.66 | 97.54 |
| To-4-1 | 283-183 | 7.35 | 0.22 | 1.31 | 54.10 | 28.61 | 0.80 | 5.03 | 97.42 |
| To-5-1 | 9-179 | 2.12 | 14.68 | 43.26 | 9.00 | 16.97 | 0.44 | 11.94 | 98.41 |
| To-5-1 | 20-1 | 2.28 | 23.67 | 31.91 | 10.71 | 16.25 | 0.32 | 13.74 | 98.88 |
| To-5-1 | 23-4 | 5.31 | 21.72 | 23.84 | 14.68 | 21.62 | 0.55 | 11.88 | 99.60 |
| To-5-1 | 4-5 | 5.38 | 5.01 | 17.12 | 36.14 | 29.85 | 1.12 | 4.35 | 98.97 |
| To-5-1 | 20-9 | 12.03 | 0.37 | 1.11 | 45.80 | 35.92 | 1.55 | 2.99 | 99.77 |
References
- Epshtein, E.M.; Anikeeva, L.I. Some Issues of Geology and Petrology of the Complex of Ultrabasic Alkaline Intrusive Rocks. Phys. Chem. Probl. Form. Rocks Ores. V 1963, 2, 182–195. (In Russian) [Google Scholar]
- Entin, A.R.; Zaitsev, A.I.; Nenashev, N.I.; Vasilenko, V.B.; Orlov, A.I.; Tyan, O.A.; Ol’khovick, Y.A.; Ol’shtinky, S.I.; Tolstov, A.V. On the Sequence of Geological Events Associated with the Intrusion of the Tomtor Massif of Ultramafic Alkaline Rocks and Carbonatites (North-Western Yakutia). Geol. Geophys. 1990, 31, 42–51. (In Russian) [Google Scholar]
- Kravchenko, S.M.; Pokrovskii, B.G. The Tomtor Alkaline Ultrabasic Massif and Related REE-Nb Deposits. Northern Siberia. Econ. Geol. 1995, 71, 676–689. [Google Scholar] [CrossRef]
- Tolstov, A.V. Mineralogical and Geochemical Features of Apatite-Magnetite Ores of Tomtor Massif (North-Western Yakutia). Geol. Geophys. 1994, 35, 91–100. (In Russian) [Google Scholar]
- Tolstov, A.V.; Tyan, O.A. Gology and Ore Potential of Tomtor Massif; YSC SB RAS: Yakutsk, Russia, 1999. (In Russian) [Google Scholar]
- Bagdasarov, Y.A. Geochemical Features of Carbonatites and Related Silicate Rocks of Alkaline-Carbonatite Tomtor Massif (Eastern Prianabarye. Yakutia). Geochemistry 1997, 35, 10–20. (In Russian) [Google Scholar]
- Panina, L.I.; Rokosova, E.Y.; Isakova, A.T.; Tolstov, A.V. Mineral composition of alkaline lamprophyres of the Tomtor massif as reflection of their genesis. Russ. Geol. Geophys. 2017, 58, 887–902. [Google Scholar] [CrossRef]
- Okrugin, A.V.; Tolstov, A.V.; Sleptsov, A.P.; Baranov, L.N. Petrochemical Features of the Association of Ultrabasic Alkali Rocks and Carbonatites of the Tomtor Massif and Interpretation of Possible Trends of Their Evolution. Arct. Subarct. Nat. Resour. 2019, 24, 7–24. (In Russian) [Google Scholar] [CrossRef]
- Vladykin, N.V.; Kotov, A.B.; Borisenko, A.S.; Yarmolyk, V.V.; Pokhilenko, N.P.; Salnikova, E.B.; Travin, A.V.; Yakovlev, S.Z. Age Boundaries of the Formation of the Tomtor Alkaline-Ultrabasic Massif: Results of Geochronological U-Pb and 40Ar/39Ar Studies. Dokl. Earth Sci. 2014, 454, 195–199. (In Russian) [Google Scholar] [CrossRef]
- Skublov, S.G.; Tolstov, A.V.; Baranov, L.N.; Melnik, A.E.; Levashova, E.V. First Data on the Geochemistry and U-Pb Age of Zircons from the Kamaphorites of the Tomtor Alkaline-Ultrabasic Massif. Arctic Yakutia. Geochemistry 2020, 80, 125505. (In Russian) [Google Scholar] [CrossRef]
- Lazareva, E.V.; Zhmodik, S.M.; Dobretsov, N.L.; Tolstov, A.V.; Shcherbov, B.L.; Karmanov, N.S.; Gerasimov, E.Y.; Bryanskaya, A.V. Major minerals of abnormally high-grade ores of the Tomtor deposit (Arctic Siberia). Russ. Geol. Geophys. 2015, 56, 844–873. [Google Scholar] [CrossRef]
- Okrugin, A.V.; Zaitsev, A.I.; Borisenko, A.S.; Zemnukhov, A.L.; Ivanov, P.O. Gold-Platinum Placers of the Basin of the Anabar River and Their Possible Relationship with Alkaline-Ultrabasic Magmatites of the North of the Siberian Platform. Otechestvennaya Geol. 2012, 11–21. (In Russian) [Google Scholar]
- Okrugin, A.; Gerasimov, B. Paragenetic Association of Platinum and Gold Minerals in Placers of the Anabar River in the Northeast of the Siberian Platform. Minerals 2023, 13, 96. [Google Scholar] [CrossRef]
- Okrugin, A.V.; Yakubovich, O.V.; Ernst, R.E.; Druzhinina, Z.Y. Platinum-bearing placers: Mineral associations and their 190Pt-4He and Re-Os ages, and potential links with large igneous provinces in the Siberian craton. Econ. Geol. 2020, 115, 1835–1853. [Google Scholar] [CrossRef]
- Okrugin, A.V.; Zhuravlev, A.I. Mineralogical Criteria for Genetic Relationship of Igneous and Carbonatite Rocks of the Tomtor Massif (Siberian Platform). IOP Conf. Ser. Earth Environ. Sci. 2021, 906, 012104. [Google Scholar] [CrossRef]
- Schairer, J.F.; Yoder, H.S. Crystal State and Melting of Simple Alkaline Basalts. In Experimental Petrology and Mineralogy; Makeev, V.I., Ed.; Nedra: Moscow, Russia, 1971; pp. 6–15. (In Russian) [Google Scholar]
- Sheinmann, U.M. Ultrabasic-Alkaline Rocks Formation. In Alkaline Intrusions. Their Location and Related Mineralization; Gosgeoltehizdat: Moscow, Russia, 1961; pp. 15–54. (In Russian) [Google Scholar]
- Milashev, V.A.; Krutoyarsky, M.A.; Rabkin, M.I.; Erlikh, E.N. Kimberlites and Picrite Porphyry of North-Eastern Part of Siberian Platform; Gosgeoltehizdat: Moscow, Russia, 1963. (In Russian) [Google Scholar]
- Koval’sky, V.V.; Nikishov, K.N.; Egorov, O.S. Kimberlites and Carbonatites of Anabar Anteclise; Nauka: Moscow, Russia, 1969. (In Russian) [Google Scholar]
- Egorov, L.S. Melilites of Maymecha-Kotuy Province; Nedra: Saint Petersburg, Russia, 1969. (In Russian) [Google Scholar]
- Marshintsev, V.K. Carbonatite Bodies of Eastern Slope of Anabar Uplift; Kn. izd-vo: Yakutsk, Russia, 1974. (In Russian) [Google Scholar]
- Frolov, A.A.; Lapin, A.V.; Tolstov, A.V.; Zinchuk, N.N.; Belov, S.V.; Burgomistrov, A.A. Carbonatites and Kimberlites (Relationship. Minerageny. Prospecting); NIA-Priroda: Moscow, Russia, 2005. (In Russian) [Google Scholar]
- Vladykin, N.V.; Torbeeva, T.S. Lamproites of Tomtor Massif (Eastern Preanabarie). Geol. Geophys. 2005, 46, 1038–1049. (In Russian) [Google Scholar]
- Belov, S.V.; Lapin, A.V.; Tolstov, A.V.; Frolov, A.A. Minerageny of Platform Magmatism (Trapps. Carbonatites. Kimberlites); SB RAS: Novosibirsk, Russia, 2008. (In Russian) [Google Scholar]
- Holmes, A. A Contribution to the Petrology of the Kimberlite and Its Inclutions. Geol. Soc. S. Afr. 1936, 39, 379–428. [Google Scholar]
- Zhuk-Pochekutov, K.A.; Gladkikh, V.S.; Leontiev, L.N. Association of Alkaline Basaltoids–Basalts of the Maymecha-Kotuy Volcanoplutonic Formation. In Petrology and Geochemical Features of Complex Ultrabasites and Carbonatites; Nauka: Moscow, Russia, 1965; pp. 5–90. (In Russian) [Google Scholar]
- Vasiliev, U.R.; Zolotukhin, V.V. Petrology Af Ultrabasites of North of Siberian Platform and Their Some Genesis Issues; Nauka: Novosibirsk, Russia, 1975. (In Russian) [Google Scholar]
- Baranov, L.N.; Tolstov, A.V.; Okrugin, A.V.; Slepcov, A.P. New Data about Mineralogy and Geochemistry of Apatite-Magnetite Ores of the Tomtor Massif. North-East of the Siberian Platform. Ores Met. 2018, 42–54, 468. (In Russian) [Google Scholar] [CrossRef]
- Vasiliev, U.R.; Kononenko, V.F.; Korolyuk, V.N. Accessory Chrome Spinels from Ultramafic Rocks of Maymecha-Kotuy Region. In Genetic and Experimental Mineralogy Materials; Nauka: Novosibirsk, Russia, 1976; pp. 7–16. (In Russian) [Google Scholar]
- Genkin, A.D.; Distler, V.V.; Laputina, I.P. Chromite Mineralization of Differentiated Trapp Intrusions and It’s Formation Parameters. In Formation Conditions of Magmatic Ore Deposits; Nauka: Moscow, Russia, 1979; pp. 105–126. (In Russian) [Google Scholar]
- Chayka, I.F.; Zhitova, L.M.; Antsiferova, T.N.; Abersteiner, A.; Shevko, A.Y.; Izokh, A.E.; Tolstykh, N.D.; Gora, M.P.; Chubarov, V.M.; Kamenetsky, V.S. In-Situ Crystallization and Continuous Modification of Chromian Spinel in the “Sulfide-Poor Platinum-Group Metal Ores” of the Norilsk-1 Intrusion (Northern Siberia. Russia). Minerals 2020, 10, 498. [Google Scholar] [CrossRef]
- Babushkina, S.A. Microcrystal Composition of Spinelids of Malokuonapskaya Pipe. Otechestvennaya Geol. 2008, 906, 85–95. (In Russian) [Google Scholar]
- Sobolev, N.V.; Pokhilenko, N.P.; Lavrentiev, Y.G.; Usova, L.V. Composition Features of Chrome Spinels from Diamonds and Kimberlites of Yakutia. Geol. Geophys. 1975, 16, 7–24. (In Russian) [Google Scholar]
- Afanasiev, V.P.; Zinchuk, N.N.; Pokhilenko, N.P. Morphology and Morphogenesis of Indicator Minerals of Kimberlites; GEO of SB RAS Publishing House: Novosibirsk, Russia, 2001. (In Russian) [Google Scholar]
- Muan, A.; Huack, J.; Loffal, T. Equilibrium Studies with a Bearing on Lunar Rocks. In Proceedings of the Third Lunar Science Conference; Lunar and Planetary Science Institute: Houston, TX, USA, 1972; pp. 158–196. [Google Scholar]
- Kushiro, I.; Schairer, J.F. New Data of Diopside-Forsterite-Silica System. In Experimental Petrology and Mineralogy; Nedra: Moscow, Russia, 1969; pp. 52–62. [Google Scholar]
- Onuma, K.; Yagi, K. The System Diopside-Akermanite-Nepheline. Am. Mineral. 1967, 52, 227–243. [Google Scholar]
- Schairer, J.F.; Yoder, H.S. The Nature of Residual Liquids from Crystallization. with Data on the System Nepheline-Diopside-Silica. Am. J. Sci. 1960, 258-A, 273–283. [Google Scholar]
- Shkodzinsky, V.S. Global Petrology According Modern Data of Hot Heterogenous Earth Accretion; NEFU Publishing House: Yakutsk, Russia, 2018. (In Russian) [Google Scholar]
- Wyllie, P.J. Problem of Formation Carbonatites in Relation with Experimental Data. Origin and Differentiation of Carbonatite Magma. In Carbonatites; MIR: Moscow, Russia, 1969; pp. 265–300. [Google Scholar]
- Solovova, I.P.; Girnis, A.V.; Kogarko, L.N.; Kononova, N.N.; Stoppa, F.; Rosatelli, G. Composition of Magmas and Carbonate–Silicate Liquid Immiscibility in the Vulture Alkaline Igneous Complex. Italy Lithos 2005, 85, 113–128. (In Russian) [Google Scholar] [CrossRef]
- Wyllie, P.J.; Lee, W.J. Kimberlites, Carbonatites, Peridotites and Silicate-Carbonate Liquid Immiscibility Explained in Parts of the System CaO-(Na2O+K2O)-(MgO+FeO)-(SiO2+Al2O3)-CO2. In Proceedings of the VIIth International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; pp. 923–932. [Google Scholar]
- Kapustin, Y.L. Differentiated Sill of Basaltoids and Excretion Features in It of Calcite. Zapiski VMO 1985, 275–288. (In Russian) [Google Scholar]
- Panina, L.I.; Motorina, I.V. Liquid Immiscibility of Deep Magmas and Nucleation of Carbonatite Melts. Geochemistry 2008, 46, 487–504. (In Russian) [Google Scholar]
- Delicin, L.M.; Melentiev, G.B.; Batenin, V.M.; Tolstov, A.V. The Coexistence of Two Immiscible Liquid Phases in Silicate-Salt Niobium-Rare Earth System. Dokl. RAS 2014, 462, 440–443. (In Russian) [Google Scholar]
- Ernst, R.E.; Hamilton, M.A.; Söderlund, U.; Hanes, J.A.; Gladkochub, D.P.; Okrugin, A.V.; Kolotilina, T.; Mekhonoshin, A.S.; Bleeker, W.; LeCheminant, A.N.; et al. Long-Lived Connection between Southern Siberia and Northern Laurentia in the Proterozoic. Nat. Geosci. 2016, 9, 464–469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okrugin, A.; Zhuravlev, A. Mineralogical and Geochemical Evidence of Paragenetic Unity of Igneous Silicate and Carbonatite Rocks of the Tomtor Massif in the North-East of the Siberian Platform. Minerals 2023, 13, 211. https://doi.org/10.3390/min13020211
Okrugin A, Zhuravlev A. Mineralogical and Geochemical Evidence of Paragenetic Unity of Igneous Silicate and Carbonatite Rocks of the Tomtor Massif in the North-East of the Siberian Platform. Minerals. 2023; 13(2):211. https://doi.org/10.3390/min13020211
Chicago/Turabian StyleOkrugin, Alexander, and Anatolii Zhuravlev. 2023. "Mineralogical and Geochemical Evidence of Paragenetic Unity of Igneous Silicate and Carbonatite Rocks of the Tomtor Massif in the North-East of the Siberian Platform" Minerals 13, no. 2: 211. https://doi.org/10.3390/min13020211









